Abstract
Neurons containing melanin-concentrating hormone (MCH) in the lateral hypothalamic area (LH) have been shown to promote rapid eye movement sleep (REMs) in mice. However, the downstream neural pathways through which MCH neurons influence REMs remained unclear. Because MCH neurons are considered to be primarily inhibitory, we hypothesized that these neurons inhibit the midbrain ‘REMs-suppressing’ region consisting of the ventrolateral periaqueductal gray and the lateral pontine tegmentum (vlPAG/LPT) to promote REMs. To test this hypothesis, we optogenetically inhibited MCH terminals in the vlPAG/LPT under baseline conditions as well as with simultaneous chemogenetic activation of MCH soma. We found that inhibition of MCH terminals in the vlPAG/LPT significantly reduced transitions into REMs during spontaneous sleep-wake cycles and prevented the increase in REMs transitions observed after chemogenetic activation of MCH neurons. These results strongly suggest that the vlPAG/LPT may be an essential relay through which MCH neurons modulate REMs.
Keywords: Paradoxical sleep, chemogenetics, optogenetics, sublaterodorsal nucleus, REM sleep transitions, Archearhodopsin T
Introduction
Neurons containing melanin-concentrating hormone (MCH) located in the lateral hypothalamic area (LH) have long been implicated in sleep-wake regulation (Peyron et al., 2011; Verret et al., 2003; Willie et al., 2008). MCH neurons fire maximally during rapid eye movement sleep (REMs) (Hassani et al., 2009) and they express c-Fos after periods of high REMs (e.g. following selective REMs deprivation) (Verret et al., 2003), indicating a crucial role for these neurons in REMs regulation. Consistent with these observations, brief optogenetic stimulations of MCH neurons in mice during non-REM sleep (NREMs) increased NREMs-REMs transitions and similar stimulations during REMs increased the duration of REMs bouts (Jego et al., 2013). On the other hand, long-term optogenetic stimulations of MCH neurons produced inconsistent results with one study reporting increases in both NREMs and REMs (Konadhode et al., 2013), while another study showed an increase in REMs but a strong decrease in NREMs (Tsunematsu et al., 2014). These divergent results are likely caused by differences in stimulation paradigms (pulse width, duration and frequency), differences in the location of the stimulated MCH neurons (variation in optical fiber placement) and/or inherent shortcomings of optogenetics as a stimulation technique (e.g. causing artificial monotonous firing, heating and damaging of brain tissues associated with long-term stimulations) (Cardin et al., 2010; Christie et al., 2013; Rogan and Roth, 2011). In contrast, activation of MCH neurons using chemogenetic methods, which do not suffer from the same shortcomings, specifically increased REMs without altering NREMs (Vetrivelan et al., 2016), thereby suggesting a specific REMs-promoting role for these neurons.
Although the above-mentioned studies indicated a causal relationship between MCH neurons and REMs, the neural pathways by which MCH neurons influence REMs are largely unclear. Studies during the last decade have identified the sublaterodorsal tegmental nucleus (SLD) in the dorsolateral pons as the principal region for REMs generation, and the ventrolateral periaqueductal gray and the adjoining lateral pontine tegmentum (vlPAG/LPT) as key regions for REMs suppression (Lu et al., 2006; Luppi et al., 2006; Vetrivelan et al., 2011). These cell groups form the primary REMs circuitry and the interaction between them is thought to control the onset/occurrence of REMs. The mesopontine cholinergic neurons, originally hypothesized to be REMs generators (McCarley and Hobson, 1975), may only modulate REMs by their actions on this primary REMs circuitry, but are not necessary for spontaneous REMs (Grace et al., 2014; Kroeger et al., 2017; Lu et al., 2006; Vetrivelan and Lu, 2019). The termination of REMs episodes is likely regulated by wake-promoting cell groups, including the monoaminergic neurons in the brainstem (Lu et al., 2006; Sakai et al., 2001; Vetrivelan et al., 2011), resuming their activity at the end of REMs (most, if not all REM episodes, terminate in wakefulness in mice). Thus, to increase REMs, MCH neurons could activate/disinhibit brainstem REMs generating networks and increase REMs transitions or inhibit wake-generating networks to delay REMs termination and extend the duration of REMs bouts. However, chemogenetic activation of MCH neurons increases only NREMs-REMs transitions but does not alter REMs bout duration (Vetrivelan et al., 2016). It is therefore likely that MCH neurons target brainstem networks gating the initiation of REMs such as the vlPAG/LPT and the SLD to promote REMs transitions.
MCH neurons are predominantly inhibitory. MCH and other neuropeptides including cocaine- and amphetamine-regulated transcript (CART) and nesfatin-1 found in the MCH neurons are inhibitory peptides (Brailoiu et al., 2007; Broberger, 1999; Elias et al., 2001; Elias et al., 1998). A majority of MCH neurons also contain glutamate decarboxylase (GAD67; necessary for GABA synthesis), suggesting that MCH neurons may use GABA as neurotransmitter. However, MCH neurons do not contain the vesicular GABA transporter (Vgat; necessary for GABA release) and the evidence for GABA release from MCH terminals is controversial (Chee et al., 2015; Jego et al., 2013). On the other hand, MCH neurons do contain an excitatory neurotransmitter, glutamate, but its release from MCH neurons may inhibit postsynaptic neurons by feed-forward inhibition (Chee et al., 2015). Nevertheless, activation of MCH neurons increases REMs even in the absence of glutamate, suggesting that the glutamate may not be necessary for REMs promotion by MCH neurons (Naganuma et al., 2019). Hence, we hypothesized that MCH neurons promote REMs primarily by inhibiting REMs-suppressing neurons in the vlPAG/LPT rather than activating REMs-promoting SLD neurons. This hypothesis is further supported by the observations that MCH infusions into the SLD indeed decreased REMs (Monti et al., 2016), and that REMs suppression induced by pharmacological inhibition of the LH (although not specifically the MCH neurons) was associated with an increase in c-Fos in the vlPAG/LPT (Clement et al., 2012). To test our above-mentioned hypothesis, we optogenetically inhibited MCH terminals in the vlPAG/LPT under baseline conditions as well as with simultaneous chemogenetic activation of MCH cell bodies, and studied changes in REMs.
Methods:
Animals:
Adult male MCH-Cre mice (n=15) on a mixed background were used in this study. These transgenic mice, expressing Cre recombinase specifically in the MCH neurons, were generated by our colleagues Drs. Bradford Lowell and Dong Kong (Department of Medicine, Division of Endocrinology, BIDMC) and are currently available at the Jacksons laboratory (Stock no# 014099). Eutopic expression of Cre in this transgenic line has been verified in our previous studies (Kong et al., 2010; Vetrivelan et al., 2016).
All animals were maintained in the vivarium under 12:12 h light:dark cycle (lights on 7 AM) with ad libitum access to food and water. Ambient temperature of the vivarium was maintained at 22 ± 1°C. Care of the animals met National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center.
