Abstract
Background
Although depression is the leading cause of disability worldwide, its pathophysiology is poorly understood. Our previous study showed that hippocampal peroxisome proliferator-activated receptor δ (PPARδ) overexpression displays antidepressive effect and enhances hippocampal neurogenesis during chronic stress. Herein, we further extended our curiosity to investigate whether downregulating PPARδ could cause depressive-like behaviors through downregulation of neurogenesis.
Methods
Stereotaxic injection of lentiviral vector, expressing short hairpin RNA complementary to the coding exon of PPARδ, was done into the bilateral dentate gyri of the hippocampus, and the depression-like behaviors were observed in mice. Additionally, hippocampal neurogenesis, brain-derived neurotrophic factor and cAMP response element-binding protein were measured both in vivo and in vitro.
Results
Hippocampal PPARδ knockdown caused depressive-like behaviors and significantly decreased neurogenesis, neuronal differentiation, levels of mature brain-derived neurotrophic factor and phosphorylated cAMP response element-binding protein in the hippocampus. In vitro study further confirmed that PPARδ knockdown could inhibit proliferation and differentiation of neural stem cells. Furthermore, these effects were mimicked by repeated systemic administration of a PPARδ antagonist, GSK0660 (1 or 3 mg/kg i.p. for 21 d).
Conclusions
These findings suggest that downregulation of hippocampal PPARδ is associated with depressive behaviors in mice through an inhibitory effect on cAMP response element-binding protein/brain-derived neurotrophic factor-mediated adult neurogenesis in the hippocampus, providing new insights into the pathogenesis of depression.
Keywords: depression, PPARδ, hippocampus, neurogenesis, BDNF
Significance Statement.
Hippocampal peroxisome proliferator-activated receptor δ (PPARδ) downregulation by genetic manipulation or pharmacological blockade induces depression-related behaviors, which is correlated with BDNF-CREB-associated neurogenesis and neuronal differentiation in the hippocampus. Downregulating PPARδ inhibits proliferation of neuronal stem cells and their differentiation into neurons. These in vivo and in vitro data strongly suggest that PPARδ plays a crucial role in neurogenesis and regulates both depression and memory.
Introduction
Depression is a common disorder worldwide, associated with an increased risk of suicide, impaired social skills, and social withdrawal (Rosenström and Jokela, 2017). Although many advances have been made in understanding the neurobiology of this complex disorder, the pathophysiological mechanisms are still unclear. Accumulating studies have supported a strong association between adult hippocampal neurogenesis, the formation of new neurons in the dentate gyrus (DG) of the adult brain, and depression (Serafini et al., 2014; Schoenfeld and Cameron, 2015). People with depression often display decreased hippocampal neurogenesis that results in hippocampal atrophy (Small et al., 2011; Fotuhi et al., 2012). Stress suppresses hippocampal neurogenesis, which can be reversed by antidepressant treatments (Dranovsky and Hen, 2006; Li et al., 2009; Boldrini et al., 2012; Schoenfeld and Gould, 2012). Inhibiting hippocampal neurogenesis blocks some behavior-modulatory effects of antidepressants (Santarelli et al., 2003), which suggests that neurogenesis might be critical for antidepressant action.
It is well known that neurotrophins serve as important regulators of depression. Brain-derived neurotrophic factor (BDNF) is the most extensively studied neurotrophin, which is upregulated in the hippocampus by antidepressant treatment and is sufficient to produce antidepressant-like effects (Wang et al., 2008; Taliaz et al., 2010; Son et al., 2012). BDNF mediates its effects by activating several intracellular pathways, such as the mitogen-activated protein kinases and/or extracellular-regulated kinase cascade (Peng et al., 2008; Xiao et al., 2011), thus leading to an increase in cAMP-response element binding protein (CREB) and promoting B-cell lymphoma-2 (Bcl-2) synthesis. Moreover, CREB is able to modify BDNF and Bcl-2 transcriptions. An increase in the BDNF/CREB/Bcl-2 regulatory pathways underlies the molecular basis for the improvement of neurogenesis, synaptic plasticity, memory, and mood (Li et al., 2009; Mariga et al., 2017).
Peroxisome proliferator-activated receptor δ (PPARδ, aka PPARβ) is one of the 3 known PPARs (the others are PPARα and PPARγ), which are part of the nuclear receptor superfamily of transcription factors. PPARδ is a critical regulator of diverse biological processes, including maintenance of lipid and glucose homeostasis, inflammation, cell proliferation, and differentiation (Feige et al., 2006; Straus and Glass, 2007; Yu et al., 2014). Interestingly, in addition to the peripheral organs, PPARδ is also expressed throughout the brain, with particularly high levels in the hippocampus, entorhinal cortex, and hypothalamus (Woods et al., 2003; Hiqashiyama et al., 2007). Neuronal expression of this subtype is relatively higher compared with that of PPARα and PPARγ (Lemberger et al., 1996). To date, the neuroprotective benefits of PPARδ agonists have been reported in several experimental models of stroke (Arsenijevic et al., 2006; Pialat et al., 2007), Alzheimer’s disease (Kalinin et al., 2009), Parkinson’s disease (Martin et al., 2013; Das et al., 2014), autoimmune encephalomyelitis (Polak et al., 2005), and spinal cord injury (Paterniti et al., 2010). Our previous study has found that chronic stress, a known risk factor for depression, could decrease the expression of PPARδ in the hippocampus, and overexpression of hippocampal PPARδ could produce antidepressant-like effects, as observed in the chronic mild stress and learned helplessness paradigms (Ji et al., 2015). Herein, we further extended our curiosity to investigate the effects of hippocampal PPARδ downregulation on mood-related behaviors and neurogenesis in vivo or in vitro.
MATERIALS AND METHODS
Animals
Male ICR mice (18–22 g, 6–8 weeks) (Yangzhou University Medical Center, Yangzhou, China) were housed under controlled temperature, humidity, and lighting (22°C ± 2°C, 55% ± 5%, and a 12-h-light/-dark cycle with lights on at 7:00 am), with food and water freely available unless otherwise noted. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Laevis and Tropicials, 1996) and approved by the Animal Care and Use Committee of China Pharmaceutical University.
