Abstract
Background:
The effectiveness of rotavirus vaccines in low and very low birth weight infants (LBW and VLBW) weighing <2500 and <1500 g at birth, respectively, a high-risk population for severe rotavirus gastroenteritis, has not been well examined.
Methods:
We analyzed inpatient commercial claims data for US children <5 years of age from July 2001 to June 2015. Claims for acute gastroenteritis (AGE) and rotavirus-coded hospitalizations and LBW, VLBW and normal birth weight (NBW) infants were identified. Receipt of rotavirus vaccine was defined using Current Procedural Terminology. Rate reductions were calculated using prevaccine (2001–2006) and postvaccine (2007–2015) annual AGE and rotavirus hospitalization rates.
Results:
As of December 2014, rotavirus vaccine coverage was 87%, 82% and 64%, for NBW, LBW and VLBW infants, respectively. For 2014–2015, among NBW, LBW and VLBW children <5 years of age, AGE hospitalization rate reductions relative to the prevaccine introduction period were 60% [95% confidence interval (CI): 58%–61%], 64% (95% CI: 57%–70%) and 55% (95% CI: 39%–67%), respectively. Rotavirus hospitalization rate reductions were 91% (95% CI: 90%–92%), 98% (95% CI: 93%–100%) and 93% (95% CI: 70%–98%). Rotavirus vaccines resulted in a 62% (95% CI: 51%–71%), 72% (95% CI: 44%–86%) and 71% (95% CI: 7%–91%) reduction in AGE hospitalization rates comparing vaccinated versus unvaccinated NBW, LBW and VLBW children 3–23 months of age, respectively.
Conclusions:
Rotavirus vaccines have substantially reduced AGE hospitalizations and are highly effective in LBW and VLBW infants, similar to NBW infants. Efforts to improve vaccination coverage, particularly in LBW and VLBW infants, should continue.
Keywords: low birth weight, rotavirus, vaccination, gastroenteritis, hospitalization rates
Before the introduction of 2 rotavirus vaccines, RotaTeq (RV5) in 2006 and Rotarix (RV1) in 2008, rotavirus was the leading cause of acute gastroenteritis (AGE) for US children <5 years of age.1 Rates of severe all-cause AGE and rotavirus AGE among the US pediatric population have declined dramatically since postvaccine introduction,2 with well-documented direct and indirect effects of vaccination.3–5
In 2015, approximately 8% of all US births were of low birth weight (LBW, <2500 g) and of those, 1.4% were of very low birth weight (VLBW, <1500 g).6 As compared with normal birth weight (NBW) infants, LBW and VLBW infants are at increased risk for severe rotavirus AGE7,8 and associated complications.9,10
Because of small sample sizes, the efficacy of rotavirus vaccines in low birth infants could not be well evaluated in prelicensure clinical trials.11,12 However, given that the vaccine was well tolerated and immunogenic in these infants and given that LBW infants are at increased risk of severe AGE, the US Advisory Committee on Immunization Practices recommends that LBW infants, including those who are premature, receive rotavirus vaccination at the same schedule as NBW infants provided they are age eligible for vaccination (6–14 weeks of age for dose 1), are clinically stable, and vaccine is administered at the time of or after discharge from the neonatal intensive care unit (NICU) or nursery. However, because some low birth infants may be >14 weeks of age at the time of discharge from the NICU or nursery, they may no longer be age eligible to receive rotavirus vaccination.1
Using a large national administrative claims database, we assessed the impact of rotavirus vaccination in reducing AGE hospitalizations comparing high risk vaccinated to high-risk unvaccinated children in the postvaccine era. Additionally, we examined if rotavirus vaccine coverage rates among LBW and VLBW infants differed from their NBW counterparts.
MATERIALS AND METHODS
We retrospectively analyzed longitudinal cohort data from Truven Health MarketScan Commercial Database (Truven Health Analytics, Ann Arbor, MI), which constitutes insurance claims from deidentified enrollees <65 years of age participating in employer-sponsored insurance plans. The MarketScan data are generalizable to the US population that are on employer-sponsored plans which encompasses approximately 58% of the US population. Medicaid recipients are not included. In 2015, enrollment data from almost 30 million primary beneficiaries and their dependents were captured. Our study population included data from approximately 3 million children <5 years of age from years 2001–2015 with data available from the monthly enrollment and inpatient Marketscan databases.
