Abstract
Chromatin is a highly regulated environment, and protein association with chromatin is often controlled by post‐translational modifications and the corresponding enzymatic machinery. Specifically, SUMO‐targeted ubiquitin ligases (STUbLs) have emerged as key players in nuclear quality control, genome maintenance, and transcription. However, how STUbLs select specific substrates among myriads of SUMOylated proteins on chromatin remains unclear. Here, we reveal a remarkable co‐localization of the budding yeast STUbL Slx5/Slx8 and ubiquitin at seven genomic loci that we term “ubiquitin hotspots”. Ubiquitylation at these sites depends on Slx5/Slx8 and protein turnover on the Cdc48 segregase. We identify the transcription factor‐like Ymr111c/Euc1 to associate with these sites and to be a critical determinant of ubiquitylation. Euc1 specifically targets Slx5/Slx8 to ubiquitin hotspots via bipartite binding of Slx5 that involves the Slx5 SUMO‐interacting motifs and an additional, novel substrate recognition domain. Interestingly, the Euc1‐ubiquitin hotspot pathway acts redundantly with chromatin modifiers of the H2A.Z and Rpd3L pathways in specific stress responses. Thus, our data suggest that STUbL‐dependent ubiquitin hotspots shape chromatin during stress adaptation.
Keywords: Cdc48/p97, chromatin remodeling, STUbL, SUMO, ubiquitin
Subject Categories: Chromatin, Epigenetics, Genomics & Functional Genomics; Post-translational Modifications, Proteolysis & Proteomics
Introduction
SUMO‐targeted ubiquitin ligases (STUbLs) modify SUMOylated proteins with ubiquitin and thereby transfer substrates from the SUMO (small ubiquitin‐like modifier) to the ubiquitin pathway (Sriramachandran & Dohmen, 2014). To achieve this, STUbLs combine binding to SUMOylated proteins via SUMO‐interacting motifs (SIMs) with ubiquitin ligase activity (Prudden et al, 2007; Sun et al, 2007; Uzunova et al, 2007; Xie et al, 2007). Apart from this defining feature, the STUbL enzyme family is highly heterogeneous, as is the regulation of each member, even though functional aspects appear to be conserved (Sriramachandran & Dohmen, 2014). Of note, thousands of proteins are SUMOylated in cells (Hendriks & Vertegaal, 2016), but only a handful of them were shown to be targeted by STUbLs. This raises the question of which features make a protein a STUbL substrate.
A hallmark of several STUbL substrates is modification with SUMO chains (polySUMOylation) (Uzunova et al, 2007; Tatham et al, 2008), but it has also been suggested for the human STUbLs RNF4 and Arkadia/RNF111, as well as for Drosophila Degringolade/Dgrn that they might use additional SUMO‐independent interactions for substrate recognition (Abed et al, 2011; Groocock et al, 2014; Kuo et al, 2014; Sun et al, 2014; Thomas et al, 2016). However, in case of the prototypical STUbL, budding yeast Slx5/Slx8, no substrate recognition elements have been characterized other than its SUMO‐interacting motifs.
STUbLs orchestrate many nuclear functions such as, but not limited to, DNA repair, quality control, and transcriptional regulation (Sriramachandran & Dohmen, 2014). Accordingly, most STUbL substrates are nuclear proteins. Human RNF4, for example, targets the PML (promyelocytic leukemia) protein, which is polySUMOylated in nuclear PML bodies upon arsenic exposure (Tatham et al, 2008). Other RNF4 substrates include transcription factors and proteins involved in different DNA repair pathways (for reviews, see Sriramachandran & Dohmen, 2014; Nie & Boddy, 2016). Budding yeast Slx5/Slx8 was initially identified for its role in genome stability as well, which manifests in a synthetic lethal phenotype with the DNA helicase Sgs1 (Mullen et al, 2001). Slx5/Slx8 is involved in the repositioning of DNA lesions to nuclear pore complexes (Nagai et al, 2008; Su et al, 2015; Churikov et al, 2016; Horigome et al, 2016). In line with an additional major function in chromatin maintenance, several DNA‐associated proteins have been described as Slx5/Slx8 substrates (Ohkuni et al, 2016; Schweiggert et al, 2016; Thu et al, 2016; Liang et al, 2017), including transcription factors (TFs) such as Mot1 (mutant variant) and Matα2 (Wang & Prelich, 2009; Xie et al, 2010). Interestingly, in the latter case, Matα2 SUMOylation is dispensable for Slx5/Slx8 targeting, but the SIMs of Slx5 and Matα2 DNA binding are required (Xie et al, 2010; Hickey et al, 2018). Matα2 ubiquitylation subsequently facilitates the recruitment of the Cdc48 complex (p97/VCP in mammalian cells) (Wilcox & Laney, 2009), a segregase that can extract ubiquitylated proteins from their local environment, such as chromatin (Rape et al, 2001; Ramadan et al, 2007; Maric et al, 2014; Franz et al, 2016).
It emerges that both sequence‐specific DNA‐binding proteins and other chromatin‐associated proteins are STUbL substrates. However, it is still unknown whether STUbLs fulfill a general role in regulating protein turnover at chromatin and to what extent other components of the ubiquitin–proteasome system (UPS), such as Cdc48, are involved. To address these questions, we obtained genome‐wide binding profiles of Slx8 and ubiquitylated proteins. Notably, Slx8 localized to surprisingly few genomic sites, and the ubiquitin signal at seven of these sites was Slx5/Slx8‐dependent and strongly enriched in cdc48 mutant strains. These data indicate that these “ubiquitin hotspots” are sites of STUbL‐ and Cdc48‐dependent protein turnover on chromatin. Ubiquitin hotspots are bound by the poorly characterized transcription factor‐like protein Ymr111c/Euc1, which is modified with SUMO and is a STUbL substrate. Notably, however, deletion of EUC1 does apparently not lead to an abrogation of transcription in the vicinity of ubiquitin hotspots, but rather results in strong genetic interactions with H2A.Z and Rpd3 pathways, which regulate expression of many genes. Euc1 and ubiquitin hotspots are part of an Rpd3S‐dependent pathway that is required to cope with cellular stress induced by suboptimal temperature. Moreover, the analysis of the Slx5/Slx8‐recruitment mechanism led to the identification of a SUMO‐independent substrate‐binding domain within Slx5, suggesting a new mode of substrate recognition by Slx5/Slx8.
Results
Slx5/Slx8 and Cdc48 control seven ubiquitin hotspots across the yeast genome
To investigate Slx5/Slx8‐catalyzed ubiquitylation of chromatin‐associated proteins, we developed chromatin immunoprecipitation (ChIP) protocols for ubiquitylated proteins (Appendix Fig S1A) and Slx5/Slx8 in Saccharomyces cerevisiae. We used the FK2 ubiquitin antibody with broad specificity toward mono‐ubiquitylated proteins and K29‐, K48‐, and K63‐linked chain types combined with genome‐wide tiling arrays (ChIP‐chip, Fig 1A). We detected ubiquitin signals at open reading frames (ORFs). These signals appear to represent histone H2B mono‐ubiquitylation that has been described to be particularly abundant on highly transcribed genes (Braun & Madhani, 2012), as they were either lost in cells that harbor a mutation of the main H2B ubiquitylation site (h2b‐K123R) or in cells that lack Rad6, the E2 enzyme for H2B ubiquitylation (Jentsch et al, 1987; Robzyk et al, 2000) (Fig 1A and Appendix Fig S1B, Dataset EV1). However, some ubiquitin signals were H2B ubiquitylation‐independent and notably enhanced in cdc48 mutants (cdc48‐6, Fig 1A, Dataset EV1).
Figure 1. The SUMO‐targeted ubiquitin ligase Slx5/Slx8 is required for the formation of seven ubiquitin hotspots across the yeast genome.
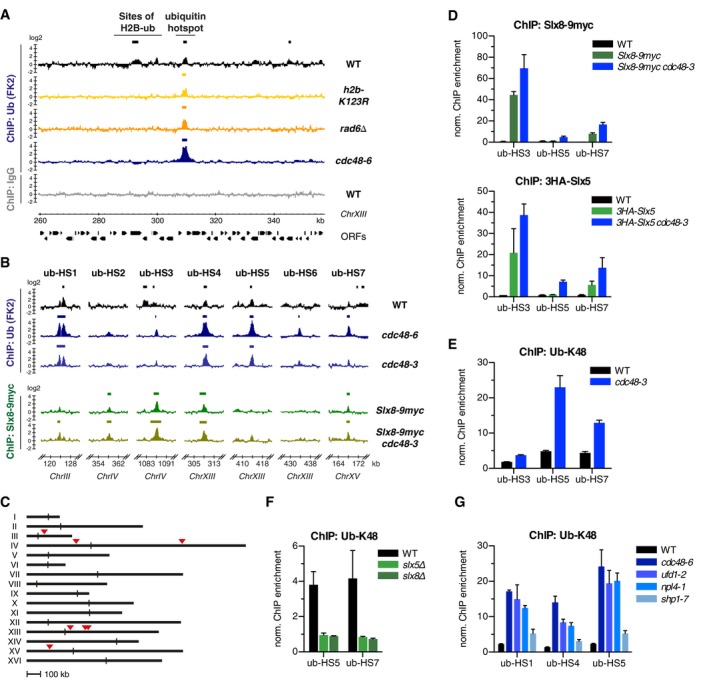
- Genome‐wide ubiquitin binding profiles identify numerous regions of histone H2B ubiquitylation in WT cells and distinct sites of non‐H2B ubiquitylation (“ubiquitin hotspot”, ub‐hotspot, ub‐HS) which persist in h2b‐K123R and rad6∆ strains, and increase in cdc48 mutants (cdc48‐6). A 90 kb stretch of chromosome XIII (ChrXIII) is depicted. Chromatin immunoprecipitation was performed using the FK2 ubiquitin antibody (see also Appendix Fig S1A), and enriched DNA was analyzed on NimbleGen arrays (Chip‐chip). DNA from non‐specific IgG ChIP experiments served as background control. Significantly enriched regions are marked by bars above the respective ChIP‐chip tracks and are summarized in Dataset EV1. Data represent means from two independent replicates, except for ubiquitin (FK2) in rad6∆ and IgG‐ChIP in WT (n = 1). All experiments, including those using cdc48‐6 and other temperature‐sensitive (ts) alleles, were performed at 30°C (semi‐permissive temperature for ts‐alleles) unless stated otherwise.
- Seven ub‐HSs show strong correlation between ubiquitin binding and Slx8 enrichment (Slx8‐9myc ChIP). 16‐kb windows of the indicated regions centered around the ub‐HSs are depicted for ubiquitin and Slx8 binding profiles. Ubiquitin (FK2) data for WT and cdc48‐6 are from the same experiment as depicted in (A). Data represent means from two independent replicates.
- Schematic representation of the 16 yeast chromosomes (I‐XVI) with positions of the ub‐HSs marked by red triangles. Vertical bars indicate positions of centromeres.
- Slx8 and Slx5 are recruited to ub‐HSs. DNA from Slx8‐9myc (top) or 3HA‐Slx5 (bottom) ChIP experiments of the indicated strains was analyzed by quantitative real‐time PCR (ChIP‐qPCR) at selected ub‐HSs. Data represent mean ± standard deviation (SD, n = 2). Consistent with a previous study, we did not observe any Slx8 enrichment at centromeres (van de Pasch et al, 2013); however, we note that we could not confirm the reported centromere binding of Slx5 (see also Appendix Fig S2E).
- K48‐linked ubiquitin chains accumulate in a cdc48 mutant (cdc48‐3). Ub‐K48 ChIP followed by qPCR for the same loci as in (D) is depicted. See also Fig EV1C and D. Data represent means ± SD (n = 2).
- Slx5 and Slx8 are required for ub‐HS formation. Ub‐K48 ChIP‐qPCR in strains lacking one subunit of the Slx5/Slx8 complex (slx5∆, slx8∆). Data represent means ± SD (n = 3).
- Ufd1 and Npl4 act in concert with Cdc48 to remove ubiquitylated proteins from ub‐HS sites. Ub‐K48 ChIP for the indicated strains grown at the semi‐permissive temperature of 30°C. Data represent means ± SD (n = 2).
In similar experiments, Slx8 bound specifically to only few sites in the genome (Slx8‐9myc, Fig 1B, Dataset EV2). Comparison of our genome‐wide profiles of regions enriched for both ubiquitin‐ and Slx8‐binding revealed a striking correlation for seven sites, which we term “ubiquitin hotspots” (ub‐hotspots, ub‐HS in figures, Figs 1B and C, and EV1A and B, Datasets EV1, EV2 and EV3). Besides these seven “ubiquitin hotspots”, we detected only two sites of major ubiquitin accumulation in cdc48 mutants without Slx8 enrichment (ub‐only‐sites), and two distinct sites of major Slx8 enrichment without ubiquitin accumulation (Datasets EV1 and EV2, see also Appendix Fig S2E).
Figure EV1. Related to Figs 1 and 2. Seven ubiquitin hotspots across the yeast genome share a Cdc48‐dependent extraction mechanism and recruit Ymr111c/Euc1.

-
AThe FK2 ubiquitin antibody and a ub‐K48 specific antibody detect the same ub‐HSs in genome‐wide ChIP‐chip experiments. 16‐kb windows from genome‐wide ChIP‐chip data using either the ubiquitin (FK2) or ub‐K48 (clone Apu2) antibodies are shown. For comparison, data for ubiquitin (FK2) ChIP in WT, cdc48‐6, cdc48‐3 are reproduced from Fig 1B and for h2b‐K123R partially (ub‐HS4) from Fig 1A. Data represent means from two independent replicates.
-
BTable summarizing the ub‐HSs identified in Fig 1B. Stretches were defined by significantly enriched regions in ub‐K48 ChIP‐chip in cdc48‐6 (all except ub‐HS2) or Slx8‐9myc‐enriched regions in the cdc48‐3 background (ub‐HS2).
