Abstract
The involvement of glucose in fundamental metabolic pathways represents a core element of biology. Late in the 20th century, a unique glucose-derived signal was discovered, which appeared to be involved in a variety of cellular processes, including mitosis, transcription, insulin signaling, stress responses, and potentially, Alzheimer’s disease, and diabetes. By definition, this glucose-fed signaling system was a posttranslational modification to proteins. However, unlike classical cotranslational N-glycosylation occurring in the endoplasmic reticulum and Golgi apparatus, this process occurs elsewhere throughout the cell in a highly dynamic fashion, similar to the quintessential posttranslational modification, phosphorylation. This more recently described posttranslational modification, the β-O-linkage of N-acetylglucosamine (i.e., O-GlcNAc) to nucleocytoplasmic proteins, represents an under-investigated area of biology. This signaling system operates in all of the tissues examined and seems to have persisted throughout all multi-cellular eukaryotes. Thus, it comes with little surprise that O-GlcNAc signaling is an integral system and viable target for biomedical investigation. This system may be a boundless source for insight into a variety of diseases and yield numerous opportunities for drug design. This Perspective will address recent insights into O-GlcNAc signaling in the cardiovascular system as a paradigm for its involvement in other biological systems.
What is O-GlcNAc?
The β-O-linkage of N-acetylglucosamine (O-GlcNAc) to nucleocytoplasmic proteins represents a unique mechanism of intracellular signaling (Zachara and Hart, 2006; Hart et al., 2007). As glucose enters a cell and becomes phosphorylated by hexokinase, it can be diverted from the main glycolytic/ glycogen pathways into accessory pathways. One such pathway is the hexosamine biosynthetic pathway (HBP) (see Fig. 1A). The first step in the HBP, catalyzed by glutamine/fructose amidotransferase (GFAT), commits glucose to this pathway and is rate-limiting. This step generally links glycolytic metabolism to amino acid metabolism via the requirement of glutamine as the amide source. Continued modification of the hexosamine core occurs through the addition of an acetyl group, thereby potentially linking this pathway to fatty acid oxidation. The HBP culminates in the formation of UDP-GlcNAc, the unique monosaccharide donor for the post-translational modification known as O-GlcNAc. Although UDP-GlcNAc is the sugar donor for the O-GlcNAc modification, it is noteworthy that UDP-GlcNAc also contributes to glycophosphatidylinositol lipids, N-glycosylation, and other cellular processes.
Fig. 1.
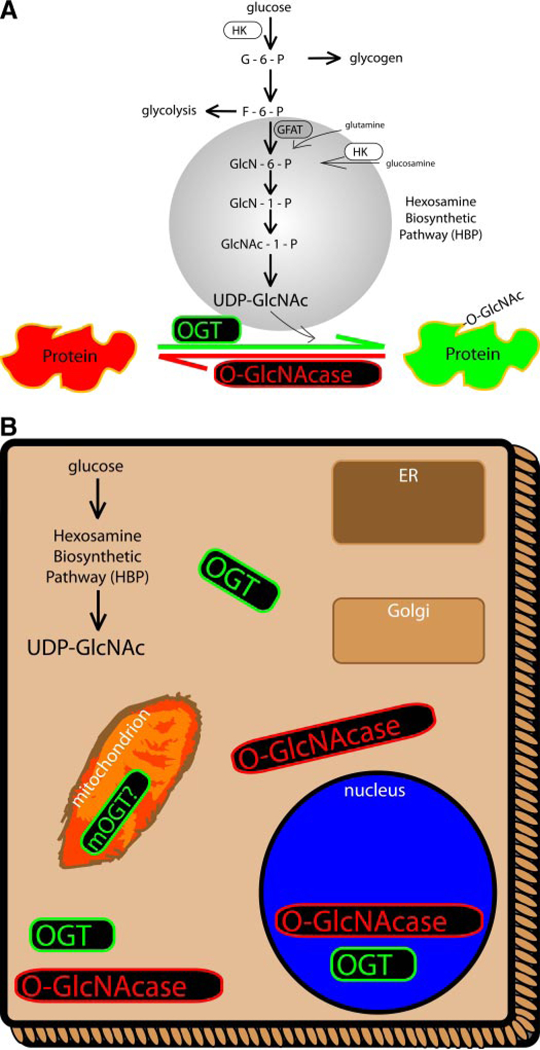
A, hexosamine biosynthetic pathway. Upon entry into the cell, glucose is phosphorylated, most of which is destined for glycolysis or glycogen storage. A small percentage of F-6-P can be diverted to the HBP, culminating in the formation of the monosaccharide, UDP-GlcNAc. Although UDP-GlcNAc pools are drawn upon by several other cellular processes, this sugar serves as the donor for the O-GlcNAc post-translational modification. B, intracellular localization of the enzymes responsible for adding (OGT) and removing (O-GlcNAcase) the O-GlcNAc post-translational modification from proteins. Both enzymes are located throughout the cytoplasm and nucleus. These enzymes are not generally associated with the endoplasmic reticulum (ER)-Golgi complex. Some evidence indicates a potential mitochondrial isoform of OGT. Such varied distribution yields numerous proteins that are O-GlcNAc-modified, including transcription factors.
Two antagonistic enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase, directly control the presence of the O-GlcNAc post-translational modification on proteins. OGT catalyzes the addition of a single GlcNAc moiety (from UDP-GlcNAc) to serine and/or threonine sites on nascent and mature proteins throughout the cell. Conversely, O-GlcNA-case (sometimes OGA, GCA, NCOAT, or mgea5 in the literature) removes O-GlcNAc from proteins. There has been no clear documentation of further elongation of the O-GlcNAc post-translational modification in mammalian systems. Both enzymes share general nucleocytoplasmic expression and lack significant endoplasmic reticulum or Golgi expression (see Fig. 1B). The sequences for both enzymes are highly conserved across all of the examined eukaryotes, and only one gene seems responsible for each enzyme. OGT has three potential isoforms arising from alternate splicing, including a mitochondrial isoform (Hanover et al., 2003; Love et al., 2003). A unique aspect of the O-GlcNAcase enzyme is the presence of a histone acetyltransferase domain, suggesting a possible role for histone acetyltransferase domain of O-Glc-NAcase in the transcriptional regulation by O-GlcNAc (Tole- man et al., 2004). Several transcription factors have been identified to be regulated by O-GlcNAc-modification (Kelly et al., 1993; Yang et al., 2001, 2002; Lamarre-Vincent and Hsieh-Wilson, 2003). Interestingly, O-GlcNAc can either compete for the same Ser or Thr hydroxyl sites as phosphorylation, such as on c-Myc (Kamemura et al., 2002) and estrogen receptor-β (Cheng et al., 2000), occupy adjacent sites such as on synapsin I (Cole and Hart, 1999) and the tumor suppressor p53 (Yang et al., 2006), or occur at distant sites from phosphorylation such as on the c-terminal domain of RNA polymerase II (Comer and Hart, 2001) and cytokeratin (Chou, 1992).