Lentivirus Generation
Lentiviral miRNA-mediated knockdown (Brummelkamp et al., 2002; Yu et al., 2002; Stegmeier et al., 2005) was used to silence the PPARδ gene. We generated lentiviral vector constructs expressing short hairpin RNA (shRNA) complementary to the coding exon of mice PPARδ tagged with a fused enhanced green fluorescent protein (EGFP) and named it as LV-PPARδ-shRNA-EGFP. We also generated a lentiviral vector expressing EGFP alone (LV-EGFP). The sequence for the PPARδ miRNA (shRNA-mir hairpin structure) was 5ʹ-tgcTAAAGAAGACGGAGAGTGACTCGAGTCACTCTCCGTCTTCTTTA Gttttttc-3ʹ. The bold sequence (first 19 nucleotide) is the antisense target sequence. The final sequence (italics) represents the sense target sequence. The normal control sequence form GeneChem was 5ʹ-ttcTCCGAACGTGTCACGT CTCGAGACGTGACACGTTCGGAGA Atttttg-3ʹ. In both PPARδ shRNA and normal control sequences, the middle 6 nucleotide (underlined) were hairpin loops. The coding sequence of PPARδ shRNA was amplified by polymerase chain reaction (PCR). The primer sequences were as follows: 5ʹ-GCCCCGGTTAATTTGCATAT-3’ (forward) and 5ʹ-GAGGCCAGATCTTGGGTG-3ʹ (reverse). The PCR fragments and the GV118 vector (U6-MCS-Ubi-EGFP) plasmid were digested with Age I and ligated with Age I to produce GV118-PPARδ-shRNA-EGFP. The plasmid was used to transform DH5α Escherichia coli for identification. For recovery of recombinant lentivirus-PPARδ-shRNA-EGFP (LV-PPARδ-shRNA-EGFP), HEK293 cells were co-transfected with 20 μg of the GV118 plasmid with a cDNA encoding PPARδ-shRNA and 15 μg pHelper 1.0 and 10 μg pHelper 2.0 plasmid to generate the recombinant lentivirus (LV), and LV-PPARδ-shRNA-EGFP. After 48 hours, the supernatant was harvested from HEK293 cells. The virus amplification was repeated thrice and the supernatant was filtered through a 0.45-μm filter. After resuspension, serially diluted LV was used to transfect HEK293 cells. Seven days later, labeled HEK293 cells were counted to calculate the viral titer (8 × 108 TU/mL). All of the lentiviral vectors contained the EGFP as a reporter to track LV-mediated expression using fluorescence microscopy.
Animal Surgery and LV Microinjection
Mice were anesthetized with chloral hydrate (350 mg/kg, i.p.) and placed on a stereotaxic device. A 30-gauge infusion cannula was inserted into the dorsal/ventral DG (dorsal: 1.5 mm posterior to bregma, 1.0 mm lateral to the midline, and 1.7 mm below dura; ventral: 3.0 mm posterior to bregma, 2.0 mm lateral to the midline, and 1.9 mm below dura) with 2 injection sites on each side (Kheirbek et al., 2013). LV (2 × 109 TU/μL, 2 μL/side) containing PPARδ-shRNA with or without the EGFP was infused (0.2 μL/min) using a micro-injection pump (CMA402 Suringo Pump, Dakumar Machinery). Injectors were left intact for 5 minutes in place after completing the injection to ensure complete diffusion from the syringe tip. Behavioral tests and immunostaining assays were performed on the 3rd week after the LV transfection or after repeated systemic administration of the PPARδ antagonist GSK0660 (1 or 3 mg/kg i.p. once daily) for 21 days.
Behavioral Tests
Open field test (OFT), tail suspension test (TST), forced swimming test (FST), novelty-suppressed feeding test (NSFT), and elevated plus maze test (EPMT) were performed as described previously (Yu et al., 2016). Detailed descriptions of these tests can be found in the supplemental Methods and Materials.
mRNA and Protein Analysis
The descriptions of reverse transcription-PCR (RT-PCR) for mRNA analysis and western blot (WB) can be found in the supplemental Methods and Materials.
Immunostaining
For analyzing hippocampal neurogenesis and neural differentiation, the mice received 4 injections of 5-Bromo-2’-Deoxyuridine (BrdU; 50 mg/kg i.p. every 2 hours) on the 3rd week after LV injection. The mice were anesthetized with chloral hydrate (350 mg/kg, i.p.) after the last BrdU administration and transcardially perfused (0.1 M phosphate buffered saline followed by 4% paraformaldehyde). The brains were post fixed in 4% paraformaldehyde overnight and dehydrated with 30% sucrose over 2 days. Serial sections (35 μm) were cut throughout the hippocampus using an oscillating tissue slicer and preserved in normal saline. Then the sections were incubated with rat polyclonal antibody anti-BrdU (1:40, Abcam), rabbit polyclonal antibody anti-NeuN (1:200, Millipore), and rabbit polyclonal antibody anti-GFAP (1:200, Millipore) under 4°C overnight. We used the following secondary antibodies: cyanin 3 (1:500, Beyotime Biotechnology), Alexa Fluor 350 (1:500, Beyotime Biotechnology), DyLight 405 (1:100, Bioworld Biotechnology), and Alexa Fluor 647 (1:500, Beyotime Biotechnology). Fluorescent signals were detected using a fluorescence microscope (Olympus DP72). The cells were counted as described previously (Jedynak et al., 2014) by another blinded experimenter. Briefly, every 9th section was kept for BrdU immunohistochemistry. The cells in the DG were counted through a 40× objective lens in each section and multiplied by 10, regarded as the total quantity of labeled cells. The quantification was carried out using Image-Pro Plus software. The percentage of differentiated cells was calculated as the number of marker-positive cells divided by the total number of cells.
In Vitro Assays for Proliferation and Differentiation of Adult Neural Stem Cells in Vitro
Adult neural stem cells (NSCs) from the DG of 8- to 9-week-old female mice were dissected and cultured as reported previously (Guo et al., 2012). The NSCs proliferation was assessed by 3-(4, 5-dimethythiazole-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) and cell counting kit (CCK-8, Beyotime Biotechnology) as well as BrdU incorporation observation. The NSC differentiation was determined using neuronal marker neuronal nuclear antigen (NeuN) or astrocytic marker glial fibrillary acidic protein (GFAP) antibodies respectively. DAPI+ cells were used to count the total number of cells. Images were analyzed by Image-Pro Plus software. The proportion of cells positive for specific markers was calculated to the total number. The detailed descriptions were provided in our previous publication (Ji et al., 2015).