LBW infants were defined as newborns with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 765.01–765.18 (excluding 765.09, 765.10) indicating a birthweight <2500 g, whereas NBW infants were defined by the absence of a LBW ICD-9-CM code; VLBW infants (ie, birthweight <1500 g) were those with codes: 765.01–765.05, 765.11–765.15. AGE hospitalizations were identified based on the presences of the following ICD-9-CM codes: viral enteritis, 008.6–008.8 (including rotavirus, 008.61); bacterial enteritis, 001.0–005.9 (excluding 003.2) and 008.0–008.5; parasitic intestinal disease, 006.0–007.9 (excluding 006.3–006.6); presumed infectious diarrhea, 009.0–009.3; presumed noninfectious diarrhea, 558.9 and diarrhea not otherwise specified, 787.91, occurring in at least 1 of 15 possible discharge diagnoses fields from the inpatient admissions database.
Rotavirus Vaccine Coverage
RV5 and RV1 coverage was assessed for 2006 to 2015 and was defined as the receipt of at least 1 dose of either vaccine type among children who were continuously enrolled from birth until at least 3 months of age. Current Procedural Terminology was used to define receipt of RV5 or RV1 based on Current Procedural Terminology codes (RV5 = 90680, RV1 = 90681). The continuous enrollment criteria ensured that infants had the opportunity to receive at least 1 dose of a rotavirus vaccine.
When assessing vaccine trends, states with universal vaccine programs at any time during the study period were removed to control for bias in vaccine coverage rates as data on receipt of vaccination may not have been captured in MarketScan in these states.13 From 2007–2012, 13 states provided RV vaccines through a state-sponsored childhood vaccine program (Alaska, Idaho, Maine, Massachusetts, New Hampshire, New Mexico, North Dakota, Oregon, Rhode Island, Vermont, Washington and Wisconsin, Wyoming). In 2013–2014, the number decreased to 11 states (same states in 2007–2012 with exception of Alaska, North Dakota, Oregon and Wisconsin and the inclusion of Connecticut and South Dakota). In 2015, 10 states provided RV vaccines as part of their universal vaccination program (Alaska, Idaho, Maine, Massachusetts, New Hampshire, New Mexico, Rhode Island, Vermont, Washington and Wyoming). According to the National Immunization Survey, the 2015 median (range) of rotavirus vaccine coverage rates among children 19–35 months of age for the 15 states excluded was 75% (71%–88%) as compared with 73% (64%–83%) in states without a state-funded vaccine program.14 To better ensure the validity of our vaccine coverage estimates, we also compared coverage trends for receipt of at least 1 dose for the diphtheria, tetanus and acellular pertussis (DTaP) vaccine. Administration of the first dose of DTaP is recommended at the same time as the rotavirus vaccines which could then be compared with well-established coverage rates captured by National Immunization Survey.
AGE and Rotavirus Hospitalization Trends
Annual rates for both all-cause AGE and rotavirus AGE hospitalization were calculated for children <5 years of age. July 2007 to June 2015 were considered postvaccine introduction years and July 2001 to June 2006 as prevaccine years. July 2006 to June 2007 was excluded from pre/postvaccine introduction analyses and was considered a transitional year because rota-virus vaccine uptake was low and uneven across the country. Enrollment data and annual rates were calculated using number of child enrollment days contributed per year divided by 365.25. Rates were adjusted for seasonality with rates calculated based on birth month-year for both annual July–June and peak January–June seasons. Poisson regression was used to assess trends in AGE and rotavirus hospitalization rates during the study period. All states, including the universal states, were included in the trends analysis.
Rotavirus Vaccine Effectiveness
For the postvaccine years from July 2008 to June 2015, we assessed the annual effectiveness of the rotavirus vaccines in eligible populations by comparing AGE hospitalization rate reductions among vaccinated infants to unvaccinated infants for LBW, VLBW and NBW infants. The population were children who were age eligible to receive at least 1 dose of RV5 or RV1 before the start of each July–June season, that is, 3–23 months of age by each July and were continuously enrolled through the rotavirus season, that is, through June of each season. Our postvaccine study period starts in July 2008 as RV5 vaccine coverage by birth month was unstable before this time. Analysis was restricted to include no more than 1 diarrheal event per enrollee per season. Rates were assessed for children <2 years of age. Children from the states with universal vaccine programs were also excluded.