Surgery:
Under anesthesia (ketamine 100 mg/kg + xylazine 10mg/kg; i.p.), MCH-Cre mice (n=4) were stereotaxically injected (Scammell et al., 1998) with 260 nL of an adeno-associated virus (AAV) carrying Archaerhodopsin T (ArchT) and a green fluorescent protein (GFP), AAV8-CAG-Flex-ArchT-GFP (University of North Carolina vector core, USA) unilaterally into the LH [−1.7 mm AP, 4.8 mm DV, ±1.0 mm ML (Franklin and Paxinos, 2007)] for tracing experiments. Another cohort of MCH-Cre mice (n=9) was injected with a mixture of two AAVs: AAV-DIO-hM3Dq-mCherry (AAV8-hSyn-DIO-hM3DqmCherry; University of North Carolina vector core, USA) and AAV-Flex-ArchT-GFP bilaterally into the LH (460 nL per side) for opto/chemogenetic experiments. Three weeks after viral injections, the second cohort underwent an additional surgery during which the mice were implanted with bilateral optical fibers (200 μm core diameter; BFH37–200 Multimode; NA 0.37; Thorlabs) targeting 0.3 mm dorsal to the vlPAG/LPT region (−4.25 mm AP, 2.2 mm DV, ±0.8 mm ML) and electrodes for recording EEG and EMG (Kroeger et al., 2018). EEG electrodes were inserted unilaterally into the frontal cortex (1.5 mm anterior to Bregma, 1.7 mm lateral to sagittal suture) and parietal cortex (2.5 mm posterior to Bregma, 1.5 mm lateral to sagittal suture), for recording fronto-parietal biploar EEG. EMG electrodes were placed bilaterally onto the nuchal muscles (Kroeger et al., 2017).
Sleep-wake recording and analysis:
After 2 weeks of postoperative recovery, mice were transferred to the recording chambers and habituated to the connecting cables for 7 days. Mice were then injected with either saline or clozapine-n-oxide (CNO; 0.3 mg/kg; i.p. at ZT6) followed by sham or yellow/orange light illumination targeting the vlPAG/LPT concurrent with EEG/EMG recordings starting at ZT7.Yellow/orange light (593.5 nm wave length; ~10 mW light power at the fiber tip / irradiance of 79.54 mW/mm2; from a DPSS laser diode, Laserglow Technologies, Canada) illuminations were applied after 30 seconds of stable NREMs and continued until the next wake bout irrespective of whether NREMs transitioned into REMs or wake. We performed 15 such inhibitions (random 15 NREMs bouts) within the 3 h recording period after CNO/saline (ZT7-ZT10). We chose this paradigm because chemogenetic activation of MCH neurons in our previous study (Vetrivelan et al., 2016) specifically increased REMs amounts by increasing REMs transitions (but did not affect wake or NREMs) and in this study we assessed whether acute inhibition of MCH terminals in the vlPAG/LPT could block this response. Moreover, because the photo-inhibitions continued until the end of REMs, our paradigm allowed us to detect changes in the duration of REMs bouts in addition to NREMs-REMs transitions.
Histology:
Four weeks after viral injections, mice in the tracing experiments were deeply anesthetized (chloral hydrate; 700 mg/kg) and transcardially perfused with 30 ml saline followed by 30 ml of 10% formalin (Chen et al., 2017; Vetrivelan et al., 2016). Mice in the opto/chemogenetic experiments were i.p. injected with saline or CNO and similarly perfused 2 h later. Brains were harvested and cut into 3 series of 40 μm sections.
One series of sections from the mice in the tracing experiments was labeled for GFP (rabbit anti-GFP, ThermoFisher Scientific, USA; Cat# A11122, 1:10000 dilution) by diaminobenzidine (DAB)-immunohistochemistry (Kroeger et al., 2017; Oishi et al., 2013; Vetrivelan et al., 2016). The number of GFP+ boutons in the vlPAG/LPT and in the SLD were counted using counting boxes (1.0 × 0.6 mm for the vlPAG/LPT and 0.5 × 0.4 mm for the SLD) placed over these regions on brain sections (vlPAG/LPT: AP −4.16, −4.36 and −4.60 mm from bregma; SLD: AP −5.02 and −5.34 mm from bregma as per the mouse atlas of Franklin and Paxinos (Franklin and Paxinos, 2007).
One series of sections from mice in the chemo/optogenetic experiments was labeled for MCH by immunofluorescence (Oishi et al., 2013; Vetrivelan et al., 2016) (Rabbit anti-MCH, generous gift from Dr. Maratos-Flier, BIDMC; 1:10,000 dilution) while we visualized hM3Dq and ArchT-expressing neurons using the native florescence of the respective tags - mCherry and GFP. A second series from these mice was processed for c-Fos (1° Ab - rabbit anti-c-Fos, Oncogene Sciences, USA; catalog # 4188; 1:30000 dilution; 2° Ab - biotin-SP-conjugated donkey anti-rabbit IgG; Jackson ImmunoResearch, USA; catalog #11–065-152; 1:1000 dilution) and mCherry immunolabeling (1° Ab - rabbit anti-DsRed, Clontech, USA; catalog # 632496; 1:10000 dilution and 2° Ab: biotin-SP-conjugated donkey anti-rabbit IgG; Jackson ImmunoResearch, USA; catalog #11–065-152; 1:1000 dilution) by DAB-immunohistochemistry (Kroeger et al., 2017; Oishi et al., 2013; Vetrivelan et al., 2016). All cell counting in the LH MCH field mentioned in the results was performed manually as described previously (Oishi et al., 2013). Finally, we mapped AAV injection sites and the location of the optical fiber tips in all mice.
In vitro electrophysiology:
Male MCH-Cre mice (n= 2; 4 week old) were injected with AAV8-CAG-Flex-ArchT-GFP into the LH as described above and sacrificed after 4 weeks. Brains were quickly removed into ice-cold cutting solution consisting of 72 mM sucrose, 83 mM NaCl, 2.5 mM KCl, 1 mM NaH2PO4, 26 mM NaHCO3, 22 mM glucose, 5 mM MgCl2, 1 mM CaCl2, carbogenated with 95% O2/5% CO2, with a measured osmolarity of 310–320 mOsm/l (Fenselau et al., 2017; Garfield et al., 2016; Resch et al., 2017). Brains were then cut into 250 μm coronal sections on a vibratome (Leica VT1000S) and the slices containing the LH were prepared for whole-cell current clamp recordings (Fenselau et al., 2017; Resch et al., 2017). ArchT-GFP expressing MCH neurons were visualized using an upright microscope (SliceScope, Scientifica) equipped with infrared-differential interference contrast and fluorescence optics. Current-clamp recordings were performed from 4 neurons using borosilicate glass microelectrodes (5–7 MΩ) filled with internal solution (pH 7.3) containing 128 mM potassium gluconate, 10 mM KCl, 10 mM HEPES, 1 mM EGTA, 1 mM MgCl2, 0.3 mM CaCl2, 5 mM Na2-ATP, 0.3 mM Na-GTP (Fenselau et al., 2017; Garfield et al., 2016; Resch et al., 2017). Kynurenate (1 mM; Sigma) and picrotoxin (100 μM; Tocris) were added to the bath to block glutamatergic and GABAergic synaptic transmission. 5–20 pA current was applied via the patch pipette if GFP+ MCH neurons did not fire action potentials. After establishing a stable baseline, ArchT was activated using yellow/orange laser light (565 nm, ~3mW/mm2, 1 mm beam width from a 880 mW LUXEON yellow light-emitting diode, #M565L3; Thorlabs, Newton, NJ, USA) applied for 5–10 seconds and the recordings continued for 1–2 minutes. We analyzed the data using Clampfit 9.0 (Molecular Devices) and IGOR Pro 6 (WaveMetrics, Lake Oswego, OR, USA).