Data and Statistical Analyses
Data shown are expressed as mean ± SEM. All data were analyzed by a 1-way ANOVA followed by a Dunnett’s post hoc analysis for multiple comparisons. All analyses were carried out using SPSS v20.0. P <.05 was considered as significant difference between the groups.
RESULTS
Hippocampal PPARδ Knockdown Causes Depressive-Like Behaviors
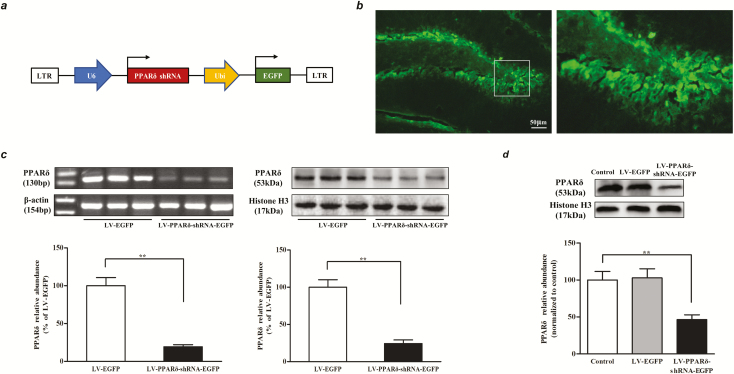
To assess the effect of hippocampal PPARδ knockdown on mood-related behaviors in mice, we generated LV encoding shRNA designed to target and downregulate PPARδ expression (Figure 1A). In vivo validation was confirmed by observation of EGFP+ cells (Figure 1B). RT-PCR and WB quantifications showed that hippocampal PPARδ levels were significantly decreased on the 7th day after infection with LV-PPARδ-shRNA-EGFP (RT-PCR: P < .01; WB: P < .01; Figure 1C). In vitro validation revealed that the PPARδ protein level in the NSCs infected with LV-PPARδ-shRNA-EGFP was significantly less than that of noninfected cells or cells infected with the LV-EGFP (F2,9 = 25.89; P < .01; Figure 1D). These results indicate that the LV-PPARδ-shRNA-EGFP is effective and can be used to knockdown PPARδ in the hippocampus or NSCs.
Figure 1.
LV-PPARδ-shRNA-EGFP induced-knockdown of peroxisome proliferator-activated receptor δ (PPARδ) expression in the hippocampus dentate gyrus (DG) of mice and the neural stem cells (NSCs). (A) The LV constructs encoding green fluorescent protein (GFP) and short hairpin RNAs (shRNAs) targeting PPARδ. (B) Shown are representative DG areas with lentivirus transfection. The right shows what is contained within the box shown in the left image. (C) In vivo measurements of nuclear PPARδ mRNA (left) or protein (right) expression on the 7th day in the DG of hippocampus of mice microinjected with LV-PPARδ-shRNA or with LV-EGFP into DG (n = 6). (D) Nuclear PPARδ protein expression was measured using western blot after infection of NSCs with LV in vitro (n = 4). Data are shown as mean ± SEM. *P < .05, **P < .01.
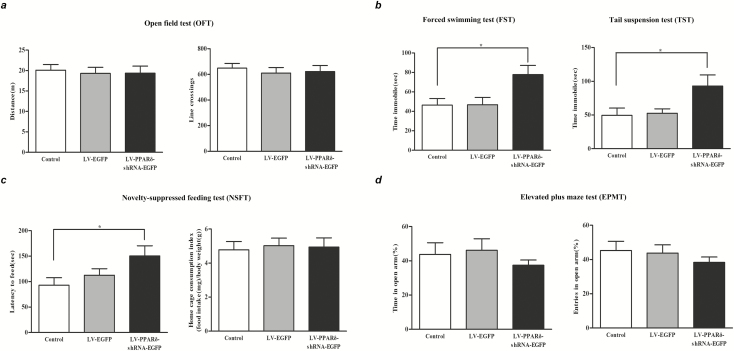
We then investigated whether knockdown of hippocampal PPARδ could affect mood-related behaviors. Three weeks after LV injection, we performed behavioral tests and analyzed the total distance traveled and line crossings in the OFT to detect locomotor activity. One-way ANOVA revealed that hippocampus-specific knockdown of PPARδ did not affect locomotor activity (distance: F2,33 = 0.082, P > .05; line crossings: F2,33 = 0.203, P > .05; Figure 2A). The data of the FST and TST showed that hippocampus-specific knockdown of PPARδ significantly increased the immobility time (FST: F2,33 = 6.708, P < .05; TST: F2,33 = 4.094, P < .05; Figure 2B). In addition, because of the frequent overlapping of symptoms of depression and anxiety in human beings (Xin et al., 2015), we examined anxiety in these mice using the NSFT and EPMT. In the NSFT, hippocampus-specific knockdown of PPARδ significantly increased the latency to feed in the novel environment (F2,33 = 5.474, P < .05; Figure 2C) but did not alter the home cage food consumption index (F2,33 = 0.070, P > .05, Figure 2C), suggesting that the PPARδ knockdown-induced changes in the latency to feed in a novel environment cannot be explained by possible changes in appetite. However, in the EPMT, neither the time spent in the open arms (F2,33 = 0.672, P > .05; Figure 2D) nor the entries into the open arms (F2,33 = 0.620, P > .05; Figure 2D) was significantly affected by hippocampus-specific knockdown of PPARδ.
Figure 2.
LV-PPARδ-shRNA-EGFP-mediated hippocampus-specific knockdown of peroxisome proliferator-activated receptor δ (PPARδ) produced depressive-like behaviors in mice. Shown are (A) the distance and line crossings in the open field test (OFT), (B) the immobility time in the forced swimming test (FST) and tail suspension test (TST), (C) the latency to feed and home cage consumption index in the novelty-suppressed feeding test (NSFT), and (D) total time spent and entries in open arm in the elevated plus maze test (EPMT) in mice. Data are shown as mean ± SEM; n = 12. *P < .05, **P < .01 vs control.