-
C, DUbiquitin signals increase around 5–10‐fold in both cdc48‐6 and cdc48‐3 mutants with ubiquitin (FK2) and ub‐K48‐chain‐specific antibodies. ChIP‐qPCR experiment using ubiquitin (FK2) (C) and ub‐K48 (D) antibodies and indicated strains. Data represent means ± SD (n = 3 for (C), n = 4 for (D)).
-
Eufd1‐2, ubx4∆, and ubx5∆ show an increase of ubiquitin conjugates at all ub‐HSs similar to cdc48‐6. Genome‐wide ChIP‐chip data using the ub‐K48 or a non‐specific IgG control antibody for the indicated strains. Data for WT and cdc48‐6 reproduced from (A) for comparison. Data represent means from two independent replicates.
-
FA single point mutation within the ub‐HS‐motif abolishes ubiquitin enrichment. A G>T mutation was introduced in one of the conserved TTGTT repeats of ub‐HS4 F7 (bottom scheme) and integrated at the LEU2 locus as described in Fig 2A. ChIP‐qPCR for ub‐K48 demonstrated that the ubiquitin enrichment is lost upon mutation of the ub‐HS‐motif (ub‐HS4 F7‐mut). Experiments were performed in cdc48‐6 strains. Data represent means ± SD (n = 5).
-
GTable summarizing the confirmed hits from two independent Y1H screens as described in Fig 2D. Protein start sites are indicated. aa: amino acid, N.D.: not determined.
-
HEndogenous Euc1 does not bind the mutated ub‐HS4‐motif. ChIP with an Euc1‐specific antibody was performed in strains with ub‐HS4 F7 or ub‐HS4 F7‐mut integrated at the LEU2‐locus as described in (F). Experiments were performed in cdc48‐6 strains. Data represent means ± SD (n = 2).
Next, we determined the accumulation of specific ubiquitin chain types at ub‐hotspots. Using a ubiquitin K48 chain‐specific antibody (clone Apu2, “ub‐K48”), we detected the same ub‐hotspots as with the FK2 antibody (Fig EV1A, Dataset EV3). Moreover, strains harboring different mutant alleles of the CDC48 gene showed an increase of ubiquitin‐ChIP signals from around 5–10‐fold (WT) to 15–50‐fold (cdc48‐6, cdc48‐3) enrichment over background, with comparable results for both antibodies (Fig EV1C and D). Since the ub‐K48 antibody was more specific for the Slx8‐bound and Cdc48‐controlled ub‐hotspots, we used this antibody for the rest of our study.
Consistent with recruitment of the Slx5/Slx8 heterodimer to chromatin, we found that both subunits were enriched to similar levels at selected ub‐hotspots, and observed a moderate increase in Slx5/Slx8 binding in cdc48‐3 mutant cells (Fig 1D). While the ubiquitin signals did not correlate with Slx5/Slx8 enrichment levels at the tested sites (compare Fig 1D and E), they dropped to background levels in slx5Δ and slx8Δ cells (Fig 1F), indicating that Slx5/Slx8 is the relevant ubiquitin E3 ligase at these sites.
Cdc48 targeting is usually facilitated by cofactors that mediate substrate specificity (Buchberger et al, 2015). Consistent with previous results for other chromatin‐bound substrates (Verma et al, 2011), we found that specifically the Cdc48Ufd1‐Npl4 complex removes ubiquitylated proteins from ub‐hotspots (Figs 1G and EV1E), assisted by additional Ubx4 and Ubx5 cofactors (Fig EV1E and Appendix Fig S1C, Dataset EV3). In contrast, impairment of Cdc48 substrate delivery to the proteasome (rad23∆ dsk2∆; Richly et al, 2005) or proteasome assembly (ump1∆; Ramos et al, 1998) did not cause accumulation of ubiquitin conjugates at ub‐hotspots (Appendix Fig S1D).
Taken together, our analysis of genome‐wide ubiquitin and Slx8 ChIP data reveals seven ubiquitin hotspots that share similar features: (i) strong accumulation of ubiquitin in cdc48 and associated cofactor mutants, (ii) recruitment of Slx5 and Slx8, and (iii) dependence on the functional Slx5/Slx8 dimer for ubiquitylation.
A sequence motif within ub‐hotspots is bound by Ymr111c/Euc1
Interestingly, all ub‐hotspots lie within intergenic regions, do not seem to be associated with any annotated features within the yeast genome, and appear to be distributed among the sixteen yeast chromosomes (Fig 1C). We could also not identify any shared pathway or function of the adjacent genes (Dataset EV4).
To investigate whether any sequence features define ub‐hotspots, we cloned a 1,038‐bp region of ub‐HS4 on chromosome XIII (ChrXIII) and inserted it into the LEU2 locus on ChrIII (ectopic ub‐HS4, Fig 2A). This fragment was sufficient to drive formation of an ectopic ub‐hotspot at the new position (Appendix Fig S2A), suggesting a role for specific DNA sequences. Indeed, we were able to map a minimal 39‐bp fragment required for ub‐hotspot formation (ub‐HS4 F7, Appendix Fig S2A, Fig 2A–C).
Figure 2. A sequence motif within ub‐hotspots is bound by Ymr111c/Euc1.
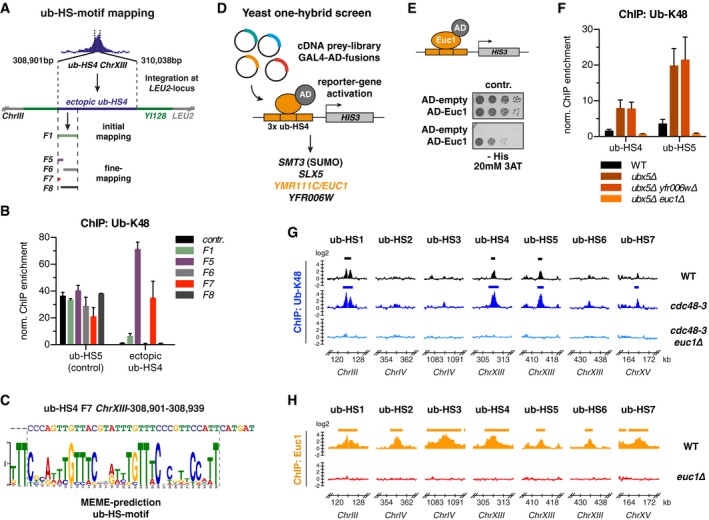
-
ASchematic of the ub‐HS‐motif mapping strategy. A 1,038‐bp stretch of ub‐HS4 (blue line) was cloned and integrated at the LEU2 locus (gray) using the integrative YIplac128 vector (green). Initial mapping led to the identification of fragment F1 (Appendix Fig S2A), which was further truncated for fine‐mapping ((B), F5–F8). qPCR primers were designed to bind within the YI128 backbone.
-
BA 39‐bp fragment of ub‐HS4 is sufficient to drive ectopic ub‐HS formation. Fragments of ub‐HS4 were integrated ectopically, and ub‐K48 ChIP‐qPCR was performed for the endogenous ub‐HS5 and the ectopic ub‐HS4 fragments as depicted in (A). contr.: control, empty YIplac128 vector was integrated at LEU2. Experiments were performed in cdc48‐6 background. Data represent means ± SD (n = 2).
-
CExperimental mapping and bioinformatic prediction identify a similar ub‐HS‐motif. Comparison between the experimentally mapped ub‐HS4 F7 (B) and the consensus motif of all ub‐HSs identified by the MEME software.
-
DA yeast one‐hybrid screening strategy to identify proteins binding to the ub‐HS‐motif. Three copies of the ub‐HS4‐motif (F7) were cloned upstream of a minimal promoter followed by a HIS3 reporter gene and integrated at the URA3 locus. A yeast cDNA library with N‐terminally fused Gal4 activation domain (AD) was used to screen for survival on media lacking histidine and multiple plasmids coding for 4 different genes were recovered (bottom). See also Fig EV1G.
-
EEuc1 binds to the ub‐HS‐motif in a Y1H assay. Gal4‐AD‐ or Gal4‐AD‐Euc1‐encoding plasmids were transformed into a Y1H reporter strain as described in (D). Serial dilutions were spotted on control plates and plates lacking histidine with 20 mM 3‐amino‐triazole (3AT). Cells were grown at 30°C for 3 days.
-
FEuc1 is required for the formation of ub‐HSs. ChIP with ub‐K48 specific antibodies was performed for the indicated strains, and enriched DNA was analyzed by qPCR. Data represent means ± SD (n = 4).
-
G, HEuc1 binds to endogenous ub‐HSs. Genome‐wide binding profiles of K48‐linked ubiquitin chains (G) or Euc1 (H) were obtained in ChIP‐chip experiments as described in Fig 1A. Euc1‐ChIP experiments were performed with a polyclonal antibody raised against Euc1 aa 292–462. Data represent means from two independent replicates.
We also used the MEME suite to predict sequence motifs within the ub‐hotspots (Bailey et al, 2009). Consistent with our experimental mapping, a sequence motif of 36 bp was identified, which largely overlapped with the experimentally mapped 39‐bp fragment (Fig 2C). This “ub‐HS‐motif” was found in all the ub‐hotspots in at least one copy (Appendix Fig S2B), suggesting that a sequence‐specific DNA‐binding protein might localize to ub‐hotspots. Supporting this notion, a single point mutation within one of the central, conserved TTGTT repeats led to a complete loss of ubiquitin from the ectopic ub‐hotspot (ub‐HS4 F7‐mut, Fig EV1F).
To identify proteins binding to the ub‐HS‐motif, we applied an unbiased yeast one‐hybrid (Y1H) screening strategy (Fig 2D). In two independent screens in either a WT or a ubx5Δ strain, which shows enriched ubiquitin at ub‐hotspots (Fig EV1E), we identified several clones of four different genes: SMT3 (encoding for SUMO), SLX5, and the uncharacterized YMR111C/EUC1 and YFR006W (Figs 2D and EV1G). Identification of SUMO suggests a SUMOylation event upstream of Slx5/Slx8 recruitment, while isolation of SLX5 clones confirms our ChIP data (Fig 1D). We confirmed the recruitment of Ymr111c/Euc1, SUMO, and Yfr006w with Gal4 activation domain (AD) fusion fragments (Fig 2E and Appendix Fig S2C). Importantly, deletion of YMR111C/EUC1 led to a complete loss of ubiquitin‐ChIP signals at ub‐hotspots, while deletion of YFR006W had no effect (Fig 2F and G, Dataset EV3). Therefore, we here name YMR111C as “EUC1” (Enriches Ubiquitin on Chromatin 1).
Consistent with a key role of Euc1 in the formation of ub‐hotspots, we found that activation of the HIS3 reporter by AD‐SUMO was Euc1‐dependent (Appendix Fig S2C), suggesting that Euc1 binding occurs before SUMO binding or a SUMOylation event. In line with this, Euc1 could not bind the mutated ub‐HS4 sequence (Appendix Fig S2D).
We raised an antibody against Euc1 to test its association with the endogenous ub‐hotspot sites in ChIP‐chip experiments (Fig 2H, Dataset EV5). As expected from the Y1H assays, Euc1 strongly accumulated at ub‐hotspots in WT but not in euc1∆ cells (Fig 2H), nor at the mutated ectopic ub‐HS4 sequence (Fig EV1H). Notably, ub‐hotspots are the major sites of Euc1 binding in the entire genome, with only three additional sites of Euc1 accumulation (two of which also enrich Slx8 and contain the ub‐HS‐motif, Appendix Fig S2E). These data indicate that Euc1 specifically localizes to ub‐HS‐motif sites and is required for the formation of the ub‐hotspots.
The transcription factor‐like Euc1 shows transactivation in reporter gene assays
Euc1 harbors a predicted coiled‐coil (CC) domain in its N‐terminal part and a GCR1 domain at its C‐terminus (Fig 3A). GCR1 domains have been shown to confer sequence‐specific DNA binding in Gcr1 and related transcription factors (TFs) (Huie et al, 1992; Hohmann, 2002). A distantly related GCR1 domain protein that also binds DNA is Cbf2, which is part of the CBF3 complex and establishes kinetochore attachment with centromeres (Espelin et al, 2003). Protein structure prediction suggested a myb‐like DNA‐binding fold within the GCR1 domain (Appendix Fig S3A) (Biedenkapp et al, 1988; Kelley et al, 2015) and our mapping results confirm that the complete GCR1 domain and C‐terminus are essential for Euc1 association with the ub‐HS‐motif (Appendix Fig S3B). Introduction of two point mutations to the predicted DNA‐binding loop (W333A, R334A, euc1‐DBD*) resulted in complete loss of association with ub‐hotspots in Y1H assays (Appendix Fig S3C) and Euc1 ChIP experiments (Appendix Fig S3D and E), suggesting that Euc1 directly binds the ub‐HS‐motif. Concomitantly, ubiquitin enrichment at ub‐hotspots was also lost in euc1‐DBD* cells (Appendix Fig S3E).
Figure 3. The transcription factor‐like Euc1 shows transactivation in reporter gene assays.

- Euc1 domain structure is reminiscent of transcription factors. Top: Schematic representation of Euc1, with a predicted coiled‐coil domain (CC) and GCR1 domain indicated. Predicted DNA binding of the C‐terminal part was confirmed in Y1H experiments (Appendix Fig S3A–C). Bottom: Euc1‐like protein sequences from closely related Saccharomyces species were aligned, and a phylogenetic tree was generated using Clustal Omega. Jalview was used to graphically display the degree of sequence conservation (see also Appendix Fig S4A).
- The ub‐HS‐motif is conserved in closely related yeast species. The 7 yeast Multiz Alignment & Conservation tool of the UCSC Genome Browser was used to retrieve alignments of sequences corresponding to ub‐HS‐motifs from other Saccharomyces species. Dot (.) indicates a conserved base.