Sleep-wake data analysis:
EEG/EMG signals were amplified (A.M systems, USA) and digitally recorded (sampling rate, 256 Hz; Filters, EEG: 0.3–70 Hz and EMG: 5–300 Hz) on a PC using Vital Recorder software (Kissei Comtek, Japan). EEG/EMG data was divided into 10-s epochs and manually scored (SleepSign 3 software, Kissei Comtek, Japan) into one of the three sleep-wake states: wake, NREMs or REMs as previously described (Lu et al., 2000). Briefly, wake was identified by the presence of desynchronized (high frequency and low amplitude) EEG and high muscle (EMG) tone with movement-related activity. NREMs was identified by the presence of synchronized (high amplitude, low frequency with predominating delta [0.5 – 4 Hz] activity) and lower muscle tone compared to wake. REMs was identified by the presence of desynchronized EEG activity with regular theta (4–6 Hz) components and muscle atonia or lower muscle tone compared to NREMs. If more than one sleep state occurred within a 10 sec epoch, the epoch was scored for the state that occupied more than 5 sec.
We first calculated the percentage of time spent in wake, NREMs and REMs and their bout numbers and mean durations during the 3-h recording period after CNO in all mice and compared them with post-saline data (Fig. 4). For the experiments involving optogenetic inhibition trials (saline/CNO + sham inhibition/laser light; Fig. 5), we calculated the duration of NREMs bouts, NREMs-REMs transitions (number of REMs bouts) and duration of REMs bouts for each trial and calculated the mean values for each condition in individual animals. These values were used to calculate the group means for each parameter for all four experimental conditions. REMs latency (time to REMs onset from when the laser was turned on) was also calculated for each trial. Trials without REMs were not included for the REMs latency calculation. We then used Fast Fourier transform (FFT) to extract the power spectra of EEG traces during each trial (0. 5 Hz resolution) and calculated the percentages of delta (0.5–4 Hz) and theta (6–9 Hz) power in NREMs and REMs periods. Integral EMG during NREMs and REMs during each trial (normalized to 20 second of NREMs prior to trial onset) for all four experimental conditions was calculated. Finally, we calculated the percentage of time spent in NREMs and REMs during the combined duration of all 15 trials. These data (number of NREMs-REMs transitions, average duration of NREMs and REMs bouts, REM latency, percentage of NREMs and REMs, EEG delta and theta power during NREMs/REMs and integral EMG) were obtained for all 4 experimental conditions (Fig. 5). We first tested these data for normality using D’Agostino & Pearson normality test. As the data were normally distributed, we then used either a paired t-test (Fig 4) or a repeated measures two-way ANOVA followed by Holm-Sidak post-hoc test for all comparisons (Fig 5). All statistical analysis was performed using Graphpad Prism (version 7.03, GraphPad Software, Inc.)
Results
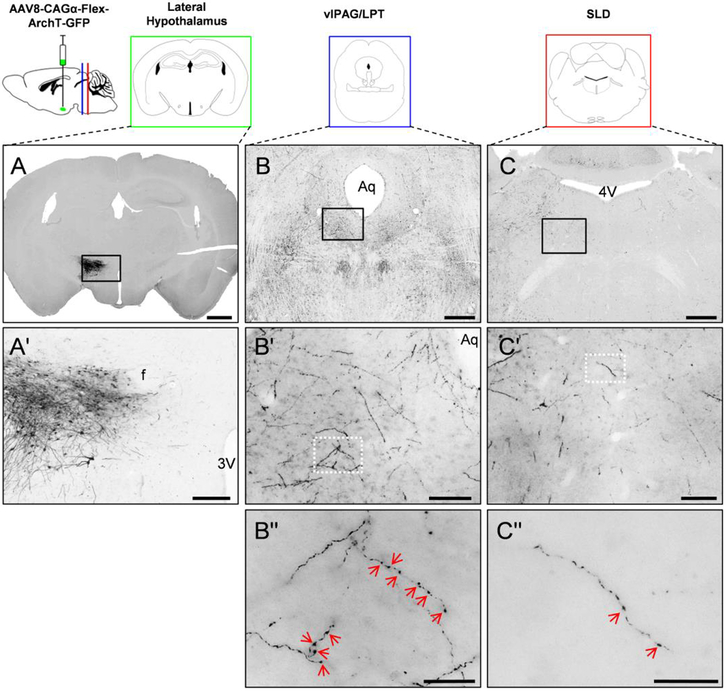
To verify that MCH neurons send projections to brainstem REMs regulatory centers, we induced expression of ArchT with a GFP tag as tracer selectively in MCH neurons by injecting an AAV8-CAG-Flex-ArchT-GFP (Kroeger et al., 2018) into the LH (Fig. 1 A, A′) of MCH-Cre mice (Kong et al., 2010; Vetrivelan et al., 2016). Histological processing of brain sections after 4 weeks of construct expression demonstrated dense innervation of the vlPAG/LPT (171.4±65.2 boutons/mm2; n=3 mice) and moderate innervation of the SLD (19.7±6.1 boutons/mm2; n=3 mice) by MCH neurons (Fig. 1 B′′,C′′).
Figure 1. Anterograde tracing from lateral hypothalamic MCH neurons to the primary sites of REMs control.
MCH neurons (A– injection site in LH) innervate the REMs-suppressing vlPAG/LPT (B) as well as the REMs-generating SLD (C) in the brain stem, as indicated by the presence of ArchT-GFP labeled axon terminals (black color) in these regions. Black squares in A, B and C represent the regions magnified in A′, B′ and C′ and white squares in B′ and C′ represent the regions magnified in the Bʹʹ and Cʹʹ. Red arrowheads in Bʹʹ and Cʹʹ indicate individual boutons. Scale bars are: (A) 1mm; (B, C) 0.5 mm; (A′) 200 μm; (B′, C′) 100 μm; (Bʹʹ and Cʹʹ) 20 μm. 3V, 3rd ventricle; 4V 4th ventricle; Aq, aqueduct; f, fornix.
As MCH neurons are primarily considered to be inhibitory and project more heavily to the vlPAG/LPT than the SLD, we hypothesized that the vlPAG/LPT is the principal relay through which MCH neurons regulate REMs. To test this hypothesis, we utilized a combination of optogenetic and chemogenetic methods. We induced Cre-dependent expression of the inhibitory light-sensitive opsin, ArchT [causes complete silencing of neurons upon yellow-orange light (593.5 nm) illumination] (Chow et al., 2010; Han et al., 2011), as well as excitatory DREADDs (Roth, 2016) [hM3Dq; activates neurons in response to an otherwise inert ligand clozapine-N-oxide (CNO)] selectively in MCH neurons by injecting a 1:1 mixture of two Cre-dependent AAVs – AAV-DIO-hM3Dq-mCherry (Vetrivelan et al., 2016) and AAV-Flex-ArchT-GFP (Kaur et al., 2017) into the LH of MCH-Cre mice. We then implanted all mice with bilateral optical fibers targeting the vlPAG/LPT for yellow/orange laser illumination and electrodes for recording EEG and EMG to assess sleep-wake states (Kaur et al., 2017). This setting allowed us to perform optogenetic inhibition of MCH terminals in the vlPAG/LPT at baseline conditions as well as during simultaneous chemogenetic activation of MCH neurons by intraperitoneal (i.p.) administration of CNO.