Although certain aspects of such regulation have been described, many questions remain unanswered in cells and tissues of the cardiovascular system. Moreover, basic questions regarding the identity of the promoters for OGT and O-GlcNAcase have gone unreported. Such insights would provide invaluable information regarding transcriptional control of O-GlcNAc signaling. The relevant question is “how does this signaling system affect cellular function in health and disease?”. To answer such questions, it is important to recognize some of the basic biological roles of the O-GlcNAc signaling system. Subsequent to its discovery by Torres and Hart (1984), O-GlcNAc has been implicated in a variety of cellular processes, both physiologic and pathologic. Such diverse roles for O-GlcNAc signaling have been reviewed elsewhere (Fülöp et al., 2007a; Hart et al., 2007) and include processes as varied as proteasomal function (Zhang et al., 2003), Alzheimer’s disease (Liu et al., 2004), insulin signaling (Yang et al., 2008), angiogenesis (Luo et al., 2008), mitosis (Slawson et al., 2005), and metabolic integration (Luo et al., 2007; Cheung and Hart, 2008; Dentin et al., 2008). In this Perspective, we address techniques to assess O-GlcNAc signaling and focus on the involvement of O-GlcNAc signaling in diabetes and in acute myocardial injury.
O-GlcNAc Detection Methods
Despite its discovery two decades ago, few protein targets have been unequivocally and site-specifically identified as carrying an O-GlcNAc modification. This is due to the lack of specific and sensitive methods, which are further limited by the relatively labile nature of the modification, its limited mass, and its lack of charge (Whelan and Hart, 2003). One of the earlier developed approaches relies upon the use of exogenous galactosyltransferase to transfer [3H]galactose from the donor UDP-galactose (uridine diphosphogalactose) to terminal GlcNAc residues (Torres and Hart, 1984; Hanover et al., 1987; Holt et al., 1987; Roquemore et al., 1992). Unfortunately, galactosyltransferase cannot easily access GlcNAc residues, and the enzyme lacks specificity for the O-GlcNAc modification. Hence, galactosyltransferase will recognize any terminal GlcNAc residue, such as those found in many N-linked oligosaccharides. Consequently, definitive identification of O-GlcNAc modification requires further carbohydrate characterization. In addition, the donor sugar UDP-[3H]galactose has very low specific radioactivity (17–20 Ci/mmol), making this method insensitive compared to radiolabeling techniques with other isotopes, such as [32P]-phosphate, with specific radioactivity as high as 6000 Ci/ mmol. Such low specific radioactivity of the sugar donor may require several weeks of exposure to detect O-GlcNAc.
Plant lectins like wheat germ agglutinin (WGA) or succinylated WGA have also been used for the detection and purification of O-GlcNAc-containing proteins (Yamamoto et al., 1981; Cheung and Hart, 2008; Taylor et al., 2008). However, WGA recognizes any terminal β-GlcNAc residue, and so far, no O-GlcNAc-specific lectin has been identified. Therefore, the samples must first be treated with peptide/N-glycosidase F to remove the N-linked sugars. Moreover, WGA does not recognize GlcNAc in a linkage-specific manner. Thus, the identification of O-GlcNAc by this existing method requires further carbohydrate analysis for confirmation. Finally, the sensitivity of the method is limited so that only proteins with multiple O-GlcNAc residues are readily detected.
Several monoclonal antibodies that recognize O-GlcNAc are commercially available, and the most commonly used ones are referred to as RL2 (Holt et al., 1987; Park et al., 1987; Snow et al., 1987) and CTD110.6 (Comer et al., 2001). CTD110.6, a murine monoclonal IgM raised against the O-GlcNAc-modified C-terminal domain of the RNA polymerase II large subunit, specifically recognizes single O-GlcNAc residues in β-O-glycosidic linkage to serine and threonine (see Fig. 2, A and C, for a typical O-GlcNAc Western blot with CTD110.6). CTD110.6 shows no apparent cross-reactivity with peptide determinants and apparently does not react with other closely related carbohydrate antigens. RL2, generated against nuclear pore glycoproteins, recognizes a limited subset of O-GlcNAc proteins and requires protein determinants in addition to O-GlcNAc (see Fig. 2, B and D, for a typical O-GlcNAc immunoblot with RL2). Both of the antibodies are somewhat restrictive in their target specificity and may require more than one site of modification. Despite their limitations, these antibody tools have proven invaluable in both early and ongoing studies of O-GlcNAc biology.
Fig. 2.
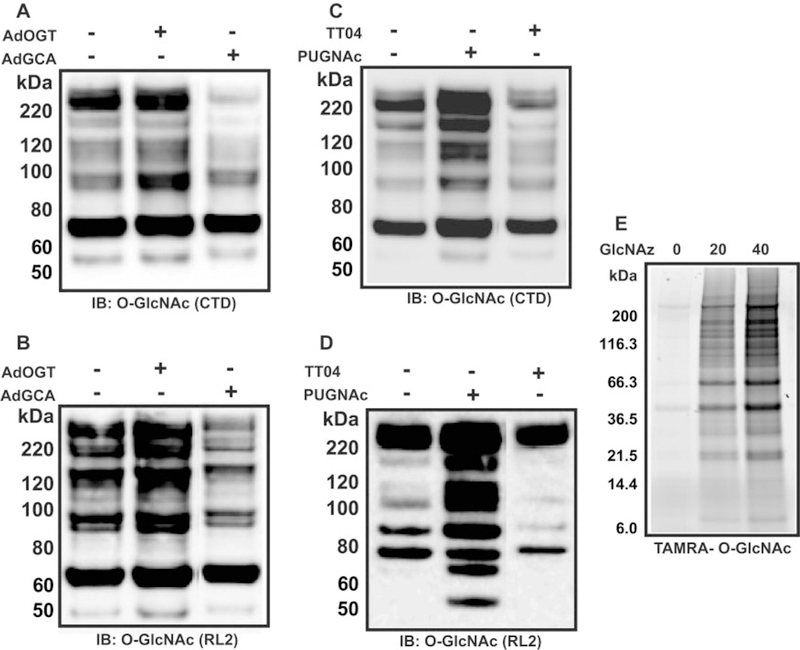
Representative images for Western blots using two O-GlcNAc antibodies and Click-chemistry techniques. A and B, lysates from neonatal rat cardiac myocytes (NRCMs) treated with nothing, AdOGT (20 m.o.i.), or AdO-GlcNAcase (20 m.o.i.) for 24 h and immunoblotted for O-GlcNAc using CTD110.6 (CTD) (A) or RL2 (B). C and D, lysates from NRCMs treated with Vehicle, putative OGT inhibitor [TT04, 1 μM 2 h before protein isolation; see Ngoh et al., 2008] or O-GlcNAcase inhibitor (PUGNAc, 50 μM overnight) and immunoblotted for O-GlcNAc using CTD (C) or RL2 (D). E, NRCMs were treated with dimethyl sulfoxide, 20 or 40 μM UDP-GlcNAz for 24 h. GlcNAz-modified proteins were then incubated with a fluorescently tagged alkyne (TAMRA-alkyne) to form a stable triazole conjugate. The proteins were separated by electrophoresis and the gel-imaged with the 532-nm excitation line of a Typhoon 9400 imager. IB, immunoblot; GCA, O-GlcNcase; TT04, compound 4 (Gross et al., 2005).