- Euc1 can induce transactivation via its N‐terminal 30 amino acids. Euc1 constructs under the endogenous EUC1 promoter were transformed in a reporter strain as described for Fig 2D, and serial dilutions were spotted on control or selective media to test HIS3 activation. Cells were grown at 30°C for 3 days.
- Quantification of HIS3 mRNA levels from strains used in (C). Cells were grown in liquid media with selection for the transformed plasmids (SC‐Leu), harvested in logarithmic growth phase, and total mRNA was prepared. After reverse transcription, HIS3 mRNA levels were quantified using qPCR (RT–qPCR), normalized first to ACT1 mRNA and then to the empty vector control strain. Data represent means ± SD (n = 4). P = 2.43 × 10−5 (Student's t‐test).
Phylogenetic analysis revealed EUC1‐like genes in several other yeast species, with most pronounced sequence homology in CC‐ and GCR1 domains (Fig 3A, Appendix Fig S4A). Conversely, ub‐hotspot sequences could be identified in those Saccharomyces species, where corresponding intergenic regions could be aligned to S. cerevisiae (Fig 3B, Appendix Fig S4B). We note that residues most conserved in the different hotspot motifs also appeared most highly conserved in related yeasts, hinting at a similar function of Euc1 proteins at these sites.
To test whether Euc1 itself could also function as a transcriptional activator, we deleted endogenous EUC1 in the ub‐HS4‐HIS3 ubx5∆ Y1H reporter strain, which reduced background activation of the HIS3 reporter (Appendix Fig S4C). Conversely, plasmid‐borne expression of Euc1 using its endogenous promoter was sufficient to drive transcription of the reporter (Fig 3C and D). To map the transactivation domain of Euc1, we introduced truncations in the N‐terminus that contains an acidic patch (aa 19–28, Appendix Fig S4D) (Sigler, 1988). Consistent with a role in transactivation, truncation of either the first 15 or 30 amino acids led to a strong decrease in HIS3 expression (Fig 3C). Moreover, the first 30 amino acids of Euc1, when fused to the Gal4 DNA‐binding domain (BD), were sufficient to drive expression of HIS3 under the control of a GAL1 promoter (Appendix Fig S4D). While these data suggest that Euc1 could act as a transcription factor at ub‐hotspots, they are also consistent with a model whereby Euc1 mediates ub‐hotspot formation to establish a specific chromatin domain or structure, like Cbf2 does at centromeres (see below).
Ub‐hotspot formation requires Euc1 SUMOylation
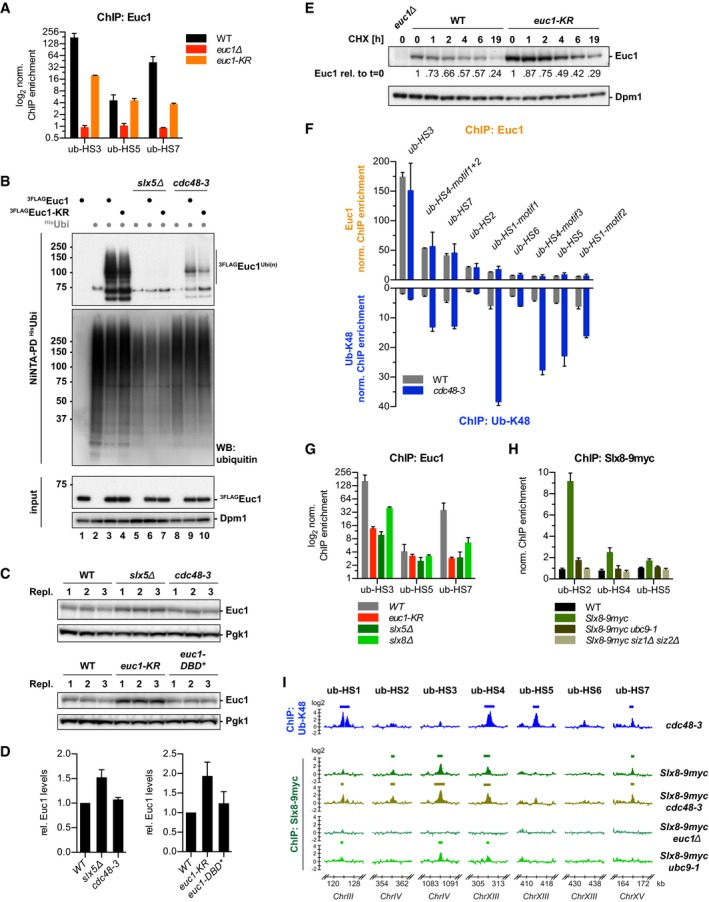
Our initial data suggested that SUMOylation (Appendix Fig S2C) and ubiquitylation (Fig 2F and G) might be crucial regulators of the ub‐hotspots. We considered Slx5/Slx8 as the prime candidate for the responsible ubiquitin ligase (Fig 1B, D and F) and we set out to investigate how this highly specific recruitment would be established and regulated. Several large‐scale proteomic studies reported Ymr111c/Euc1 as a putative SUMOylation substrate (Zhou et al, 2004; Denison et al, 2005; Hannich et al, 2005) and we found Euc1 to interact with conjugatable SUMO (SUMO‐GG) as well as the SUMOylation enzymes Ubc9 (E2), Siz1, and Siz2 (E3s) in a yeast two‐hybrid experiment (Y2H, Fig 4A). Immunoprecipitation of Euc1 revealed an up‐shifted band that was detected by an anti‐SUMO antibody. This band was diminished in ubc9‐1 and lost in siz1∆ siz2∆ cells (Fig 4B). Mutation of putative SUMOylation consensus sites identified a single point mutation (euc1‐K231R, hereafter euc1‐KR) that affected Euc1 SUMOylation (Fig 4B). In denaturing NiNTA pull‐downs (NiNTA‐PDs) of His‐tagged SUMO, we detected a band corresponding to monoSUMOylated Euc1 and a weaker band further up‐shifted, presumably representing diSUMOylation (Fig 4C). In turn, SUMOylation was strongly reduced in euc1‐KR cells (Fig 4C), indicating that K231 is the major SUMOylation site in Euc1.
Figure 4. Euc1 is SUMOylated, recruits Slx5 to ub‐hotspots, and is ubiquitylated in a Slx5/Slx8‐dependent manner.

-
AEuc1 interacts with SUMO pathway proteins in a yeast two‐hybrid (Y2H) assay. A Y2H reporter strain (PJ69‐7a) was transformed with Gal4 DNA‐binding domain (BD) and Gal4 activation domain (AD) fusion constructs in the indicated combinations. AD‐SUMO‐GG can be conjugated to SUMOylation substrates, while AD‐SUMO‐AA is conjugation‐deficient. Cells were spotted on control media or selective media (‐ His) and grown for 3 and 6 days, respectively.
-
BEuc1 is SUMOylated by Ubc9, Siz1, and Siz2 on lysine 231. Euc1 was immunoprecipitated from the indicated strains, and eluates were probed by WB against SUMO (top) and Euc1 (bottom). An up‐shifted band corresponding to Euc1SUMO was detected with both antibodies. Asterisks denote non‐specific bands.
-
CEuc1 is predominantly monoSUMOylated on lysine 231. Denaturing NiNTA pull‐downs (NiNTA‐PD) with strains expressing His‐tagged SUMO (HisSUMO) as indicated and 3FLAGEuc1 constructs under the control of an ADH promoter. Covalently SUMO‐modified proteins were enriched and eluates probed with a FLAG antibody to visualize SUMOylated Euc1. Eluates were probed for SUMO to control for equal pull‐down, and Pgk1 was probed as input control. Euc1‐KR denotes the K231R mutation here and hereafter.
-
DEuc1 SUMOylation is required for ub‐HS formation. Ub‐K48 ChIP quantified by qPCR for the indicated strains. The ubx5∆ background was used to enhance the ubiquitin signal, and similar results were obtained in a WT background. Data represent means ± SD (n = 3).
-
EEuc1 ubiquitylation depends on Slx8 and partly on Euc1 SUMOylation. Denaturing NiNTA‐PDs as described for (C) but with strains expressing His‐ubiquitin (HisUbi) and 3FLAGEuc1 under the control of the ADH promoter. WBs were developed with a FLAG antibody to probe for 3FLAGEuc1, a ubiquitin blot served as PD‐control and Dpm1 as input control. The slx8‐CD allele carries the C206S and C209S mutations (Xie et al, 2007).
-
F, GSUMOylated Euc1 is required to recruit Slx5 and Slx8 to ub‐HSs. ChIP against Slx5 and Slx8 in the indicated genetic backgrounds was quantified by qPCR. Note that Slx5 is still recruited in the absence of Slx8 (3HA‐Slx5 slx8∆), but not vice versa (Slx8‐9myc slx5∆). Data for WT and 3HA‐Slx5 in (F) and WT and Slx8‐9myc in (G) are from Fig 1D and are shown here for comparison. Data represent means ± SD (n = 2).
Source data are available online for this figure.
Of note, SUMOylation of Euc1 at K231 is required for the formation of ub‐hotspots (Fig 4D). Interestingly, however, Euc1 enrichment itself was also diminished by up to 10‐fold in euc1‐KR cells for two out of three tested sites (Fig EV2A). These data therefore suggest that Euc1 SUMOylation is involved in ub‐hotspot function, by regulating Euc1 DNA binding and/or Slx5/Slx8 recruitment.
Figure EV2. Related to Fig 4. Slx5/Slx8‐mediated ubiquitylation does not lead to fast Euc1 degradation.
-
AEuc1 binding to ub‐HSs drops in euc1‐KR cells. ChIP against Euc1 was quantified by qPCR. Data represent means ± SD (n = 2).
-
BSlx5 is required for Euc1 ubiquitylation. Denaturing NiNTA‐PDs with HisUbi as in Fig 4E. Note that Euc1 ubiquitylation levels were abolished in slx5∆ cells, but also reduced in cdc48‐3 cells.
-
C, DEuc1 levels increase in slx5∆ and for euc1‐KR. Euc1 levels were quantified from WBs of three replicate samples (C) using a LI‐COR Odyssey Fc imaging system and normalized to Pgk1 and wild‐type levels (D). Pgk1 served as loading control. Data represent means ± SD (n = 3).
-
EEuc1 shows slow degradation kinetics. Cells were treated with 0.5 mg/ml cycloheximide (CHX), and samples were taken at the indicated times. Quantification was done as in (C–D). Relative Euc1 signals are normalized to Dpm1 levels and to t = 0.
-
FEuc1 and ub‐K48 ChIP signals do not correlate. ChIP against Euc1 (top) and ub‐K48 (bottom) analyzed by qPCR for all ub‐HSs. Separate primer pairs were used for distinct motif occurrences within ub‐HS1 and ub‐HS4. Note that Euc1 signals did not increase in a cdc48‐3 strain. Data for ub‐K48 ChIP for ub‐HS3/HS5/HS7 are reproduced from Fig 1E for comparison. Data represent means ± SD (n = 2).
-
GEuc1 binding to ub‐HS sites is reduced—rather than increased—in slx5∆ and slx8∆ cells. ChIP against Euc1 was quantified by qPCR in the indicated strains. Note that the binding defect for Euc1 is similar for the euc1‐KR and slx5∆ strains. Data represent means ± SD (n = 2).
-
HSUMOylation is required for recruitment of Slx8‐9myc to ub‐HSs. ChIP against Slx8‐9myc was quantified by qPCR. Data represent means ± SD (n = 3).
-
IGenome‐wide binding profiles of Slx8 in euc1∆ and ubc9‐1 mutant cells. ChIP‐chip was performed as described in Fig 1A. Binding profiles for ub‐K48 (cdc48‐3) and for Slx8‐9myc (WT, cdc48‐3) are reproduced for comparison from Figs EV1A and 1B, respectively. Data represent means from two independent replicates.
Source data are available online for this figure.
SUMOylated Euc1 recruits Slx5/Slx8 to ub‐hotspots
To test whether Euc1 is a ubiquitylation substrate of Slx5/Slx8, we performed denaturing NiNTA‐PDs of His‐ubiquitin to reveal covalently modified ubiquitylation substrates (Fig 4E). Probing for 3FLAGEuc1, we detected an up‐shifted smear, presumably corresponding to polyubiquitylated Euc1 species (Fig 4E). Notably, Euc1 ubiquitylation was almost entirely lost in a strain harboring a catalytically dead Slx8 RING domain variant (slx8‐CD, Fig 4E) and also in slx5∆ cells (Fig EV2B), indicating STUbL‐dependent ubiquitylation. Although steady‐state levels of Euc1 were mildly enhanced in slx5∆ and SUMOylation‐deficient euc1‐KR cells, both WT Euc1 and Euc1‐KR were stable in cycloheximide chase experiments (Fig EV2C–E). We therefore conclude that Slx5/Slx8‐catalyzed ubiquitylation either does not lead to Euc1 degradation or that only a small fraction of Euc1 is regulated by Slx5/Slx8.
Euc1 appears to not be the only ubiquitylation substrate at ub‐hotspots, as Euc1 signals at ub‐hotspots did not correlate with ubiquitylation signals and Euc1 also did not accumulate in cdc48 mutant strains—in contrast to ubiquitylation—or in ubiquitylation‐deficient slx5∆ and slx8∆ strains (Fig EV2F and G, see also Fig EV2B). We therefore hypothesized that Euc1 and its SUMOylation act as cofactors required for Slx5/Slx8 recruitment. Indeed, when we tested enrichment of Slx5 and Slx8 at ub‐hotspot sites in euc1∆ or euc1‐KR strains, both proteins were entirely lost (Fig 4F and G). Consistently, Slx8 recruitment also depended on a functional SUMOylation machinery (Fig EV2H and I). Furthermore, our ChIP data suggested that while Slx5 was recruited in the absence of Slx8 (3HA‐Slx5 slx8∆, Fig 4F), Slx8 recruitment to ub‐hotspots was Slx5‐dependent (Slx8‐9myc slx5∆, Fig 4G).