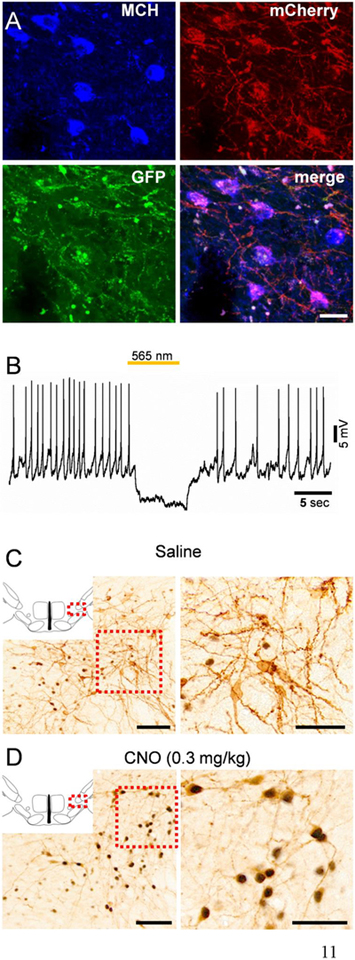
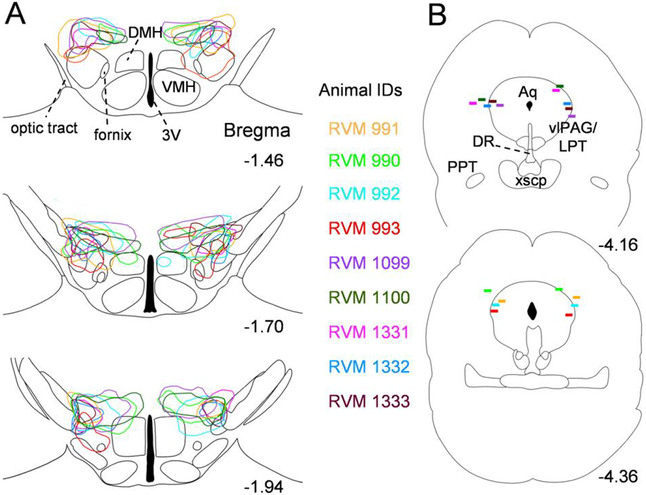
In post-mortem histology, we observed a large number of triple-labelled neurons in the LH MCH field (mCherry marking hM3Dq expression, GFP marking ArchT expression and the MCH peptide marking MCH cell bodies; Fig. 2A), indicating that both hM3Dq and ArchT were expressed in MCH neurons (transfection efficiency 45±4.6% in the perifornical lateral hypothalamus). On the other hand, 91.6 ± 2.8% of mCherry+ neurons and 88.6 ± 3.1% of GFP+ neurons (n=4 mice) were also positive for MCH indicating a high specificity of AAV-DIO-hM3Dq-mCherry and AAV-Flex-ArchT-GFP, respectively. Current clamp recordings from ArchT-expressing (GFP+) neurons showed that yellow/orange light illumination hyperpolarized and completely silenced these neurons (Fig. 2B). In addition, i.p. injections of CNO, 2 h before perfusions, induced c-Fos in hM3Dq-expressing (mCherry+) neurons demonstrating that MCH neurons were activated by CNO (89.4 ± 5.8% of mCherry+ neurons expressed c-Fos after CNO vs. 11.6 ± 2.7% after saline; n=3 mice; Fig. 2C). We further analyzed histological sections and mapped the extent of AAV-injections in the LH (Fig. 3A) and the location of optical fiber tips in the vlPAG/LPT (Fig. 3B) in each mouse. As expected, expression of viral constructs was restricted to the LH and the zona incerta MCH field (Fig. 3A), while optical fiber tips were located approximately 0.3 mm dorsal to the vlPAG/LPT (Fig. 3B).
Figure 2. Chemoactivation and optogenetic inhibition of MCH neurons.
(A) Triple-labeled neurons in the LH indicating the expression of hM3Dq-mCherry (red; native florescence) and ArchT-GFP (green; native florescence) in MCH neurons (blue; immunofluorescence labeling with rabbit anti-MCH and goat anti-rabbit antibodies). (B) Representative whole cell, current clamp recording from an ArchT-expressing MCH neuron (identified by GFP fluorescence) indicating hyperpolarization and complete cessation of action potentials induced by 565 nm light illumination (yellow/orange line above the trace). (C,D) c-Fos activity (black staining) in hM3Dq- and ArchT-expressing MCH neurons (mCherry labeling in brown) 2 h after saline (C) or CNO (0.3 mg/kg) application (D) indicating activation of MCH neurons by CNO. Scale bars are: (A) 20 μm; (C,D) overview images 100 μm; magnified images 50 μm.
Figure 3: Map of injection sites and optical fiber tip locations in MCH-Cre mice.
(A) Outlines of AAV injection sites in three levels of the hypothalamus [AP −1.46, −1.70 and −1.94 (Franklin and Paxinos, 2007)]. (B) Location of optical fiber tips mapped onto two levels of the brainstem [AP −4.16 and −4.36 (Franklin and Paxinos, 2007)]. Animal IDs of all mice (n = 9) are listed in corresponding colors with injection site outlines and fiber placement markings. 3V, 3rd ventricle; Aq, aqueduct; DMH, dorsomedial hypothalamic nucleus; DR, dorsal raphe nucleus; vlPAG/LPT, ventrolateral periaqueductual gray/lateral pontine tegmentum; PPT, pedunculopontine tegmental nucleus; VMH, ventromedial hypothalamic nucleus; xscp, decussation of the superior cerebellar peduncle.
