Abstract
Purpose:
Theaim of this peper was to retrospectively review our experience utilising protective fluid instillation techniques during percutaneous microwave ablation of liver tumours to determine if fluid instillation prevents non-target injuries and allows a more aggressive case selection.
Materials and methods:
This institute review board-approved, US Health Insurance Portability and Accountability Act-compliant, retrospective study reviewed percutaneous microwave ablation of 151 malignant hepatic tumours in 87 patients, comparing cases in which protective fluid instillation was performed with those where no fluid was utilised. In cases utilising hydrodisplacement for bowel protection, a consensus panel evaluated eligibility for potential ablation without hydrodisplacement. Patient age, tumour size, local tumour progression rate, length of follow-up, complications, displacement distance/artificial ascites thickness, and treatment power/time were compared.
Results:
Fluid administration was utilised during treatment in 29/151 of cases: 10/29 for protection of bowel (8/10 cases not possible without fluid displacement), and 19/29 for body wall/diaphragm protection. Local tumour progression was higher when hydrodisplacement was used to protect bowel tissue; this may be due to lower applied power due to operator caution. Local tumour progression was not increased for artificial ascites. There was no difference in complications between the fluid group and controls.
Conclusion:
Intraperitoneal fluid administration is a safe and effective method of protecting non-target structures during percutaneous hepatic microwave ablation. While hydrodisplacement for bowel protection allows more aggressive case selection, these cases were associated with higher rates of local tumour progression.
Keywords: Clinical trials – thermal ablation, radiofrequency/microwave, thermal ablation
Introduction
Percutaneous tumour ablation is rapidly becoming accepted for the treatment of primary and metastatic liver tumours. To date, the greatest clinical experience is with radio-frequency (RF) ablation, but substantial physical limitations have resulted in a high local recurrence rate and a lack of effectiveness when treating tumours above 3.0 cm in size [1]. Recently, the introduction of high power microwave (MW) ablation systems has led to a renewed interest in MW technology. In contrast to RF, MW is relatively impervious to differences in tissue type, operates at higher applied temperatures, penetrates more deeply into target tissues, and is less affected by perfusion or vascular heat sinks [2]. This results in the creation of faster, larger, and more consistent ablation zones when MW is applied at high power [3]. While head-to-head comparisons are sparse, early clinical results with MW have compared favourably to historical RF series [4,5].
The larger ablation zones possible with MW have raised concerns related to unwanted collateral damage. This is particularly problematic as many MW systems create long, ovoid ablation zones (up to 6–7 cm in length) depending on antenna design, wavelength, and applied power [6]. Ideally, an ablation zone would cover only the targeted tumour and an ablative margin. Treating small tumours with devices that create long ablation zones can result in significant collateral damage proximal or distal to the target.
Fluid instillation (hydrodissection) has been used in multiple ways to limit collateral damage during percutaneous tumour ablation. The technique was first described for use with RF ablation and involves instillation of fluid into the peritoneal space, fat, or other body cavities [7–9]. Instillation of 5% dextrose in water (D5W) into the peritoneal space (artificial ascites) has been shown to decrease diaphragm and gastric injuries during percutaneous RF ablation of hepatic tumours [10–12]. It has also been shown to improve visualisation of hepatic dome lesions and to decrease post-procedural body wall pain [13,14]. Fluid has also been used as a true displacement technique for bowel protection during RF ablation procedures [8]. Alternative protective techniques such as gas instillation, intraperitoneal catheter balloon displacement, and blunt needle displacement have also been described [15–18].