In summary, our data demonstrate that Euc1 plays a major role in regulating ub‐hotspots by facilitating Slx5/Slx8 recruitment. However, while Euc1 is a Slx5/Slx8 ubiquitylation substrate, it seems likely that there are additional Slx5/Slx8 substrates bound to ub‐hotspots that are cleared from DNA by Cdc48.
Specific interaction sites mediate SUMO‐SIM‐independent Euc1‐Slx5 binding
Substrate targeting of STUbLs is thought to rely on multiple SUMO‐SIM contacts to confer specificity for polySUMOylated substrates (Sriramachandran & Dohmen, 2014). In contrast, Slx5/Slx8 recruitment to ub‐hotspots appears to involve monoSUMOylated Euc1 (Fig 4C). Moreover, ubiquitin enrichment at the ub‐hotspots was unchanged in polySUMO chain‐defective cells expressing only lysine‐free SUMO (SUMO‐KRall) (Fig EV3A). What then underlies the specificity of Slx5/Slx8 recruitment to ub‐hotspots, given the high prevalence of SUMOylation on chromatin (Chymkowitch et al, 2015)?
Figure EV3. Related to Fig 5. Euc181–183 interacts with the Slx5 middle domain (Slx5‐Md, aa 201–335)† .
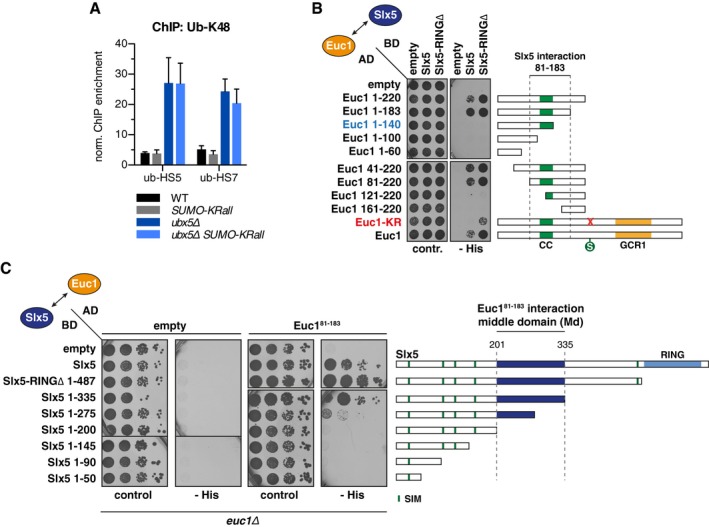
- SUMO‐chain formation is not required for ub‐HS formation. ChIP against ub‐K48 in strains expressing a SUMO variant with all lysines mutated to arginines (SUMO‐KRall) as the only source of SUMO. Enriched DNA was analyzed by qPCR. Data represent means ± SD (n = 3).
- The region of Euc1 required for interaction with Slx5 maps to aa 81–183. Y2H assay to map the Slx5 interaction site on Euc1. Note that SUMOylation‐deficient Euc1‐KR still interacts with Slx5‐RING∆, albeit weaker than wild‐type Euc1 (bottom 2 rows). Cells were grown at 30°C for 2 days.
- The region of Slx5 required to interact with Euc1 maps to aa 201–335. C‐terminal Gal4‐BD‐Slx5 truncation constructs were probed for interaction with Euc181–183 in Y2H. Note that the interaction gradually decreases when truncations between aa 201 and 487 were made. We defined aa 201–335 (middle domain, Slx5‐Md) to be the minimal region required for robust interaction with Euc1 (Fig 5F); however, the region between aa 336 and 487 also contributes to the interaction (compare Slx5‐RING∆ and Slx5‐Md in Fig 5D and G). Cells were grown at 30°C for 3 days.
Our ChIP data suggested that SUMOylated Euc1 interacts with Slx5 to recruit the Slx5/Slx8 complex (Fig 4F), and we could confirm an annotated interaction between Euc1 and Slx5 in Y2H experiments (Figs 5A and EV3B, Appendix Fig S5A and B) (Yu et al, 2008). The Euc1‐Slx5 interaction was further enhanced when we used a Slx5 fragment lacking the C‐terminal RING domain (Slx5‐RING∆). Interestingly, however, Slx5‐RING∆ still interacted with an Euc1‐KR construct lacking the SUMO target site (Fig EV3B). Moreover, recombinant, non‐SUMOylated Euc1 co‐precipitated Slx5 in vitro (Fig 5B), indicating that Euc1 SUMOylation is not strictly required for interaction. We mapped a minimal interacting fragment to aa 81–183 of Euc1 (Figs EV3B and 5A), a stretch lacking the SUMOylation site, but comprising the CC domain, which is crucial for Euc1 dimerization (Appendix Fig S5C and D). Also, co‐immunoprecipitation (co‐IP) experiments with 3FLAGEuc1 showed a robust in vivo interaction with Slx5 that was independent of Euc1 SUMOylation at K231 (Fig 5C, lanes 2–3). Overall, this indicates additional, SUMO‐independent Euc1‐Slx5 interaction sites. Recently, binding of Matα2 to DNA has been shown to be required for Slx5/Slx8‐mediated degradation (Hickey et al, 2018). Euc1, however, was still able to co‐precipitate Slx5 when its DNA‐binding domain was mutated (Euc1‐DBD*, Fig 5C, lane 4).
Figure 5. Specific substrate–ligase interaction sites mediate SUMO‐SIM‐independent Euc1‐Slx5 binding.

- Euc181–183 binds to Slx5 in Y2H assays. Y2H assay performed as in Fig 4A. Serial dilutions were spotted for each plasmid combination, and cells were grown at 30°C for 2 days. The Slx5‐RING∆ construct is deleted for the Slx5 C‐terminus from the beginning of the RING domain (∆aa 488–619). See also Fig EV3B.
- Euc1 binds to Slx5 in vitro. Recombinant purified GST or GSTEuc1 was used in GST pull‐down assays to co‐precipitate recombinant 6HisSlx5.
- Euc1 binds to Slx5 in vivo. Cell lysates from an euc1∆ strain expressing the indicated 3FLAGEuc1 constructs from plasmids (under EUC1 promoter) were subjected to immunoprecipitation using anti‐FLAG beads. IP eluates were probed by WB with Slx5, SUMO, and Euc1 antibodies, and inputs were probed with Slx5, FLAG, and Dpm1 antibodies (top to bottom). Note that the Euc1‐Slx5 interaction is independent of the Slx8 ligase activity (slx8‐CD, lanes 5–8).
- Euc1 Slx5‐binding mutants (SBM1, SBM2) affect Euc1‐Slx5 interaction in Y2H assays. AD‐Euc181‐183 constructs harboring mutations in the region required for Slx5 binding were probed for interaction with BD‐Slx5 constructs as described in Fig 4A. Mutations: SBM1: aa 139–143 ENQKK>ANAAA, SBM2: aa 162–165 KEVF>AAAA. Serial dilutions were spotted, and cells were grown at 30°C for 2 days. See Appendix Fig S6A for expression levels.
- Euc1‐SBM constructs show defective Slx5 binding in vivo. Mutations described in (D) were introduced into full‐length 3FLAGEuc1 constructs (with or without the K231R mutation), and FLAG IPs were performed as described in (C). WB analysis showed strong Slx5‐binding defects for the SBM1/SBM2 and SBM1+2 constructs (top panel, immunoprecipitated Slx5). WBs were probed as in (C).
- The Slx5 middle domain (Slx5‐Md) is required for interaction with Euc1. Y2H assays with AD‐Euc181–183 and BD‐Slx5 constructs. Slx5‐Md: aa 201–335, Slx5‐Md∆: ∆aa 201–338. Serial dilutions were spotted, and cells were grown at 30°C for 4 days. See also Fig EV3C.
- Euc1‐SBM constructs show defective binding to the Slx5‐Md. Y2H assay with the indicated constructs as in (D). See Appendix Fig S6A for expression levels.
- Schematic representation of Euc1, Slx5, and Slx8, domain features, and interactions. Domains and protein lengths are drawn to scale, and numbers below each bar denote amino acid positions. The mapped interaction between Euc1 and Slx5 is indicated by an arrow. CC: coiled‐coil domain, SBM: Slx5‐binding mutant, S: SUMO, SIM: SUMO‐interacting motif.
Source data are available online for this figure.
Truncation of the Euc1 CC domain abolished the interaction with Slx5 (Fig EV3B) as well as Euc1 dimerization in Y2H (Appendix Fig S5C and D); however, Slx5 interaction was also lost by truncation of the region between aa 140 and 183, indicating a potential Slx5‐binding site in this region (compare Fig EV3B and Appendix Fig S5C, Euc1 1–140). To guide our search, we used HH‐MOTiF for de novo motif prediction (Prytuliak et al, 2017) using Euc1 aa 81–183 and a set of putative Slx5 substrates as query. We introduced mutations in predicted binding sites downstream of the CC domain: Two of these strongly diminished binding to Slx5 while leaving dimerization intact (Slx5‐binding mutant 1 and 2, SBM1: aa 139–143 ENQKK>ANAAA, SBM2: aa 162–165 KEVF>AAAA, Fig 5D and Appendix Fig S6A and B). Notably, both mutations strongly reduced the interaction between Euc1 and Slx5 in co‐IP experiments (Fig 5E, lanes 2–5), while additional mutation of the Euc1 SUMOylation site had little effect (KR mutation, Fig 5E, lanes 6–9).
We also mapped the region of Slx5 that mediates Euc1 binding to a previously uncharacterized region between aa 200 and 335 (Fig EV3C). We designate this region, which does not contain any predicted SIMs, Slx5 middle domain (Slx5‐Md). Slx5‐Md is required and sufficient for interaction with the Euc81–183 fragment (Fig 5F) and Slx5‐binding‐deficient mutants of Euc1 strongly diminished the interaction (Fig 5G). We conclude that interaction with Euc1 involves the Slx5‐Md and that SUMO‐SIM contacts are not strictly required, but likely contributing, suggesting bipartite substrate recognition (Fig 5H).
Specific Euc1‐Slx5 interaction sites are required for ub‐hotspots
We tested whether ubiquitylation of Euc1 or ub‐hotspot formation would be affected by mutations that specifically abolish SUMO‐independent contacts between Euc1 and Slx5. Using NiNTA‐PDs with His‐ubiquitin, we found that both Euc1 Slx5‐binding mutants (SBM1 and SBM2), as well as the double mutant, showed a reduction in Euc1 ubiquitylation (Fig 6A, lanes 3–6). When we additionally mutated the Euc1 SUMOylation site (KR), Euc1 ubiquitylation was further decreased (Fig 6A, lanes 7–10). Furthermore, the WT Slx5‐construct complemented the loss of ubiquitylation in an slx5∆ strain (Fig 6B, lane 2), whereas expression of the Slx5‐SIM* construct with all SIMs mutated yielded reduced Euc1 ubiquitylation, and expression of the Slx5‐Md∆ construct abolished Euc1 ubiquitylation (Fig 6B, lanes 3–4).
Figure 6. Specific Euc1‐Slx5 interactions are required for ub‐hotspots and Euc1 ubiquitylation.

- Euc1 Slx5‐binding mutations impair Euc1 ubiquitylation. His‐ubiquitin‐modified proteins were enriched by denaturing NiNTA‐PDs as described in Fig 4E. 3FLAG‐tagged Euc1 constructs were expressed from plasmids under the control of the ADH promoter. NiNTA‐PD eluates were probed with FLAG and ubiquitin (P4D1) antibodies. Whole cell lysates (input) were probed with FLAG and Dpm1 antibodies. Asterisk denotes a non‐specific band.
- The Slx5‐SIMs and Slx5‐Md are required for Euc1 ubiquitylation. NiNTA‐PDs for His‐ubiquitin as in (A). All strains expressed wild‐type 3FLAGEuc1 from plasmids under the ADH promoter and His‐ubiquitin. Slx5 constructs were expressed from plasmids under the control of the endogenous promoter. WBs were probed as described in (A), and Slx5‐levels were probed using an HA antibody. Slx5‐SIM*: SIMs 1–4 were mutated as described (Xie et al, 2010); for SIM5, aa 477–479 (IIV) were mutated to alanines. To avoid possible mislocalization by deletion of the Slx5‐Md, which overlaps with a putative NLS (Westerbeck et al, 2014), an N‐terminal NLS was fused to all constructs. See Appendix Fig S7C for HU complementation.
- Euc1 Slx5‐binding mutations (SBM1/SBM2) reduce/abolish ub‐HSs and recruitment of Slx5 to ub‐HSs. ChIP‐qPCR for ub‐K48 (top) or Slx5 (using Slx5 antibody, bottom) in euc1∆ ubx5∆ cells expressing the indicated constructs from plasmids under the control of the EUC1 promoter. Data represent means ± SD (n = 3). See also Appendix Fig S7E–G.
- Slx5‐SIMs and the Slx5‐Md are required for the formation of ub‐HSs and recruitment of Slx5. ChIP‐qPCR for ub‐K48 (left) or Slx5 (anti‐HA ChIP, right) in ubx5∆ cells or slx5∆ ubx5∆ cells complemented with plasmids expressing the indicated Slx5 constructs under the control of the SLX5 promoter. Data represent means ± SD (n = 2). See also Appendix Fig S7H.
- Schematic model for the proposed sequence of events at ub‐hotspots. See main text (Discussion) for details. SIM: SUMO‐interacting motif, Md: middle domain, S: SUMO, Ub: ubiquitin.
Source data are available online for this figure.
