Abstract
Intranasal transmission of hepatitis C virus (HCV) via contaminated drug-sniffing implements is a potential but unconfirmed source of viral infection. We demonstrate the virological plausibility of intranasal transmission by confirming that blood and HCV RNA are present in the nasal secretions and drug-sniffing implements of HCV-infected intranasal drug users recruited from a community health clinic in New York City.
INTRODUCTION
Hepatitis C virus (HCV) is the most common bloodborne pathogen in the United States and is a major cause of liver-related morbidity, mortality, and liver transplantation [1]. HCV is transmitted through contact with infected blood [2] (mostly via shared needles and other drug injection paraphernalia); however, a large proportion (up to 20%) of HCV infections remain unexplained, especially among noninjection drug users [3]. One hypothesis to account for these unexplained cases involves intranasal transmission of HCV via contaminated implements, such as straws, used to snort cocaine, heroin, and other powdered drugs [4]. Implements inserted into the nasal cavity, which has been eroded by long-term drug sniffing, might come into contact with HCV-infected mucus or blood, which might then be transmitted to a susceptible individual sharing the same implement [5]. Epidemiological studies of intranasal transmission of HCV have produced inconsistent findings [6, 7], in part because of the high correlation between drug sniffing and other risk factors for HCV infection. Here, we attempt to refute the intranasal transmission hypothesis by invalidating ≥1 of its virological preconditions. Specifically, we address 2 primary research questions: (1) Does HCV RNA exist in the nasal secretions of serum-positive drug sniffers? (2) If so, can HCV RNA be transferred onto the sniffing implements shared by intranasal drug users. A secondary aim was to examine clinical nasal pathologies that might facilitate intranasal HCV transmission.
METHODS
Our sample included low-income, urban intra-nasal drug users with chronic, active HCV infection. Subjects were primarily Hispanic and African American and were recruited from a neighborhood health clinic in East Harlem, New York City, an area with a high prevalence of HCV infection (up to 29%) among noninjection drug users [3]. Eligibility criteria included (1) age, ≤ 18 years; (2) self-reported intranasal drug use; and (3) a positive result of a quantitative HCV PCR blood test. Overall, 38 patients enrolled in the study and pro-vided informed consent. Study protocols were approved by 3 institutional review boards.
The following medical information was obtained from sub-jects: quantitative HCV RNA test result and viral load, hepatitis B antibody test results, liver enzyme levels (i.e., alanine aminotransferase level), and liver biopsy history. Subjects completed a brief survey, in either Spanish or English, that covered demographic characteristics, risk factors for HCV infection, injection and noninjection drug use, health status, and nasal pathology symptoms.
Blood samples were collected for quantitative PCR. Two nasal secretion samples (1 from each nostril) were collected with Dacron nasal swabs and placed in (1) 1 mL of TRIzol reagent (Gibco BRL) for RNA detection or (2) 1 mL of OBTI solution for blood detection. Similarly, 2 experimental sniffing implements, which consisted of new (packaged) soda straws commonly used by drug sniffers, were collected from each subject. To avoid harmful effects of sniffing powdered substances, sub-jects were instructed to “snort air” while mimicking their nor-mal drug-sniffing behavior.
HCV RNA was isolated from 200 uL of serum by use of the QIAamp MinElute kit (Qiagen); HCV RNA was isolated from nasal secretions and sniffing implements using the TRIzol (Gibco BRL) on the basis of established protocols [8]. The first strand of cDNA was synthesized by ImProm-IITM Reverse Transcription System (Promega) using gene-specific down-stream primers targeting the HCV p22 core region, with minor modification of the upstream primer (410R-5’-ATGTACCCCA-TGAGGTCGGC-3’). HCV cDNA was amplified by PCR with 40 cycles of denaturation (94C for 30 s), annealing (58C for 30 s), and elongation (72C for 45 s) with primers 406F-5’-TAGACCGTGCACCATGAGC-3’ and 410R. PCR products were detected by Southern blot using 32P-labeled probe (5’-AGGAAGACTTCCGAGCGGTCGCAA-3’).