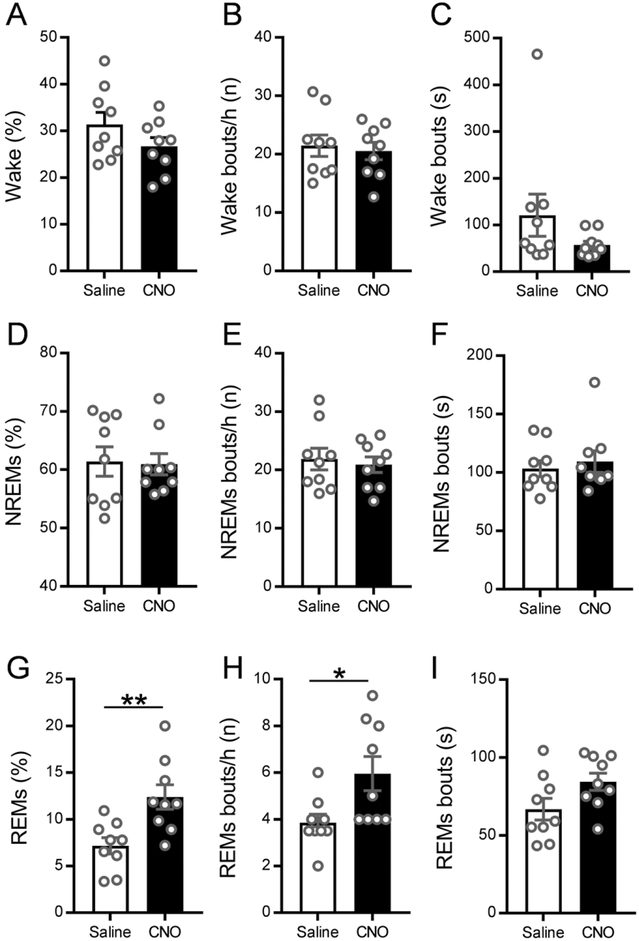
Prior to performing inhibition of MCH terminals in the vlPAG/LPT, we sought to confirm that chemoactivation of MCH neurons increases REMs as we previously reported (Vetrivelan et al., 2016). Therefore, we i.p. injected MCH-Cre mice expressing hM3Dq and ArchT in MCH neurons with CNO (0.3 mg/kg) or saline (vehicle) at ZT6 and recorded sleep-wake from ZT7 to ZT10. We found that CNO activation of MCH neurons specifically increased REMs without significantly altering NREMs or wake (Fig 4 A–I). The amount of REMs was increased by 63% during the 3 h recording period after CNO when compared to saline (11.7± 1.3% REMs after CNO vs. 7.2±0.9% after saline, Fig. 4G). The increased REMs amounts after CNO were due to a significant increase in REMs bout numbers (6.0± 0.7 bouts/h after CNO vs. 3.9±0.4 bouts/h after saline; Fig. 4H), but not due to an increase in REMs bout duration (Fig. 4I). In contrast, NREMs and wake parameters (amounts, bout number and durations) after CNO were not significantly different from those after saline (Fig. 4A–F). These data confirm our previous findings (Vetrivelan et al., 2016) that MCH neurons specifically promote REMs by increasing NREMs-REMs transitions.
Figure 4: Chemoactivation of MCH neurons specifically increases REMs.
Percentages of time spent in wake, NREMs and REMs (A, D, G), as well as number of bouts (B, E, H) and mean bout duration (C, F, I) during the 3 h recording period after saline (white bars) or CNO (black bars) injections in MCH-Cre mice (n = 9). Saline or CNO (0.3 mg/kg, i.p.) was administered at ZT6 and sleep-wake recordings were conducted between ZT7-ZT10. CNO injections specifically increased the amount of REMs (p=0.0021, 2-tailed paired t-test) and the number of REMs bouts (p=0.0106, 2-tailed paired t-test) without affecting other stages. Data are Mean ± SEM; * P < 0.05, ** P < 0.01.
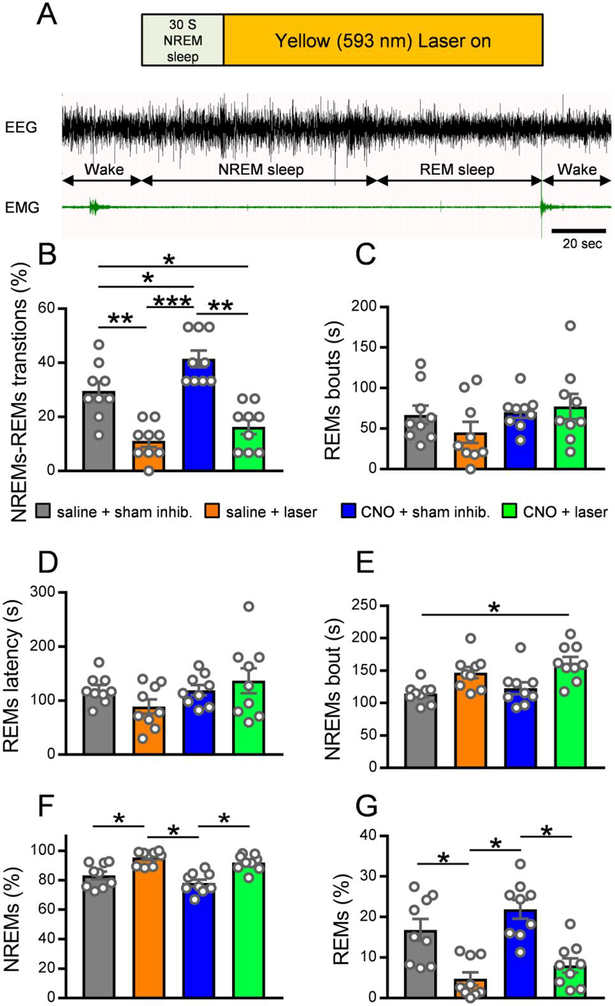
Next, to assess the importance of MCH terminals in the vlPAG/LPT in NREMs-REMs transitions, we optogenetically inhibited these terminals during baseline conditions as well as during concurrent chemoactivation of MCH soma. We injected MCH-Cre mice expressing both hM3Dq and ArchT in MCH neurons with saline (vehicle) or CNO i.p. (i.e. ‘control’ or ‘stimulation’ conditions, respectively, for MCH cell bodies expressing DREADDs) followed by sham or yellow light illumination (i.e. ‘control’ or ‘inhibition’ conditions for MCH terminals expressing ArchT) through the implanted optical fibers targeting the vlPAG/LPT. We performed different combinations of injections and photo-inhibitions (saline + sham inhibition; saline + laser light; CNO + sham inhibition; CNO + laser light) on 4 separate days in a randomized crossover design with ‘wash-out’ days (at least 3 days) in-between. We injected saline or CNO at ZT6 and performed sham or laser light illuminations from ZT7-ZT10 with simultaneous sleep-wake recordings. Specifically, we applied photo-inhibition (yellow/orange light; 593.5 nm wave length; ~10 mW light power at the fiber tip / irradiance of 79.54 mW/mm2) after 30 s of stable NREMs and continued until the next wake bout was observed (irrespective of whether NREMs transitioned into REMs or directly into wake) (Fig. 5A). We performed 15 such inhibition trials in each mouse within the 3 h recording period (ZT7-ZT10). During sham inhibition, the pulse generator remained “on” and was activated for each trial, but the light source was turned “off” so that yellow/orange light was not delivered into the brain tissue during these trials.
Figure 5: Optogenetic inhibition of MCH terminals in the vlPAG/LPT decreases NREMs-REMs transitions.