To further verify the role of PPARδ in the pathogenesis of depression, we next investigated the effect of GSK0660, a selective PPARδ antagonist that can penetrate the blood–brain barrier (Savage et al., 2015), on the depressive behaviors in mice. GSK0660 treatment increased the immobility time in the FST (F2,27 = 5.339, P < .05; supplemental Figure 1A) and the TST (F2,27 = 3.850, P < .05; supplemental Figure 1A). In the NSFT, GSK0660 treatment markedly increased the latency to feed (F2,27 = 7.103, P < .05; supplemental Figure 1B) without changing mice home cage consumption index (F2,27 = 0.012, P > .05; supplemental Figure 1B). The OFT showed that GSK0660 treatment did not affect the locomotor activity (distance: F2,27 = 0,691, P > .05; line crossings: F2,27 = 0.305, P > .05; supplemental Figure 1C). In addition, neither the time spent in the open arms (F2,27 = 1.308, P > .05; supplemental Figure 1D) nor the entries into the open arms (F2,27 = 1.753, P > .05; supplemental Figure 1D) was significantly affected by GSK0660 treatment in the EPMT.
Collectively, we found that downregulation of hippocampal PPARδ through specific gene knockdown or using a selective PPARδ antagonist induced depressive behaviors in mice.
Hippocampal PPARδ Knockdown Decreases Neurogenesis and Neuronal Differentiation
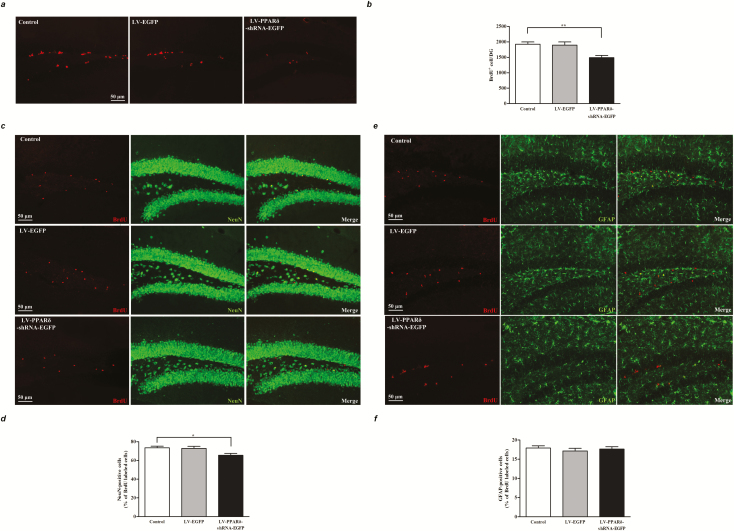
Next, we were curious about the regulatory role of PPARδ on neurogenesis and neuronal differentiation that are suppressed in depression. BrdU+ cells in the DG were examined on the 16th day after the first BrdU injection in mice. Mice injected with the LV-PPARδ-shRNA-EGFP displayed a significant decrease in the number of BrdU-labled cells in the DG (F2,15 = 8.436, P < .01; Figure 3A–B) compared with the mice injected with the LV-EGFP.
Figure 3.
Hippocampus-specific knockdown of peroxisome proliferator-activated receptor δ (PPARδ) decreased hippocampal neurogenesis and neuronal differentiation in mice. (A) Representative micrographs and (B) quantification of 5-Bromo-2’-Deoxyuridine (BrdU)-labeled cells (red) in the dentate gyrus (DG) of the mice. (C) Representative micrographs of cells double-labeled for BrdU (red, left) and the neuronal marker NeuN (green, middle). (D) Percentages of neurons labeled by BrdU in the DG of the mice injected with LV-EGFP or LV-PPARδ-shRNA-EGFP. (E) Representative micrographs of cells double-labeled for BrdU (red, left) and the astrocyte marker glial fibrillary acidic protein (GFAP) (green, middle). (F) Percentages of glial cells labeled by BrdU in the DG of the mice injected with LV-EGFP or LV-PPARδ-shRNA-EGFP. Data shown are mean ± SEM; n = 6. *P < .05, **P < .01 vs control.
To examine the phenotype of BrdU+ cells in the DG, double labeling for BrdU and NeuN, a neuronal marker, or GFAP, an astrocyte marker, was performed after BrdU injection. The results indicated that hippocampus-specific knockdown of PPARδ decreased the percentage of NeuN+/BrdU+ cells (F2,15 = 4.882, P < .05; Figure 3C–D), but did not affect the percentage of GFAP+/BrdU+ cells (F2,15 = 0.353, P > .05; Figure 3E–F). In addition, GSK0660 treatment also showed significant decrease in the number of the BrdU-labled cells in the DG (F2,15 = 8.743, P < .05; supplemental Figure 2A).