Baseline rates for prevaccine years were calculated for children <2 years of age. We used Poisson regression to adjust for hospitalization rates by birth quarter and reported the rate reductions and 95% confidence intervals (CIs) for receipt of at least 1 dose of either rotavirus vaccine.
RESULTS
Rotavirus Vaccination Coverage
Our final cohort included approximately 2 million children <5 years of age of which 96% were identified as NBW and 4% were defined as LBW. Among the LBW infants, 24% also met the VLBW criteria. RV vaccination coverage trends for all weight groups (that is, NBW, LBW and VLBW) were similar with increasing coverage rates since introduction of RV5 in 2006 and RV1 in 2008. As of December 2014, coverage among infants who had received at least 1 dose of RV5 or RV1 was near 90% for the NBW cohort and 82% and around 64% for the LBW and VLBW groups, respectively. The difference in RV coverage among the LBW and NBW was statistically significant (p < 0.0001). Comparatively, DTaP coverage was over 90% for both the NBW and LBW and 85% in the VLBW group (Fig. 1).
FIGURE 1.
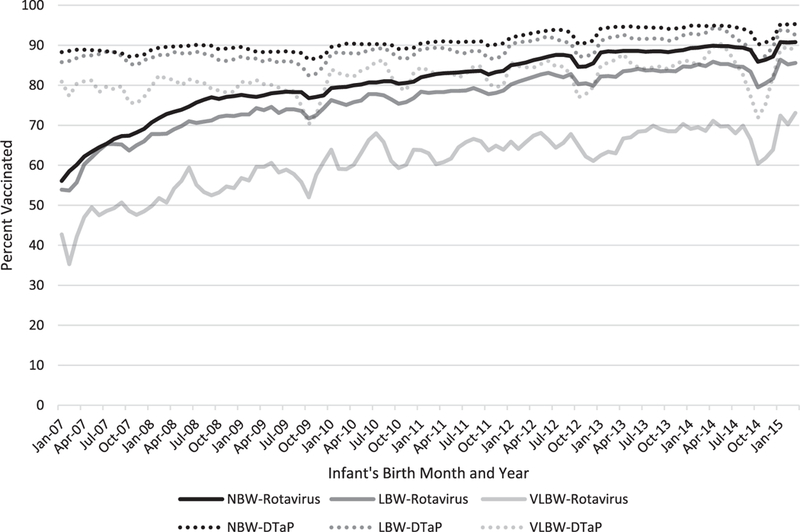
Three-month moving average of rotavirus and DTaP vaccine coverage among eligible NBW, LBW and VLBW infants enrolled in MarketScan, 2007–2015 (eligible infants were those who were continuously enrolled from birth until at least 3 months of age while coverage was defined as receipt of at least 1 dose of vaccine. Children from universal vaccine states were excluded).
Of the 12,421 eligible VLBW infants who received at least 1 dose of a rotavirus vaccine, 77% received their first dose at or before 14 weeks of age compared with over 90% for both the NBW and LBW infants (Table 1). Results were comparable when reviewing DTaP coverage among the same cohort. Among all children <5 years of age, as of December 2014, RV vaccine coverage rates were similar for both NBW and LBW at 84% and 80%, respectively, while the VLBW coverage rate was 65% (Table, Supplemental Digital Content 1, http://links.lww.com/INF/C977). Overall RV vaccine coverage has steadily increased since the vaccines were introduced for all age and weight groups.
TABLE 1.
Timing of First Rotavirus Dose According to Weight Group, 2007–2015*
| Vaccinated Infants No. |
Recommended Schedule† No. (%) |
Delayed Schedule‡ No. (%) |
|
|---|---|---|---|
| Normal birth weight | 1,541,183 | 1,469,847 (95) | 71,336 (5) |
| Low birth weight | 65,867 | 60,073 (91) | 5794 (9) |
| Very low birth weight | 12,421 | 9536 (77) | 2885 (23) |
Vaccinated infants were those who were continuously enrolled from birth until at least 3 months of age and had receipt of at least 1 dose of a rotavirus vaccine. Children who were from a universal vaccine state were excluded.
Recommended schedule was defined as receipt of the first dose of a rotavirus vaccine ≤104 days.
Delayed schedule was defined as receipt of the first dose of a rotavirus vaccine >104 days.