(A) Schematic illustration of the photo-inhibition paradigm during NREMs and REMs in MCH-Cre mice. Yellow/orange light (593.5 nm wave length) illuminations were applied after 30 seconds of stable NREMs and continued until the next wake bout irrespective of whether NREMs transitioned into REMs or wake. (B) Percentage of NREMs bouts transitioning to REMs. Repeated measures (RM) two-way ANOVA for ‘Drug treatment’ (F(1,8)=32.95, p=0.0004) and ‘Laser treatment’ (F(1,8)=49.73, p=0.0001), followed by Holm-Sidak’s multiple comparison test (saline + sham inhibition vs. saline + laser light, p=0.0068; saline + sham inhibition. vs. CNO + sham inhibition, p=0.0363; saline + sham inhibition vs. CNO + laser light, p=0.0309; saline + laser light vs. CNO + sham inhibition, p=0.0004; CNO + sham inhibition vs. CNO + laser light, p=0.0012). RM two-way ANOVAs for the duration of REMs bouts (C) and the latency to REMs onset (D) were not statistically significant (REMs bout duration ‘Drug treatment’ F(1,8)=2.853, p=0.1297, ‘Laser treatment’ F(1,8)=0.452, p=0.5203; REMs latency ‘Drug treatment’ F(1,8)=1.797, p=0.2169, ‘Laser treatment’ F(1,8)=0.5166, p=0.4928). (E) NREMs bout duration. RM two-way ANOVA for ‘Drug treatment’ (F(1,8)=8.766, p=0.0181) and ‘Laser treatment’ F(1,8)=19.3, p=0.0023, followed by Holm-Sidak’s multiple comparison test (saline + sham inhibition. vs. CNO + laser light, p=0.0282). (F) Percentage of NREMs during the 15 trials (starting with NREMs) within the 3h recording period. RM two-way ANOVA for ‘Drug treatment’ (F(1,8)=6.879, p=0.0305) and ‘Laser treatment’ (F(1,8)=61.61, p=0.≤0.0001), followed by Holm-Sidak’s multiple comparisons test (saline + sham inhibition vs. saline + laser light, p=0.05; saline + laser light vs. CNO + sham inhibition, p=0.0127; CNO + sham inhibition vs. CNO + laser light, p=0.0346). (G) Percentage of REMs during the 15 trials (starting with NREMs) within the 3h recording period. RM two-way ANOVA for ‘Drug treatment’ (F(1,8)=6.879, p=0.0305) and ‘Laser treatment’ (F(1,8)=61.61, p≤0.0001), followed by Holm-Sidak’s multiple comparisons test (saline + sham inhibition vs. saline + laser light, p=0.05; saline + laser light vs. CNO + sham inhibition, p=0.0127; CNO + sham inhibition vs. CNO + laser light, p=0.0346). Saline or CNO (0.3 mg/kg; i.p.) was injected at ZT6 and sham/laser light illumination was applied between ZT7-ZT10 (n = 9 mice). Data are Mean ± SEM, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. Scale bar in (A) is 20 sec.
We observed that during baseline conditions (saline + sham inhibition), 29.6 ± 3.4% of NREMs bouts transitioned into REMs in MCH-Cre mice and that chemoactivation of MCH neurons (CNO + sham inhibition) significantly increased these NREMs-REMs transitions (41.5 ± 3.1%, Fig. 5B). However, chemoactivation of MCH neurons (CNO + sham inhibition) did not alter the average duration of REMs bouts (Fig. 5C) or the latency to REMs onset (Fig. 5D). Finally, CNO + sham inhibition also did not significantly affect NREMs bout durations (Fig. 5E), indicating that chemoactivation of MCH neurons may not decrease the NREMs duration to promote REMs but biases the system in favor of transitions into REMs instead of wake.
Photo-inhibition of MCH terminals in the vlPAG/LPT during baseline conditions significantly reduced NREMs-REMs transitions and only 11.1 ± 2.2% of NREMs bouts ended in REMs after saline + laser light compared to 29.6 ± 3.4% after saline + sham inhibition (Fig. 5B). Photo-inhibition, however, did not affect the average duration of REMs bouts (Fig. 5C) or the REMs latency (Fig. 5D), but significantly decreased theta power during REMs (32.25 ± 2.3% vs. 35.50 ± 2.5% after saline + sham inhibition, p=0.0435). In addition, the percentage of REMs during the combined duration of all 15 trials was significantly reduced (4.8 ± 1.6% vs. 16.8 ± 2.7% after saline + sham inhibition, Fig. 5G) with a concurrent increase in the percentage of NREMs (95.2 ± 1.6% vs. 83.2 ± 2.7% after saline + sham inhibition, Fig. 5F). The increased NREMs during photoinhibition was also accompanied by an increase in delta power (44.97 ± 0.9% vs. 39.84 ± 1.5% after saline + sham inhibition, p=0.0054) and a corresponding decrease in theta power (18.76 ± 0.7% vs. 21.93 ± 0.8% after saline + sham inhibition, p=0.0021). These findings indicate that MCH terminals in the vlPAG/LPT could be tonically active and exert an inhibitory influence on REMs-suppressing vlPAG/LPT neurons which may aid in NREMs-REMs transitions. Therefore, the removal of this influence by photoinhibition increased NREMs and reduced NREMs-REMs transitions.
When chemoactivation of MCH neurons was combined with optogenetic inhibition of MCH terminals at the vlPAG/LPT, the previously observed increase in REMs transitions after CNO was completely blocked. During CNO + laser light, only 16.3 ± 2.7% of NREMs episodes transitioned into REMs, which was significantly lower than after CNO + sham inhibition (41.5 ± 3.1%) or saline + sham inhibition (29.6 ± 3.4%), but was not significantly different from saline + laser light (11.1 ± 2.2%, Fig. 5B). On the other hand, the average duration of REMs bouts remained unaffected (Fig. 5C). Consistent with these findings, we observed that the amount of REMs during the combined duration of all 15 trials in the CNO + laser light condition was significantly decreased (8.1 ± 1.8% vs. 21.9 ± 2.3% in the CNO + sham inhibition condition, Fig. 5G), while the percent time spent in NREMs increased (91.9 ± 1.7% vs. 78.1 ± 2.2%, Fig. 5F) compared to the CNO + sham inhibition condition. The increase in NREMs was presumably due to decreases in REMs transitions and a delayed onset of REMs. These results demonstrate that the vlPAG/LPT is a necessary relay through which MCH neurons promote NREMs-REMs transitions.
We next examined if MCH terminals in the vlPAG/LPT also modulate muscle tone during REMs in addition to REMs transitions. We therefore compared integral EMG activity during NREMs and REMs within the trial period across all four experimental paradigms, but did not find any significant differences (REMs: RM two-way ANOVA; ‘Drug treatment’: F(1,4)=1.073, p=0.3588 and ‘Laser treatment’ F(1,4)=3.991, p=0.1156; NREMs: ‘Drug treatment’: F(1,4)=0.7126, p=0.4461 and ‘Laser treatment’ F(1,4)=1.041, p=0.3652; n = 5 mice), suggesting that neither chemoactivation of MCH neurons nor photoinhibition of MCH terminals in the vlPAG/LPT modulate muscle tone.
Discussion:
Our experiments are the first to demonstrate that optogenetic inhibition of MCH terminals in the vlPAG/LPT is sufficient to prevent the increase in NREMs-REMs transitions following chemogenetic activation of MCH neurons and thus marking the vlPAG/LPT as a primary relay through which MCH neurons promote REMs transitions.
