Abstract
Background.
Psychosocial and health-related risk factors for depressive symptoms are known. It is unclear if these are associated with depressive symptom patterns over time. We identified trajectories of depressive symptoms and their risk factors among midlife women followed over 15 years.
Methods.
Participants were 3300 multiracial/ethnic women enrolled in a multisite longitudinal menopause and aging study, Study of Women’s Health Across the Nation. Biological, psychosocial, and depressive symptom data were collected approximately annually. Group-based trajectory modeling identified women with similar longitudinal patterns of depressive symptoms. Trajectory groups were compared on time-invariant and varying characteristics using multivariable multinomial analyses and pairwise comparisons.
Results.
Five symptom trajectories were compared (50% very low; 29% low; 5% increasing; 11% decreasing; 5% high). Relative to whites, blacks were less likely to be in the increasing trajectory and more likely to be in the decreasing symptom trajectory and Hispanics were more likely to have a high symptom trajectory than an increasing trajectory. Psychosocial/health factors varied between groups. A rise in sleep problems was associated with higher odds of having an increasing trajectory and a rise in social support was associated with lower odds. Women with low role functioning for 50% or more visits had three times the odds of being in the increasing symptom group.
Conclusions.
Changes in psychosocial and health characteristics were related to changing depressive symptom trajectories. Health care providers need to evaluate women’s sleep quality, social support, life events, and role functioning repeatedly during midlife to monitor changes in these and depressive symptoms.
Keywords: Asians, blacks, depressive symptom trajectories, Hispanics, midlife women, risk factors
Introduction
Previous longitudinal analyses from studies of midlife women, including the Study of Women’s Health Across the Nation (SWAN), have identified psychosocial and health-related factors, such as life events, low social support, and vasomotor symptoms (VMS), associated with the risk for developing depressive symptoms (Woods et al. 2008; Bromberger et al. 2010). These findings, however, describe mean levels of depressive symptoms observed in study populations usually at one point in time and do not address the heterogeneity of different patterns of depressive symptoms experienced by midlife women over time or about the characteristics of women experiencing these varied patterns. Elucidating varying depressive symptom trajectories in midlife women and their risk factors may help identify women who are at greatest risk of high or increasing depressive symptom courses.
Although prior studies have examined trajectories of depressive symptoms and their predictors in cohorts, these studies may not generalize to midlife women. Most were conducted in individuals over 65 years of age (e.g. Andreescu et al. 2008; Byers et al. 2012; Kuchibhatla et al. 2012; Sutin et al. 2013) or across a wide age range, such as 25–80 years (Lincoln & Takeuchi, 2010) or in mixed cohorts. In addition, most were conducted in largely white samples and evaluated baseline characteristics only as potential predictors of varying depressive symptom trajectories (Byers et al. 2012; Montagnier et al. 2014). Although several studies found that depressive symptom trajectories varied in men and women (Hsu, 2012; Kuchibhatla et al. 2012; Montagnier et al. 2014), few estimated trajectories by gender or in females only (e.g. Byers et al. 2012; Melchior et al. 2013), in middle-aged women specifically (Liang et al. 2011), or in racially or ethnically diverse populations.
To our knowledge, only two studies examined patterns of depressive symptoms longitudinally in midlife women (Melchior et al. 2013; Hickey et al. 2016). Neither study, however, examined associations of depressive symptom trajectory groups with time-varying predictors. Assessment of time-varying factors is important in evaluating the potential for changing circumstances or exposures to influence or be correlated with specific trajectory patterns.
Additionally, we know that a single clinical depressive episode or brief period of subthreshold depressive symptoms has less impact on the functioning and well-being of an individual than has persistent, recurrent episodes or chronic subthreshold depressive symptoms (Vandeleur et al. 2017). Thus, it is important to know something about the factors that contribute to long-term depressive symptom trajectories, especially to consistently elevated symptoms or trajectories that change over time. Specifically, we want to know if changes in these risk factors over time will exacerbate or diminish depressive symptoms trajectories depending on the pattern of risk factor changes. Such knowledge has the potential to enhance or expand clinical assessments and to identify modifiable factors that warrant monitoring in order to intervene in the development of persistent elevated or increasing depressive symptoms.
The current study had two primary purposes. Based on the data from SWAN, we first sought to identify trajectories of depressive symptoms assessed approximately annually over 15 years. Second, we aimed to evaluate whether baseline time-invariant as well as time-varying factors previously shown to be associated with risk for depression at one or a few points in time are also associated with the longitudinal course of depressive symptoms. Specifically, we hypothesized that (1) baseline time-invariant sociodemographic and health characteristics, such as race/ethnicity or prior medical conditions, would predict having increasing or high depressive symptoms over time (Bromberger et al. 2010); and (2) within-woman time-varying VMS, number of life events, low role functioning, sleep problems, and little physical activity would distinguish among the groups of women with different trajectories of depressive symptoms (Kessler, 2003). Importantly, these characteristics have potential to be modified.
Method
Participants and study design
The SWAN cohort was recruited between 1995 and 1997 with a telephone screening interview to determine study eligibility. Study design details and sampling procedures have been described previously (Sowers et al. 2000). Community-based samples of women were selected at seven sites across the United States using a variety of recruitment strategies and sampling frames. Each site recruited non-Hispanic white women and women from one specified minority group (black in Pittsburgh, Boston, the Detroit area, and Chicago; Japanese in Los Angeles; Chinese in the Oakland region; and Hispanic in Newark). Eligibility included aged 42–52 years, an intact uterus, at least one menstrual period and not pregnant/breastfeeding and no use of reproductive hormones in the previous 3 months, and self-identification with the designated race/ethnic group at the site. Of the 16 065 women screened, approximately 50% of eligible women (3302) were enrolled in the longitudinal cohort and followed approximately annually. Participants did not differ statistically by age, marital status, parity, or menopausal status from those eligible women who did not enroll. Women with a high school education or less and those who reported that it was hard or very hard to pay for basics were less likely to participate.
The Institutional Review Boards at all participating sites approved the study protocol, and written informed consent was obtained from all participants at each visit. The current study includes 3300 women who provided depressive symptom data at any time over 15 years. Two women who had not completed the depression symptoms scale at any visit were excluded. Study participants were assessed approximately annually from baseline through 13 follow-up visits. The protocol included extensive self-reported reproductive, health, psychosocial, lifestyle, physical, and psychological symptoms, and collections of biologic data. Study retention rate at visit 13 was 76%; 3% died.
Measures
Outcome: depressive symptoms
Depressive symptoms were assessed at each visit with the Center for Epidemiologic Studies Depression (CES-D) scale, a 20-item scale measuring frequency of depressive symptoms during the previous week (Radloff, 1977). Items are rated on a four-point scale of 0 (rarely) to 3 (most or all the time) and summed to obtain an overall score with a range from 0 to 60. The CES-D has been shown to be valid and reliable in diverse race/ethnic populations (Ying, 1988; Guarnaccia et al. 1989; Jones-Webb & Snowden, 1993). CES-D scores at each visit were used to identify CES-D trajectories as described below.