AGE and Rotavirus Hospitalization Trends
Similar to the coverage estimates, this analysis included approximately 2,256,000 children <5 years of age with comparable proportions in each weight group. Rates of all-cause AGE and rotavirus AGE hospitalization were greatest among VLBW infants, followed by LBW infants, and lowest in NBW infants in both pre- and postvaccine years. The rates of AGE hospitalizations in all groups have continued to decline since the introduction of the RV vaccines though variations in the impact of the vaccines can be seen from year to year (Fig. 2). Additionally, a biennial pattern of rotavirus seasonality emerges for the cohorts which is also evident among the AGE hospitalizations though the pattern is less distinct among the LBW and VLBW cohorts.
FIGURE 2.
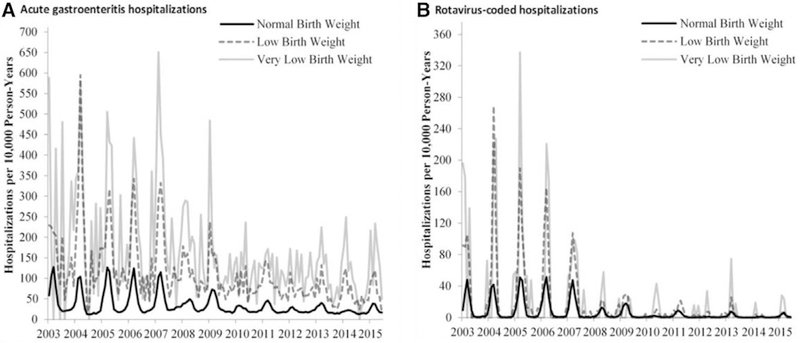
Acute gastroenteritis and rotavirus-coded hospitalization rates among children <5 years of age, 2003–2015.
In the prevaccine years, 2001–2006, the rate of both rotavirus-coded and AGE hospitalizations among the LBW and VLBW cohorts was higher than the rates in the postvaccine years, 2014–2015. The effect was evident across all age groups though the magnitude and significance of the impact varied (Table 2). For <5-year olds, the mean prevaccine rotavirus-coded hospitalization rate for LBW infants was 38 per 10,000 person-years as compared with 1 per 10,000 person-years in 2014–2015 for an overall reduction of 98% (95% Cl: 93%–100%). Comparatively, the VLBW prevaccine rate was 43 per 10,000 person-years and in 2014–2015, the rate was 3 per 10,000 person-years for an overall reduction of 93% (95% CI: 70%–98%).
TABLE 2.
Rates and Rate Reduction of Acute Gastroenteritis and Rotavirus-coded Hospitalizations Among Normal, Low and Very Low Birth Weight Children Comparing Prevaccine Years (2001–2006) to Postvaccine Years (2007–2015) — July-June
| July 2001– June 2006* |
July 2007– June 2008 |
July 2008– June 2009 |
July 2009– June 2010 |
July 2010– June 2011 |
July 2011– June 2012 |
July 2012– June 2013 |
July 2013– June 2014 |
July 2014– June 2015 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate (Ref)† |
Rate † | Rate Red. (95% CI)‡ |
Rate† | Rate Red. (95% CI)‡ |
Rate† | Rate Red. (95% CI)‡ |
Rate† | Rate Red. (95% CI)‡ |
Rate† | Rate Red. (95% CI) |
Rate† | Rate Red. (95% CI)‡ |
Rate† | Rate Red. (95% CI)‡ |
Rate† | Rate Red. (95% CI)‡ |
|
| Rotavirus-coded hospitalizations | |||||||||||||||||
| Normal birth weight | |||||||||||||||||
| <1 yr§ | 15 | 3 | 79 (75–83) | 4 | 76 (71–79) | 1 | 95(93–96) | 2 | 87(84–89) | 0 | 98(96–98) | 1 | 91(88–92) | 1 | 96 (94–97) | 1 | 92(90–94) |
| 1 yr | 29 | 9 | 69(65–73) | 8 | 72(68–75) | 1 | 96(94–97) | 4 | 85 (83–87) | 1 | 98(97–99) | 3 | 90(88–91) | 0 | 98(98–99) | 2 | 93(91–94) |
| 2–4 yr | 7 | 2 | 68(63–72) | 6 | 16 (8–23) | 1 | 88(85–90) | 3 | 59(54–63) | 0 | 96(94–97) | 1 | 78(74–81) | 0 | 97(95–98) | 1 | 87(84–90) |