PPARδ Knockdown Inhibits Proliferation and Differentiation of NSCs
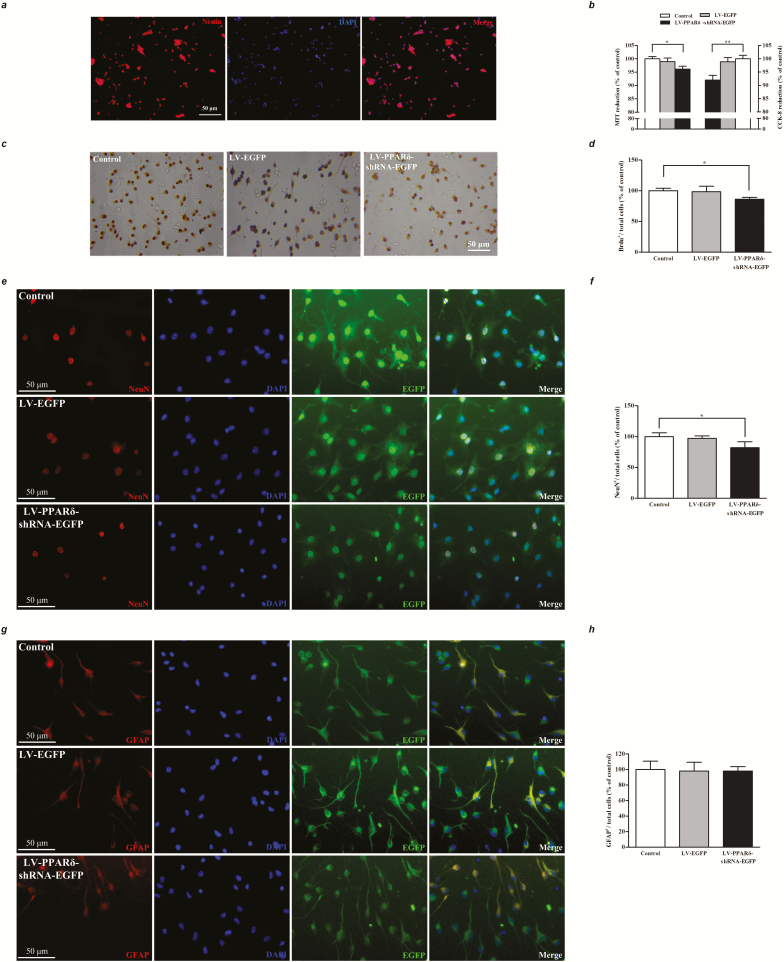
To further confirm the in vivo results, we observed the effect of PPARδ knockdown or blockade on the proliferation and differentiation of NSCs in vitro. In a floating culture medium, the NSCs from mouse hippocampus showed neurosphere formation with obvious nestin expression (Figure 4A). CCK-8 and MTT reduction assays revealed that cell proliferation was significantly decreased in the NSCs transfected with LV-PPARδ-shRNA-EGFP (MTT: F2,15 = 5.199, P < .05; CCK-8: F2,15 = 7.570, P < .01; Figure 4B). GSK0660 also produced similar effects with LV (MTT: F2,15 = 12.20, P < .01, supplemental Figure 2B; CCK-8: F2,15 = 6.504, P < .01; supplemental Figure 2C). BrdU incorporation experiment showed a significant decrease of the BrdU+ cells in the monolayer-cultured NSCs treated with LV-PPARδ-shRNA-EGFP (F2,15 = 5.011, P < .05; Figure 4C–D) or GSK0660 (F2,15 = 4.776, P < .05; supplemental Figure 2D–E).
Figure 4.
Knockdown of peroxisome proliferator-activated receptor δ (PPARδ) inhibited proliferation and differentiation of NSCs. (A) Neural stem cells (NSCs) from mice hippocampus expressed nestin (red), a protein marker for the NSCs. (B) Cell proliferation was determined by 3-(4, 5-dimethythiazole-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) and cell counting kit (CCK-8) assays. (C) Representatives of 5-Bromo-2’-Deoxyuridine (BrdU)-labeled cells of the NSCs. (D) Statistical graph showed declined BrdU+ cells in monolayer-cultured NSCs. (E) Representatives of immunofluorescence for neuronal marker, NeuN (red). (F) Percentages of neurons labeled by NeuN in the NSCs. (G) Representatives of immunofluorescence for astrocytic marker glial fibrillary acidic protein (GFAP) (red). (H) Percentages of glial cells labeled by GFAP in the NSCs. Data are shown as mean ± SEM; n = 6. *P < .05, **P < .01 vs control.
We also investigated the effect of PPARδ knockdown or blockade on cell differentiation in the cultured NSCs. The results showed that PPARδ knockdown significantly reduced the percentage of NeuN+/total cells (F2,15 = 4.098, P < .05; Figure 4E–F). Similarly, GSK0660 treatment (0.1 or 10 μM) substantially decreased the percentage of NeuN+/total cells (F2,15 = 6.174, P < .05; supplemental Figure 2F–G). Neither PPARδ knockdown nor blockade changed the percentage of GFAP+/total cells (LV-PPARδ knockdown: F2,15 = 0.016, P > .05; Figure 4G–H; GSK0660: F2,15 = 0.622, P > .05; supplemental Figure 2H–I). These results indicate that downregulating PPARδ inhibits NSCs differentiated into neurons in vitro.
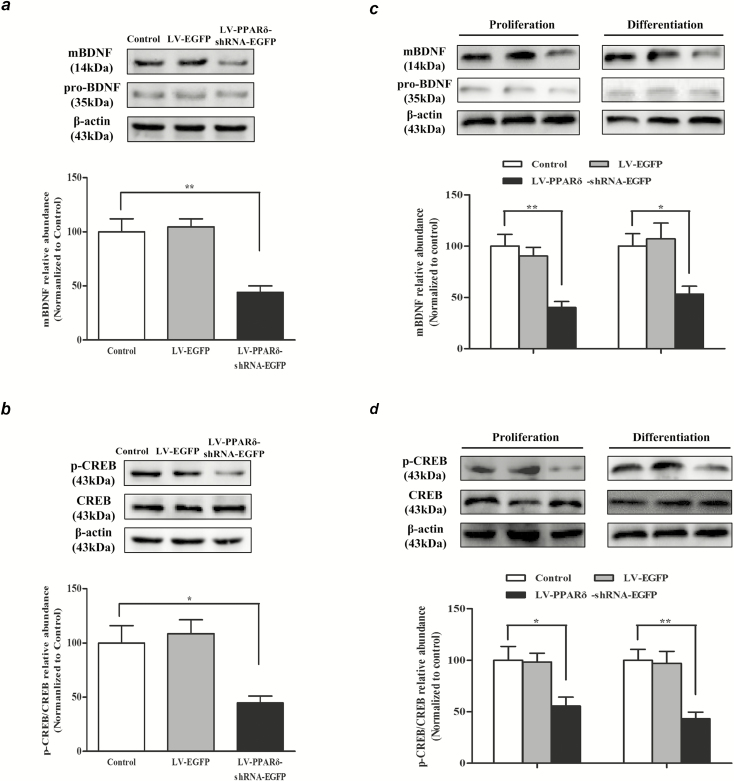
PPARδ Knockdown Decreases mBDNF Generation and CREB Phosphorylation
It is well known that BDNF-CREB signaling plays a crucial role in neurogenesis (Mariga et al., 2017). As shown in Figure 5, the mice with LV-PPARδ-shRNA-EGFP treatment displayed a significant decrease in hippocampal mBDNF, but not in pro-BDNF (mBDNF: F2,15 = 14.42, P < .01; Figure 5A). Assay for pCREB using an antibody directed against pCREB (Ser133) showed much lower phosphorylation of CREB at Ser133 in the hippocampus of mice treated with the LV-PPARδ-shRNA-EGFP (F2,15 = 7.775, P < .05; Figure 5B). Decreases of mBDNF and pCREB were also observed in the in vitro NSCs after treatment with LV-PPARδ-shRNA-EGFP (proliferation: mBDNF: F2,15 = 13.45, P < .01; Figure 5C; pCREB/CREB: F2,15 = 5.760, P < .05; Figure 5D) (differentiation: mBDNF: F2,15 = 11.34, P < .05; Figure 5C; pCREB/CREB: F2,15 = 19.76, P < .01; Figure 5D). In addition, decreases of mBDNF and pCREB were found in the mice (mBDNF: F2,15 = 9.496, P < .05; pCREB/CREB: F2,15 = 12.72, P < .05; supplemental Figure 3A) or NSCs treated with GSK0660 (proliferation: mBDNF: F2,15 = 15.81, P < .05; supplemental Figure 3B; pCREB/CREB: F2,15 = 6.729, P < .05; supplemental Figure 3C) (differentiation: mBDNF: F2,15 = 9.130, P < .05; supplemental Figure 3B; pCREB/CREB: F2,15 = 13.81, P < .05; supplemental Figure 3C). These results suggest that PPARδ downregulation inhibits BDNF-CREB signaling, which is involved in hippocampal neurogenesis.