Baseline characteristics
Demographic factors included race/ethnicity, educational attainment, marital status, and difficulty paying for basics. Health-related factors reported at baseline included number of lifetime medical conditions, smoking, and health limitations on daily activities (see Table 1 for details). Menopausal status was based on menstrual bleeding patterns in the past 3 months and categorized as premenopausal (no change in regularity in the past year) or early perimenopausal (at least one menstrual period in the past 3 months and change in regularity in the past year) (WHO Scientific Group, 1996).
Table 1.
Baseline characteristics by CES-D trajectory group
| Characteristic | Very low n=1617 N (%) | Low n = 955 N (%) | Increasing n = 152 N (%) | Decreasing n = 359 N (%) | High n = 163 N (%) | Total N = 3246 N (%) | p χ2 |
|---|---|---|---|---|---|---|---|
| Race | <0.0001 | ||||||
| Asian: Chinese/Japanese | 280 (17) | 151 (16) | 32 (21) | 49 (14) | 13 (8) | 525 (16) | |
| Black | 465 (29) | 251 (26) | 30 (20) | 120 (33) | 53 (33) | 919 (28) | |
| Hispanic | 68 (4) | 110 (12) | 14 (9) | 51 (14) | 31 (19) | 274 (8) | |
| White | 804 (50) | 443 (46) | 76 (50) | 139 (39) | 66 (40) | 1528 (47) | |
| Education | <0.0001 | ||||||
| ⩽HS diploma | 302 (19) | 259 (27) | 39 (26) | 125 (35) | 66 (41) | 791 (25) | |
| >HS/college/degree | 1300 (81) | 687 (73) | 111 (74) | 233 (65) | 94 (59) | 2425 (75) | |
| Financial strain | <0.0001 | ||||||
| Somewhat/very hard | 449 (28) | 435 (46) | 67 (44) | 202 (57) | 116 (71) | 1267 (39) | |
| Not very hard paying for basics | 1160 (72) | 513 (54) | 84 (56) | 152 (43) | 47 (29) | 1956 (61) | |
| Marital status | <0.0001 | ||||||
| Never/sep/divorce/widow | 463 (29) | 329 (35) | 53 (36) | 147 (42) | 77 (48) | 1069 (33) | |
| Married/partnered | 1124 (71) | 615 (65) | 94 (64) | 206 (58) | 85 (52) | 2124 (67) | |
| Smoking | <0.0001 | ||||||
| Current smoker | 200 (13) | 178 (19) | 27 (18) | 86 (25) | 47 (29) | 538 (17) | |
| Never/past smoker | 1375 (87) | 762 (81) | 120 (82) | 264 (75) | 115 (71) | 2636 (83) | |
| Health limits activity | <0.0001 | ||||||
| Yes | 186 (12) | 147 (16) | 43 (29) | 90 (25) | 53 (33) | 519 (16) | |
| No | 1396 (88) | 795 (84) | 104 (71) | 263 (75) | 108 (67) | 2666 (84) | |
| Menopausal status | <0.0001 | ||||||
| Premenopause | 919 (58) | 513 (54) | 80 (53) | 152 (43) | 71 (44) | 1735 (54) | |
| Early perimenopause | 676 (42) | 436 (46) | 71 (47) | 198 (57) | 89 (56) | 1470 (46) | |
| Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | ANOVA | |
| Age, years | 46.4 (2.7) | 46.3 (2.6) | 46.2 (2.7) | 46.3 (2.8) | 46.2 (2.6) | 46.3 (2.7) | .87 |
| CES-D sum (0–60) | 5.1 (4.9) | 12.1 (7.0) | 11.7 (7.4) | 21.2 (9.3) | 26.6 (9.8) | 10.3 (9.2) | <0.0001 |
| BMI | 27.5 (6.8) | 28.3 (7.0) | 29.3 (8.1) | 29.7 (8.0) | 30.4 (8.6) | 28.2 (7.2) | <0.0001 |
| No. LT medical conditions (0–13) | 1.3 (1.2) | 1.6 (1.3) | 1.9 (1.5) | 1.8 (1.4) | 2.0 (1.4) | 1.5 (1.3) | <0.0001 |
| Vasomotor symptoms (0–8) | 0.6 (1.1) | 0.9 (1.5) | 1.3 (1.9) | 1.2 (1.7) | 1.4 (1.8) | 0.8 (1.4) | <0.0001 |
| Sleep problems (0–12) | 2.4 (2.4) | 3.6 (3.0) | 4.2 (3.1) | 4.8 (3.5) | 5.9 (3.7) | 3.3 (3.0) | <0.0001 |
| Physical activity (3–15) | 7.9 (1.8) | 7.6 (1.8) | 7.4 (1.8) | 7.3 (1.7) | 7.3 (1.9) | 7.7 (1.8) | <0.0001 |
| Social support (0–16) | 13.3 (2.7) | 12.1 (3.3) | 11.7 (3.3) | 10.7 (3.8) | 9.3 (3.9) | 12.4 (3.3) | <0.0001 |
| Number of life events (0–18) | 3.1 (2.3) | 4.0 (2.6) | 4.3 (2.6) | 4.3 (2.9) | 4.8 (2.9) | 3.7 (2.6) | <0.0001 |
BMI, body mass index, BMI was calculated from measured weight and height (kg/m2). CES-D, Center for Epidemiologic Studies Depression; HS, high school. Because only four Chinese and nine Japanese women were included in the ‘consistently high depressive symptoms’ trajectory group, these two race/ethnic groups were combined into a single Asian group for analyses. Chinese and Japanese women were similar or nearly so on all baseline and time-varying characteristics, including baseline status, total years in study, and slopes of time-varying characteristics.
Lifetime (LT) medical conditions included ever having anemia, diabetes, high blood pressure, arthritis/osteoarthritis, thyroid disease, heart attack, angina, fibroids, cancer (other than skin cancer), migraines, hypercholesterolemia, osteoporosis, and stroke. The number of conditions reported was summed and treated as a continuous variable. Frequency of vasomotor symptoms (VMS) in the past 2 weeks (0, 1–5, 6–8, 9–13, or all days) (Matthews et al. 1994) were self-reported at each visit and summed [ranging from zero (both symptoms ‘not at all’) to eight (both occurred ‘everyday’)]. Sleep problems were determined from self-reported number of nights of difficulty falling asleep, staying asleep, or early morning awakening during each of the previous 2 weeks (Kravitz et al. 2008). Frequency of sleep problems was summed and ranged from zero (no symptoms) to 12 (all three symptoms ‘5 + times per week’). Physical activity level was measured at baseline and 50% of follow-up visits (visits 3, 5, 6, 9, 12, 13) with a 19-item composite continuous measure (range 3–15) (Baecke et al. 1982; Sternfeld et al. 1999) that assessed physical activity in four domains. Social support was assessed at 11 visits using a summed score (range 0–16) of the frequency of availability of four types of needed emotional and instrumental supports (Sherbourne & Stewart, 1991) with higher scores indicating more supports. The number of life events occurring in the previous 12 months were totaled from a checklist of 18 life events (Bromberger et al. 2013).