| <5 yr | 13 | 4 | 72(69–74) | 6 | 56(53–59) | 1 | 93(92–94) | 3 | 78(76–79) | 0 | 97(97–98) | 2 | 86(85–88) | 0 | 97(97–98) | 1 | 91(90–92) |
| Low birth weight | |||||||||||||||||
| <1 yr§ | 26 | 3 | 87(60–96) | 4 | 84(60–94) | 2 | 94(75–99) | 5 | 81(59–92) | 2 | 93 (78–98) | 2 | 93 (76–98) | 1 | 97(80–100) | 1 | 97(78–100) |
| 1 yr | 75 | 33 | 57 (4–81) | 38 | 49(7–72) | 6 | 92(73–97) | 6 | 93(76–98) | 3 | 96 (83–99) | 13 | 83 (64–92) | 0 | NA | 0 | NA |
| 2–4 yr | 42 | 0 | NA | 7 | 84 (46–95) | 0 | NA | 11 | 75(41–89) | 2 | 95 (79–99) | 3 | 93 (77–98) | 1 | 98(83–100) | 1 | 98(82–100) |
| <5 yr | 38 | 6 | 83(67–91) | 11 | 71(55–82) | 2 | 94(86–98) | 7 | 83(71–89) | 2 | 94 (88–97) | 4 | 89(80–94) | 1 | 98(93–100) | 1 | 98(93–100) |
| Very low birth weight | |||||||||||||||||
| <l yr‡ | 35 | 4 | 87(5–98) | 7 | 80(13–95) | 7 | 81(20–96) | 0 | NA | 3 | 93 (46–99) | 0 | NA | 0 | NA | 3 | 90 (29–99) |
| 1 yr | 51 | 58 | −13 (−327 to 70) | 12 | 77 (−84 to 97) | 18 | 65 (−64 to 93) | 0 | NA | 14 | 72 (−34 to 94) | 29 | 44 (−85 to 83) | 0 | NA | 0 | NA |
| 2–4 yr | 65 | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 5 | 92 (39–99) | 4 | 93(44–99) | 4 | 94(48–99) | 5 | 93 (43–99) |
| <5 yr | 43 | 11 | 74(26–91) | 7 | 85(50–95) | 7 | 83 (51–94) | 0 | NA | 5 | 87(64–95) | 7 | 84(59–94) | 1 | 97(76–100) | 3 | 93 (70–98) |
| Acute gastroenteritis hospitalizations | |||||||||||||||||
| Normal birth weight | |||||||||||||||||
| <1 yr§ | 60 | 48 | 20(16–24) | 44 | 27(23–30) | 33 | 45(42–48) | 34 | 43(40–46) | 30 | 49(47–52) | 33 | 45(42–48) | 27 | 55(52–57) | 26 | 56(53–59) |
| lyr | 88 | 55 | 37 (34–40) | 58 | 34(31–37) | 34 | 61(59–64) | 38 | 57(54–59) | 28 | 69(67–71) | 33 | 62(60–64) | 22 | 75 (73–77) | 29 | 67(65–69) |
| 2–4 yr | 30 | 21 | 29(26–32) | 29 | 3 (−1 to 6) | 17 | 45(43–48) | 20 | 33 (31–36) | 14 | 55(52–57) | 17 | 44(41–46) | 12 | 59(56–61) | 13 | 57(54–59) |
| <5 yr | 48 | 34 | 29(27–31) | 38 | 20(18–22) | 24 | 51(49–52) | 27 | 44(43–46) | 20 | 58(57–59) | 24 | 50(49–52) | 18 | 63 (62–64) | 19 | 60(58–61) |
| Low birth weight | |||||||||||||||||
| <1 yr§ | 142 | 136 | 5 (−18 to 23) | 104 | 27(10–41) | 94 | 34(18–47) | 95 | 33(18–45) | 80 | 44(31–54) | 69 | 52(40–61) | 77 | 46 (32–57) | 68 | 52(39–62) |
| lyr | 229 | 158 | 31 (−1 to 53) | 186 | 19 (−8 to 40) | 87 | 62(46–73) | 104 | 54(38–67) | 82 | 64(51–74) | 112 | 51(35–63) | 56 | 76(65–83) | 69 | 70(57–79) |
| 2–4 yr | 117 | 39 | 67(41–81) | 51 | 57(29–74) | 31 | 74(55–85) | 68 | 42(14–61) | 40 | 66(47–78) | 48 | 59(39–72) | 35 | 70(54–81) | 36 | 70(53–80) |
| <5 yr | 156 | 113 | 27(14–39) | 107 | 32(20–42) | 78 | 50(41–58) | 90 | 43(33–51) | 69 | 56(48–63) | 70 | 55(47–62) | 58 | 63(56–69) | 57 | 64(57–70) |
| Very low birth weight | |||||||||||||||||
| <1 yr§ | 203 | 213 | −5 (−48 to 25) | 142 | 30 (−1 to 51) | 151 | 26 (−5 to 48) | 114 | 44(19–61) | 132 | 35(9–53) | 92 | 55(34–69) | 122 | 40(14–58) | 91 | 55(32–71) |
| lyr | 217 | 328 | −51 (−170 to 16) | 259 | −19 (−104 to 30) | 125 | 43 (−7 to 69) | 150 | 31 (−22 to 61) | 145 | 33 (−16 to 62) | 249 | −15 (−84 to 28) | 113 | 48(6–71) | 133 | 39 (−10 to 66) |