The Slx5‐Md (aa 201–335) overlaps with a stretch that has been implicated in nuclear localization, Slx8 and Slx5 interaction (Westerbeck et al, 2014), as well as an auto‐ubiquitylation‐protective “lysine desert” (Sharma et al, 2017). To exclude hypomorphic effects, we first tested the ability of the SLX5 alleles to complement hypersensitivity of slx5∆ cells on replication stress induced by hydroxyurea (HU) (Xie et al, 2007). The slx5‐Md∆ allele complemented the growth phenotype on HU, while the slx5‐SIM* did not (Appendix Fig S7A–C). Complementation was not influenced by an N‐terminally fused NLS. Second, we monitored turnover of a fragment of the known Slx5/Slx8‐substrate Matα2 (Hickey & Hochstrasser, 2015), which was not affected in slx5‐Md∆ cells, suggesting that the involvement of the Md is substrate‐specific (Appendix Fig S7D).
Last, we tested whether the ubiquitin signal at ub‐hotspots was influenced and found it to be reduced in cells that expressed Euc1‐SBM1 as only copy of Euc1, while it was completely lost in euc1‐SBM2 and euc1‐SBM1+2 cells (Fig 6C, top). As expected, the recruitment of Slx5 to ub‐hotspots was also diminished for euc1‐SBM1 cells and lost for euc1‐SBM2 and euc1‐SBM1+2 cells (Fig 6C, bottom), as was Euc1 binding (Appendix Fig S7E–G), suggesting mutually dependent binding of Euc1 and Slx5/Slx8 to ub‐hotspots. Conversely, we also tested whether the Slx5‐Md and SIMs would be required for ubiquitylation at the ub‐hotspots. When we complemented an slx5∆ ubx5∆ strain with plasmids encoding Slx5 variants, we observed that WT SLX5 was able to restore ub‐hotspots, while the slx5‐SIM* and the slx5‐Md∆ alleles were not (Fig 6D, left). Similar to Euc1‐SBM1/2, mutation of Slx5‐SIMs or deletion of Slx5‐Md led to loss of Slx5 recruitment (Fig 6D, right), and to strongly diminished recruitment of Euc1 itself (Appendix Fig S7H), indicating the presence of an Euc1‐Slx5/Slx8 complex at ub‐hotspots.
Overall, our data point toward a bipartite or multivalent interaction between Euc1 and Slx5/Slx8 where not only Slx5‐SIMs and Euc1 SUMOylation, but also substrate‐specific contacts between Euc1 and Slx5 are critical for Euc1 ubiquitylation and the formation of ub‐hotspots on chromatin (summarized in Fig 6E).
The Slx5/Slx8‐dependent ub‐hotspot pathway controls Euc1 function
The sophisticated STUbL recruitment mechanism suggested that Slx5/Slx8 could regulate Euc1 and ub‐hotspot function. To determine whether Euc1 might be active as a transcriptional activator at ub‐hotspots, we performed transcriptome analysis in euc1∆, euc1‐KR cells and in cells overexpressing EUC1 (pGAL‐EUC1). In euc1∆ cells, only 2 of 15 genes in direct proximity of ub‐hotspots were significantly up‐ or downregulated in expression, with opposing trends (RCO1 up, SSF2 down, Fig 7A, Appendix Fig S8A). More than 150 genes showed a significant change in euc1∆ (P < 0.01), suggesting that Euc1 may play a widespread function in the regulation of gene expression or reflecting a cellular adaptation to loss of EUC1 (Dataset EV6). Gene ontology (GO) term enrichments suggest an upregulation of small molecule metabolic processes (e.g., carboxylic acid and amino acid metabolism, Dataset EV6). Overall, these data do therefore not provide support for a role of Euc1 as direct transcriptional activator of ub‐hotspot adjacent genes, at least not in the tested conditions.
Figure 7. The ub‐hotspot pathway is required to regulate aberrant Euc1 function.

- Deletion of EUC1 does not lead to ub‐HS adjacent gene deregulation, but rather widespread transcriptome changes. Total RNA isolated from WT and euc1∆ cells was polyA‐enriched and sequenced (RNAseq, triplicates). Significance testing was based on the Wald test, see Materials and Methods for details. See also Appendix Fig S8A for quantification of selected transcripts by qPCR. baseMean: mean expression levels across all samples.
- RNAseq transcriptome analysis of EUC1 overexpression as in (A). ∆euc1 cells with pGAL‐EUC1 or pEUC1‐EUC1 (control) integrated at the URA3 locus (YIplac211) were grown to mid‐log phase, and 2% galactose was added for 3 h. See also Appendix Fig S8E.
- EUC1 overexpression is toxic at elevated temperatures and upon exposure to the membrane‐fluidizing drug benzyl alcohol (BenzAlc). For (C, D, F), serial dilutions of the indicated strains were spotted and grown on YPD control (or selective media) plates or under conditions as indicated. See also Fig EV4A and B.
- EUC1 overexpression toxicity depends on DNA binding and transactivation. The indicated EUC1 alleles with endogenous or galactose‐inducible promoters were integrated at the URA3 locus (YIplac211, euc1∆ background) and spotted on glucose control or galactose plates to induce EUC1 overexpression. See also Appendix Fig S9A–C.
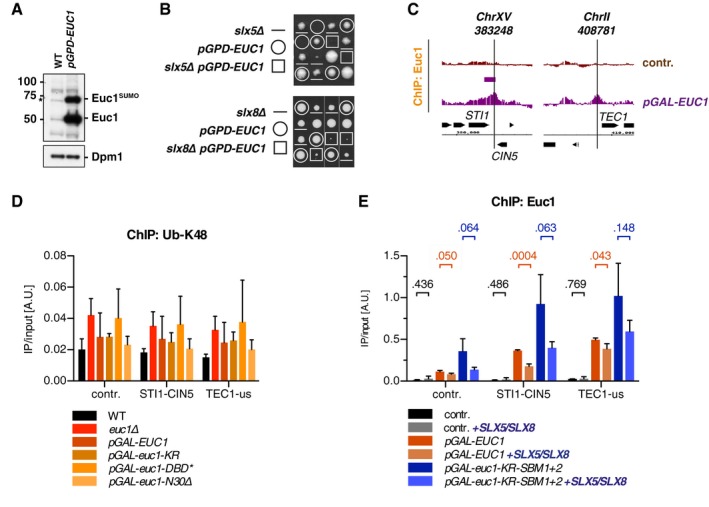
- Aberrant binding of overexpressed EUC1 partially depends on Euc1 DNA binding. ChIP‐qPCR of Euc1 after 3‐h galactose induction. Note that IP/input ratios of Euc1 signals are shown, also for the control locus (contr., TOS1 promoter) to highlight Euc1 binding at non‐ub‐HS sites. STI1‐CIN5: intergenic region. TEC1‐us: upstream (promoter) region. Data represent means ± SD (n = 3). A.U.: arbitrary units. See also Fig EV4C and D.
- Simultaneous overexpression of SLX5 and SLX8 rescue EUC1 toxicity. Experiment as in (D), but with concomitant plasmid‐borne overexpression (ADH promoter) of SLX5 and SLX8 (bottom panels) and on media selecting for SLX5/SLX8 plasmids. See also Fig EV4E and Appendix Fig S9D.
We noticed that mutations affecting Euc1 SUMOylation (euc1‐KR, siz1∆ siz2∆) led to stronger reporter gene activation using Euc1‐N‐Gal4‐BD reporter constructs (Appendix Fig S8B and C). Importantly, however, we failed to detect strongly deregulated genes in a transcriptome analysis of euc1‐KR cells (Appendix Fig S8D, Dataset EV7) putting further in question whether Euc1 acts as a transcription factor in cells.
Supporting this notion, none of the ub‐hotspot adjacent genes were strongly deregulated upon EUC1 overexpression (Fig 7B, Appendix Fig S8E, Dataset EV8). Four of them showed mild up‐ or downregulation, but many other genes showed stronger expression changes, in particular genes involved in cellular metabolism. Interestingly, however, overexpression of EUC1 (pGPD‐EUC1, pGAL‐EUC1) led to lethality at elevated temperatures or upon exposure to the membrane‐fluidizing drug benzyl alcohol (Lone et al, 2015), while deletion of EUC1 did not impair growth under these conditions (Figs 7C and EV4A). In line with a strict control by Slx5/Slx8, overexpressed EUC1 also led to a strong growth phenotype when paired with slx5∆ or slx8∆ (Fig EV4B). Overexpression toxicity was not influenced by mutations that abolish ub‐hotspot formation, such as SUMOylation‐deficient euc1‐KR or Slx5‐binding‐deficient euc1‐SBM1+2 (Fig 7D, Appendix Fig S9A), suggesting that these phenotypes are not caused by over‐active ub‐hotspots. In contrast, overexpression toxicity was dependent on DNA binding and at least in part on the N‐terminal transactivation domain, as well as on the CC domain, that also is essential for transactivation (DBD*, N30∆, CC∆ alleles, Fig 7D, Appendix Fig S9A and B). This effect was not due to impaired nuclear localization, as Euc1‐WT, Euc1‐KR, and Euc1‐DBD* all localized to the nucleus (Appendix Fig S9C). Compared to endogenous Euc1, the overexpressed protein bound to additional loci as tested in ChIP‐chip and confirmed in ChIP‐qPCR (STI1‐CIN5 intergenic, TEC1 upstream (us), Figs 7E and EV4C), and this additional binding was strongly reduced in euc1‐DBD* and euc1‐N30∆ cells. Importantly, binding to these additional loci did not lead to the formation of additional ub‐hotspots, collectively indicating that effects of Euc1 overexpression appear unrelated to ub‐hotspots (Figs 7E and EV4D).
Figure EV4. Related to Fig 7. Slx5/Slx8 alleviates EUC1 overexpression toxicity by curbing ectopic Euc1 localization.
- Western blot against Euc1 (and Dpm1 as loading control) to compare expression levels in WT and pGPD‐EUC1 cells. Asterisk denotes a non‐specific band.
- EUC1 overexpression leads to strong phenotypes or lethality in slx5∆ and slx8∆ cells. Individual cells from tetrads (arranged in vertical columns) were grown on YPD plates at 30°C for 3 days.
- ChIP‐chip tracks from regions showing additional EUC1‐binding signals upon EUC1‐overexpression (pGAL‐EUC1, 3 h induction). Data represent means from two independent replicates.
- Ubiquitin (ub‐K48) ChIP‐qPCR for the same samples as shown in Fig 7E. Data represent means ± SD (n = 3). A.U.: arbitrary units.
- Overexpression of SLX5/SLX8 leads to a reduction of aberrant Euc1 binding to non‐ub‐HS loci. ChIP‐qPCR analysis of selected strains from Fig 7F as indicated, after 3‐h galactose induction. Data represent means ± SD (n = 3). P‐values from Student's t‐tests for the indicated comparisons are shown.
Widespread deregulation of genes upon EUC1 overexpression could reflect direct Euc1‐dependent transcriptional regulation of these genes, but indirect effects are possible as well, given that EUC1 overexpression deregulated other transcription factors, such as CIN5 (a TF mediating pleiotropic drug resistance and salt tolerance) and TEC1 (a TF targeting filamentation genes) (Fig 7B, Appendix Fig S8E, Dataset EV8).
Importantly, overexpression of SLX5/SLX8 (pADH‐SLX5+SLX8) rescued Euc1 toxicity and prevented aberrant binding at STI1‐CIN5 and TEC1‐us loci (Figs 7F and EV4E), but overexpression of the SUMOylation‐ and Slx5‐binding‐deficient euc1‐KR‐SBM1+2 could not be compensated by additional SLX5/SLX8 overexpression (Figs 7F and EV4E, Appendix Fig S9D). Taken together, we conclude that Euc1 needs to be tightly controlled by the STUbL pathway in order to restrict its action to sites of ub‐hotspots and that spurious Euc1 leads to changes in gene expression, likely via its transactivation function. In contrast, endogenous Euc1 seems to have no or only a limited effect on transcription of ub‐hotspot adjacent genes.
EUC1 shows genetic interactions with regulators of gene expression upon thermostress
To explore the function of Euc1 and ub‐hotspots, we investigated genetic interactors of EUC1. Previous high‐throughput studies have suggested candidate genetic interactors that show GO term enrichments for chromatin organization, negative regulation of transcription, histone deacetylation, and related processes (Dataset EV9; Zheng et al, 2010; Costanzo et al, 2010, 2016). Indeed, we observed a negative genetic interaction of EUC1 with HTZ1, encoding for histone H2A.Z and the functionally linked nucleosome remodeling complex SWR1‐C (SWR1 and YAF9 genes), in particular upon thermostress (heat or cold), DMSO, or HU treatment (Fig 8A, Appendix Fig S10A and B). Histone H2A.Z deposition by SWR1‐C has been implicated in the regulation of inducible promoters, heterochromatin maintenance, and genome maintenance (Billon & Côté, 2013). Genetic studies previously revealed sensitivity and interactions of mutants in the H2A.Z‐SWR1‐C pathway upon heat and DMSO stress, and H2A.Z was proposed as a nucleosomal “thermosensor” in A. thaliana and budding yeast (Zhang et al, 2005; Lindstrom et al, 2006; Kumar & Wigge, 2010; Gaytán et al, 2013). The precise molecular basis for DMSO toxicity is unclear. However, interference with membrane organization and inhibition of histone deacetylases (HDACs) have been described (Gurtovenko & Anwar, 2007; Marks & Breslow, 2007). Other negative genetic interactions became apparent with NPL3, an mRNA splicing and processing factor, and STB5, a transcription factor involved in oxidative and multidrug stress responses (Fig 8A, Appendix Fig S10A and C–F).
Figure 8. EUC1 shows genetic interactions with regulators of gene expression upon thermo‐ and DMSO stress.