Time-varying variables
Longitudinal variables were assessed at baseline and each follow-up visit unless otherwise stated (see Table 2). These included body mass index (BMI), presence of 13 medical conditions in the last year, number of nights with sleep problems (difficulty falling and staying asleep or early morning awakening) per week for each of the last 2 weeks, and physical activity level was measured at baseline and 50% of follow-up visits. Other health-related factors included use of medication for nerves (e.g. antidepressants, antipsychotics, and other psychotherapeutic agents) and hormone use. Bodily pain, role limitations, and social function related to physical health or emotional problems were assessed using subscales from the SF-36 (Ware & Sherbourne, 1992) and dichotomized using the 25th percentile of the sample as the cut point for impaired functioning (Rose et al. 1999). Frequency of hot flashes and night sweats in the last 2 weeks were summed and ranged from zero (both symptoms ‘not at all’) to eight (both occurred ‘everyday’) (see Tables 1 and 2 for details.)
Table 2.
Across all visits summary characteristics by CES-D trajectory group
| Characteristic | Very low n = 1617 N (%) | Low n = 955 N (%) | Increasing n = 152 N (%) | Decreasing n = 359 N (%) | High n = 163 N (%) | Total N = 3246 N (%) | P χ2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Take medications for nerves | <0.0001 | ||||||||
| For 0–1 visit | 1355 (84) | 692 (72) | 76 (50) | 200 (56) | 71 (44) | 2394 (74) | |||
| For 2 or more visits | 262 (16) | 263 (28) | 76 (50) | 159 (44) | 92 (56) | 852 (26) | |||
| High bodily pain | |||||||||
| For ⩾50% of visits | 184 (11) | 245 (26) | 63 (41) | 154 (43) | 106 (65) | 752 (23) | <0.0001 | ||
| For <50% of visits | 1433 (89) | 710 (74) | 89 (59) | 205 (57) | 57 (35) | 2494 (77) | |||
| Low role emotion | |||||||||
| For ⩾ 50% of visits | 42 (3) | 133 (14) | 55 (36) | 153 (43) | 102 (63) | 485 (15) | <0.0001 | ||
| For <50% of visits | 1574 (97) | 822 (86) | 97 (64) | 206 (57) | 61 (37) | 2760 (85) | |||
| Low role physical | |||||||||
| For ⩾ 50% of visits | 142 (9) | 248 (26) | 69 (45) | 160 (45) | 99 (61) | 718 (22) | <0.0001 | ||
| For <50% of visits | 1475 (91) | 707 (74) | 83 (55) | 199 (55) | 64 (39) | 2528 (78) | |||
| Low social function | |||||||||
| For ⩾50% of visits | 90 (6) | 230 (24) | 64 (42) | 208 (58) | 130 (80) | 722 (22) | <0.0001 | ||
| For <50% of visits | 1527 (94) | 725 (76) | 88 (58) | 151 (42) | 33 (20) | 2524 (78) | |||
| Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | ANOVA | |||
| Slope BMI | 0.11 (0.18) | 0.11 (0.20) | 0.09 (0.26) | 0.12 (0.24) | 0.10 (0.24) | 0.11 (0.20) | 0.37 | ||
| Slope FU medical conditions | 0.06 (0.05) | 0.07 (0.06) | 0.09 (0.07) | 0.08 (0.06) | 0.08 (0.06) | 0.07 (0.06) | <0.0001 | ||
| Slope vasomotor symptoms | 0.03 (0.09) | 0.03 (0.09) | 0.04 (0.11) | 0.02 (0.09) | 0.03 (0.09) | 0.03 (0.09) | 0.22 | ||
| Slope sleep problems | 0.08 (0.09) | 0.08 (0.09) | 0.12 (0.11) | 0.07 (0.11) | 0.07 (0.10) | 0.08 (0.09) | <0.0001 | ||
| Slope physical activity | −0.00 (0.04) | −0.01 (0.04) | −0.02 (0.05) | −0.01 (0.05) | −0.01 (0.05) | −0.01 (0.04) | 0.0004 | ||
| Slope social support | 0.04 (0.06) | 0.04 (0.07) | 0.00 (0.08) | 0.06 (0.08) | 0.06 (0.08) | 0.04 (0.07) | <0.0001 | ||
| Slope number life events | −0.04 (0.06) | −0.05 (0.06) | −0.02 (0.07) | −0.06 (0.07) | −0.06 (0.07) | −0.05 (0.06) | <0.0001 | ||
| % Completed visits | 82.0 (30.2) | 79.3 (31.7) | 91.6 (16.1) | 77.7 (32.0) | 74.0 (33.9) | 80.8 (30.7) | <0.0001 | ||
| Total time in study, years | 12.9 (5.1) | 12.5 (5.4) | 14.9 (2.1) | 12.3 (5.3) | 11.8 (5.8) | 12.7 (5.2) | <0.0001 | ||
| Total # visits (w/CES-D) | 10.5 (4.3) | 10.0 (4.4) | 11.4 (3.1) | 9.5 (4.4) | 9.0 (4.4) | 10.2 (4.3) | <0.0001 | ||
BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression; FU, follow-up.
Medication for nerves was assessed via self-report and verification of prescription bottles and coded as taking these at none or one visit v. two or more visits. Bodily pain and each role function scale was dichotomized using the 25th percentile of the sample as the cut point for impaired functioning as previously established (Rose et al. 1999; Avis et al. 2003). Visits were aggregated and participants were categorized as those having high pain or poor function at 50% or more of attended visits v. not.
Slope represents the annual change in a characteristic.
Social support was assessed based on the frequency of availability of four types of needed emotional and instrumental supports (Sherbourne & Stewart, 1991) with higher scores indicating more support. The number of life events occurring in the previous 12 months were totaled with a checklist of 18 life events (Bromberger et al. 2013).
For binary time-varying characteristics – medication for nerves, bodily pain, and role and social function – within-woman summaries were created as noted in Table 2. For continuous time-varying predictors, we summarized them using woman-specific slopes, which quantify annual within-woman change in the predictor over time (Fitzmaurice et al. 2011).
Statistical analysis
To identify clusters of women with similar longitudinal age-related patterns of depressive symptoms, we analyzed the data using group-based trajectory modeling with SAS PROC TRAJ (Jones et al. 2001; Nagin, 2005; Jones & Nagin, 2007; Andruff et al. 2009). Models for continuous CES-D utilized a censored normal distribution and considered two to nine trajectory groups. Participant age at each assessment was used to quantify time. Each trajectory was examined for linear, quadratic, or cubic patterns and the best function was determined via significance and fit statistics. To determine the optimal number of trajectory groups, we compared Bayesian Information Criterion (BIC) among models, evaluated groups from a clinical perspective to ensure that groups with changing patterns were represented and considered whether parsimonious groups were denoted.