| 2–4 yr | 120 | 38 | 69 (−10 to 91) | 53 | 56 (−23 to 84) | 41 | 66(5–88) | 98 | 18 (−70 to 61) | 63 | 47 (−14 to 76) | 85 | 30 (−42 to 65) | 87 | 28 (−44 to 64) | 59 | 51 (−5 to 77) |
| <5 yr | 195 | 191 | 2 (−31 to 26) | 145 | 25(0–44) | 120 | 38(17–54) | 117 | 40 (20–55) | 115 | 41(23–55) | 120 | 39(20–53) | 108 | 44 (27–58) | 88 | 55 (39–67) |
For 2001–2006, we reported the average annual rates.
Rates are reported as n/10,000 person-years.
Rate reductions were not reported because of small number of events.
Infants <1 year of age were restricted to those who were vaccine eligible (ie, 3–11 months).
NA indicates not applicable; Red, reduction; ref., reference group.
For the same time frame, similar rotavirus-coded hospitalization reductions were noted in all age groups (ie, <1, 1, 2–4 and <5 years) showing declines ranging from 90% to 100% in the VLBW groups and 97% to 100% in the LBW children. AGE hospitalization rates also declined among all birth weight groups. Among <5- year olds, the mean prevaccine rate in the LBW group was 156 per 10,000 person-years as compared with 195 per 10,000 person-years in the VLBW infants. The 2014–2015 LBW rate reduction was 64% (95% CI: 57%−70%) and 55% (95% CI: 39%−67%) in the VLBW cohort.
Across all postvaccine annual seasons, age groups and weight classes, the LBW age groups for 1-year olds and 2- to 4-year olds reported the largest declines in 2014–2015 with an overall reduction in AGE hospitalizations of 70% (95% CI: 57%–79% and 95% CI: 53%–80%, respectively), when compared with the prevaccine rate. Similar trends were evident during the peak rota-virus months of January to June with annual rate reductions for both rotavirus-coded and AGE hospitalizations in all weight groups for children <5 years old (Table, Supplemental Digital Content 2, http://links.lww.com/INF/C978).
Rotavirus Vaccine Effectiveness
For season 2014–2015, among the 1,300,000 age-eligible children <2 years of age, AGE hospitalization rates for those who received at least 1 dose of a rotavirus vaccine were lower compared with their unvaccinated counterparts (Table 3). In 2008–2009, the first full season, there was a rate reduction for all weight classes with a decline of 49% (95% CI: 42%–55%), 60% (95% CI: 36%–75%) and 75% (95% CI: 17%–93%) in the NBW, LBW and VLBW groups, respectively. During 2014–2015 season, among LBW children who had received at least 1 dose of RV5 or RV1, there was 72% (95% CI: 44%–86%) reduction and a 71% (95% CI: 7%–91%) decline among the VLBW infants as compared with infants who did not receive a dose of either RV vaccine. Among the NBW infants, similar trends were noted with a 62% (95% CI: 51%–71%) reduction which is the greatest reported decline among this group since introduction of the rotavirus vaccine. Because of small numbers, we did not report on rate reductions for rotavirus- coded hospitalizations.