-
A, BEUC1 displays negative genetic interactions with genes involved in general and specific transcriptional regulation (A), and in particular with members of the Rpd3L histone deacetylase (HDAC) complex (B). For (A–D), serial dilutions of the indicated strains were spotted and grown on YPD control or selective media plates, or under conditions as indicated. For (B), plates were incubated for 2 days (22°C, 30°C, 4% DMSO) or 3 days (37°C). Note that all strains used in (A–D) contained an extra copy of MED11, see Appendix Fig S10C–F for details.
-
CPlasmid‐borne EUC1 complements genetic interactions with Rpd3L subunits. Empty vector (–) or a plasmid encoding EUC1 with its endogenous promoter were transformed in the indicated strains and spotted on selective media.
-
DEUC1 and Rpd3S act in a common pathway which is redundant with Rpd3L. rco1∆ and eaf3∆ (Rpd3S) show similar phenotypes when paired with sds3∆ (Rpd3L) as euc1∆ and are epistatic with euc1∆.
-
EGraphic summary of EUC1 genetic interactions as tested in (A–D) and Appendix Fig S10. Arrows indicate negative genetic interactions upon cold (blue), heat (red), or DMSO stress (black), and dashed gray arrows indicate epistatic relationships.
Most strikingly, deletion of EUC1 led to a pronounced aggravation of the described heat sensitivity of cells deficient in the Rpd3L histone deacetylase complex (dep1∆, rxt2∆, sds3∆, Fig 8B and C) (Ruiz‐Roig et al, 2010). Rpd3 is an orthologue of human class I HDACs and is part of two major HDAC complexes in yeast (Carrozza et al, 2005): (i) Rpd3L, which is primarily recruited to promoter regions for transcriptional repression, but also activation, e.g., upon heat stress; and (ii) Rpd3S, which is recruited to transcribed regions by Set2‐mediated histone H3K36 methylation and has been described to control cryptic intragenic transcription by deacetylating histones within coding regions (de Nadal et al, 2004; Carrozza et al, 2005; Ruiz‐Roig et al, 2010). We note that the pronounced heat and DMSO sensitivity of shared Rpd3L and Rpd3S subunit mutants (rpd3∆, sin3∆) was not further increased by euc1∆ (Appendix Fig S10G and H) and that phenotypes of Rpd3S subunit mutants (rco1∆, eaf3∆) were not further increased by euc1∆ (Fig 8D). Interestingly, combination of mutations in Rpd3S (rco1∆, eaf3∆) and Rpd3L (sds3∆) showed strongly increased sensitivity to heat stress and DMSO, suggesting functional redundancy of the Rpd3L and Rpd3S complexes (Fig 8D). In this background, the effect of Rpd3S mutations was not further aggravated by deletion of EUC1, indicating epistasis and that Euc1 acts with Rpd3S in a pathway that mediates thermotolerance and resistance to DMSO stress (Fig 8D and E). In agreement with our assignment of EUC1 to an Rpd3S‐related function, we found significant correlations of genes downregulated in euc1∆ with those downregulated in published rpd3∆ transcriptomes, but also with datasets for set2∆, which acts as an upstream regulator in the Rpd3S pathway (Appendix Fig S11A) (McKnight et al, 2015; McDaniel et al, 2017).
Euc1‐mediated ub‐hotspots are crucial during stress responses when gene expression control is impaired
To corroborate a potential role of Euc1 in the response to heat stress, we performed ChIP in cells shifted to 37°C and observed a significant increase of Euc1 recruitment to ub‐hotspots, in particular in sds3∆ cells (Fig 9A, Appendix Fig S11B). Similar trends were observed in htz1∆ and npl3∆ cells (Appendix Fig S11C–E). Notably, ubiquitin signals decreased at 37°C, but this effect was also seen for an Euc1‐independent ubiquitin‐bound region (ub‐only‐site1), possibly reflecting a decrease in available free ubiquitin at elevated temperatures (Fig 9A) (Finley et al, 1987). Overall, these data support a model whereby Euc1‐ and Slx5/Slx8‐dependent ub‐hotspots serve a role in gene expression control, which becomes critical upon exposure to thermo‐ and other stress conditions.
Figure 9. Euc1‐mediated ub‐hotspots and Slx5/Slx8 are crucial for a functional stress response under impaired gene expression control.

- Euc1 is recruited to ub‐HSs upon mild heat stress (37°C, 90 min). ChIP‐qPCR experiments with strains and conditions as indicated. Data represent means ± SD (n = 4), see Appendix Fig S11B for statistical analysis.
- The ability to form ub‐hotspots is crucial for endogenous EUC1 function. The genetic interaction of EUC1 with SDS3 (Rpd3L complex) was rescued with plasmid‐borne EUC1 alleles, and serial dilutions were spotted on selective media. (–) denotes empty vector. See also Appendix Fig S12A and B.
- SLX5 also shows negative genetic interactions with SDS3 upon heat stress. Serial dilutions of the indicated strains were spotted on YPD and incubated as indicated. See also Appendix Fig S12C and D. Note that all strains used in (A–C) contained an extra copy of MED11, see Appendix Fig S10C–F for details.
Therefore, we wondered whether transactivation or ub‐hotspot functions would be required in the context of endogenous Euc1 function. Plasmid‐borne expression of Euc1 was used to complement euc1Δ phenotypes. Notably, transactivation‐deficient euc1‐N30∆ and WT EUC1 could both rescue temperature and DMSO sensitivity in sds3∆ and npl3∆ backgrounds (Fig 9B, Appendix Fig S12A). In contrast, euc1 mutants deficient in ub‐hotspot formation (KR, SBM1+2, KR‐SBM1+2, DBD*, CCΔ, Figs 4D and 6C, Appendix Figs S3E and S12B) behaved like euc1∆, suggesting that specifically ub‐hotspots are required for thermotolerance and resistance against DMSO in the context of the Rpd3S pathway (Fig 9B, Appendix Fig S12A). Consistently, we found that deletion of SLX5 and SLX8 enhanced sds3∆ heat sensitivity as well (Fig 9C, Appendix Fig S12C) and that deletion of EUC1 did not further aggravate these phenotypes. Also, these genetic interactions are likely dependent on ub‐hotspot function, as they could be rescued by plasmid‐borne expression of WT SLX5 but only to a minor extent by slx5‐SIM* and slx5‐Md∆ (Appendix Fig S12D).
We therefore conclude that Euc1‐ and Slx5/Slx8‐dependent ub‐hotspots are critical for thermotolerance and the response to other stresses, and that Euc1 is functionally linked to gene regulation by the Rpd3S histone deacetylase.
Discussion
A complex cascade controls proteins bound at ub‐hotspots
Chromatin is a tightly controlled cellular environment. In recent years, STUbLs and the Cdc48/p97 segregase have emerged as components of the ubiquitin–proteasome system that critically control protein–DNA transactions across species (Ramadan et al, 2007; Wilcox & Laney, 2009; Ndoja et al, 2014; Maric et al, 2014; Franz et al, 2016; Nie & Boddy, 2016). By generating genome‐wide ubiquitin and Slx8 binding profiles, in this work we identified specific intergenic sites in the yeast genome—ub‐hotspots—where the SUMO and ubiquitin pathways converge to control the abundance of proteins on DNA.
We elucidated a cascade of events controlling ub‐hotspots suggesting the following model (summarized in Fig 6E): (i) The DNA‐encoded ub‐HS‐motif is bound by Euc1 via its GCR1 domain. (ii) Ubc9, Siz1, or Siz2 modify Euc1 with SUMO, and SUMOylation stabilizes DNA binding of Euc1. (iii) Euc1 recruits Slx5/Slx8 via specific contacts between Euc1 and the middle domain of Slx5 (Slx5‐Md), as well as an additional SUMO‐SIM‐mediated interaction. (iv) Slx5/Slx8 ubiquitylate Euc1 and presumably other targets at ub‐hotspots, and (v) the Cdc48Ufd1‐Npl4 complex, together with Ubx4 and Ubx5, removes K48‐linked ubiquitylated proteins from chromatin. Such extracted, ubiquitylated proteins could be either degraded by the proteasome or recycled by deubiquitylation as it has been found for other TFs (Inui et al, 2011), even if marked with K48‐linked ubiquitin chains (Flick et al, 2004). Euc1 itself does not seem to underlie extensive turnover, as it is a very stable protein (Fig EV2E).
Sequence‐specific binding and the resulting highly localized ChIP signals offer an attractive explanation as to why signals at ub‐hotspots appear so strongly enriched over other events of STUbL binding and ubiquitylation, which are known to occur on chromatin. Importantly, ub‐hotspots exhibit a considerable variability in the quantity of Euc1, Slx5/Slx8, and ubiquitin enrichment (Fig 1B, D and E), with an apparent correlation of Euc1 and Slx5/Slx8 enrichment, but not ubiquitin enrichment (Fig EV2F). This phenomenon could be explained by either variable efficiency of ubiquitylation at different ub‐hotspots or the association of other proteins with the Euc1 complex. These proteins might then become targets of Slx5/Slx8‐dependent ubiquitylation. In line with such an “in trans ubiquitylation” model, other studies have found that STUbLs can also target interaction partners of SUMOylated proteins (Abed et al, 2011; Schweiggert et al, 2016). Notably, this model implies that ubiquitylation substrates may not necessarily be the same for all ub‐hotspots. Overall, such a cascade of binding and modification events at the ub‐hotspots offers ample possibility for regulation and fine‐tuning.
Euc1 and ub‐hotspots function in tolerance to cellular stress
What could be the cellular function of Euc1? Euc1 shares similarity in domain architecture with transcription factors (TFs) of the GCR1 domain family, including DNA‐binding, transactivation, and coiled‐coil dimerization domains (Fig 3A) (Holland et al, 1987; Hohmann, 2002). All three previously characterized GCR1 domain TFs function as transcriptional regulators during the adaptation of cells to changing environmental conditions, such as glucose availability and osmotic stress (reviewed in Hohmann, 2002). Notably, while we find that Euc1 can activate transcription in reporter gene assays, our evidence so far does not suggest that Euc1 would function as transcription factor at ub‐hotspots. It is possible that Euc1's role as transcription factor is only activated upon a specific, currently unknown stimulus, but we favor a model whereby Euc1 exerts its major function through the formation of ub‐hotspots, which seems independent of transactivation (Fig 9B, Appendix Fig S12A).
Our data highlight negative genetic interactions with other players regulating gene expression (H2A.Z‐SWR1‐C pathway, NPL3, STB5, and Rpd3L complex), in particular under stress conditions such as exposure to cold, heat, or DMSO. Consistently, several of the EUC1 interactors have well‐described functions in stress adaptation, apparently by their widespread functions in gene expression (de Nadal et al, 2004; Kumar & Wigge, 2010; Ruiz‐Roig et al, 2010; Moehle et al, 2012; Gaytán et al, 2013). Importantly, our genetic analysis places Euc1 in a functional pathway with the Rpd3S complex (Fig 8D and E). Rpd3, an orthologue of mammalian HDAC1 family enzymes, which generally repress transcription, was shown to be recruited to promoters of osmo‐ and heat stress‐responsive genes, mostly as part of the Rpd3L complex, to activate their expression under stress conditions (de Nadal et al, 2004; Yang & Seto, 2008; Ruiz‐Roig et al, 2010). On the other hand, the Rpd3S complex mainly acts within transcribed regions and is recruited by Set2‐mediated H3K36 methylation to deacetylate histone H4 and establish a repressed state after transcription to prevent spurious cryptic transcription (Carrozza et al, 2005). More recently, cryptic transcripts regulated by this pathway have been demonstrated to also regulate promoter states and transcription of coding transcripts, in particular upon changing nutrient or stress conditions and in aged cells (Sen et al, 2015; Kim et al, 2016; McDaniel et al, 2017).
While the precise mechanism by which Euc1 and Rpd3S cooperate is currently elusive, our data provide clues to guide future research: In euc1∆ cells, several genes are misregulated, including RCO1 of the Rpd3S complex (upregulated) and SIR2, a sirtuin family HDAC (downregulated). Strikingly, HSP12 was downregulated in euc1∆ and upregulated upon EUC1 overexpression. Hsp12 is involved in maintaining membrane organization and is a direct target of Rpd3‐dependent activation upon osmotic stress (de Nadal et al, 2004). Deregulation of cellular membrane homeostasis could also provide a rationale for the observed phenotypes of EUC1 overexpression strains upon exposure to benzyl alcohol, which has been described to interfere with membrane organization (Lone et al, 2015). Additionally, DMSO, which enhances some genetic interactions, can interfere with membrane organization and cells require the H2A.Z pathway for DMSO tolerance (Gurtovenko & Anwar, 2007; Gaytán et al, 2013). Of note, DMSO was also found to cause weak HDAC inhibition (reviewed in Marks & Breslow, 2007), and might thereby also aggravate gene expression defects in already partially compromised backgrounds. In all, our data suggest a model whereby several gene regulatory mechanisms, including ub‐hotspots and Rpd3S, have overlapping functions to allow adaptation to different conditions of cellular stress.
Specificity in the STUbL pathway is achieved by multivalent substrate–ligase contacts
Slx5‐SIMs are required for ubiquitylation of all currently known Slx5/Slx8 substrates. In yeast, several phenotypes such as hydroxyurea sensitivity, as well as accumulation of high‐molecular‐weight SUMO conjugates, can be complemented by the distantly related mammalian RNF4 or even Arkadia/RNF111 variants (Prudden et al, 2007; Sun et al, 2007, 2014; Uzunova et al, 2007). Because complementation is strictly SIM‐dependent, these functions may rely on a “polySUMO‐SIM interaction mode”, which would exclusively involve recognition of polySUMO chains by multiple SIMs, but not necessarily additional substrate recognition features within the different STUbLs. Also in the case of Matα2, intact SIMs of Slx5 have been shown to be required for Matα2 turnover, even though SUMOylation is not required for STUbL‐dependent ubiquitylation of Matα2, possibly because Slx5‐SIMs recognize hydrophobic features on Matα2 (Xie et al, 2010).