Technical considerations
We adapted a combination of opto-and chemogenetic methods to determine the importance of MCH terminals in the vlPAG/LPT in promoting REMs. While this is the first time such an approach is used in the sleep literature, we find that this methodology is robust and perfectly suited to address this kind of research question. Previous studies investigating neural circuits relied upon either optogenetic stimulation or inhibition of neural terminals in the target region (Jego et al., 2013; Kaur et al., 2017; Qiu et al., 2016). However, optogenetic stimulation of axon terminals has one major drawback in addition to the drawbacks associated with optogenetic soma stimulations mentioned earlier (monotonous artificial firing, tissue heating and damage), – stimulation of terminals may lead to antidromic activation of cell somata and/or axon collaterals and confound the results. The stimulation of axon terminals in one target region may therefore cause the release of neurotransmitter(s) in other downstream targets and hence the observed physiological effects cannot be attributed to the stimulated region. On the other hand, inhibition of axon terminals does not suffer from this drawback, but may not be appropriate for neurons playing only modulatory roles in physiological functions. We have previously shown that MCH neurons are not necessary for baseline REMs (Vetrivelan et al., 2016), but activation of MCH neurons may be necessary for the homeostatic and allostatic regulation of REMs. Thus, the approach we used i.e. chemogenetic cell soma stimulation paired with optogenetic inhibition of MCH terminals in the vlPAG/LPT takes advantage of the merits of both techniques while minimizing the drawbacks associated with optogenetic methods and is therefore perfectly suitable for this study. Nevertheless, one issue to consider is that inhibition of MCH terminals in the vlPAG/LPT in the current study could have inhibited axons passing through this region and thereby caused inhibition of other downstream targets (e.g. the SLD). However, we found a ten-fold higher density of MCH terminals in the vlPAG/LPT than in the SLD (Fig. 1) and it is also unclear whether MCH projections to the SLD even pass through the vlPAG/LPT. Moreover, a previous study has shown that inhibition of parabrachial terminals in the basal forebrain produced stronger and more robust physiological effects compared to inhibition of parabrachial terminals in the LH, although parabrachial projections to the basal forebrain may traverse the LH (Kaur et al., 2017). Thus, the REMs changes observed after inhibition of MCH terminals in the vlPAG/LPT in this study were unlikely due to any non-specific inhibition of MCH fibers passing through this region.
MCH-vlPAG/LPT circuitry in REMs control
We found that focal optogenetic inhibition of MCH terminals in the vlPAG/LPT prevents the increase in REMs transitions resulting from chemoactivation of MCH neurons. Considering that chemoactivation increases the firing rate of MCH neurons (Vetrivelan et al., 2016) while MCH terminals in other regions (including the SLD) remain uninhibited, our results strongly indicate that the vlPAG/LPT is a necessary relay through which MCH neurons promote REMs.
In contrast, a previous study (Jego et al., 2013) reported that REMs regulation by MCH neurons was mediated through the tuberomammillary nucleus (TMN) and medial septum (MS). In their study, optogenetic stimulation of MCH terminals in the TMN and MS during REMs increased the duration of REMs bouts while similar stimulations during NREMs were ineffective (Jego et al., 2013). The resulting inhibition of TMN wake-promoting neurons by these neuropeptides/neurotransmitters may then prevent wake initiation and thereby delay REMs termination. It is even possible that similar effects may be induced by stimulating MCH terminals in other wake promoting regions, such as the locus coeruleus and dorsal raphe nucleus, since MCH injections into these regions also increases REMs (Lagos et al., 2009; Monti et al., 2015). However, optogenetic activation of MCH cell bodies during NREMs increased NREMs-REMs transitions, which was not observed after stimulation of terminals in the TMN or MS (Jego et al., 2013). Finally, chemoactivation of MCH cell bodies specifically increases REMs transitions but not REMs bout duration (Naganuma et al., 2019; Naganuma et al., 2018; Vetrivelan et al., 2016). In contrast, Varin and colleagues (Varin et al., 2018) reported increases in both REMs transitions and duration. Based on a strong reduction in REMs latency and altered slow-wave dynamics in NREMs after chemoactivation, they nevertheless concluded that MCH neurons promote REMs primarily by accelerating the termination of NREMs and thereby facilitating NREMs-REMs transitions. Thus, MCH terminals in the TMN and other wake-promoting cell groups may not contribute to NREMs-REMs transitions, the primary means by which MCH neurons promote REMs, and therefore these cell groups may not be ‘necessary’ for REMs regulation by MCH neurons. Nevertheless, a potential contribution of MCH terminals in these regions for extending the duration of REMs (once initiated) cannot be ruled out completely.
There are other potential targets for MCH modulation of REMs including GABAergic neurons in the SLD and orexin neurons in the LH (Hassani et al., 2009). MCH neurons could initiate REMs by inhibiting GABAergic interneurons within the SLD and thereby disinhibiting ‘REM-on’ SLD glutamatergic neurons. However, lesions of the SLD including these interneurons decreases REMs (Lu et al., 2006), while selective elimination of GABA release from the SLD neurons has no effect on REMs (Krenzer et al., 2011). In addition, MCH application into the SLD indeed decreases REMs (Monti et al., 2016), making the GABAergic SLD neurons an unlikely target for MCH modulation of REMs. Similarly, as firing of orexin is reciprocal to MCH neurons, it is reasonable to hypothesize that MCH neurons inhibit orexin neurons to promote REMs transitions. However, chemoactivation of MCH neurons promotes REMs even in mice lacking orexins, ruling out this possibility (Naganuma et al., 2018).
In contrast, we found that the increased NREMs-REMs transitions following chemoactivation of MCH neurons were abolished when MCH terminals in the vlPAG/LPT were optogenetically inhibited. The frequency of NREMs-REMs transitions during our photo-inhibition experiments was even lower than under baseline conditions, indicating that MCH terminals in the vlPAG/LPT may also promote spontaneous NREMs-REMs transitions. Interestingly, MCH neurons increase their activity during NREMs even though they fire maximally during REMs (Hassani et al., 2009) and thus may aid in NREMs-REMs transitions. Increased NREMs-REMs transitions after activation of MCH neurons may or may not be accompanied by a decrease in NREMs bout durations. For example, chemoactivation of MCH neurons with 0.3 mg CNO did not alter NREMs bout duration (Vetrivelan et al., 2016), but chemoactivation with 0.5 mg CNO (Varin et al., 2018) or photoactivation of MCH neurons (which produced higher increases in REMs), reduced NREMs bout durations (Tsunematsu et al., 2014). It is likely that a higher drive for REMs (e.g. after REMs deprivation) may cause a reduction in NREMs to accommodate the increased demand for REMs and thus stronger activation of MCH neurons under such conditions may reduce NREMs bout duration to further increase REMs. Consistent with this, inhibition of MCH terminals in the vlPAG/LPT in the current study decreased REMs transitions and increased NREMs amounts. Interestingly, activation of GABAergic neurons in the vlPAG/LPT also produced similar effects on REMs and NREMs (Hayashi et al., 2015; Weber et al., 2018). Taken together, our current results strongly suggest that MCH neurons inhibit GABAergic neurons in the vlPAG/LPT to promote REMs.
Limitations
Although our current results using photoinhibition convincingly demonstrate the necessity of MCH terminals in the vlPAG/LPT in promoting REMs, in the future, it may be required to demonstrate their sufficiency by photoactivation. A second limitation is that our study could not identify the specific neurotransmitter/peptide in MCH neurons responsible for inhibition of vlPAG/LPT neurons since ArchT inhibition likely decreased the release of all neurotransmitters/peptides by MCH neurons. However, activation of MCH neurons that are unable to release glutamate still increased REMs suggesting that MCH or other peptides, rather than the glutamate (via feed forward inhibition), may directly inhibit the GABAergic neurons in the vlPAG/LPT to promote REMs, although direct MCH projections to these GABAergic neurons need to be verified in future tracing studies.