Figure 5.
Knockdown of peroxisome proliferator-activated receptor δ (PPARδ) decreased the production of mBDNF and the phosphorylation of cAMP-response element binding protein (CREB). The protein levels of (A) mBDNF and pro-BDNF, (B) pCREP and CREB in the hippocampus DG of the mice were detected by western blot using respective antibodies; β-actin was used loading control. Relative expression of mBDNF/control or pCREP/CREB was quantified by densitometric analysis. (C) The mBDNF and pro-BDNF and (D) pCREB and CREB were detected by western blot using respective antibodies in the neural stem cells (NSCs) maintained in proliferation or differentiation medium; β-actin was used loading control, and relative expression of mBDNF/control or pCREP/CREB was quantified by densitometric analysis. Data are shown as mean ± SEM; n = 6. *P < .05, **P < .01 vs control.
DISCUSSISON
The present study showed that downregulating hippocampal PPARδ by intra-hippocampal microinfusion of LV, expressing shRNA complementary to the coding exon of PPARδ, or by repeated systemic administration of PPARδ antagonist GSK0660 induced depressive-like behaviors in mice. These treatments also resulted in a reduction of hippocampal neurogenesis and neuronal differentiation as well as decreases in mBDNF and pCREB, both in vivo and in vitro.
PPARδ is expressed throughout the brain, with prominent localization in mouse hippocampus, entorhinal cortex, and hypothalamus, but lower levels in the corpus callosum and caudate putamen (Woods et al., 2003; Hiqashiyama et al., 2007). The expression patterns of PPARβ/δ support the idea that this receptor has important constitutive roles in these brain subregions. While there are no significant differences between PPAR subtypes distribution in stress-related brain subregions (i.e., prefrontal cortex, paraventricular nucleus of hypothalamus) (Moreno et al., 2004), PPARδ shows a relatively high neuronal expression compared with the other PPAR subtypes (Lemberger et al., 1996). Notably, PPARδ plays an important role in modulating the activities of the other 2 PPAR subtypes (Shi et al., 2002). Our previous study showed that acute or chronic stress downregulated hippocampal PPARδ expression and induced depressive-like phenotype in mice, whereas hippocampal PPARδ overexpression reversed such a phenomenon (Ji et al., 2015). Therefore, in the present study, we extended our curiosity to find the effects of PPARδ downregulation in mouse hippocampus. We found that hippocampal PPARδ downregulation induced several behavioral impairments associated with depression, including increased immobility time in the TST and FST and latency to feed in the NSF test. Moreover, findings from EPMT indicated that knockdown of hippocampal PPARδ had the potential to induce anxiety-like behaviors. Moreover, these effects were mimicked by repeated administration with the selective PPARδ antagonist. All data indicated that PPARδ could be a key molecule in the hippocampus that might have potential regulatory roles in the pathophysiology of depression.
It is well known that hippocampal volume is decreased in people with recurrent depression relative to age- and sex-matched controls (Videbech and Ravnkilde, 2004; Geerlings and Gerritsen, 2017). Moreover, the hippocampus is very susceptible to stress and contains high levels of glucocorticoid receptors and glutamate. The optimal function of the hippocampus is critical for modulation of the hypothalamus-pituitary-adrenal axis, and its dysregulation is observed in almost one-half of all depressed patients (Sapolsky, 2000). Therefore, the hippocampus is one of the most commonly studied brain regions in depression. In the past decade, researchers have established that the hippocampus is one of the few brain regions in the healthy mammalian brain where neurogenesis occurs throughout adult life (Kempermann et al., 2004), and it plays central roles in the formation of memory and emotional processes (Egan et al., 2003; Drevets et al., 2008). Adult hippocampal neurogenesis is known to contribute to the behavioral modulatory effects of antidepressant treatments (Surget et al., 2011; Snyder et al., 2011). Recent studies showed that neurogenesis-related changes specific to a dorsal/ventral subregion are associated with observed behavioral phenotypes, and dorsal hippocampus is associated with cognitive functions, while the ventral hippocampus is related with stress, and emotion (Fanselow and Dong, 2010; O’Leary and Cryan, 2014). However, it is difficult to induce changes in PPARδ at some specific subregion by microinfusion of LV into the hippocampus because of its infectious diffusion. Less BrdU+ cells in dorsal and ventral sub-region were observed after downregulation or antagonism of hippocampal PPARδ in the present study, and on the contrary, more BrdU+ cells in the subregions were displayed after its upregulation or activation (Ji et al., 2015). Furthermore, the in vitro study showed that PPARδ downregulation inhibited the proliferation of NSCs as well as their differentiation into neurons. These in vivo and in vitro data strongly suggest that PPARδ plays a crucial role in neurogenesis and makes a plausible explanation that PPARδ regulates both depression and memory.