Given the large ablation zones possible with MW, the need for measures to protect against non-target injury becomes more important. Also, the technological differences between MW and RF may lead to significant differences in the heating physics, heat generation rate, and water vaporisation which would affect displacement techniques and outcomes [2]. While multiple studies have shown fluid instillation techniques effective at non-target organ protection during RF ablation, these techniques are not well established in the literature for MW ablation. Ohmoto et al. first described fluid instillation techniques with MW ablation in two technical innovation reports [19,20]. Zhang et al. were the first group to scientifically study the use of fluid instillation protective techniques for MW ablation in which they treated 36 hepatic tumours with high technical success rates and no evidence of bowel injury [21]. However, their rate of local tumour progression of 16.1% is higher than several series investigating the use of fluid instillation with RF ablation. The purpose of this study is to retrospectively review our experience performing percutaneous MW ablation of the liver in combination with fluid instillation techniques to determine if the use of fluid instillation prevents injuries to non-target structures and allows a more aggressive case selection due to a decreased risk of collateral injury. Also, given the recent results of Zhang et al., we wished to investigate whether there was any association between fluid protective techniques and long-term treatment outcomes [21].
Materials and methods
Patient selection
This retrospective study was approved by our institutional human subjects review board and complied with all aspects of the US Health Insurance Portability and Accountability Act. A single institution retrospective review of all percutaneous MW ablation procedures for malignant hepatic lesions yielded a total of 151 hepatic tumours in 87 patients (70 men, 17 women) treated between 2008 and 2012 that had at least 1 month of subsequent imaging follow-up. A total of 87 hepatocellular carcinomas (HCC) were treated in 59 patients, while 64 liver metastases were treated in an additional 28 patients.
MW ablation procedure
All ablation procedures were performed under general anaesthesia via percutaneous approach by one of four radiologists experienced in tumour ablation. One to three high-powered gas-cooled antennas (LK or PR antennas, Certus 140, NeuWave Medical, Madison, WI) were utilised in each procedure. The antennas were placed under real-time ultrasound (GE E9, GE Medical, Waukesha, WI) or CT-fluoroscopy (GE Optima 580, GE Medical) guidance, with CT reserved for lesions not visualised by ultrasound. If multiple tumours were present they were treated in the same session (1.55 ± 1.16 tumours per case, 1.32 for HCC, 2.06 for metastases). While the general practice preference was to perform the ablation cycle for 5 min at 65 W, the performing physician was free to deviate from that based upon lesion size, proximity to vulnerable structures, and realtime ultrasound or CT-fluoroscopy intraprocedural monitoring for ablation zone adequacy or extension of the ablation zone to non-target organs. Likewise, the decision on whether protective intraperitoneal fluid (hydrodisplacement or artificial ascites) was used was made by the performing physician. In patients eligible to receive intravenous contrast, a contrast-enhanced biphasic (late-arterial and portal venous) CT of the abdomen was performed at the completion of each procedure to determine adequacy of ablation and evaluate for immediate complications. Otherwise, non-contrast CT was performed. If there was suspicion for residual untreated tumour on the post-procedure CT, repeat ablation was performed during the same session. Peri-procedural complications were monitored and recorded during an overnight stay and via telephone contact 5–7 days after the procedure. Delayed complications were evaluated with follow-up imaging and at clinical visits. Complications were classified according to the Society of Interventional Radiology (SIR) Classification system for Complications by Outcome [22].
Hydrodisplacement vs. artificial ascites
Fluid can be instilled into specific locations, such as between solid organs and bowel (hydrodisplacement) to displace and protect specific vulnerable structures. Alternatively, fluid can be placed into the general peritoneal cavity (artificial ascites) to decrease post-procedural body wall and diaphragmatic pain associated with treating peripheral liver tumours. Given their differing goals, the cases in which fluid instillation was utilised were divided into these two groups (Figures 1 and 2).
Figure 1.