Trajectories of depressive symptoms were modeled from 33 562 CES-D assessments with a mean (S.D.) of 10.2 (4.3) assessments per participant (range 1–14). The seven trajectory model was initially selected based on the BIC (see online Supplementary Figure and Tables), because it captured two groups with changing patterns over time, and was more parsimonious than models with a larger number of groups. The seven trajectory model exhibited good average posterior probability (⩾0.79) for each group indicating the likelihood of an individual belonging to each trajectory, with the highest probability determining group assignment. Estimated group membership distributions were ⩾5% for all but the highest symptom group. The latter consisted of <2% and was excluded for that reason (Andruff et al. 2009). We combined the two lowest trajectories because both remained flat with very low mean CES-D scores (<6 over time). Thus, the remaining sample of 3246 women was represented by five CES-D trajectories (see online Supplementary Figure). The final five trajectories were chosen based on conceptual models of change and model fit.
Unadjusted associations of characteristics with the five trajectory groups were estimated using χ2 tests and analysis of variance. Two tailed p values <0.05 were considered statistically significant for individual predictors. Multivariate associations were estimated with multinomial logistic regression. In the case of VMS, a woman-specific quadratic term also was included.
Several multinomial regression models were estimated: (1) a baseline model including demographic and time-invariant baseline characteristics that were significant in initial bivariate analyses, (2) a full model added all time-varying characteristics that were significant in initial bivariate analyses to the previous model, and (3) a final model using backward elimination to produce the most parsimonious model ( p < 0.05). For within-woman slope predictors, corresponding baseline characteristics were kept in the model to facilitate estimation of both cross-sectional (baseline) and longitudinal (within-woman slopes) effects on trajectory grouping (Diggle et al. 2013). p Values from pairwise comparisons employed a Bonferroni correction for multiple comparisons (0.05/5 comparisons, p < 0.01). Evaluation of collinearity between characteristics via Spearman correlation and Phi coefficients showed small-to-moderate associations (all <0.42). Analyses were run using SAS (Version 9.3, SAS Institute, Inc., Cary, NC, USA).
Results
Description of five trajectory groups
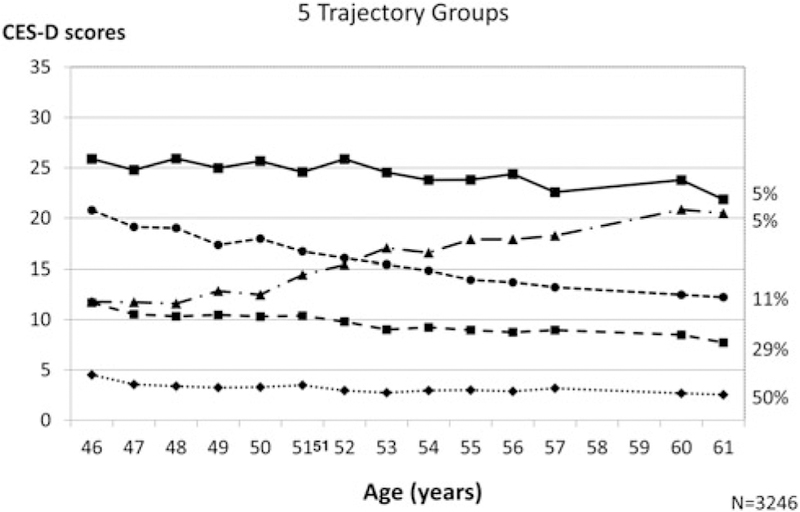
Three of the trajectories were fairly stable with differing levels of depressive symptoms, one group had an increase, and another group had a decrease in symptoms over time: very low (N = 1617, 50%), low (N = 955, 29%), increasing symptoms (N = 152, 5%), decreasing symptoms (N = 359, 11%), and high depressive symptoms (N = 163, 5%) (see Fig. 1).
Fig. 1.
Depressive Symptoms by Age5 Trajectory Groups
Unadjusted associations of characteristics with trajectory groups
Baseline characteristics associated with trajectory groups
The bottom of Table 2 shows that 74–91% of women in each trajectory group completed all 14 visits. The majority of women (about 79%) had low and flat trajectories (Table 1). Mean baseline CES-D scores were highest in the decreasing (21.2) and high (26.6) trajectory groups. Except for age, all baseline characteristics were significantly different across CES-D trajectory groups (overall p < 0.001). In general, unfavorable compared with favorable baseline characteristics were more prevalent in the increasing, decreasing, and high symptom trajectory groups.
Longitudinal time-varying characteristics associated with trajectory groups
The upper section of Table 2 shows the prevalence of the categorical variables over time, and the lower section presents the mean annual changes in slopes across time of the continuous characteristics measured repeatedly according to the trajectory groups. Except for BMI and VMS, all slopes differed significantly across trajectory groups. Characteristics that worsened over time were most prevalent in the increasing symptom trajectory.
Multinomial logistical regression analyses
Table 3 shows the global p values for each characteristic in the base-line model, full model, and final model that included variables that remained significant at p values <0.05. In the latter, baseline financial strain and baseline smoking differed significantly overall among the five trajectory groups. All three race/ethnic groups, Asians, blacks, and Hispanics, each differed significantly from whites in trajectory membership. Education, number of medical conditions prior to baseline, and baseline health limitations on activity were not significantly related to the symptom trajectory group when time-varying characteristics were included.
Table 3.
Multinomial logistic models, overall p values for each characteristic
| Characteristic | Baseline model N = 3119 | Full model N = 2792 | Final model N = 3038 |
|---|---|---|---|
| Asian v. white | 0.30 | 0.01 | 0.003 |
| Black v. white | 0.046 | 0.01 | 0.01 |
| Hispanic v. white | 0.05 | 0.03 | 0.02 |
| Baseline age | 0.72 | 0.86 | 0.48 |
| Overall comparison of sites | 0.02 | 0.03 | .06 |
| Baseline ⩽HS education v. not | 0.05 | 0.17 | - |
| Baseline financial strain v. not | <0.0001 | 0.003 | 0.0002 |
| Base not married v. married | 0.02 | 0.83 | - |
| Baseline current smoker v. not | 0.0003 | 0.04 | 0.008 |
| Baseline health limits activity v. not | <0.0001 | 0.40 | - |
| Baseline early peri- v. premenopause | 0.0002 | 0.19 | - |
| Two + visits taking medication for nerves | - | <0.0001 | <0.0001 |
| Baseline BMI | - | 0.50 | - |
| Slope BMI | - | 0.10 | - |
| Baseline LT # medical conditions | - | 0.21 | - |
| Slope number FU medical conditions | - | 0.29 | - |
| Baseline vasomotor symptoms | - | 0.08 | 0.04 |
| Slope vasomotor symptoms | - | 0.80 | - |
| Slope squared vasomotor symptoms | - | 0.46 | - |
| Baseline sleep problems | - | <0.0001 | <0.0001 |
| Slope sleep problems | - | 0.0003 | <0.0001 |
| Baseline physical activity | - | 0.08 | 0.005 |
| Slope physical activity | - | 0.02 | 0.02 |
| Baseline social support | - | <0.0001 | <0.0001 |
| Slope social support | - | <0.0001 | <0.0001 |
| Baseline number life events | - | <0.0001 | <0.0001 |
| Slope number life events | - | <0.0001 | <0.0001 |
| ⩾50% of visits with high bodily pain | - | 0.32 | - |
| ⩾50% of visits with low role emotional | - | <0.0001 | <0.0001 |
| ⩾50% of visits with low role physical | - | 0.002 | 0.0004 |
| ⩾50% of visits with low social function | - | <0.0001 | <0.0001 |
Sample size (N) for each model varies due to random missing data for individual characteristics. p Values are bolded and represent the overall effect, which includes comparison of all five trajectory groups.