Several chemical approaches have also been developed to analyze O-GlcNAc residues (Whelan and Hart, 2003). Alkaline β-elimination can be used to generate radiochemically labeled O-GlcNAc moieties (Torres and Hart, 1984; Hanover et al., 1987) and introduce a radiochemical label if borotritide is used instead of borohydride in the reduction stage of the reaction. Another more recent approach, mild BEMAD (β-elimination followed by Michael addition with dithiothreitol), is useful for mass spectrometry (Wells et al., 2002). Bertozzi’s group (Vocadlo et al., 2003) recently developed a purely chemical means of detection involving the incorporation of an azido derivative of GlcNAc (GlcNAz) into protein targets of O-GlcNAc. Because the enzymes of O-GlcNAc modification (OGT and O-GlcNAcase) tolerate analogs of their natural substrates in which the N-acyl side chain has been modified to a bio-orthogonal azide moiety (GlcNAz), cells can be metabolically labeled or the reaction can be performed in vitro with recombinant OGT (see Fig. 2E for representative gel fluorescence using GlcNAz/TAMRA-alkyne). These O-azidoacetylglucosamine-modified proteins can be covalently derivatized with various biochemical probes at the site of protein glycosylation using Staudinger ligation. This strategy should have general application for both the identification of O-GlcNAc-modified proteins and mapping protein target sites that bear O-GlcNAc modification. Because UDP-GlcNAc is incorporated into several classes of glycoconjugates, specificity must be demonstrated with properly controlled experiments. Tagging-via-substrate (Nandi et al., 2006) and Click-chemistry (Gurcel et al., 2008) also use the same principle. These techniques are highly sensitive and especially useful for lower molecular weight proteins. Despite the development of such techniques, the lack of a recognizable consensus motif somewhat complicates the analyses of O-GlcNAc function and limits predictive capabilities.
Diabetes
O-GlcNAc earned the moniker of a nutrient “signal” or “sensor” based on numerous studies demonstrating a relationship between extracellular glucose and intracellular O-GlcNAc levels. The idea was that as extracellular glucose levels rose, the HBP would be flooded by excess F-6-P and, consequently, UDP-GlcNAc. Because UDP-GlcNAc may positively influence OGT activity, it followed that O-GlcNAc levels would concomitantly rise. Until recently, the converse was universally accepted as true. Furthermore, free fatty acids may augment flux through the HBP, resulting in elevated levels of O-GlcNAc (Hawkins et al., 1997). Other nutrients, such as the essential amino acid glutamine (Liu et al., 2007a), promote flux through the HBP, resulting in increased O-GlcNAc levels.
Recently, McClain’s group showed that glucose deprivation in human hepatoma cells (HepG2) induced dramatic increases in O-GlcNAc modification that were independent of HBP pathway (Taylor et al., 2008). At the same time, Cheung and Hart (2008) found similar results in Neuro-2a neuroblastoma cells. These results tempt one to conclude that O-GlcNAc may not simply be a nutrient sensor as previously thought. Because both studies were executed in cancer cell lines, which are primarily glycolytic, it would be interesting to evaluate the response of primary cells that are less reliant on glucose as a fuel source (e.g., cardiac myocytes). Nevertheless, the seemingly paradoxical results emphasize that high O-GlcNAc levels do not simply reflect high glucose levels, a theme that will be revisited.
In light of such ideas and possibly because other glucose accessory pathways had been implicated in diabetes, several groups have examined the influence of O-GlcNAc on insulin signaling and for its potential role in diabetes. One of the earliest implications for the HBP in deranged insulin signaling (Marshall et al., 1991) identified GFAT as a potentially important regulatory point in adipocytes, diverting F-6-P into the HBP. Subsequent work from McClain’s group (Hebert et al., 1996) demonstrated that overexpression of GFAT, the first step in the HBP, sufficed to produce insulin resistance in mice. Although such augmentation might affect several other biochemical pathways, the subsequent conclusions involved an increase in O-GlcNAc signaling in the development of such a characteristically diabetic trademark. Such insights support the hypothesis that elevated HBP flux participates in the pathogenesis of diabetes.
More recent evidence supports participation of O-GlcNAc signaling per se in the pathogenesis of diabetes. Using differentiated NIH-3T3 and L1 adipocyte cells, Hart’s group (Vosseller et al., 2002) found that prolonged augmentation of O-GlcNAc levels (using the O-GlcNAcase inhibitor, PUGNAc) interfered with insulin-induced phosphorylation of Akt (protein kinase B) and insulin receptor substrate 1, but not insulin receptor substrate 2. Thus, it appeared that augmented O-GlcNAc signaling could participate in the generation of insulin desensitization. Such work extends the aforementioned studies focusing on HBP by indicating O-GlcNAc per se in the development of impaired insulin signaling. Using similar cells, Buse’s group has questioned the central role of O-GlcNAc signaling in the disruption of insulin signaling (Nelson et al., 2000; Robinson et al., 2007). Buse’s group found that glucose-and glucosamine-induced insulin resistance occurred through potentially distinct pathways (Nelson et al., 2000). Moreover, the enhanced O-GlcNAc levels seen with high glucose/glucosamine were not necessary for insulin signaling defects (Robinson et al., 2007). How O-GlcNAc signaling precisely fits into the context of insulin dysregulation remains to be definitively established.