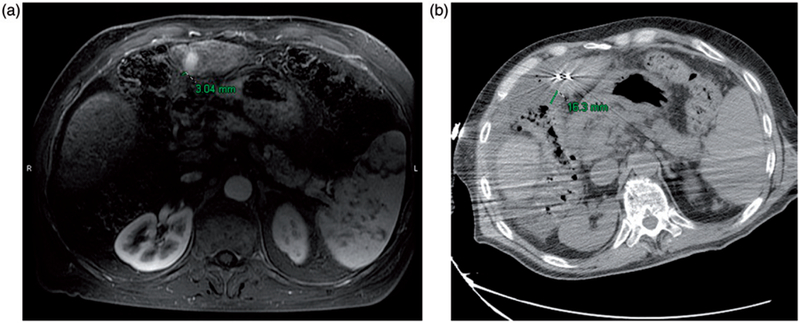
(a) Preablation contrast enhanced MRI demonstrates an arterially enhancing HCC. The liver capsule adjacent to the HCC is 3 mm from the adjacent colon. (b) Intraablation noncontrast CT performed after hydrodisplacement demonstrates that the colon has moved, now 16 mm from the liver capsule. The fluid thickness in this case is 13 mm. Notice also the two microwave antennae which have been placed.
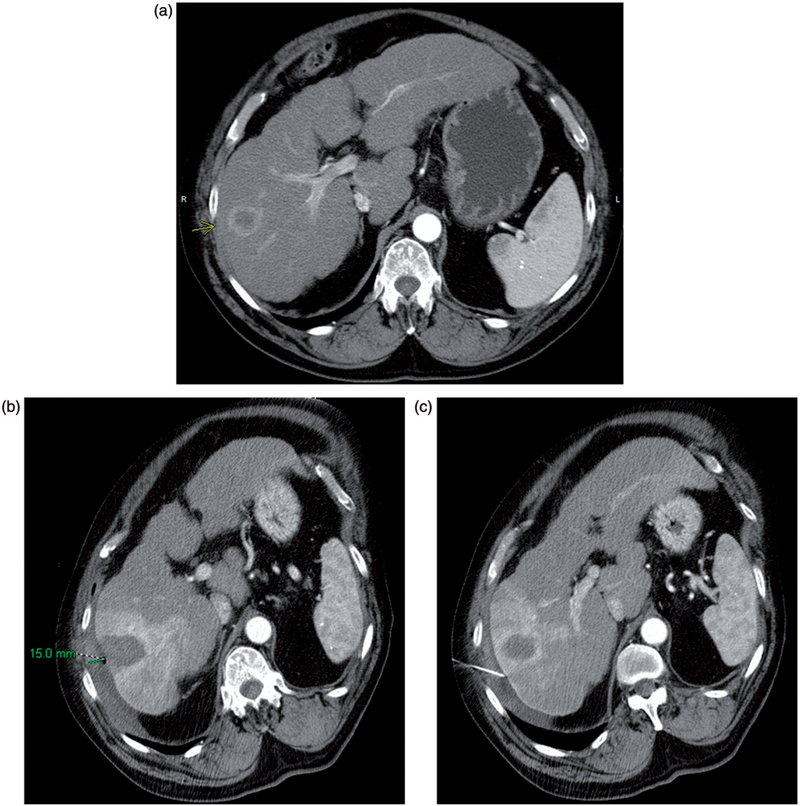
Figure 2.
(a) Preablation contrast enhanced CT demonstrates an arterially enhancing right lobe HCC. The overlying liver capsule directly abuts the body wall, putting this patient at risk for postprocedural pain. (b) Postablation contrast enhanced CT demonstrates postablation changes as well as instillation of artificial ascites, measuring 15 mm in thickness between the body wall and the liver capsule. (c) Postablation contrast enhanced CT demonstrates the artificial ascites needle still in place, allowing for continuous instillation of fluid during the ablation procedure.
To determine whether the use of hydrodisplacement increases the aggressiveness of case selection, two board certified radiologists experienced in tumour ablation (M.G.L. and T.Z., with 4 and 3 years of MW ablation experience respectively) reviewed all hydrodisplacement cases to determine whether a given case could have been safely attempted without the use of pre-ablation fluid displacement or other thermoprotective technique. In all cases where hydrodisplacement was utilised, it was performed to displace and protect a nearby bowel loop. Determinations of the possibility of attempting those cases without hydrodisplacement were made in consensus after reviewing the pre-ablation images (either CT or MRI) closest to the date of the ablation procedure. The consensus panel did not review the artificial ascites cases as it was administered to decrease post-procedural body wall/ diaphragmatic pain, a far less severe complication than bowel injury and one that would not preclude percutaneous ablation.