HCV cDNA was amplified from randomly selected HCV-positive blood samples with use of high-fidelity Pfu polymerase (Perkin Elmer) using 410R and 406F primers and cloned into a TA cloning vector (Invitrogen). The pTA_HCV was used to prepare standard curves ranging from 1 × 106 to 10 copies of HCV mRNA, which were run in parallel to each set of samples. The intensity of DNA bands was evaluated by densitometry using the Kodak Image Analysis System; the HCV load for the test sample was calculated on the basis of the numeric value derived from the HCV titration curve. HCV load was calculated as the number of copies per milliliter for blood specimens and as the number of copies per sample for nasal secretions and implements.
Traces of blood in nasal secretions and sniffing implements were detected by Hexagon OBTI Kit (BLUESTAR Forensic). Titration curves were prepared using human hemoglobin (Sigma) in 2-fold dilutions ranging from 10 to 0.1 ug/mL. The concentration of blood in each sample was established by com-paring the OBTI intensity between the sample and the hemoglobin titration curve.
Nasal cavity pathology was assessed for each patient by anterior nasal examination, rendering diagnoses on 8 nasal pathologies. Rhinitis was diagnosed on the basis of the classic symptoms of mucosal and nasal secretion appearance [9]. Rhinosinusitis was defined by symptomatic inflammation of the paranasal sinuses and nasal cavity [10].
Sample prevalences of HCV RNA and occult blood in nasal secretions and on sniffing implements were estimated. Ninety-five percent CIs were calculated around point estimates using the adjusted Wald method. Descriptive statistics were calculated for sample descriptors and measures of nasal pathology. Our limited sample size precluded statistical tests of significance (e.g., associations between virological and clinical variables).
RESULTS
All 38 patients had chronic, active hepatitis C. The serum HCV load ranged from 250 to 5,000,000 copies/mL (median, 5000 copies/mL). Recent liver biopsies had been per-formed for 6 patients; all indicated chronic liver disease, with stages ranging from 1 to 4. Recent alanine aminotransferase levels were available for 17 patients; the mean level (±SD) was 46.7 ± 26.7 U/L (range, 16 –118 U/L). Antibody screening revealed that 34% of subjects were positive for antibodies to HIV, and 45% were positive for antibodies to hepatitis B virus.
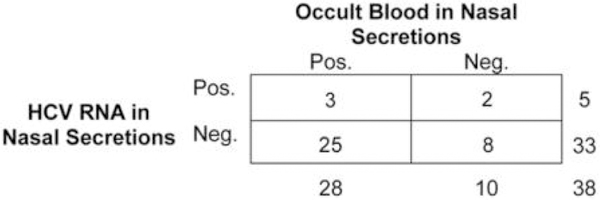
Trace amounts of blood were detected in 28 (74%) of 38 nasal secretion samples (range, 0.1–10 ug/mL) and on 3 (8%) of the 38 sniffing implements (range, 0.1–2 ug/mL). HCV RNA was detected in 5 nasal secretion samples (13%; HCV RNA level range, 10–100 copies/sample) and on 2 sniffing implements (5%; HCV RNA level, 50 and 100,000 copies/sample). Prevalence estimates suggest a wide discrepancy between the presence of blood (74%) and the presence of HCV RNA (13%) in the nasal secretion samples (table 1). Of the 5 HCV RNA–positive nasal secretion samples, only 3 had traces of occult blood; of the 28 samples containing occult blood, 25 were negative for HCV RNA (figure 1).
Table 1.
Detection of hepatitis C virus (HCV) RNA and blood in biological specimens obtained from 38 patients with HCV-positive serum specimens.
| Assay | No. (%) of persons (n = 38) | 95% CI |
|---|---|---|
| Blood detection with OBTI | ||
| Nasal secretions | 28 (73.7) | 57.8 – 85.2 |
| Sniffing straws | 3 (7.9) | 2.0 – 21.5 |
| HCV RNA detection with PCR | ||
| Nasal secretions | 5 (13.2) | 5.3 – 27.8 |
| Sniffing straws | 2 (5.3) | 0.5 – 18.2 |
Figure 1.