Indeed, others have shown that various tissues from diabetic animals have elevated O-GlcNAc levels, presumably due to high extracellular glucose levels, although it is difficult to assign temporal priority. One such in vivo study by Dillmann’s group (Hu et al., 2005) examined diabetic mice that had elevated cardiac O-GlcNAc levels, impaired calcium handling, and blunted cardiac performance. The authors delivered adenoviral-driven O-GlcNAcase (which removes O-GlcNAc from proteins) to diabetic mouse hearts and documented a reduction in O-GlcNAc levels, improved calcium handling, and rescued cardiac performance. Although no potential targets of O-GlcNAc were directly identified, enhanced phosphorylation of phospholamban was noted. Because of the difficulty in proving causality in such models, it remains to be established whether excessive O-GlcNAc signaling is the direct culprit or whether a generalized imbalance in O-GlcNAc signaling is to blame. This should be examined more closely, particularly considering the diabetogenic capacity of putative inhibitors (alloxan and streptozo- tocin) of the antagonistic enzymes, OGT (Konrad et al., 2002), and O-GlcNAcase (Roos et al., 1998), respectively. Furthermore, Hanover’s group demonstrated that deletion of either OGT (Hanover et al., 2005) or O-GlcNAcase (Forsythe et al., 2006) produced “insulin resistance” in worms. Nevertheless, a potential clinical link has also been shown between elevated O-GlcNAc levels and diabetes. Mutations in the locus corresponding to human O-GlcNAcase have been reported from the Framingham cohort in Caucasian Americans (Meigs et al., 2002) and other populations (Lehman et al., 2005), although some have questioned this link in certain groups (Farook et al., 2002).
Cardiomyocyte Ischemia/Hypoxia
O-GlcNAc and Cell Stress
Disruption of blood flow to the myocardium exerts profound metabolic dysfunction. Myocardial ischemia is an acute and direct metabolic insult. Thus, metabolic defects represent plausible potential mechanisms operative during acute myocardial infarction. However, for the context of the present discussion, such discourse will not take the form of according-to-Hoyle investigations of ATP levels, glucose oxidation, and fatty acid oxidation. Instead, it will focus on the influence of the O-GlcNAc metabolic signal on the development of postischemic myocardial and posthypoxic cardiac myocyte damage.
The idea that cells might recruit O-GlcNAc signaling to cope with cellular stress was recently posited (Zachara et al., 2004). In this study, the authors subjected various cell lines to diverse forms of cell stress and uniformly observed a global elevation of O-GlcNAc levels. Furthermore, if the authors augmented O-GlcNAc levels, they rescued their cells from death. Several laboratories (Liu et al., 2006, 2007a,b; Cham- pattanachai et al., 2007, 2008; Fülöp et al., 2007b; Jones et al., 2008; Ngoh et al., 2008) subsequently evaluated the potential involvement of O-GlcNAc signaling in acute cardiac injury. How O-GlcNAc signaling changes in the acute ischemic-reperfused tissue represents an area of significant importance. Work in isolated, perfused rat hearts indicates that no-flow global ischemia augments O-GlcNAc levels during the reflow phase (Liu et al., 2006). Other studies from this group also show that low-flow global ischemia augments O-GlcNAc levels early in the low-flow phase, which then decline during reflow (Fülöp et al., 2007b). The differential response of O-GlcNAc levels to different models of isolated heart ischemia may provide insights into the regulation of O-GlcNAc signaling in the hypoxic myocardium. Other studies in isolated neonatal cardiomyocytes indicate that O-GlcNAc levels rise following hypoxia (Champattanachai et al., 2007) and were confirmed in a follow-up study by the same group (Champattanachai et al., 2008). Whether such findings are uniformly true at all durations of hypoxia and reoxygenation remains to be seen. Moreover, the changes in the in situ heart may differ significantly from isolated cells/ hearts because of the more complex metabolic substrates presented to the heart in vivo.
Oxidative stress is a significant culprit in posthypoxic myocyte death. Recent work suggests that short-term hydrogen peroxide exposure augments O-GlcNAc levels (Jones et al., 2008). Whether such dynamic changes, addressed in one-dimensional immunoblots, are true of all proteins modified by O-GlcNAc may not be true. That is, the individual O-GlcNAcylation of specific proteins may follow unique and disparate trends. Although this is an important issue to address in future studies, such collective insights during hypoxia and oxidant stress are reminiscent of original findings by Zachara et al. (2004) of augmented O-GlcNAc signaling during cell stress. Thus, the myocardium, like various cell lines, possesses the ability to recruit the O-GlcNAc signaling system in times of stress. Such a conserved and stress-responsive system suggests an endogenous self-defense system operative in the heart.
The phenomenon of ischemic preconditioning exemplifies such a transient mechanism of self-defense operative in the heart and most other organs examined. Ischemic preconditioning occurs when brief, nonlethal bouts of ischemia precede a long and otherwise lethal duration of ischemia. The ability of ischemic preconditioning to reduce myocardial infarct size is significant and reproducible and serves as the gold standard for studies of cardioprotection (Jones and Bolli, 2006). Although some mechanisms responsible for the protection of preconditioning continue to be debated, previous work from Steenbergen and colleagues demonstrated enhanced glucose uptake during ischemic preconditioning (Tong et al., 2000). Considering the protective effect of augmented O-GlcNAc signaling shown by Zachara et al. (2004), it is plausible that the increase in glucose uptake occurring during preconditioning may boost flux through the HBP and consequently augment O-GlcNAc levels. Indeed, a recent report showed that either early or delayed ischemic preconditioning can augment net O-GlcNAc levels in the intact heart in vivo (Jones et al., 2008). Whether such changes are in fact necessary for the protective effects of preconditioning remains unknown, although it is clear that such augmentation (through pharmacologic means) is sufficient to reduce infarct size in vivo (Jones et al., 2008). Future studies should help delineate whether ischemic preconditioning relies upon O-GlcNAc signaling for the generation of its autoprotective phenotype.
Hexosamine Biosynthetic Flux
Beyond the general associations between cellular stress and O-GlcNAc levels, a significant body of work has amassed, indicating that flux through the hexosamine biosynthetic pathway can augment O-GlcNAc levels and exert cytoprotection in vitro (Liu et al., 2006, 2007a,b; Champattanachai et al., 2007, 2008; Fulop et al., 2007b). Champattanachai et al. (2007) showed that high glucose did not significantly change global O-GlcNAc levels under normoxic conditions; however, during hypoxia, hyperglycemia augmented O-GlcNAcylation and reduced apoptosis. Because the hyperglycemia-mediated increase in posthypoxic O-GlcNAc levels was blocked by azerserine (i.e., a GFAT inhibitor), it seemed that the protective effect required HBP flux and subsequent O-GlcNAc modification of proteins.
Using glucosamine to augment HBP flux and, consequently, O-GlcNAc levels, recent studies in the isolated perfused rat heart have convincingly demonstrated the capacity of such an approach to reduce cardiac damage and preserve posthypoxic contractile function (Liu et al., 2006, 2007b; Fülöp et al., 2007b). Glucosamine presumably enters the HBP downstream of GFAT, subsequent to phosphorylation by hexokinase, and consequently contributes to the formation of UDP-GlcNAc, thereby driving the O-GlcNAc modification of proteins. Using a related approach, Chatham’s group also perfused isolated rat hearts with glutamine, which donates the amido group to F-6-P in the GFAT reaction. Such an approach, like glucosamine perfusion, did indeed exert protection in their model (Liu et al., 2007a). The general cardioprotective effect of glutamine is supported by prior evidence from others using an isolated heart model to indicate glutamine as a potential cardioprotective agent (Khogali et al., 1998), although the mechanism was not attributed to alterations in HBP or O-GlcNAc signaling. However, results from an in vivo porcine model of myocardial ischemia-reperfusion injury question the efficacy of glutamine as a cardioprotective approach (Kristensen et al., 2005). Clearly, more work is required in this regard.