BMI, body mass index; FU, follow-up; HS, high school; LT, lifetime.
Longitudinal time-varying measures of number of medications for nerves and changes in sleep problems, physical activity, social support, and life events and their baseline values differed significantly among trajectory groups. Additionally, all three low functioning characteristics (i.e. role emotional, role physical, and social function) were significant. Contrary to our hypotheses, changes in BMI, number of medical conditions in the past year, VMS, and majority of visits with bodily pain did not significantly vary among symptom trajectories.
Pairwise comparisons of the characteristics of the trajectory groups are displayed in Table 4 and, as noted above, were considered significant for a multiple comparison corrected p value ⩽0.01. All women in the five groups were included in the analyses of pairwise comparisons; however, in the interest of focusing on the trajectory groups with high symptoms and those with changing trajectories, not all comparisons are displayed. Therefore, for some predictors, the global p value for group comparisons is significant ( p < 0.05), but the significance is due to the comparison of the very low symptom group with other groups.
Table 4.
Pairwise comparisons from the final multinomial logistic model evaluating association with CESD trajectory group
| Increasing group v. low group | Increasing group v. high group | Increasing group v. decreasing group | Decreasing group v. low group | Decreasing group v. high group | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | P | |
| Asian v. White | 1.86 (0.94–3.71) | 0.07 | 1.23 (0.44–3.46) | 0.70 | 1.03 (0.47–2.27) | 0.94 | 1.81 (1.03–3.19) | 0.04 | 1.19 (0.47–3.05) | 0.71 | 0.003 |
| Black v. White | 0.60 (0.33–1.07) | 0.09 | 0.70 (0.34–1.44) | 0.33 | 0.43 (0.23–0.80) | 0.008 | 1.40 (0.94–2.07) | 0.10 | 1.64 (0.92–2.90) | 0.09 | 0.01 |
| Hispanic v. White | 0.30 (0.10–0.93) | 0.04 | 0.08 (0.01–0.47) | 0.006 | 0.42 (0.13–1.35) | 0.15 | 0.72 (0.35–1.48) | 0.38 | 0.19 (0.04–0.89) | 0.04 | 0.02 |
| Baseline age | 1.00 (0.93–1.08) | 0.97 | 0.98 (0.89–1.07) | 0.61 | 0.98 (0.90–1.06) | 0.64 | 1.02 (0.97–1.08) | 0.43 | 1.00 (0.92–1.08) | 0.89 | 0.48 |
| Baseline financial strain v. not | 0.78 (0.52–1.18) | 0.23 | 0.53 (0.31–0.92) | 0.02 | 0.75 (0.48–1.17) | 0.20 | 1.04 (0.78–1.40) | 0.78 | 0.71 (0.45–1.12) | 0.14 | 0.0002 |
| Baseline current smoker v. not | 0.99 (0.59–1.66) | 0.97 | 0.74 (0.39–1.37) | 0.33 | 0.82 (0.47–1.40) | 0.46 | 1.22 (0.87–1.70) | 0.26 | 0.90 (0.56–1.46) | 0.67 | 0.008 |
| Two + visits with medication for nerves | 1.59 (1.04–2.45) | 0.03 | 0.68 (0.39–1.19) | 0.18 | 0.80 (0.50–1.27) | 0.34 | 2.00 (1.47–2.71) | <0.0001 | 0.86 (0.55–1.35) | 0.51 | <0.0001 |
| Baseline VMS | 1.11 (0.99–1.25) | 0.08 | 1.10 (0.95–1.27) | 0.19 | 1.10 (0.97–1.25) | 0.13 | 1.01 (0.92–1.11) | 0.85 | 1.00 (0.88–1.13) | 0.98 | 0.04 |
| Base sleep problems | 1.07 (0.99–1.15) | 0.07 | 0.91 (0.83–0.99) | 0.03 | 0.99 (0.92–1.07) | 0.81 | 1.08 (1.03–1.13) | 0.003 | 0.92 (0.86–0.98) | 0.01 | <0.0001 |
| Slope sleep problemsa | 1.55 (1.29–1.86) | <0.0001 | 1.22 (0.96–1.56) | 0.10 | 1.45 (1.19–1.77) | 0.0002 | 1.07 (0.93–1.23) | 0.37 | 0.84 (0.69–1.04) | 0.11 | <0.0001 |
| Base physical activity | 0.86 (0.76–0.97) | 0.01 | 0.95 (0.81–1.11) | 0.53 | 0.91 (0.80–1.04) | 0.16 | 0.94 (0.86–1.03) | 0.18 | 1.05 (0.92–1.19) | 0.50 | 0.005 |
| Slope physical activitya | 0.80 (0.65–0.97) | 0.03 | 0.93 (0.72–1.20) | 0.57 | 0.82 (0.66–1.02) | 0.07 | 0.97 (0.84–1.12) | 0.68 | 1.13 (0.91–1.40) | 0.27 | 0.02 |
| Base social support | 0.88 (0.82–0.94) | 0.0001 | 1.11 (1.03–1.20) | 0.007 | 0.99 (0.92–1.06) | 0.66 | 0.89 (0.85–0.94) | <0.0001 | 1.13 (1.06–1.21) | 0.0001 | <0.0001 |
| Slope social supporta | 0.55 (0.46–0.66) | <0.0001 | 0.64 (0.51–0.81) | 0.0002 | 0.55 (0.45–0.68) | <0.0001 | 0.99 (0.86–1.16) | 0.99 | 1.16 (0.94–1.43) | 0.16 | <0.0001 |
| Baseline # life events | 1.13 (1.04–1.24) | 0.007 | 1.05 (0.94–1.18) | 0.38 | 1.15 (1.04–1.26) | 0.006 | 0.99 (0.92–1.06) | 0.73 | 0.92 (0.83–1.01) | 0.09 | <0.0001 |
| Slope # life eventsa | 1.67 (1.33–2.09) | <0.0001 | 1.40 (1.04–1.88) | 0.03 | 1.81 (1.41–2.32) | <0.0001 | 0.92 (0.77–1.10) | 0.36 | 0.77 (0.60–1.00) | 0.05 | <0.0001 |
| ⩾50% of visits with low role emotional | 2.84 (1.76–4.58) | <0.0001 | 0.52 (0.29–0.95) | 0.03 | 0.92 (0.56–1.52) | 0.75 | 3.08 (2.18–4.35) | <0.0001 | 0.57 (0.35–0.92) | 0.02 | <0.0001 |
| ⩾50% of visits with low role physical | 1.40 (0.87–2.27) | 0.17 | 1.50 (0.80–2.80) | 0.21 | 1.60 (0.95–2.70) | 0.08 | 0.87 (0.62–1.22) | 0.43 | 0.93 (0.56–1.54) | 0.79 | 0.0004 |
| ⩾50% of visits with low social function | 1.66 (1.01–2.73) | 0.04 | 0.31 (0.16–0.59) | 0.0004 | 0.58 (0.34–0.99) | 0.046 | 2.85 (2.05–3.94) | <0.0001 | 0.53 (0.31–0.90) | 0.02 | <0.0001 |
Site also in model ( p = 0.06). Significant p values are bolded. p Values in the final column represent the overall effect, which includes comparison of all five trajectory groups. Significant p values for trajectory group pairwise comparisons are based on a Bonferroni correction for five comparisons, p < 0.01. Base, baseline; CES-D, Center for Epidemiologic Studies Depression; CI, confidence interval; OR, odds ratio; VMS, vasomotor symptoms.