Hepatitis C virus (HCV) RNA and occult blood in nasal secretions.
The prevalence of rhinitis in this cohort was high (71%)(table 2). In contrast, the prevalence of rhinosinusitis (11%) is consistent with that of the general population. More than 40%of subjects experienced rhinorrhea or nasal congestion at least once per week, 8% reported nose bleeds at least once per week, and 8% and 16% reported mucosal lesions and crusting, respectively. Approximately one-half of the subjects attributed these symptoms to intranasal drug use. Four persons (11%) were observed to have nasal septal perforations; 1 (3%) had a nasopalatal perforation; and 6 (16%) displayed symptoms of saddlenose deformation. These pathologies have been associated with advanced nasal cavity deterioration associated with chronic intranasal drug use [11].
Table 2.
Frequency of nasal pathology symptoms among intranasal drug users.
| Symptom | No. (%) of subjects (n=38) | |
|---|---|---|
| Findings of an anterior nasal clinical examination | ||
| Loss of nasal hairs | 4 (10.5) | |
| Rhinitis | 27 (71.1) | |
| Rhinosinusitis | 4 (10.5) | |
| Presence of nasal crusting and/or scabbing | 6 (15.8) | |
| Sores or erosion of nasal mucosa | 3 (7.9) | |
| Saddlenose deformation | 6 (15.8) | |
| Nasopalatal perforation | 1 (2.6) | |
| Nasal septum perforation | 4 (10.5) | |
| Self-reported nasal pathology: | ||
| Frequency of nose bleeds in the past year | ||
| Never or rarely | 26 (68.4) | |
| Once or a few times a month | 9 (23.7) | |
| Once or a few times a week | 2 (5.3) | |
| Once or more a day | 1 (2.6) | |
| Experienced a runny or stuffy nose in the past year | ||
| Never or rarely | 16 (42.1) | |
| Once or a few times a month | 6 (15.8) | |
| Once or a few times a week | 13 (34.2) | |
| Once or more a day | 3 (7.9) | |
| Reason for nasal symptoms | ||
| Allergies | 19 (50.0) | |
| Cold or Flu | 10 (26.3) | |
| Drug sniffing | 21 (55.3) | |
| “Have you ever noticed any of the following problems with your nose due to drug sniffing?” | ||
| Scabs in your nose | 14 (36.8) | |
| Sores in your nose | 8 (21.1) | |
| Poor sense of smell | 13 (34.2) | |
| Sinus pain | 13 (34.2) | |
| Headaches located in your forehead | 16 (42.1) | |
| Double vision | 5 (13.2) | |
| “Has a doctor or other health care professional ever told you that the inside of your nose is damaged in any way from sniffing drugs?” | 7 (18.4) |
DISCUSSION
Our findings revealed a high prevalence of blood (74%) in the nasal secretions of HCV-positive long-term drug sniffers. We also confirmed that HCV RNA was present in the nasal secretions of a substantial proportion (13%) of this cohort. Most significantly, this study demonstrated that both blood and HCV particles can be transferred onto sniffing implements (i.e., straws) during simulated intranasal drug use. Studies have shown that HCV can remain viable on environ-mental surfaces for up to 16 h, but little is known about the quantity of virus required for transmission [12]. The prevalences of HCV in the nasal secretions and on sniffing straws are likely conservative estimates. It is reasonable to assume that HCV will be present in the nasal secretions with greater frequency and quantity during episodes of active drug sniffing, which may exacerbate discharge of nasal fluids and blood.