O-GlcNAc Transferase and O-GlcNAcase in Cardiomyocyte Survival
Additional support for cytoprotection associated with global augmentation of O-GlcNAc levels can be found in studies using enetic and molecular (see Table 1) approaches to evaluate the enzymes controlling the presence of O-GlcNAc on proteins. Alloxan, a uracil and UDP-GlcNAc analog, may be an irreversible inhibitor of OGT (Konrad et al., 2002). In one study, alloxan not only blocked the glucosamine-mediated increase in O-GlcNAc levels but also its inhibition of angiotensin II-induced increase in [Ca2+]i in isolated neonatal cardiomyocytes (Nagy et al., 2006). Moreover, in isolated perfused hearts, alloxan blocked glucosamine-mediated tolerance to injury resulting from calcium paradox and ischemia/reperfusion. Although results from these studies are cautiously interpreted due to the high concentration of alloxan and because of the recent revelation that alloxan may also inhibit O-GlcNAcase (Lee et al., 2006), they provide important insight into the necessity of OGT signaling in the context of elevated hexosamine biosynthesis.
TABLE 1.
Commonly used and emerging pharmacologic inhibitors of O-GlcNAc transferase and O-GlcNAcase
| O-GlcNAc Transferase | O-GlcNAcase |
|---|---|
| Alloxan (Konrad et al., 2002) | PUGNAc (Horsch et al., 1991) |
| Compound 4 (Gross et al., 2005) | Streptozotocin (Roos et al., 1998) |
| Compound 5 (Gross et al., 2005) | Alloxan (Konrad et al., 2002) |
| NButGT (Macauley et al., 2005) | |
| GlcNAcstatin (Dorfmueller et al., 2006) | |
| Thiamet-G (Yuzwa et al., 2008) | |
In two recent in vitro studies of isolated neonatal cardiac myocytes (Champattanachai et al., 2008; Ngoh et al., 2008), adenoviral (Ad) overexpression of OGT significantly elevated protein expression, augmented O-GlcNAc levels, and reduced posthypoxic cardiac myocyte death. In addition, posthypoxic mitochondrial membrane potential was also better preserved in the AdOGT group compared with the posthypoxic Ad-green fluorescent protein (control virus) group. Conversely, RNA interference against OGT reduced its apparent activity, according to diminished O-GlcNAc levels, and exacerbated posthypoxic cell death. Inhibition of OGT also exaggerated the posthypoxic collapse of mitochondrial membrane potential. Thus, OGT seems essential in the constitutive, as well as inducible, abilities of the cell to withstand lethal stressors.
There are several pharmacologic inhibitors of O-GlcNAcase, including streptozotocin (Roos et al., 1998), PUGNAc (Horsch et al., 1991), and 1,2-dideoxy-2-methyl-D-glucopyranoso[2,1-d]-2-thiazoline (Macauley et al., 2005). Streptozotocin (STZ), a diabetogenic compound and GlcNAc analog, inhibits O-GlcNAcase by binding to its active site, leading to the formation of an energetically more stable transition state ligand (Toleman et al., 2006). Results from studies using STZ as an O-GlcNAcase inhibitor should be interpreted with caution because of its cytotoxic effects on pancreatic p cells and its ability to reduce cellular NAD+ levels (Burkart et al., 1999). Whether the STZ-mediated increase in O-GlcNAc levels is due to secondary or other toxic effects remains debatable. Furthermore, Macauley et al. (2005) showed that STZ had no effect on O-GlcNAcase activity in vitro. PUGNAc, a GlcNAc analog, prevents the binding of O-GlcNAcase to GlcNAc. Even though PUGNAc lacks the cytotoxic effects of STZ (Haltiwanger et al., 1998), it inhibits other lysosomal hydrolases and shows little specificity for O-GlcNAcase over β-hexosaminidase (Macauley et al., 2005). In isolated cardiac myocytes, augmentation of O-GlcNAc levels using PUGNAc attenuates posthypoxic (Champattanachai et al., 2007) and oxidative stress injury (Jones et al., 2008). Similar findings were observed in isolated perfused hearts (Liu et al., 2007b), and PUGNAc reduced infarct size following acute in vivo myocardial ischemia reperfusion (Jones et al., 2008). NAG-thiazoline (Macauley et al., 2005) inhibits O-GlcNAcase and has 1500-fold greater specificity for O-GlcNAcase over β-hex-osaminidase than PUGNAc. Champattanachai et al. (2008) showed that NButGT attenuates cardiac myocyte death following hypoxia and oxidative stress. GlcNAcstatin (Dorfmueller et al., 2006), a glucoimidazole-based inhibitor, has been shown to inhibit O-GlcNAcase in human human embryonic kidney 293 and SH-SY5Y neuroblastoma cell lines but has not been as widely studied as PUGNAc. Thiamet-G, another O-GlcNAcase inhibitor, has also recently been developed (Yuzwa et al., 2008). Again, few studies have addressed the role of O-GlcNAcase inhibition beyond the use of PUGNAc or NButGT. As with inhibitors used in the OGT work above, concern remains regarding the off-target effects of PUGNAc, STZ, and other putative inhibitors of O-GlcNAcase. Thus, the use of other approaches, such as RNA interference or adenoviruses, could clarify some of the off-target effects of the aforementioned pharmacologic approaches.
How Does O-GlcNAc Signal Protection?
The question of the mechanism(s) involved with alterations in O-GlcNAc signaling is not trivial. O-GlcNAc signaling apparently involves numerous intracellular targets, which may contribute to varying extents during myocardial injury. The limited tools to address O-GlcNAc targets also limit the success of this task. Nevertheless, two recent studies also provide evidence that modulating O-GlcNAc levels may alter O-GlcNAc modification on (at least) the mitochondrial voltage-dependent anion channel (VDAC), which may represent a unique mechanism of cytoprotection (Jones et al., 2008; Ngoh et al., 2008). Although the molecular identity of the mitochondrial permeability transition pore (mPTP) remains debatable, VDAC is widely recognized as a putative component. Examination of mice given PUGNAc (an O-GlcNAcase inhibitor) to augment O-GlcNAc levels indicated an increase in O-GlcNAc modification of VDAC and resistance of isolated, adult cardiac mitochondria to calcium-induced swelling, which is in index of mPTP formation (Jones et al., 2008). Conversely, a purported OGT inhibitor reduced O-GlcNAc modification of VDAC (and other targets) and sensitized isolated, adult cardiac mitochondria to mPTP formation (Ngoh et al., 2008). Potential beneficial effects of augmented O-GlcNAc signaling, according to preserved posthy-poxic mitochondrial membrane potential, have also been reported in isolated neonatal rat cardiac myocytes by two groups (Champattanachai et al., 2008; Jones et al., 2008; Ngoh et al., 2008). Thus, there is a potential mechanistic link among a likely target of O-GlcNAc signaling, mitochondrial preservation, and cell viability. Whether such modes of protection require O-GlcNAc modification of mPTP components (Jones et al., 2008; Ngoh et al., 2008) remains to be further verified.