In case of Euc1, we have not observed any evidence for long SUMO chains attached to Euc1, suggesting that a “polySUMO‐SIM interaction mode” is unlikely to account for Euc1 recognition. In contrast, our data suggest that not only the SUMO‐SIM interaction, but also additional specific contacts between the central part of Euc1 and the Slx5‐Md are needed for ubiquitylation of Euc1 and possibly other substrates at the ub‐hotspots. It seems intuitive that these interaction surfaces will collectively allow a “bipartite recognition mode” and provide the required affinity/avidity for specific Slx5/Slx8 recruitment. Bipartite substrate recognition has so far not been demonstrated for Slx5/Slx8. However, it is a well‐known mode of interaction in SUMO‐regulated pathways, a prominent example being recognition of PCNA‐SUMO by the helicase Srs2 (Papouli et al, 2005; Pfander et al, 2005; Armstrong et al, 2012).
Notably, bipartite substrate recognition is a more general theme for STUbL enzymes: RNF4 has a basic patch that mediates targeting to nucleosomes (Groocock et al, 2014), as well as an arginine‐rich motif required for interaction with phosphorylated substrates (Kuo et al, 2014; Thomas et al, 2016). Arkadia/RNF111 uses its Mn/Mc domains for substrate interaction and localization (Sun et al, 2014). Degringolade/Dgrn uses its RING domain for interaction with Hairy (Abed et al, 2011). Euc1 recognition by Slx5/Slx8 may therefore serve as a guide for future research elucidating how different substrate recognition domains control the diverse STUbL functions from the response to DNA damage to early embryonic development.
Materials and Methods
Yeast and molecular biology methods
All yeast and molecular biology methods followed standard procedures and are specified in the Appendix Supplementary Methods.
ChIP and ChIP‐on‐chip (ChIP‐chip) analysis
ChIP experiments were performed as described previously with minor modifications (Aparicio et al, 2005; Kalocsay et al, 2009). Briefly, cells were grown to mid‐log phase, crosslinked with formaldehyde, and chromatin was isolated and enriched for the indicated proteins with specific antibodies. Subsequently, input and enriched DNA were quantified by qPCR on a Light Cycler 480 System (Roche) or analyzed on yeast tiling arrays (NimbleGen) for genome‐wide binding data. Genome‐wide binding profiles (ChIP‐chip) were generated from two independent experiments including a dye‐swap, except for ubiquitin (FK2) rad6∆ and IgG WT profiles in Fig 1A. All ChIP‐qPCR data presented are means ± SD from 2 to 5 independent experiments with > 109 cells and triplicate qPCR quantification. Where P‐values are given, an unpaired, two‐tailed Student's t‐test was applied. See Appendix Supplementary Methods for details.
Yeast one‐hybrid (Y1H) screen and reporter gene assays
To isolate proteins binding to the ub‐HS‐motif, two independent Y1H screens were performed. For screen 1, the reporter strain carrying a ubx5∆ allele (MJK391) was derived from YM4271 (Clontech). For screening, three copies of the mapped ub‐HS4‐motif (chromosome XIII 308901‐308939) were cloned upstream of a minimal promoter followed by HIS3 (cloned from pHISi‐1 (Clontech)) and integrated at the URA3 locus. A yeast cDNA library cloned into a vector with an N‐terminally fused Gal4 activation domain (AD) (Dualsystems) was transformed into the strain, and clones were selected on media lacking histidine supplemented with 50 mM 3‐amino‐triazole (3AT). Positive clones were isolated, retested, and sequenced. Screen 2 was performed by Hybrigenics Services SAS (Paris, ULTImate Y1H Screen), with three copies of chromosome XIII 308898‐308939 upstream of a HIS3 reporter gene in UBX5 cells and 2 mM 3AT selection.
For Y1H/reporter gene assays, either plasmids encoding AD‐fusion constructs or untagged proteins were transformed, several clones were mixed, adjusted to OD600 = 0.5, and fivefold serial dilutions were spotted on control or selective media and incubated at 30°C for 2–5 days as indicated.
Transcriptome analysis (RNAseq)
Total RNA was isolated as detailed in Appendix Supplementary Methods, polyA RNA was enriched (NEBNext Poly(A) mRNA Magnetic Isolation Module #E7490), and libraries were prepared for sequencing (NEBNext Ultra II RNA library prep Set for Illumina #E7770L) and barcoded (NEBNext Multiplex Oligo for Illumina #E7335L, #E7500L, #E7710L, #E7730L), all according to the manufacturer's instructions. 75‐bp single‐end reads were obtained by sequencing on an Illumina NextSeq 500 instrument using a NextSeq 500/550 High Output Kit v2 (75 cycles, Illumina). Sequencing reads were aligned to the yeast transcriptome (ENSEMBL R64‐1‐1, annotation version 94) using STAR (v. 2.6.0a). Read counts per gene were provided by STAR, and TPM expression values were calculated with RSEM (v. 1.3.0). We used the unfiltered count table for differential expression analysis in DESeq2 (v. 1.22.2). Based on the standard pipeline, we estimated size factors and dispersion values for each gene and fitted a generalized linear model with a single factor “genotype”. Significance testing was based on the Wald test (default parameters). The results were extracted with an alpha of 0.1, an lfcThreshold of 0, and independent filtering (default parameters).
Microarray‐based transcriptome analysis was performed using GeneChIP Yeast Genome 2.0 arrays (Affymetrix) and is detailed in the Appendix Supplementary Methods.
Biochemical methods
For co‐immunoprecipitation, cells were lysed under native buffer conditions and cleared lysate was subjected to immunoprecipitation with either specific Euc1 antibody or anti‐FLAG resin (M2, Sigma). Eluted proteins were probed by Western blot (WB). See Appendix Supplementary Methods for details.
To detect proteins covalently modified by either SUMO or ubiquitin, yeast strains expressing either N‐terminally histidine‐tagged SUMO (7 histidines) or ubiquitin (6 histidines) under the control of the ADH1 promoter, integrated at the URA3 locus, were used. Subsequently, NiNTA‐PDs using either NiNTA agarose (Figs 4E and EV2B) or magnetic agarose beads (all others, both Qiagen) were performed as described (Psakhye & Jentsch, 2016).
Additional materials and methods can be found in the Appendix Supplementary Methods; for yeast strains, see Appendix Table S1, plasmids Appendix Table S2, qPCR primers Appendix Table S3.
Author contributions
MH, MJK, BP, and SJ designed experiments and analyzed data. MH performed experiments for Figs 1B and D–F, and 2E, G and H, and 3 and 4C, E and F, and 4, 5, 6, 7 and EV1H and EV2A–G and I, and EV3B and C, and EV4, Appendix Figs S2E, S3–S7, S8A–C and E, and S9–S12. MJK performed experiments for Figs 1A and B, and F–G and 2A–D and F, and 4A and B, and D, and EV1A–G and EV2H and EV3A and B, Appendix Figs S1, S2A–D and S8D. TS performed analysis of ChIP‐chip and RNAseq data. RP and BHH performed analysis of de novo motif searches. MH and BP wrote the manuscript, and all authors commented on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Dataset EV4
Dataset EV5
Dataset EV6
Dataset EV7
Dataset EV8
Dataset EV9
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Acknowledgements
We thank S. Brill, A. Buchberger, and M. Hochstrasser for materials; Sven Schkölziger, Alexander Strasser, Katrin Strasser, Jochen Rech, Florian Paasch, and Ludwig Rieger for technical assistance; Kerstin Maier and Assa Yeroslaviz for help with microarray analysis; Marja Driessen for help with RNA sequencing; Julian Stingele and Florian Wilfling for providing data; Johannes Heipke for plasmid construction; Julian Stingele, Ivan Psakhye, Neysan Donnelly, Florian Paasch, and members of the Jentsch and Pfander laboratories for fruitful discussions and critical reading of the manuscript.
Research in the B.P. laboratory is supported by Max Planck Society, the collaborative research cluster (SFB1064), and project grants from the German Research Council (DFG). Research in the S.J. laboratory is supported by Max Planck Society, Deutsche Forschungsgemeinschaft, Center for Integrated Protein Science Munich, Louis‐Jeantet Foundation, and a European Research Council (ERC) Advanced Grant (#339176). M.J.K. was supported by a fellowship of the Boehringer Ingelheim Fonds (BIF).
The EMBO Journal (2019) 38: e100368
Data availability
Yeast strains and plasmids are available on request. ChIP‐chip, RNAseq, and microarray data are available from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) entry GSE118818.
References
- Abed M, Barry KC, Kenyagin D, Koltun B, Phippen TM, Delrow JJ, Parkhurst SM, Orian A (2011) Degringolade, a SUMO‐targeted ubiquitin ligase, inhibits Hairy/Groucho‐mediated repression. EMBO J 30: 1289–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio O, Geisberg JV, Sekinger E, Yang A, Moqtaderi Z, Struhl K (2005) Chromatin immunoprecipitation for determining the association of proteins with specific genomic sequences in vivo . Curr Protoc Mol Biol Chapter 21: Unit 21.3 [DOI] [PubMed] [Google Scholar]
- Armstrong AA, Mohideen F, Lima CD (2012) Recognition of SUMO‐modified PCNA requires tandem receptor motifs in Srs2. Nature 483: 59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37: W202–W208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenkapp H, Borgmeyer U, Sippel AE, Klempnauer KH (1988) Viral myb oncogene encodes a sequence‐specific DNA‐binding activity. Nature 335: 835–837 [DOI] [PubMed] [Google Scholar]
- Billon P, Côté J (2013) Precise deposition of histone H2A.Z in chromatin for genome expression and maintenance. Biochim Biophys Acta 1819: 290–302 [DOI] [PubMed] [Google Scholar]
- Braun S, Madhani HD (2012) Shaping the landscape: mechanistic consequences of ubiquitin modification of chromatin. EMBO Rep 13: 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchberger A, Schindelin H, Hänzelmann P (2015) Control of p97 function by cofactor binding. FEBS Lett 589: 2578–2589 [DOI] [PubMed] [Google Scholar]
- Carrozza MJ, Li B, Florens L, Suganuma T, Swanson SK, Lee KK, Shia W‐J, Anderson S, Yates J, Washburn MP et al (2005) Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123: 581–592 [DOI] [PubMed] [Google Scholar]
- Churikov D, Charifi F, Eckert‐Boulet N, Silva S, Simon M‐N, Lisby M, Géli V (2016) SUMO‐dependent relocalization of eroded telomeres to nuclear pore complexes controls telomere recombination. Cell Rep 15: 1–13 [DOI] [PubMed] [Google Scholar]
- Chymkowitch P, Nguéa PA, Aanes H, Koehler CJ, Thiede B, Lorenz S, Meza‐Zepeda LA, Klungland A, Enserink JM (2015) Sumoylation of Rap1 mediates the recruitment of TFIID to promote transcription of ribosomal protein genes. Genome Res 25: 897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M, Baryshnikova A, Bellay J, Kim Y, Spear ED, Sevier CS, Ding H, Koh JLY, Toufighi K, Mostafavi S et al (2010) The genetic landscape of a cell. Science 327: 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M, VanderSluis B, Koch EN, Baryshnikova A, Pons C, Tan G, Wang W, Usaj M, Hanchard J, Lee SD et al (2016) A global genetic interaction network maps a wiring diagram of cellular function. Science 353: aaf1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison C, Rudner AD, Gerber SA, Bakalarski CE, Moazed D, Gygi SP (2005) A proteomic strategy for gaining insights into protein sumoylation in yeast. Mol Cell Proteomics 4: 246–254 [DOI] [PubMed] [Google Scholar]
- Espelin CW, Simons KT, Harrison SC, Sorger PK (2003) Binding of the essential Saccharomyces cerevisiae kinetochore protein Ndc10p to CDEII. Mol Biol Cell 14: 4557–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Ozkaynak E, Varshavsky A (1987) The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell 48: 1035–1046 [DOI] [PubMed] [Google Scholar]
- Flick K, Ouni I, Wohlschlegel JA, Capati C, McDonald WH, Yates JR, Kaiser P (2004) Proteolysis‐independent regulation of the transcription factor Met4 by a single Lys 48‐linked ubiquitin chain. Nat Cell Biol 6: 634–641 [DOI] [PubMed] [Google Scholar]
- Franz A, Ackermann L, Hoppe T (2016) Ring of change: CDC48/p97 drives protein dynamics at chromatin. Front Genet 7: 73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytán BD, Loguinov AV, La Rosa De VY, Lerot J‐M, Vulpe CD (2013) Functional genomics indicates yeast requires Golgi/ER transport, chromatin remodeling, and DNA repair for low dose DMSO tolerance. Front Genet 4: 154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groocock LM, Nie M, Prudden J, Moiani D, Wang T, Cheltsov A, Rambo RP, Arvai AS, Hitomi C, Tainer JA et al (2014) RNF4 interacts with both SUMO and nucleosomes to promote the DNA damage response. EMBO Rep 15: 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtovenko AA, Anwar J (2007) Modulating the structure and properties of cell membranes: the molecular mechanism of action of dimethyl sulfoxide. J Phys Chem B 111: 10453–10460 [DOI] [PubMed] [Google Scholar]
- Hannich JT, Lewis A, Kroetz MB, Li S‐J, Heide H, Emili A, Hochstrasser M (2005) Defining the SUMO‐modified proteome by multiple approaches in Saccharomyces cerevisiae . J Biol Chem 280: 4102–4110 [DOI] [PubMed] [Google Scholar]
- Hendriks IA, Vertegaal ACO (2016) A comprehensive compilation of SUMO proteomics. Nat Rev Microbiol 17: 581–595 [DOI] [PubMed] [Google Scholar]