Conclusions
Our results argue against the possibility of wake-promoting neurons (including TMN and orexin neurons) being the primary targets of MCH neurons for REMs regulation. Instead, our present findings strongly suggest that MCH neurons inhibit REMs-suppressing vlPAG/LPT neurons and increase NREMs-REMs transitions to promote REMs.
Highlights.
Chemoactivation of MCH neurons increases REM sleep transitions
Photoinhibition of MCH terminals in the vlPAG/LPT decreases REM sleep transitions
Photoinhibition prevents chemoactivation-induced increases in REM sleep transitions
The vlPAG/LPT is a necessary relay for REM sleep regulation by MCH neurons
Acknowledgements:
We thank Quan Ha, Gianna Absi and Sam Bragg for excellent technical assistance. We also thank Drs. Eleftheria Maratos-Flier and Melissa Chee (Department of Medicine, Beth Israel Deaconess Medical Center, Boston) for providing the rabbit anti-MCH antibody.
Funding
This work was supported by National Institutes of Health Grants R21-NS074205, R01- NS088482 (to RV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare that they have no competing interests.
References
- Brailoiu GC, et al. , 2007. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 148, 5088–94. [DOI] [PubMed] [Google Scholar]
- Broberger C, 1999. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 848, 101–13. [DOI] [PubMed] [Google Scholar]
- Cardin JA, et al. , 2010. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 5, 247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MJ, et al. , 2015. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 35, 3644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, et al. , 2017. Ventral medullary control of rapid eye movement sleep and atonia. Exp Neurol. 290, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, et al. , 2010. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 463, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie IN, et al. , 2013. fMRI response to blue light delivery in the naive brain: implications for combined optogenetic fMRI studies. Neuroimage. 66, 634–41. [DOI] [PubMed] [Google Scholar]
- Clement O, et al. , 2012. The lateral hypothalamic area controls paradoxical (REM) sleep by means of descending projections to brainstem GABAergic neurons. J Neurosci. 32, 16763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, et al. , 2001. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol. 432, 1–19. [DOI] [PubMed] [Google Scholar]
- Elias CF, et al. , 1998. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 402, 442–59. [PubMed] [Google Scholar]
- Fenselau H, et al. , 2017. A rapidly acting glutamatergic ARC-->PVH satiety circuit postsynaptically regulated by alpha-MSH. Nat Neurosci. 20, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G, 2007. The mouse brain in stereotaxic coordinates Elsevier.
- Garfield AS, et al. , 2016. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci. 19, 1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace KP, et al. , 2014. Endogenous cholinergic input to the pontine REM sleep generator is not required for REM sleep to occur. J Neurosci. 34, 14198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, et al. , 2011. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, et al. , 2009. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 106, 2418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, et al. , 2015. Cells of a common developmental origin regulate REM/non-REM sleep and wakefulness in mice. Science. 350, 957–61. [DOI] [PubMed] [Google Scholar]
- Jego S, et al. , 2013. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 16, 1637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, et al. , 2017. A Genetically Defined Circuit for Arousal from Sleep during Hypercapnia. Neuron. 96, 1153–1167 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konadhode RR, et al. , 2013. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 33, 10257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, et al. , 2010. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 12, 545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenzer M, et al. , 2011. Brainstem and spinal cord circuitry regulating REM sleep and muscle atonia. PLoS One. 6, e24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger D, et al. , 2018. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun. 9, 4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger D, et al. , 2017. Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice. The Journal of Neuroscience. 37, 1352–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos P, et al. , 2009. Effects on sleep of melanin-concentrating hormone (MCH) microinjections into the dorsal raphe nucleus. Brain Res. 1265, 103–10. [DOI] [PubMed] [Google Scholar]
- Lu J, et al. , 2000. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 20, 3830–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, et al. , 2006. A putative flip-flop switch for control of REM sleep. Nature. 441, 589–94. [DOI] [PubMed] [Google Scholar]
- Luppi PH, et al. , 2006. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 100, 271–83. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA, 1975. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 189, 58–60. [DOI] [PubMed] [Google Scholar]
- Monti JM, et al. , 2015. Increased REM sleep after intra-locus coeruleus nucleus microinjection of melanin-concentrating hormone (MCH) in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 56, 185–8. [DOI] [PubMed] [Google Scholar]
- Monti JM, et al. , 2016. Microinjection of the melanin-concentrating hormone into the sublaterodorsal tegmental nucleus inhibits REM sleep in the rat. Neurosci Lett. 630, 66–69. [DOI] [PubMed] [Google Scholar]
- Naganuma F, et al. , 2019. Melanin-concentrating hormone neurons promote rapid eye movement sleep independent of glutamate release. Brain Struct Funct. 224, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma F, et al. , 2018. Melanin-concentrating hormone neurons contribute to dysregulation of rapid eye movement sleep in narcolepsy. Neurobiol Dis. 120, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, et al. , 2013. Role of the medial prefrontal cortex in cataplexy. J Neurosci. 33, 9743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, et al. , 2011. Melanin concentrating hormone in central hypersomnia. Sleep Med. 12, 768–72. [DOI] [PubMed] [Google Scholar]
- Qiu MH, et al. , 2016. Nigrostriatal Dopamine Acting on Globus Pallidus Regulates Sleep. Cereb Cortex. 26, 1430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch JM, et al. , 2017. Aldosterone-Sensing Neurons in the NTS Exhibit State-Dependent Pacemaker Activity and Drive Sodium Appetite via Synergy with Angiotensin II Signaling. Neuron. 96, 190–206 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL, 2011. Remote control of neuronal signaling. Pharmacol Rev. 63, 291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, 2016. DREADDs for Neuroscientists. Neuron. 89, 683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, et al. , 2001. Pontine structures and mechanisms involved in the generation of paradoxical (REM) sleep. Arch Ital Biol. 139, 93–107. [PubMed] [Google Scholar]
- Scammell TE, et al. , 1998. Microinjection of a cyclooxygenase inhibitor into the anteroventral preoptic region attenuates LPS fever. Am J Physiol. 274, R783–9. [DOI] [PubMed] [Google Scholar]
- Tsunematsu T, et al. , 2014. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci. 34, 6896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varin C, et al. , 2018. Melanin-concentrating hormone-expressing neurons adjust slow-wave sleep dynamics to catalyze paradoxical (REM) sleep. Sleep. 41. [DOI] [PubMed] [Google Scholar]
- Verret L, et al. , 2003. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivelan R, et al. , 2011. Muscle tone regulation during REM sleep: neural circuitry and clinical significance. Arch Ital Biol. 149, 348–66. [DOI] [PubMed] [Google Scholar]
- Vetrivelan R, et al. , 2016. Melanin-concentrating hormone neurons specifically promote rapid eye movement sleep in mice. Neuroscience. 336, 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivelan R, Lu J, Neural Circuitry Regulating REM Sleep and Its Implication in REM Sleep Behavior Disorder In: Schenck C, et al. , (Eds.), Rapid-Eye-Movement Sleep Behavior Disorder. Springer, Cham, 2019, pp. 559–577. [Google Scholar]
- Weber F, et al. , 2018. Regulation of REM and Non-REM Sleep by Periaqueductal GABAergic Neurons. Nat Commun. 9, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, et al. , 2008. Abnormal response of melanin-concentrating hormone deficient mice to fasting: hyperactivity and rapid eye movement sleep suppression. Neuroscience. 156, 819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]