TABLE 3.
Annual Rates and Rate Reductions of Acute Gastroenteritis Hospitalizations Among Children 3–23 Months of Age Who Received At Least 1 Dose of RV5 or RV1 Versus Unvaccinated Age-eligible Children*
| Normal Birth Weight |
Low Birth Weight |
Very Low Birth Weight |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vax+ | Vax- | Vax+ | Vax- | Vax+ | Vax- | ||||
| Season | Rate/10,000 PY |
Rate Reduction (95% CI) |
Rate/10,000 PY |
Rate Reduction (95% CI) |
Rate/10,000 PY |
Rate Reduction (95% CI) |
|||
| Acute gastroenteritis hospitalizations | |||||||||
| 2008–2009 | 43 | 81 | 49 (42–55) | 66 | 164 | 60 (36–75) | 40 | 141 | 75 (17–93) |
| 2009–2010 | 25 | 46 | 47 (36–56) | 56 | 138 | 62 (35–78) | 39 | 203 | 80 (37–93) |
| 2010–2011 | 27 | 56 | 52 (43–59) | 46 | 170 | 72 (53–83) | 71 | 76 | 15 (−140 to 70) |
| 2011–2012 | 23 | 35 | 32 (17–44) | 31 | 144 | 80 (65–88) | 47 | 212 | 82 (57–93) |
| 2012–2013 | 24 | 59 | 59 (51–65) | 55 | 147 | 66 (43–80) | 70 | 244 | 71 (35–87) |
| 2013–2014 | 20 | 31 | 34 (14–50) | 43 | 128 | 66 (38–81) | 78 | 209 | 61 (14–82) |
| 2014–2015 | 23 | 62 | 62 (51–71) | 40 | 144 | 72 (44–86) | 54 | 166 | 71 (7–91) |
Eligibility was restricted to children who were 3–23 months of age by July 1 of each season (July 1, 2008, July 1, 2009, etc) and continuously enrolled from birth through end of each corresponding season (June 30, 2009, June 30, 2010; etc). Rates were adjusted for quarter of birth. Children who were from a universal vaccine state were excluded.
PY indicates person-year; RV5, RotaTeq; RV1, Rotarix; Vax +, vaccinated; Vax-, unvaccinated.
DISCUSSION
RV vaccination coverage increased for all age groups over the study period. As of 2014–2015, <1-year olds were covered at 89%, 84% and 68% for the NBW, LBW and VLBW infants, respectively, the highest coverage achieved since the introduction of the rotavirus vaccines. However, disparities were evident when comparing coverage among the different weight classes with the VLBW group consistently lagging behind the NBW children. While DTaP vaccination coverage showed similar trends, the disparities among the weight groups were less pronounced. One possible reason for the lower RV compared with DTaP coverage, especially among the VLBW infants, is that many of these infants may not be age eligible to receive the first dose of rotavirus vaccine if they were older than 14 weeks when discharged from the NICU. This hypothesis is supported by our data that among eligible infants who received at least 1 dose of the RV vaccine, only 5% of the NBW infants received their first RV dose after 14 weeks of age as compared with 23% in the VLBW groups. Additionally, it is important to note that almost a quarter of the vaccinated VLBW cohort received their first RV dose outside the US Advisory Committee on Immunization Practices recommended vaccination schedule. Our data suggest that when addressing the risk of exposure and burden of rotavirus disease, clinicians might be opting to administer the first RV dose off recommendation versus risking a missed opportunity for vaccination.
Overall hospitalizations among US children <5 years of age caused by AGE and rotavirus have declined substantially since the introduction of the 2 US-licensed rotavirus vaccines. Throughout the study period, we found that AGE hospitalization rates were higher among VLBW infants as compared with LBW and NBW cohorts. However, the hospitalization rate reductions were slightly greater among LBW infants relative to the VLBW cohort, possibly because of lower rotavirus vaccine coverage among VLBW compared with LBW infants. Nevertheless, large declines in AGE hospitalization rates of 64% and 55% were reported in 2014–2015 among LBW and VLBW infants, respectively, demonstrating that rotavirus vaccines have had a substantial health impact in these high-risk infants, similar to the general population of US infants. Our results also demonstrate the high effectiveness of rotavirus vaccines in reducing AGE hospitalizations in vaccinated infants as compared with their unvaccinated counterparts among LBW and VLBW infants, at levels similar to those in NBW infants. Taken together, these data support the use of rotavirus vaccines in all US infants, including LBW and VLBW infants.