BDNF, like other neurotrophins, is synthetized as a pro-BDNF that is proteolytically processed into mBDNF by intracellular and/or extracellular proteases (Seidah et al., 1996). It is a key signaling molecule involved in a wide range of central functions such as the maintenance of neuronal plasticity, learning, memory, neurogenesis, and mood control (Malcangio and Lessmann, 2003; Duman and Monteggia, 2006; Castren, 2014; Lu et al., 2014; Hempstead, 2015). Over the last decade, several studies have consistently highlighted BDNF as a key player in antidepressant action, and it serves as a transducer, acting as the link between the antidepressant drugs and the neuroplastic changes that result in the improvement of the depressive symptoms (Hempstead, 2015; Björkholm and Monteggia, 2016). CREB was described as one of the components downstream of the signaling pathways of BDNF in response to stress (Finkbeiner, 2000). Some stressful stimuli can induce the phosphorylation of CREB at serine-133 site by means of an intracellular signal transduction pathway (Lessmann et al., 1994; Otten et al., 2000). Phosphorylation of CREB subsequently results in the transcriptional regulation of c-fos, c-jun, and bcl-2, which play important roles in the processes of regeneration, survival, and neuronal repair (Marmigere et al., 2001; Arthur-Farraj et al., 2012; Harris et al., 2013; Li et al., 2013). Interestingly, our work showed that PPARδ downregulation decreased levels of CREB phosphorylation (serine-133) and mBDNF, while PPARδ upregulation increased their levels. All such evidence indicates that the role of PPARδ in depression is involved in BDNF-CREB signaling. Further elucidation of the specific mechanism will enable us to better understand what is required to trigger antidepressant effects in hope of developing better treatment options.
Taken together, the present study provides a persuasive demonstration for the role of hippocampal PPARδ in depression and further reinforces the interesting finding that hippocampal PPARδ downregulation by genetic manipulation or a pharmacological blockade displays depressive-like effects through BDNF/CREB-associated adult neurogenesis in the hippocampus. Overall, this study strongly supports the idea that the hippocampal PPARδ is critically involved in mood regulation and its dysfunction underlies the manifestation of depressive-like behaviors. Hopefully, hippocampal PPARδ could be a novel and promising target for developing new drugs for the treatment of depressive disorders.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81573413 and 81773714 to Hao Hong, 81603113 to Susu Tang), the Natural Science Foundation of Jiangsu Province (BK20150705 to Susu Tang), and the Fundamental Research Funds for the Central Universities (2632017PT01).
Interest Statement
None.
References
- Arsenijevic D, de Bilbao F, Plamondon J, Paradis E, Vallet P, Richard D, Langhans W, Giannakopoulos P (2006) Increased infarct size and lack of hyperphagic response after focal cerebral ischemia in peroxisome proliferator-activated receptor beta-deficient mice. J Cereb Blood Flow Metab 26:433–445. [DOI] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) C-jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkholm C, Monteggia LM (2016) BDNF - a key transducer of antidepressant effects. Neuropharmacology 102:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V (2012) Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry 72:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550–553. [DOI] [PubMed] [Google Scholar]
- Castrén E. (2014) Neurotrophins and psychiatric disorders. Handb Exp Pharmacol 220:461–479. [DOI] [PubMed] [Google Scholar]
- Das NR, Gangwal RP, Damre MV, Sangamwar AT, Sharma SS (2014) A PPAR-β/δ agonist is neuroprotective and decreases cognitive impairment in a rodent model of Parkinson’s disease. Curr Neurovasc Res 11:114–124. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R (2006) Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry 59:1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W (2006) From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45:120–159. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. (2000) Calcium regulation of the brain-derived neurotrophic factor gene. Cell Mol Life Sci 57:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings MI, Gerritsen L (2017) Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: A systematic review and meta-analysis. Biol Psychiatry 82:339–350. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Do D, Jack C (2012) Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 8:189–202. [DOI] [PubMed] [Google Scholar]
- Guo W, Patzlaff NE, Jobe EM, Zhao X (2012) Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat Protoc 7:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NG, Nogueira MS, Verley DR, Sutton RL (2013) Chondroitinase enhances cortical map plasticity and increases functionally active sprouting axons after brain injury. J Neurotrauma 30:1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL. (2015) Brain-derived neurotrophic factor: three ligands, many actions. Trans Am Clin Climatol Assoc 126:9–19. [PMC free article] [PubMed] [Google Scholar]
- Higashiyama H, Billin AN, Okamoto Y, Kinoshita M, Asano S (2007) Expression profiling of peroxisome proliferator-activated receptor-delta (PPAR-delta) in mouse tissues using tissue microarray. Histochem Cell Biol 127:485–494. [DOI] [PubMed] [Google Scholar]
- Jedynak P, Kos T, Sandi C, Kaczmarek L, Filipkowski RK (2014) Mice with ablated adult brain neurogenesis are not impaired in antidepressant response to chronic fluoxetine. J Psychiatr Res 56:106–111. [DOI] [PubMed] [Google Scholar]
- Ji MJ, Yu XB, Mei ZL, An YQ, Tang SS, Hu M, Long Y, Miao MX, Hu QH, Sun HB, Kong LY, Hong H (2015) Hippocampal PPARδ overexpression or activation represses stress-induced depressive behaviors and enhances neurogenesis. Int J Neuropsychopharmacol 19: pyv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Richardson JC, Feinstein DL (2009) A ppardelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res 6:431–437. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH (2004) Functional significance of adult neurogenesis. Curr Opin Neurobiol 14:186–191. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, Hen R (2013) Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77:955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laevis Tropicials X. (1996) Environment, housing, and management. In: National Research Council, 8th ed (Janet CG, ed), pp 80–103. Washington, DC: The National Academic Press. [Google Scholar]
- Lemberger T, Braissant O, Juge-Aubry C, Keller H, Saladin R, Staels B, Auwerx J, Burger AG, Meier CA, Wahli W (1996) PPAR tissue distribution and interactions with other hormone-signaling pathways. Ann N Y Acad Sci 804:231–251. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R (1994) BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. Neuroreport 6:21–25. [DOI] [PubMed] [Google Scholar]