Other protective mechanisms, which impinge upon the mitochondria, may also be involved. Champattanachai et al. (2008) found that glucosamine treatment promoted mitochondrial translocation of bcl2 during hypoxia reoxygenation in isolated myocytes. Blc2 is thought to interact with VDAC and reduce cell death. Hence, another potential mechanism through which enhanced O-GlcNAc levels protect at the level of the mitochondria may be through the prevention of mPTP formation due to interaction of bcl2 and VDAC. Additional work from this group has implicated dynamic p38 MAPK activation (Fülöp et al., 2007b) and/or reduced calpain proteolytic activity (Liu et al., 2007b). Such mitochondrial effects could be related to alterations in calcium handling (Hu et al., 2005; Liu et al., 2006) and/or heat shock protein activation (Zachara et al., 2004; Jones et al., 2008). Indeed, just as the targets of O-GlcNAc modification are numerous, so too are the potential mechanisms responsible for cytoprotection.
Future Directions
Our understanding of O-GlcNAc signaling in most of cardiovascular pathophysiology remains limited. However, several recent studies indicate its potential impact extends beyond the heart. Oparil’s group tested the hypothesis that in vivo arterial injury may be affected by alterations in O-GlcNAc signaling (Xing et al., 2008). Using ovariecto-mized rats, balloon injury of the carotid artery produced the expected inflammation and vascular pathology in this model. However, treatment with either glucosamine or PUGNAc (which inhibits O-GlcNAcase and increases O-GlcNAc levels) reduced leukocyte infiltration, indicating anti-inflammatory effects of augmented O-GlcNAc signaling in this model. In addition to vascular injury, other recent reports indicate glucosamine (Not et al., 2007) or PUGNAc (Zou et al., 2007), apparently via augmented O-GlcNAc signaling, can improve survival of hemorrhagic-traumatic shock in rats. In these shock studies, the authors also found evidence of reduced inflammation. Such clinically relevant in vivo studies of shock (Chatham et al., 2008) may provide new therapeutic avenues in a pathologic condition that has become a veritable dead end for many pharmacotherapeutics.
It is clear that the duration of changes in O-GlcNAc signaling may be associated with therapeutic effects (i.e., acute myocardial infarction) versus pathologic effects (i.e., diabetes). Nevertheless, O-GlcNAc signaling represents a unique mechanism for the cell to integrate metabolic changes with other aspects of cellular function. The evidence for participation of O-GlcNAc signaling in general cell biology (Zachara and Hart, 2006), particularly in acute myocardial infarction, is growing, although many additional studies are required to tease out the precise contribution of various pathways. It will be exciting to see additional developments of O-GlcNAc in other areas of cardiovascular biology, including hypertension, arrhythmia, hypertrophy, heart failure, and stem cells. The limitations of tools to site-specifically identify O-GlcNAc-modified proteins remain an important area for development. Several issues yet to be addressed include cell- and/or tissue-specific effects and intracellular localization of such changes in O-GlcNAc signaling. In a much broader sense, little is known about O-GlcNAc signaling in other organ systems. Investigation of O-GlcNAc signaling in other systems will provide not only direct insights for the others systems examined but will synergize with work in the cardiovascular and nervous systems. O-GlcNAcylation of proteins represents a truly unique post-translational modification at the crossroads of metabolism and survival.
Acknowledgments
This work is supported by National Institutes of Health Grant R01 HL083320 (to S.P.J.), American Heart Association National Center Scientist Development Grant 0535270N (to S.P.J), and Kentucky Science and Engineering Foundation Grant KSEF-1677-RDE-011 (to S.P.J.).
G.A.N is an American Heart Association Predoctoral Fellow (Great Rivers Affiliate).
ABBREVIATIONS:
- O-GlcNAc
β-O-linkage of N-acetylglucosamine
- HBP
hexosamine biosynthetic pathway
- GFAT
glutamine/fructose amidotransferase
- OGT
O-GlcNAc transferase
- GlcNAz
azido derivative of GlcNAc
- VDAC
voltage-dependent anion channel
- mPTP
mitochondrial permeability transition pore
- WGA
wheat germ agglutinin
- F-6-P
fructose 6-phosphate
- PUGNAc
O-(2-acetamido-2-deoxy-D-glucopyranosylidene) amino-N-phenylcarbamate
- Ad
adenoviral
- STZ
streptozotocin
References
- Burkart V, Wang ZQ, Radons J, Heller B, Herceg Z, Stingl L, Wagner EF, and Kolb H (1999) Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozo- cm. Nat Med 5:314–319. [DOI] [PubMed] [Google Scholar]
- Champattanachai V, Marchase RB, and Chatham JC (2007) Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein- associated O-GlcNAc. Am J Physiol Cell Physiol 292:C178–C187. [DOI] [PubMed] [Google Scholar]
- Champattanachai V, Marchase RB, and Chatham JC (2008) Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol 294: C1509–C1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham JC, Nöt LG, Fülöp N, and Marchase RB (2008) Hexosamine biosynthesis and protein O-glycosylation: The first line of defense against stress, ischemia, and trauma. Shock 29:431–440. [DOI] [PubMed] [Google Scholar]
- Cheng X, Cole RN, Zaia J, and Hart GW (2000) Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry 39:11609–11620. [DOI] [PubMed] [Google Scholar]
- Cheung WD and Hart GW (2008) AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem 283:13009–13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CF, Smith AJ, and Omary MB (1992) Characterization and dynamics of O-linked glycosylation of human cytokeratin 8 and 18. J Biol Chem 267:3901–3906. [PubMed] [Google Scholar]
- Cole RN and Hart GW (1999) Glycosylation sites flank phosphorylation sites on synapsin I: O-linked N-acetylglucosamine residues are localized within domains mediating synapsin I interactions. J Neurochem 73:418–428. [DOI] [PubMed] [Google Scholar]
- Comer FI and Hart GW (2001) Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry 40:7845–7852. [DOI] [PubMed] [Google Scholar]
- Comer FI, Vosseller K, Wells L, Accavitti MA, and Hart GW (2001) Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal Biochem 293:169–177. [DOI] [PubMed] [Google Scholar]
- Dentin R, Hedrick S, Xie J, Yates J 3rd, and Montminy M (2008) Hepatic glucose sensing via the CREB coactivator CRTC2. Science 319:1402–1405. [DOI] [PubMed] [Google Scholar]
- Dorfmueller HC, Borodkin VS, Schimpl M, Shepherd SM, Shpiro NA, and van Aalten DM (2006) GlcNAcstatin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-glcNAcylation levels. J Am Chem Soc 128:16484–16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farook VS, Bogardus C, and Prochazka M (2002) Analysis of MGEA5 on 10q24.1- q24.3 encoding the beta-O-linked N-acetylglucosaminidase as a candidate gene for type 2 diabetes mellitus in Pima Indians. Mol Genet Metab 77:189–193. [DOI] [PubMed] [Google Scholar]