Data in table 1 contradict the assumption that, in persons with HCV-positive serum specimens, detection of blood implies the presence of HCV. This discrepancy may be explained by 2 factors. First, the 2 assays (PCR and OBTI) were not performed on the same samples. Second, the OBTI assay for blood detects immune complexes between human hemoglobin (hHb) and monoclonal anti-hHb antibodies, which can occur even in the absence of viable cells. In contrast, PCR can only detect HCV RNA from intact particles. Therefore, the discrepancy between the high prevalence of occult blood and relatively low detection of HCV RNA in nasal secretions may be associated with the rapid deterioration of viral RNA in the nasal environment or the destruction of viral particles by mucosal immunity. If the viability of HCV particles in nasal secretions is moderated by nasal pathology or immunity, this might help explain conflicting epidemiological findings in which these moderating factors are not considered.
This study establishes the validity of 2 primary virological preconditions necessary for intranasal HCV transmission: (1) the presence of blood and HCV in the nasal secretions of intranasal drug users, and (2) the transference of blood and HCV from the nasal cavity onto sniffing implements, which are often shared by intranasal drug users. Moreover, the frequency and severity of nasal pathologies observed in this cohort might aggravate conditions that facilitate intranasal HCV transmission. Consequently, these findings lend important virological and clinical support to the intranasal HCV transmission hypothesis. In addition, detection of HCV in nasal secretions advances the debate regarding potential iatrogenic and nosocomial trans-mission of HCV in the context of ear, nose, and throat and related clinical practices. More research is needed to confirm intranasal transmission as a mode of viral infection and to determine its impact on the wider epidemic of HCV infection.
ACKNOWLEDGEMENTS
We thank Enrique Pouget for assistance with data management and Jeanine Botta for providing clerical and technical assistance. Dr. K. K. Lam provided guidance on manuscript revision.
Financial support. The National Institutes of Health, National Institute on Drug Abuse, and the National Institute of Diabetes and Digestive and Kidney Diseases (R21 DA019834 to J.M.M.)
Footnotes
Potential conflicts of interest: All authors: no conflicts.
References:
- 1.Hollinger FB. Factors contributing to the evolution and outcome of cirrhosis in hepatitis C. Clin Liver Dis 1999; 3:741–55 [DOI] [PubMed] [Google Scholar]
- 2.Memon MI, Memon MA. Hepatitis C: an epidemiological review. J Viral Hepat 2002; 9:84–100. [DOI] [PubMed] [Google Scholar]
- 3.Tortu S, Neaigus A, McMahon JM, Hagen D. Hepatitis C among non-injecting drug users: a report. Subst Use Misuse 2001; 36:523–34. [DOI] [PubMed] [Google Scholar]
- 4.Conry-Cantilena C, van Raden M, Gibble J, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med 1996; 334:1691–6. [DOI] [PubMed] [Google Scholar]
- 5.McMahon JM, Simm M, Milano D, Clatts M. Detection of hepatitis C virus in the nasal secretions of an intranasal drug-user. Ann Clin Microbiol Antimicrob 2004; 3:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon JM, Tortu S. A potential hidden source of hepatitis C infection among noninjecting drug users. J Psychoactive Drugs 2003; 35: 455–60. [DOI] [PubMed] [Google Scholar]
- 7.Scheinmann R, Hagan H, Lelutiu-Weinberger C, et al. Non-injection drug use and Hepatitis C virus: a systematic review. Drug Alcohol Depend 2007; 89:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162:156–9. [DOI] [PubMed] [Google Scholar]
- 9.Dykewicz M Clinical approach to diagnosis and treatment of nonallergic rhinitis. Clin Allergy Immunol 2007; 19:335–50. [PubMed] [Google Scholar]
- 10.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg 2007; 137(Suppl 3): S1–31. [DOI] [PubMed] [Google Scholar]
- 11.Villa PD. Midfacial complications of prolonged cocaine snorting. J Can Dent Assoc 1999; 65:218–23. [PubMed] [Google Scholar]
- 12.Krawczynski K, Alter MJ, Robertson BH, Lu L, Spelbring JE, Mc-Caustland KA. Environmental stability of hepatitis C virus (HCV): viability of dried/stored HCV in chimpanzee infectivity studies. Hepatol 2003; 38(Suppl 4):428. [Google Scholar]