ORs for slope variables represent a one-standard deviation change (instead of a one-unit change).
Predictors of membership in the increasing symptom trajectory compared with the low symptom trajectory
At baseline, 1107 women had a mean CES-D score ∼12. However, of these, 152 (13%) had increasing symptoms over the subsequent 15 years, whereas 955 sustained low symptom scores. The odds of being in the increasing symptom group compared with being in the low symptom group was related to a number of baseline values of time-varying characteristics: odds ratios rose 13% for every additional baseline life event and decreased 12% and 14% for each one-point higher physical activity and social support at baseline, respectively. In other words, women in the increasing symptom trajectory already could be identified at baseline by having more life events, less social support, and lower physical activity. The odds of being in the increasing symptom group also were related to the changes in these time-varying factors: 55% greater odds with each one-standard deviation rise per year in sleep problems and 67% greater odds with each one-standard deviation smaller decline per year in the number of life events from baseline. The odds of being in the increasing symptom group declined by 45% for each standard deviation rise per year in social support. Stated differently, being in the increasing symptom group was accompanied by increasing sleep problems, decreasing social support, and also smaller decreases in life events from baseline (−2%) for each standard deviation rise per year than for the low trajectory (−5%). Compared with women reporting no low role emotional functioning for 50% or more visits, women with low role functioning for 50% or more of visits had nearly three times the odds of being in the increasing symptom group.
Predictors of membership in the increasing symptom trajectory compared with the high symptom trajectory
Hispanic women had a 92% lower odds than whites of being in the increasing group compared with the high symptom group. In other words, Hispanics were more likely than whites to sustain high symptom levels over time than to have increasing symptom levels. Compared with the high symptom group, the odds of being in the increasing symptom group was 11% greater per one-point higher score in baseline social support but 36% lower with each standard deviation rise per year in time-varying social support, and 69% lower in women with v. those without low social function at least 50% of visits. Stated differently, women in the increasing symptom trajectory group attained a high level of symptoms at the end of follow-up, but they had lower social support and lower social function over time compared with women in the high symptom group.
Predictors of membership in the decreasing symptom trajectory compared with the high symptom trajectory
When compared with the high symptom trajectory, the odds of being in the decreasing symptom group was 8% lower for each one-point higher score in baseline sleep problems and 13% greater for each one-point higher score in baseline social support. Changes in psychosocial or health-related factors over time were not significant. Stated differently, only baseline sleep problems and high social support were related to having a decreasing symptom trajectory over time.
Predictors of membership in the increasing symptom trajectory compared with the decreasing symptom trajectory group
The odds of being in the increasing symptom group compared with the decreasing symptom group was 57% lower for blacks compared with whites and 15% higher for each additional life event reported at baseline. Compared with being in the decreasing symptom group, the odds of being in the increasing symptom group rose 45% with each one-standard deviation per year rise in sleep problems, 81% for each one-standard deviation per year for each smaller decrease in the number of life events from baseline and decreased by 45% for each one-standard deviation per year rise in social support. In other words, a smaller reduction in life events from baseline, more sleep problems, and less social support over time were more predictive of an increasing v. a decreasing trajectory.
Discussion
We compared five trajectories of depressive symptoms during 15 follow-up years across midlife in 3300 women from diverse race/ ethnic groups. Approximately 80% had low or very low levels of symptoms throughout the study and a small group (5%) sustained high symptom levels. The remaining 15% had either increasing (4.7%) or decreasing symptom levels (11%). Although many studies have used trajectories based on longitudinal data to describe the course of depressive symptoms, these data cover varying age periods, select samples such as the elderly, length of time covered and variation in time between measurements (Byers et al. 2012; Kuchibhatla et al. 2012; Sutin et al. 2013; Andreescu et al. 2008). In consequence, it is difficult to compare our depressive symptom trajectory results with the general female population data. The two longitudinal studies of midlife women described above, the ALSWH and GAZEL studies which followed women for 13–15 years, identified four trajectories of depressive symptoms with differing proportions in each.
The Australian Longitudinal Study of Women’s Health (ALSWH: Hickey et al. 2016) and the French GAZEL study (Melchior et al. 2013) of employees of the national gas and electricity company followed large groups of different populations of mid-life women. The ALSWH identified a stable low (80%), increasing (9.0%), decreasing (8.5%), and stable high (2.5%) trajectories (Hickey et al. 2016). The French GAZEL study also identified four trajectories of depressive symptoms, but the proportions in each differed from both the ALSWH and the current study: stable low/none (58%), decreasing (14%), primarily increasing (21.8%), persistent (6.1%) (Melchior et al. 2013). Numbers of trajectory groups and distribution of women among these may be due to differences in the ethnic background of our participants and the fact that the GAZEL participants were all employed. In the ALSWH study (Hickey et al. 2016), the group with consistently high symptom levels was more likely to have night sweats, a history of depression, be current smokers, obese, and not have a paid job, compared with the group with consistently low depressive symptoms. In the GAZEL study (Melchior et al. 2013), the consistently high symptom group was more likely to be in a low occupational grade. Importantly, these studies assessed baseline characteristics only, not those that varied over time.