- Forsythe ME, Love DC, Lazarus BD, Kim EJ, Prinz WA, Ashwell G, Krause MW, and Hanover JA (2006) Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc Natl Acad Sci USA 103:11952–11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülöp N, Marchase RB, and Chatham JC (2007a) Role of protein O-linked N-acetyl- glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res 73:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülöp N, Zhang Z, Marchase RB, and Chatham JC (2007b) Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglu- cosamine protein modification and altered p38 activation. Am JPhysiol Heart Circ Physiol 292:H2227–H2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross BJ, Kraybill BC, and Walker S (2005) Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc 127:14588–14589. [DOI] [PubMed] [Google Scholar]
- Gurcel C, Vercoutter-Edouart AS, Fonbonne C, Mortuaire M, Salvador A, Michalski JC, and Lemoine J (2008) Identification of new O-GlcNAc modified proteins using a click-chemistry-based tagging. Anal Bioanal Chem 390:2089–2097. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Grove K, and Philipsberg GA (1998) Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-β-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D- glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem 273:3611–3617. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Cohen CK, Willingham MC, and Park MK (1987) O-Linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem 262:9887–9894. [PubMed] [Google Scholar]
- Hanover JA, Forsythe ME, Hennessey PT, Brodigan TM, Love DC, Ashwell G, and Krause M (2005) A Caenorhabditis elegans model of insulin resistance: Altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci USA 102:11266–11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, Kochran J, and Love DC (2003) Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys 409:287–297. [DOI] [PubMed] [Google Scholar]
- Hart GW, Housley MP, and Slawson C (2007) Cycling of O-linked [beta]-N- acetylglucosamine on nucleocytoplasmic proteins. Nature 446:1017–1022,. [DOI] [PubMed] [Google Scholar]
- Hawkins M, Barzilai N, Liu R, Hu M, Chen W, and Rossetti L (1997) Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest 99:2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LF Jr, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, and McClain DA (1996) Overexpression of glutamine:fructose- 6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest 98:930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, and Hart GW (1987) Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol 104:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch M, Hoesch L, Vasella A, and Rast DM (1991) N-Acetylglucosaminono-1,5- lactone oxime and the corresponding (phenylcarbamoyl)oxime. Novel and potent inhibitors of beta-N-acetylglucosaminidase. Eur J Biochem 197:815–818. [DOI] [PubMed] [Google Scholar]
- Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, and Dillmann WH (2005) Adenovirus-mediated overexpression Of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 96:1006–1013. [DOI] [PubMed] [Google Scholar]
- Jones SP and Bolli R (2006) The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol 40:16–23. [DOI] [PubMed] [Google Scholar]
- Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, and Marban E (2008) Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117:1172–1182. [DOI] [PubMed] [Google Scholar]
- Kamemura K, Hayes BK, Comer FI, and Hart GW (2002) Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem 277:19229–19235. [DOI] [PubMed] [Google Scholar]
- Kelly WG, Dahmus ME, and Hart GW (1993) RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem 268: 10416–10424. [PubMed] [Google Scholar]
- Khogali SE, Harper AA, Lyall JA, and Rennie MJ (1998) Effects of L-glutamine on post-ischaemic cardiac function: protection and rescue. JMol Cell Cardiol 30:819–827. [DOI] [PubMed] [Google Scholar]
- Konrad RJ, Zhang F, Hale JE, Knierman MD, Becker GW, and Kudlow JE (2002) Alloxan is an inhibitor of the enzyme O-linked N-acetylglucosamine transferase. Biochem Biophys Res Commun 293:207–212. [DOI] [PubMed] [Google Scholar]
- Kristensen J, Maeng M, Mortensen UM, Berg J, Rehling M, and Nielsen TT (2005) Lack of cardioprotection from metabolic support with glutamine or glutamate in a porcine coronary occlusion model. Scand Cardiovasc J 39:115–120. [DOI] [PubMed] [Google Scholar]
- Lamarre-Vincent N and Hsieh-Wilson LC (2003) Dynamic glycosylation of the transcription factor CREB: a potential role in gene regulation. J Am Chem Soc 125: 6612–6613. [DOI] [PubMed] [Google Scholar]
- Lee TN, Alborn WE, Knierman MD, and Konrad RJ (2006) Alloxan is an inhibitor of O-GlcNAc-selective N-acetyl-[beta]-D-glucosaminidase. Biochem Biophys Res Com- mun 350:1038–1043. [DOI] [PubMed] [Google Scholar]
- Lehman DM, Fu DJ, Freeman AB, Hunt KJ, Leach RJ, Johnson-Pais T, Hamlington J, Dyer TD, Arya R, Abboud H, et al. (2005) A single nucleotide polymorphism in MGEA5 encoding O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase is associated with type 2 diabetes in Mexican Americans. Diabetes 54:1214–1221. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, and Gong CX (2004) O-GlcNAcylation regulates phosphorylation of tau: A mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci U S A 101:10804–10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Marchase RB, and Chatham JC (2007a) Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hex- osamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol 42:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Marchase RB, and Chatham JC (2007b) Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am J Physiol Heart Circ Physiol 293:H1391–H1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, and Marchase RB (2006) Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 40:303–312. [DOI] [PubMed] [Google Scholar]
- Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA, and Kochran J (2003) Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci 116:647–654. [DOI] [PubMed] [Google Scholar]
- Luo B, Parker GJ, Cooksey RC, Soesanto Y, Evans M, Jones D, and McClain DA (2007). Chronic hexosamine flux stimulates fatty acid oxidation by activating AMP-activated protein kinase in adipocytes. J Biol Chem 282:7172–7180. [DOI] [PubMed] [Google Scholar]
- Luo B, Soesanto Y, and McClain DA (2008) Protein modification by O-linked GlcNAc reduces angiogenesis by inhibiting Akt activity in endothelial cells. Arterioscler Thromb Vasc Biol 28:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley MS, Whitworth GE, Debowski AW, Chin D, and Vocadlo DJ (2005) O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem 280:25313–25322. [DOI] [PubMed] [Google Scholar]