- Hickey CM, Hochstrasser M (2015) STUbL‐mediated degradation of the transcription factor MATα2 requires degradation elements that coincide with corepressor binding sites. Mol Biol Cell 26: 3401–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey CM, Xie Y, Hochstrasser M (2018) DNA binding by the MATα2 transcription factor controls its access to alternative ubiquitin‐modification pathways. Mol Biol Cell 29: 542–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66: 300–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland MJ, Yokoi T, Holland JP, Myambo K, Innis MA (1987) The GCR1 gene encodes a positive transcriptional regulator of the enolase and glyceraldehyde‐3‐phosphate dehydrogenase gene families in Saccharomyces cerevisiae . Mol Cell Biol 7: 813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horigome C, Bustard DE, Marcomini I, Delgoshaie N, Tsai‐Pflugfelder M, Cobb JA, Gasser SM (2016) PolySUMOylation by Siz2 and Mms21 triggers relocation of DNA breaks to nuclear pores through the Slx5/Slx8 STUbL. Genes Dev 30: 931–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie MA, Scott EW, Drazinic CM, Lopez MC, Hornstra IK, Yang TP, Baker HV (1992) Characterization of the DNA‐binding activity of GCR1: in vivo evidence for two GCR1‐binding sites in the upstream activating sequence of TPI of Saccharomyces cerevisiae . Mol Cell Biol 12: 2690–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui M, Manfrin A, Mamidi A, Martello G, Morsut L, Soligo S, Enzo E, Moro S, Polo S, Dupont S et al (2011) USP15 is a deubiquitylating enzyme for receptor‐activated SMADs. Nat Cell Biol 13: 1368–1375 [DOI] [PubMed] [Google Scholar]
- Jentsch S, McGrath JP, Varshavsky A (1987) The yeast DNA repair gene RAD6 encodes a ubiquitin‐conjugating enzyme. Nature 329: 131–134 [DOI] [PubMed] [Google Scholar]
- Kalocsay M, Hiller NJ, Jentsch S (2009) Chromosome‐wide Rad51 spreading and SUMO‐H2A.Z‐dependent chromosome fixation in response to a persistent DNA double‐strand break. Mol Cell 33: 335–343 [DOI] [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10: 845–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee BB, Oh YM, Zhu C, Steinmetz LM, Lee Y, Kim WK, Lee SB, Buratowski S, Kim T (2016) Modulation of mRNA and lncRNA expression dynamics by the Set2‐Rpd3S pathway. Nat Commun 7: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA (2010) H2A.Z‐containing nucleosomes mediate the thermosensory response in Arabidopsis . Cell 140: 136–147 [DOI] [PubMed] [Google Scholar]
- Kuo C‐Y, Li X, Kong X‐Q, Luo C, Chang C‐C, Chung Y, Shih H‐M, Li KK, Ann DK (2014) An arginine‐rich motif of ring finger protein 4 (RNF4) oversees the recruitment and degradation of the phosphorylated and SUMOylated Krüppel‐associated box domain‐associated protein 1 (KAP1)/TRIM28 protein during genotoxic stress. J Biol Chem 289: 20757–20772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Singh N, Carlson CR, Albuquerque CP, Corbett KD, Zhou H (2017) Recruitment of a SUMO isopeptidase to rDNA stabilizes silencing complexes by opposing SUMO targeted ubiquitin ligase activity. Genes Dev 31: 802–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom KC, Vary JC, Parthun MR, Delrow J, Tsukiyama T (2006) Isw1 functions in parallel with the NuA4 and Swr1 complexes in stress‐induced gene repression. Mol Cell Biol 26: 6117–6129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone MA, Atkinson AE, Hodge CA, Cottier S, Martínez‐Montañés F, Maithel S, Mène‐Saffrané L, Cole CN, Schneiter R (2015) Yeast integral membrane proteins Apq12, Brl1, and Brr6 form a complex important for regulation of membrane homeostasis and nuclear pore complex biogenesis. Eukaryot Cell 14: 1217–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric M, Maculins T, De Piccoli G, Labib K (2014) Cdc48 and a ubiquitin ligase drive disassembly of the CMG helicase at the end of DNA replication. Science 346: 1253596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PA, Breslow R (2007) Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 25: 84–90 [DOI] [PubMed] [Google Scholar]
- McDaniel SL, Hepperla AJ, Huang J, Dronamraju R, Adams AT, Kulkarni VG, Davis IJ, Strahl BD (2017) H3K36 methylation regulates nutrient stress response in Saccharomyces cerevisiae by enforcing transcriptional fidelity. Cell Rep 19: 2371–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight JN, Boerma JW, Breeden LL, Tsukiyama T (2015) Global promoter targeting of a conserved lysine deacetylase for transcriptional shutoff during quiescence entry. Mol Cell 59: 732–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle EA, Ryan CJ, Krogan NJ, Kress TL, Guthrie C (2012) The yeast SR‐like protein Npl3 links chromatin modification to mRNA processing. PLoS Genet 8: e1003101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ (2001) Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae . Genetics 157: 103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadal E, Zapater M, Alepuz PM, Sumoy L, Mas G, Posas F (2004) The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes. Nature 427: 370–374 [DOI] [PubMed] [Google Scholar]
- Nagai S, Dubrana K, Tsai‐Pflugfelder M, Davidson MB, Roberts TM, Brown GW, Varela E, Hediger F, Gasser SM, Krogan NJ (2008) Functional targeting of DNA damage to a nuclear pore‐associated SUMO‐dependent ubiquitin ligase. Science 322: 597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndoja A, Cohen RE, Yao T (2014) Ubiquitin signals proteolysis‐independent stripping of transcription factors. Mol Cell 53: 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie M, Boddy M (2016) Cooperativity of the SUMO and ubiquitin pathways in genome stability. Biomolecules 6: 14–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuni K, Takahashi Y, Fulp A, Lawrimore J, Au W‐C, Pasupala N, Levy‐Myers R, Warren J, Strunnikov A, Baker RE et al (2016) SUMO‐targeted ubiquitin ligase (STUbL) Slx5 regulates proteolysis of centromeric histone H3 variant Cse4 and prevents its mislocalization to euchromatin. Mol Biol Cell 27: 1500–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouli E, Chen S, Davies AA, Huttner D, Krejci L, Sung P, Ulrich HD (2005) Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol Cell 19: 123–133 [DOI] [PubMed] [Google Scholar]
- van de Pasch LAL, Miles AJ, Nijenhuis W, Brabers NACH, van Leenen D, Lijnzaad P, Brown MK, Ouellet J, Barral Y, Kops GJPL et al (2013) Centromere binding and a conserved role in chromosome stability for SUMO‐dependent ubiquitin ligases. PLoS ONE 8: e65628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfander B, Moldovan G‐L, Sacher M, Hoege C, Jentsch S (2005) SUMO‐modified PCNA recruits Srs2 to prevent recombination during S phase. Nature 436: 428–433 [DOI] [PubMed] [Google Scholar]
- Prudden J, Pebernard S, Raffa G, Slavin DA, Perry JJP, Tainer JA, McGowan CH, Boddy MN (2007) SUMO‐targeted ubiquitin ligases in genome stability. EMBO J 26: 4089–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prytuliak R, Volkmer M, Meier M, Habermann BH (2017) HH‐MOTiF: de novo detection of short linear motifs in proteins by hidden markov model comparisons. Nucleic Acids Res 45: W470–W477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psakhye I, Jentsch S (2016) Identification of substrates of protein‐group SUMOylation. Methods Mol Biol 1475: 219–231 [DOI] [PubMed] [Google Scholar]
- Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, Meyer HH (2007) Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature 450: 1258–1262 [DOI] [PubMed] [Google Scholar]
- Ramos PC, Höckendorff J, Johnson ES, Varshavsky A, Dohmen RJ (1998) Ump1p is required for proper maturation of the 20S proteasome and becomes its substrate upon completion of the assembly. Cell 92: 489–499 [DOI] [PubMed] [Google Scholar]
- Rape M, Hoppe T, Gorr I, Kalocay M, Richly H, Jentsch S (2001) Mobilization of processed, membrane‐tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin‐selective chaperone. Cell 107: 667–677 [DOI] [PubMed] [Google Scholar]
- Richly H, Rape M, Braun S, Rumpf S, Hoege C, Jentsch S (2005) A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell 120: 73–84 [DOI] [PubMed] [Google Scholar]
- Robzyk K, Recht J, Osley MA (2000) Rad6‐dependent ubiquitination of histone H2B in yeast. Science 287: 501–504 [DOI] [PubMed] [Google Scholar]
- Ruiz‐Roig C, Viéitez C, Posas F, de Nadal E (2010) The Rpd3L HDAC complex is essential for the heat stress response in yeast. Mol Microbiol 76: 1049–1062 [DOI] [PubMed] [Google Scholar]
- Schweiggert J, Stevermann L, Panigada D, Kammerer D, Liakopoulos D (2016) Regulation of a spindle positioning factor at kinetochores by SUMO‐targeted ubiquitin ligases. Dev Cell 36: 415–427 [DOI] [PubMed] [Google Scholar]
- Sen P, Dang W, Donahue G, Dai J, Dorsey J, Cao X, Liu W, Cao K, Perry R, Lee JY et al (2015) H3K36 methylation promotes longevity by enhancing transcriptional fidelity. Genes Dev 29: 1362–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Mullen JR, Li M, Zaratiegui M, Bunting SF, Brill SJ (2017) A lysine desert protects a novel domain in the Slx5‐Slx8 SUMO targeted Ub ligase to maintain sumoylation levels in Saccharomyces cerevisiae . Genetics 206: 1807–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler PB (1988) Transcriptional activation. Acid blobs and negative noodles. Nature 333: 210–212 [DOI] [PubMed] [Google Scholar]
- Sriramachandran AM, Dohmen RJ (2014) SUMO‐targeted ubiquitin ligases. Biochim Biophys Acta 1843: 75–85 [DOI] [PubMed] [Google Scholar]
- Su XA, Dion V, Gasser SM, Freudenreich CH (2015) Regulation of recombination at yeast nuclear pores controls repair and triplet repeat stability. Genes Dev 29: 1006–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Leverson JD, Hunter T (2007) Conserved function of RNF4 family proteins in eukaryotes: targeting a ubiquitin ligase to SUMOylated proteins. EMBO J 26: 4102–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Liu Y, Hunter T (2014) Multiple Arkadia/RNF111 structures coordinate its polycomb body association and transcriptional control. Mol Cell Biol 34: 2981–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham MH, Geoffroy M‐C, Shen L, Plechanovová A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT (2008) RNF4 is a poly‐SUMO‐specific E3 ubiquitin ligase required for arsenic‐induced PML degradation. Nat Cell Biol 10: 538–546 [DOI] [PubMed] [Google Scholar]
- Thomas JJ, Abed M, Heuberger J, Novak R, Zohar Y, Lopez APB, Trausch‐Azar JS, Ilagan MXG, Benhamou D, Dittmar G et al (2016) RNF4‐dependent oncogene activation by protein stabilization. Cell Rep 16: 3388–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu YM, Van Riper SK, Higgins L, Zhang T, Becker JR, Markowski TW, Nguyen HD, Griffin TJ, Bielinsky AK (2016) Slx5/Slx8 promotes replication stress tolerance by facilitating mitotic progression. Cell Rep 15: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova K, Göttsche K, Miteva M, Weisshaar SR, Glanemann C, Schnellhardt M, Niessen M, Scheel H, Hofmann K, Johnson ES et al (2007) Ubiquitin‐dependent proteolytic control of SUMO conjugates. J Biol Chem 282: 34167–34175 [DOI] [PubMed] [Google Scholar]
- Verma R, Oania R, Fang R, Smith GT, Deshaies RJ (2011) Cdc48/p97 mediates UV‐dependent turnover of RNA Pol II. Mol Cell 41: 82–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Prelich G (2009) Quality control of a transcriptional regulator by SUMO‐targeted degradation. Mol Cell Biol 29: 1694–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerbeck JW, Pasupala N, Guillotte M, Szymanski E, Matson BC, Esteban C, Kerscher O (2014) A SUMO‐targeted ubiquitin ligase is involved in the degradation of the nuclear pool of the SUMO E3 ligase Siz1. Mol Biol Cell 25: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Laney JD (2009) A ubiquitin‐selective AAA‐ATPase mediates transcriptional switching by remodelling a repressor‐promoter DNA complex. Nat Cell Biol 11: 1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Kerscher O, Kroetz MB, McConchie HF, Sung P, Hochstrasser M (2007) The yeast Hex3.Slx8 heterodimer is a ubiquitin ligase stimulated by substrate sumoylation. J Biol Chem 282: 34176–34184 [DOI] [PubMed] [Google Scholar]
- Xie Y, Rubenstein EM, Matt T, Hochstrasser M (2010) SUMO‐independent in vivo activity of a SUMO‐targeted ubiquitin ligase toward a short‐lived transcription factor. Genes Dev 24: 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X‐J, Seto E (2008) The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol 9: 206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Braun P, Yildirim MA, Lemmens I, Venkatesan K, Sahalie J, Hirozane‐Kishikawa T, Gebreab F, Li N, Simonis N et al (2008) High‐quality binary protein interaction map of the yeast interactome network. Science 322: 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Roberts DN, Cairns BR (2005) Genome‐wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123: 219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Benschop JJ, Shales M, Kemmeren P, Greenblatt J, Cagney G, Holstege F, Li H, Krogan NJ (2010) Epistatic relationships reveal the functional organization of yeast transcription factors. Mol Syst Biol 6: 420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ryan JJ, Zhou H (2004) Global analyses of sumoylated proteins in Saccharomyces cerevisiae . J Biol Chem 279: 32262–32268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Dataset EV4
Dataset EV5
Dataset EV6
Dataset EV7
Dataset EV8
Dataset EV9
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Data Availability Statement
Yeast strains and plasmids are available on request. ChIP‐chip, RNAseq, and microarray data are available from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) entry GSE118818.