Importantly, we identified characteristics of midlife women that rendered them susceptible to an increasing symptom course rather than a relatively stable low level of symptoms or a decreasing symptom course over 15 years. Women whose depressive symptoms increased over time were more likely to have experienced a smaller reduction in the number of life events than those in other trajectories, increasing sleep problems and low role functioning due to emotional problems over time. Furthermore, women who were more physically active and whose social support was higher at baseline and increased over time were less likely to have trajectories of increasing symptoms than low symptoms. Although previous research has shown that such factors are associated with subsequent depressive symptoms, our findings extend the literature by demonstrating that the risk factors also predict the course of symptoms. We also show that independent of traditional risk factors measured at one point in time, changes in these factors over time are associated with depressive symptom course longitudinally and should be monitored as we discuss below.
Our a priori hypotheses regarding race differences were partially supported. Compared with white women, Hispanic women were more likely to sustain a high level of symptoms over time. Asian women had patterns of depressive symptoms similar to those of white women, i.e. they were as likely as whites to be in any of the symptom groups. Unexpectedly, black women were less likely than whites to be in the increasing than in the decreasing symptom trajectory. Although blacks were more likely than whites to have high depressive symptom trajectories in unadjusted analyses, after adjustment for education, financial strain, smoking, and bodily pain, there were no significant differences in odds of being in the high symptom trajectory between black and white women. The results were similar to those from prior longitudinal studies (e.g. the National Longitudinal Survey for Mature Women aged 52–80 years, Spence et al. 2011; the Health and Retirement study of men and women over age 50; Liang et al. 2011), which also showed that blacks were more likely than whites to have a persistent course of high depressive symptoms, but after adjusting for indicators of socioeconomic status, the differences typically became smaller and non-significant (Liang et al. 2011; Spence et al. 2011). Data from the previous studies and our own reinforce the influence of socioeconomic and health factors on any observed racial disparities in the course of depressive symptoms.
Although VMS over time did not distinguish depressive symptomatology trajectories, baseline VMS was more prevalent in the high symptom group. Studies of menopause (Cohen et al. 2006; Freeman et al. 2006) including our earlier report (Bromberger et al. 2009) have shown that VMS are associated with depressive symptoms. However, studies that distinguished night sweats from daytime hot flashes (Burleson et al. 2010; Joffe et al. 2016) have observed that night-time but not daytime hot flashes are associated with the emergence of depressive symptoms, and the only study that examined predictors of depressive symptom trajectories during the MT (Hickey et al. 2016) found that night sweats (but not hot flashes) were significantly associated with being in the stable high depressive symptom group. Unfortunately, our data did not differentiate between daytime and night-time hot flashes.
Study results suggest midlife risk factors for sustained or increases in depressive symptoms as women age. Monitoring women who have these risk factors and targeting them for intervention may help reduce risk for later depression. In addition, results suggest that depressive symptoms may change over midlife and continual monitoring is important. Potentially modifiable risk factors such as poor sleep quality, low physical activity, low social support, and life events should be monitored by health care providers. There is increasing evidence of the beneficial effect of behavioral modifications to sleep patterns (Germain et al. 2006; Baglioni et al. 2011; McCurry et al. 2016) or regular physical activity (Blumenthal et al. 1999; Dugan et al. 2015) on mood. Cognitive behavioral therapy for insomnia has been shown to be effective in improving sleep and mood (Germain et al. 2006). Health care providers can share with women the benefits of social engagement, managing role function in the presence of adverse health conditions, and maintaining healthy lifestyles for maintaining good mood as well as good health.
The study has several limitations. Although the detailed repeated measures of risk factors and depressive symptoms allowed us to identify different general patterns of these over time, we lack information on depressive symptoms during the period of time between assessments. This limited our ability to ascertain the temporal relationship between assessments. The scales we used were self-report and we did not have data on psychiatric diagnoses, which would have provided additional information about the historical context for the midlife observations. The group with very high symptoms throughout follow-up was too small to support analyses that identified risk factors for the persistence of very high levels of symptoms. Although important for midlife women, study findings cannot be generalized to men or young women. The latter may have an increased risk of affective disorders.
Despite the limitations, the study has many strengths, including the longitudinal repeated measurement of predictors and out-come and adjustment for multiple confounders. The sample was large and diverse and we used standardized measures. Our findings add to our knowledge of the course of and risk factors for depressive symptoms over time in middle-aged women, especially in relation to time-varying factors, something, to our knowledge, no other study has done.
In summary, we identified key risk factors for high depressive symptoms at one point in time that were also associated with continuation of or increases in these symptom patterns over time. We have shown that changes in these risk factors also impact change in depressive symptoms. We have added predictive information beyond baseline/time-invariant characteristics as both baseline measures and measures that vary over time were informative of risk for various longitudinal trajectories of depressive symptoms. These results refine and confirm the importance of sleep problems, low social support, life events, low levels of physical activity, and low role function as risk factors for long-term depressive symptoms.
Supplementary Material
Acknowledgements.