Limitations of this study include the lack of descriptive information for enrolled children, including race and socioeconomic status. We also only assessed infants who were privately insured thereby excluding LBW and VLBW infants who are on Medicaid, under-insured or uninsured. Given that approximately 8% of all US infants are born at a LBW6 and that among them over 50% are on Medicaid15; our results may not be generalizable to the entire US population of LBW infants. Finally, because of small numbers, we were unable to describe the hospitalization rates among infants born at extremely LBWs (<1000 g).
CONCLUSIONS
Our study illustrates the substantial impact the rotavirus vaccines have had on dramatically reducing the rates of AGE and rotavirus hospitalizations among US children <5 years of age, and provide the first evidence of the large impacts in LBW and VLBW children. Efforts to improve vaccination coverage, particularly in LBW and VLBW infants, should continue.
Supplementary Material
ACKNOWLEDGMENTS
We thank Fangjun Zhou for providing data on states with universal vaccine programs.
Footnotes
The authors have no funding or conflicts of interest to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Cortese MM, Parashar UD. Centers for the Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58(RR-2):1–25. Available from:http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5802a1.htm Accessed August 3, 2016. [PubMed] [Google Scholar]
- 2.Leshem E, Moritz R, Curns AT. Rotavirus vaccines and health care utilization for diarrhea in the United States, 2007–2011 Pediatrics. 2014;134:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gastanaduy PA, Curns AT, Parashar UD, et al. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA. 2013;310:851–853. [DOI] [PubMed] [Google Scholar]
- 4.Lopman BA, Curns AT, Yen C, et al. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis. 2011;204:980–986. [DOI] [PubMed] [Google Scholar]
- 5.Payne DC, Selvarangan R, Azimi PH, et al. Long-term consistency in rota-virus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012–2013. Clin Infect Dis. 2015;61:1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin JA, Hamilton BE, Osterman MJK, et al. National Center for Health Statistics. Births: final data for 2015. National Vital Statistics Reports. 2017;66 Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01.pdf Accessed April 10, 2017. [PubMed] [Google Scholar]
- 7.Dennehy PH, Cortese MM, Begue RE, et al. A case-control study to determine risk factors for hospitalization for rotavirus gastroenteritis in U.S. children. Pediatr Infect Dis J. 2006;25:1123–1131. [DOI] [PubMed] [Google Scholar]
- 8.Newman RD, Grupp-Phelan J, Shay DK, et al. Perinatal risk factors for infant hospitalization with viral gastroenteritis. Pediatrics. 1999;103:E3. [DOI] [PubMed] [Google Scholar]
- 9.Borah M, Baruah R. Morbidity status of low birth weight babies in rural areas of Assam: a prospective longitudinal study. J Family Med Prim Care. 2015;4:380–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruijning-Verhagen P, Mangen MJ, Felderhof M, et al. Targeted rotavirus vaccination of high-risk infants; a low cost and highly cost-effective alternative to universal vaccination. BMC Med. 2013;11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goveia MG, Rodriguez ZM, Dallas MJ, et al. ; REST Study Team. Safety and efficacy of the pentavalent human-bovine (WC3) reassortant rotavirus vaccine in healthy premature infants. Pediatr Infect Dis J. 2007;26:1099–1104. [DOI] [PubMed] [Google Scholar]
- 12.Omenaca F, Sarlangue J, Szenborn L, et al. ; ROTA-054 Study Group. Safety, reactogenicity and immunogenicity of the human rotavirus vaccine in preterm European Infants: a randomized phase IIIb study. Pediatr Infect Dis J. 2012;31:487–93. [DOI] [PubMed] [Google Scholar]
- 13.Cortes JE, Curns AT, Tate JE, et al. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med. 2011;365:1108–1117. [DOI] [PubMed] [Google Scholar]
- 14.Estimated vaccination coverage with individual vaccines by 3 months of age. National Immunization Survey. 2015. Available at: www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/data-reports/rotavirus/reports/2015.html Accessed October 12, 2017.
- 15.Healthcare Cost and Utilization Project (HCUP). 2014. Available at: https://hcupnet.ahrq.gov Accessed April 28, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