- Marshall S, Bacote V, and Traxinger RR (1991) Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 266:4706–4712. [PubMed] [Google Scholar]
- Meigs JB, Panhuysen CI, Myers RH, Wilson PW, and Cupples LA (2002) A genome- wide scan for loci linked to plasma levels of glucose and HbA(1c) in a community- based sample of Caucasian pedigrees: The Framingham Offspring Study. Diabetes 51:833–840. [DOI] [PubMed] [Google Scholar]
- Nagy T, Champattanachai V, Marchase RB, and Chatham JC (2006) Glucosamine inhibits angiotensin II-induced cytoplasmic Ca2+ elevation in neonatal cardiomy-ocytes via protein-associated O-linked N-acetylglucosamine. Am J Physiol Cell Physiol 290:C57–C65. [DOI] [PubMed] [Google Scholar]
- Nandi A, Sprung R, Barma DK, Zhao Y, Kim SC, Falck JR, and Zhao Y (2006) Global identification of O-GlcNAc-modified proteins. Anal Chem 78:452–458. [DOI] [PubMed] [Google Scholar]
- Nelson BA, Robinson KA, and Buse MG (2000) High glucose and glucosamine induce insulin resistance via different mechanisms in 3T3-L1 adipocytes. Diabetes 49: 981–991. [DOI] [PubMed] [Google Scholar]
- Ngoh GA, Watson LJ, Facundo HT, Dillmann W, and Jones SP (2008) Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol 45:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Not LG, Marchase RB, Fulop N, Brocks CA, and Chatham JC (2007) Glucosamine administration improves survival rate after severe hemorrhagic shock combined with trauma in rats. Shock 28:345–352. [DOI] [PubMed] [Google Scholar]
- Park MK, D’Onofrio M, Willingham MC, and Hanover JA (1987) A monoclonal antibody against a family of nuclear pore proteins (nucleoporins): O-linked N-acetylglucosamine is part of the immunodeterminant. Proc Natl Acad Sci U S A 84:6462–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KA, Ball LE, and Buse MG (2007) Reduction of O-GlcNAc protein modification does not prevent insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 292:E884–E890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos MD, Xie W, Su K, Clark JA, Yang X, Chin E, Paterson AJ, and Kudlow JE (1998) Streptozotocin, an analog of N-acetylglucosamine, blocks the removal of O-GlcNAc from intracellular proteins. Proc Assoc Am Physicians 110:422–432. [PubMed] [Google Scholar]
- Roquemore EP, Dell A, Morris HR, Panico M, Reason AJ, Savoy LA, Wistow GJ, Zigler JS Jr, Earles BJ, and Hart GW (1992) Vertebrate lens alpha-crystallins are modified by O-linked N-acetylglucosamine. J Biol Chem 267:555–563. [PubMed] [Google Scholar]
- Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, and Hart GW (2005) Perturbations in O-linked p-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem 280:32944–32956. [DOI] [PubMed] [Google Scholar]
- Snow CM, Senior A, and Gerace L (1987) Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol 104:1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RP, Parker Gj , Hazel Mw , Soesanto Y, Fuller W, Yazzie MJ, and McClain DA (2008) Glucose deprivation stimulates O-GlcNAc modification of proteins through up-regulation of O-linked N-acetylglucosaminyltransferase. J Biol Chem 283: 6050–6057. [DOI] [PubMed] [Google Scholar]
- Toleman C, Paterson AJ, Shin R, and Kudlow JE (2006) Streptozotocin inhibits O-GlcNAcase via the production of a transition state analog. Biochem Biophys Res Commun 340:526–534. [DOI] [PubMed] [Google Scholar]
- Toleman C, Paterson AJ, Whisenhunt TR, and Kudlow JE (2004) Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with acti- vable O-GlcNAcase and HAT activities. J Biol Chem 279:53665–53673. [DOI] [PubMed] [Google Scholar]
- Tong H, Chen W, London RE, Murphy E, and Steenbergen C (2000) Preconditioning enhanced glucose uptake is mediated by p38 MAP kinase not by phosphatidylinositol 3-kinase. J Biol Chem 275:11981–11986. [DOI] [PubMed] [Google Scholar]
- Torres CR and Hart GW (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 259:3308–3317. [PubMed] [Google Scholar]
- Vocadlo DJ, Hang HC, Kim EJ, Hanover JA, and Bertozzi CR (2003) A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc Natl Acad Sci USA 100:9116–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosseller K, Wells L, Lane MD, and Hart GW (2002) Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3–L1 adipocytes. Proc Natl Acad Sci U S A 99:5313–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, and Hart GW (2002) Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteomics 1:791–804. [DOI] [PubMed] [Google Scholar]
- Whelan SA and Hart GW (2003) Proteomic approaches to analyze the dynamic relationships between nucleocytoplasmic protein glycosylation and phosphorylation. Circ Res 93:1047–1058. [DOI] [PubMed] [Google Scholar]
- Xing D, Feng W, Nöt LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, and Oparil S (2008) Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol 295:H335–H342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Tsuji T, Matsumoto I, and Osawa T (1981) Structural requirements for the binding of oligosaccharides and glycopeptides to immobilized wheat germ agglutinin. Biochemistry 20:5894–5899. [DOI] [PubMed] [Google Scholar]
- Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, and Cho JW (2006) Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol 8:1074–1083. [DOI] [PubMed] [Google Scholar]
- Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, et al. (2008) Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451:964–969. [DOI] [PubMed] [Google Scholar]
- Yang X, Su K, Roos MD, Chang Q, Paterson AJ, and Kudlow JE (2001) O-Linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci U S A 98:6611–6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang F, and Kudlow JE (2002) Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell 110:69–80. [DOI] [PubMed] [Google Scholar]
- Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, et al. (2008) A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol 4:483–490. [DOI] [PubMed] [Google Scholar]
- Zachara NE and Hart GW (2006) Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta 1761:599–617. [DOI] [PubMed] [Google Scholar]
- Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, and Hart GW (2004) Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress: A survival response in mammalian cells. J Biol Chem 279:30133–30142. [DOI] [PubMed] [Google Scholar]
- Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, and Kudlow JE (2003) O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell 115:715–725. [DOI] [PubMed] [Google Scholar]
- Zou L, Yang S, Hu S, Chaudry IH, Marchase Rb , and Chatham JC (2007) The protective effects of PUGNAc on cardiac function after trauma-hemorrhage are mediated via increased protein O-GlcNAc levels. Shock 27:402–408. [DOI] [PubMed] [Google Scholar]