- Li Q, Wu D, Li R, Zhu X, Cui S (2013) Valproic acid protects neurons and promotes neuronal regeneration after brachial plexus avulsion. Neural Regen Res 8:2838–2848. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li YF, Huang Y, Amsdell SL, Xiao L, O’Donnell JM, Zhang HT (2009) Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology 34:2404–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Lu Y (2014) BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol 220:223–250. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Lessmann V (2003) A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkb receptors. Trends Pharmacol Sci 24:116–121. [DOI] [PubMed] [Google Scholar]
- Mariga A, Mitre M, Chao MV (2017) Consequences of brain-derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiol Dis 97:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigère F, Choby C, Rage F, Richard S, Tapia-Arancibia L (2001) Rapid stimulatory effects of brain-derived neurotrophic factor and neurotrophin-3 on somatostatin release and intracellular calcium rise in primary hypothalamic cell cultures. Neuroendocrinology 74:43–54. [DOI] [PubMed] [Google Scholar]
- Martin HL, Mounsey RB, Sathe K, Mustafa S, Nelson MC, Evans RM, Teismann P (2013) A peroxisome proliferator-activated receptor-δ agonist provides neuroprotection in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neuroscience 240:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Cerù MP (2004) Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 123:131–145. [DOI] [PubMed] [Google Scholar]
- O’Leary OF, Cryan JF (2014) A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends Pharmacol Sci 35:675–687. [DOI] [PubMed] [Google Scholar]
- Otten U, März P, Heese K, Hock C, Kunz D, Rose-John S (2000) Cytokines and neurotrophins interact in normal and diseased states. Ann N Y Acad Sci 917:322–330. [DOI] [PubMed] [Google Scholar]
- Paterniti I, Esposito E, Mazzon E, Galuppo M, Di Paola R, Bramanti P, Kapoor A, Thiemermann C, Cuzzocrea S (2010) Evidence for the role of peroxisome proliferator-activated receptor-beta/delta in the development of spinal cord injury. J Pharmacol Exp Ther 333:465–477. [DOI] [PubMed] [Google Scholar]
- Peng CH, Chiou SH, Chen SJ, Chou YC, Ku HH, Cheng CK, Yen CJ, Tsai TH, Chang YL, Kao CL (2008) Neuroprotection by imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol 18:128–140. [DOI] [PubMed] [Google Scholar]
- Pialat JB, Cho TH, Beuf O, Joye E, Moucharrafie S, Moucharaffie S, Langlois JB, Nemoz C, Janier M, Berthezene Y, Nighoghossian N, Desvergne B, Wiart M (2007) MRI monitoring of focal cerebral ischemia in peroxisome proliferator-activated receptor (PPAR)-deficient mice. NMR Biomed 20:335–342. [DOI] [PubMed] [Google Scholar]
- Polak PE, Kalinin S, Dello Russo C, Gavrilyuk V, Sharp A, Peters JM, Richardson J, Willson TM, Weinberg G, Feinstein DL (2005) Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. J Neuroimmunol 168:65–75. [DOI] [PubMed] [Google Scholar]
- Rosenström T, Jokela M (2017) Reconsidering the definition of major depression based on collaborative psychiatric epidemiology surveys. J Affect Disord 207:38–46. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. (2000) Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57:925–935. [DOI] [PubMed] [Google Scholar]
- Savage SR, McCollum GW, Yang R, Penn JS (2015) RNA-seq identifies a role for the PPARβ/δ inverse agonist GSK0660 in the regulation of TNFα-induced cytokine signaling in retinal endothelial cells. Mol Vis 21:568–576. [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Cameron HA (2015) Adult neurogenesis and mental illness. Neuropsychopharmacology 40:113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E (2012) Stress, stress hormones, and adult neurogenesis. Exp Neurol 233:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA (1996) Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett 379:247–250. [DOI] [PubMed] [Google Scholar]
- Serafini G, Hayley S, Pompili M, Dwivedi Y, Brahmachari G, Girardi P, Amore M (2014) Hippocampal neurogenesis, neurotrophic factors and depression: possible therapeutic targets? CNS Neurol Disord Drug Targets 13:1708–1721. [DOI] [PubMed] [Google Scholar]
- Shi Y, Hon M, Evans RM (2002) The peroxisome proliferator-activated receptor delta, an integrator of transcriptional repression and nuclear receptor signaling. Proc Natl Acad Sci USA 9:2613–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, Banasr M, Choi M, Chae SY, Licznerski P, Lee B, Voleti B, Li N, Lepack A, Fournier NM, Lee KR, Lee IY, Kim J, Kim JH, Kim YH, Jung SJ, Duman RS (2012) Neuritin produces antidepressant actions and blocks the neuronal and behavioral deficits caused by chronic stress. Proc Natl Acad Sci U S A 109:11378–11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ (2005) A lentiviral microrna-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci U S A 102:13212–13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DS, Glass CK (2007) Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol 28:551–558. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C (2011) Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 16:1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A (2010) Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry 15:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B (2004) Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 161:1957–1966. [DOI] [PubMed] [Google Scholar]
- Wang JW, Dranovsky A, Hen R (2008) The when and where of BDNF and the antidepressant response. Biol Psychiatry 63:640–641. [DOI] [PubMed] [Google Scholar]
- Woods JW, Tanen M, Figueroa DJ, Biswas C, Zycband E, Moller DE, Austin CP, Berger JP (2003) Localization of ppardelta in murine central nervous system: expression in oligodendrocytes and neurons. Brain Res 975:10–21. [DOI] [PubMed] [Google Scholar]
- Xiao L, Shu C, Tang J, Wang H, Liu Z, Wang G (2011) Effects of different CMS on behaviors, BDNF/CREB/Bcl-2 expression in rat hippocampus. Biomed Aging Pathol 1:138–146. [Google Scholar]
- Xin LM, Chen L, Ji ZP, Zhang SY, Wang J, Liu YH, Chen DF, Yang FD, Wang G, Fang YR, Lu Z, Yang HC, Hu J, Chen ZY, Huang Y, Sun J, Wang XP, Li HC, Zhang JB, Si TM (2015) Risk factors for anxiety in major depressive disorder patients. Clin Psychopharmacol Neurosci 13:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, DeRuiter SL, Turner DL (2002) RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci U S A 99:6047–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Levi L, Casadesus G, Kunos G, Noy N (2014) Fatty acid-binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator-activated receptor β/δ (PPARβ/δ) in the brain. J Biol Chem 289:12748–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XB, Dong RR, Wang H, Lin JR, An YQ, Du Y, Tang SS, Hu M, Long Y, Sun HB, Kong LY, Hong H (2016) Knockdown of hippocampal cysteinyl leukotriene receptor 1 prevents depressive behavior and neuroinflammation induced by chronic mild stress in mice. Psychopharmacology (Berl) 233:1739–1749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.