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH. Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994– 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD – Chanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD – Program Officers. Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA – Sonja McKinlay, PI 1995–2001. Steering Committee: Susan Johnson, Current Chair, Chris Gallagher, Former Chair. The authors thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Supplementary Material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718000703
References
- Andreescu C, Chang CC, Mulsant BH and Ganguli M (2008) Twelve-year depressive symptom trajectories and their predictors in a community sample of older adults. International Psychogeriatrics 20(2), 221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andruff H, Carraro N, Thompson A, Gaudreau P and Louvet B (2009) Latent class growth modeling: a tutorial. Tutorials in Quantitative Methods for Psychology 5(1), 11–24. [Google Scholar]
- Avis NE, Assmann SF, Ory M, Matthews KA, Schocken M and Bromberger JT (2003) Health-related quality of life among a multi-ethnic sample of middle-aged women. Medical Care 41(11), 1262–1276. [DOI] [PubMed] [Google Scholar]
- Baecke JAH, Burema J and Frijters ER (1982) A short questionnaire for measurement of habitual physical activity in epidemiological studies. American Journal of Clinical Nutrition, 36, 936–942. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U et al. (2011) Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders 135(1–3), 10–19. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P et al. (1999) Effects of exercise training on older patients with major depression. Archives of Internal Medicine 159, 2349–2356. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Chang Y, Randolph JF Jr., Avis NE, Gold EB et al. (2013) Does risk for anxiety increase during the menopausal transition? Study of Women’s Health Across the Nation. Menopause (New York, N. Y.) 20 (5), 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Matthews KA, Youk A, Brown C and Feng W (2009) Predictors of first lifetime episodes of major depression in midlife women. Psychological Medicine 39, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Schott LL, Kravitz HM, Sowers M, Avis NE, Gold EB et al. (2010) Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN). Archives of General Psychiatry 67(6), 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleson MH, Todd M and Trevathan WR (2010) Daily vasomotor symptoms, sleep problems, and mood: using daily data to evaluate the domino hypothesis in middle-aged women. Menopause (New York, N. Y.) 17(1), 87–95. [DOI] [PubMed] [Google Scholar]
- Byers AL, Vittinghoff E, Lui LY, Hoang T, Blazer DG, Covinsky KE et al. (2012) Twenty-year depressive trajectories among older women. Archives of General Psychiatry 69(10), 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Vitonis AF, Otto MW and Harlow BL (2006) Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Archives of General Psychiatry 63(4), 385–390. [DOI] [PubMed] [Google Scholar]
- Diggle P, Heagerty PJ, Liang KL and Zeger SL (2013) Analysis of Longitudinal Data Oxford, UK: Oxford University Press. [Google Scholar]
- Dugan SA, Bromberger JT, Segawa E, Avery E and Sternfeld B (2015) Association between physical activity and depressive symptoms: midlife women in SWAN. Medicine & Science in Sports & Exercise 47(2), 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM and Ware JH (2011) Applied Longitudinal Analysis, 2nd edn. New Jersey, USA: Wiley. [Google Scholar]
- Freeman EW, Sammel MD, Lin H and Nelson DB (2006) Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of General Psychiatry 63(4), 375–382. [DOI] [PubMed] [Google Scholar]
- Germain A, Moul DE, Franzen PL, Miewald JM, Reynolds CF 3rd, Monk TH et al. (2006) Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. Journal of Clinical Sleep Medicine 2(4), 403–406. [PubMed] [Google Scholar]
- Guarnaccia PJ, Angel R and Worobey JL (1989) The factor structure of the CES-D in the Hispanic Health and Nutrition Examination Survey: the influences of ethnicity, gender and language. Social Science and Medicine 29(1), 85–94. [DOI] [PubMed] [Google Scholar]
- Hickey M, Schoenaker DA, Joffe H and Mishra GD (2016) Depressive symptoms across the menopause transition: findings from a large population-based cohort study. Menopause (New York, N. Y.) 23(12), 1287–1293. [DOI] [PubMed] [Google Scholar]
- Hsu HC (2012) Group-based trajectories of depressive symptoms and the predictors in the older population. International Journal of Geriatric Psychiatry 27(8), 854–862. [DOI] [PubMed] [Google Scholar]
- Joffe H, Crawford SL, Freeman MP, White DP, Bianchi MT, Kim S et al. (2016) Independent contributions of nocturnal hot flashes and sleep disturbance to depression in estrogen-deprived women. Journal of Clinical Endocrinology and Metabolism 101(10), 3847–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Nagin D and Roeder K (2001) A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research 29, 374–393. [Google Scholar]
- Jones BL and Nagin DS (2007) Advances in group-based trajectory modeling and SAS procedure for estimating them. Sociological Methods and Research 35, 542–571. [Google Scholar]
- Jones-Webb RJ and Snowden LR (1993) Symptoms of depression among blacks and whites. American Journal of Public Health 83(2), 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2003) Epidemiology of women and depression. Journal of Affective Disorders 74, 5–13. [DOI] [PubMed] [Google Scholar]
- Kravitz H, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA et al. (2008) Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. SLEEP 31, 979–990. [PMC free article] [PubMed] [Google Scholar]
- Kuchibhatla MN, Fillenbaum GG, Hybels CF and Blazer DG (2012) Trajectory classes of depressive symptoms in a community sample of older adults. Acta Psychiatrica Scandinavica 125(6), 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Xu X, Quinones AR, Bennett JM and Ye W (2011) Multiple trajectories of depressive symptoms in middle and late life: racial/ethnic variations. Psychological Aging 26(4), 761–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln KD and Takeuchi DT (2010) Variation in the trajectories of depressive symptoms: results from the Americans’ Changing Lives Study. Biodemography Social Biology 56(1), 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Wing RR, Kuller LH, Meilahn EN and Plantinga P (1994) Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Archives of Internal Medicine 154, 2349–2355. [PubMed] [Google Scholar]
- McCurry SM, Guthrie KA, Morin CM, Woods NF, Landis CA, Ensrud KE et al. (2016) Telephone-based cognitive behavioral therapy for insomnia in perimenopausal and postmenopausal women with vasomotor symptoms: a MsFLASH randomized clinical trial. JAMA Internal Medicine 176(7), 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior M, Chastang JF, Head J, Goldberg M, Zins M, Nabi H, et al. (2013) Socioeconomic position predicts long-term depression trajectory: a 13-year follow-up of the GAZEL cohort study. Molecular Psychiatry 18(1), 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnier D, Dartigues JF, Rouillon F, Peres K, Falissard B and Onen F (2014) Ageing and trajectories of depressive symptoms in community-dwelling men and women. International Journal of Geriatric Psychiatry 29(7), 720–729. [DOI] [PubMed] [Google Scholar]
- Nagin DS (2005) Group-based Modeling of Development London: Harvard University Press. [Google Scholar]
- Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1, 385–401. [Google Scholar]
- Rose MS, Koshman ML, Spreng S and Sheldon R (1999) Statistical issues encountered in the comparison of health-related quality of life in diseased patients to published general population norms: problems and solutions. Journal of Clinical Epidemiology 52(5), 405–412. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD and Stewart AL (1991) The MOS social support survey. Social Science and Medicine 32(6), 705–714. [DOI] [PubMed] [Google Scholar]
- Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G et al. (2000) SWAN: a multi-center, multi-ethnic community-based cohort study of women and the menopausal transition. In Lobo R, Marcus R and Kelsey J. (eds). Menopause: Biology and Pathology New York: Academic Press, pp. 175–188. [Google Scholar]
- Spence NJ, Adkins DE and Dupre ME (2011) Racial differences in depression trajectories among older women: socioeconomic, family, and health influences. Journal of Health and Social Behaviors 52(4), 444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternfeld B, Ainsworth BE and Queensberry CP (1999) Physical activity patterns in a diverse population of women. Preventive Medicine 28(3), 313–323. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A, Milaneschi Y, An Y, Ferrucci L and Zonderman AB (2013) The trajectory of depressive symptoms across the adult life span. JAMA Psychiatry 70(8), 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleur CL, Fassassi S, Castelao E, Glaus J, Strippoli MF, Lasserre AM et al. (2017) Prevalence and correlates of DSM-5 major depressive and related disorders in the community. Psychiatry Research 205(2017), 50–58. [DOI] [PubMed] [Google Scholar]
- Ware JE Jr. and Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 30(6), 473–483. [PubMed] [Google Scholar]
- Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Mariella A and Mitchell ES (2008) Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause (New York, N. Y.) 15, 223–232. [DOI] [PubMed] [Google Scholar]
- WHO Scientific Group (1996) WHO Technical Report Series: Research on the Menopause in the 1990’s, 866 edn. Geneva: World Health Organization. [PubMed] [Google Scholar]
- Ying YW (1988) Depressive symptomatology among Chinese-Americans as measured by the CES-D. Journal of Clinical Psychology 44(5), 739–746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.