Fluid instillation and displacement distance calculation
Dextrose-in-water 5% (D5W) was used as the fluid medium in all cases except one, where 0.9% (normal) saline (NS) was used during treatment of a single HCC. Fluid instillation was performed after placement of an 18- or 20-gauge spinal needle into the peritoneal space or into the plane separating the target and vulnerable structure, utilising either ultrasound or CT guidance. Pre-procedural ultrasound or multiplanar CT and MR images were reviewed by a single board-certified radiologist (D.R.K.), and the distance between the liver capsule closest to the hepatic tumour and the vulnerable structure (i.e. bowel, stomach, gallbladder, diaphragm, body wall) was measured. Intraprocedural ultrasound and CT images were then reviewed to measure that same distance after the instillation of fluid (Figures 1 and 2).
Patient follow-up
The patient’s follow-up imaging reports (CT or MRI) and pathology data were reviewed to determine whether there was evidence of local tumour progression using standard criteria [23] Complications were obtained by reviewing the electronic medical record. Follow-up imaging was scheduled for 1, 3, 6, 9, 12, 18, and 24 months post-procedure with contrast-enhanced CT or MR to evaluate for local tumour progression, development of new foci of disease, and signs of delayed complications.
Statistical analysis
Cases in which no fluid was used were designated as the control group. The patient’s age, tumour size, length of follow-up, displacement distance/artificial ascites thickness, and power and time of treatment cycles of the study and control groups were compared statistically utilising an unpaired Student’s t-test and Fisher’s exact test. Rates of local tumour progression were compared utilising a log rank analysis. A p value of 50.05 was considered statistically significant.
Results
Patient population
Fluid administration was utilised in the treatment of 29/151 (19.2%) of all hepatic tumours, 17/87 (19.5%) HCCs, and 12/ 65 (18.5%) metastases. Of the 29 lesions where intraperitoneal fluid was administered, 10 utilised hydrodisplacement of vulnerable structures and 19 utilised generalised artificial ascites. These patients were considered the study group. There were no significant differences in the mean patient age or imaging follow up between the control and fluid administration groups. Although not meeting statistical significance, tumours in the fluid instillation group trended larger than the control group (2.55 cm vs. 2.12 cm, p = 0.076) (Tables 1 and 2).
Table 1.
Population statistics.
| Fluid administration (n = 29) | Control (n = 122) | p | |
|---|---|---|---|
| Age | 60.8 ± 10.6 (44–82) | 62.2 ± 7.9 (35–84) | 0.425 |
| Average tumour size (cm) | 2.55 ± 1.41 (0.5–6.0) | 2.12 ± 1.10 (0.4–6.0) | 0.076 |
Table 2.
Progression and follow-up.
| Fluid administration all (n = 29) | Hydrodisplacement (n = 10) | Artificial ascites (n = 19) | Control (n = 122) | |
|---|---|---|---|---|
| Local progression | 5/29 (17.2%) | 3/10 (30%) | 2/19 (10.5%) | 6/122 (4.9%) |
| p | 0.02 | 0.001 | 0.4 | |
| Follow-up (months) | 7.7 ± 4.6 (1–18) | 5.6 ± 2.8 (1–9) | 8.8 ± 5.1 (1–18) | 8.2 ± 6.1 (1–24) |
| p | 0.680 | 0.185 | 0.685 |
Hydrodisplacement for tumours near bowel
Fluid administration was performed in 10 of 29 cases to displace and protect bowel in proximity to the ablation zone. The consensus panel retrospectively deemed eight of these 10 tumours (80%) as unsafe to treat without pre-ablation displacement based on the pre-ablation imaging review. The panel would have been willing to attempt the remaining two cases without utilising hydrodisplacement, but with the caveat that only limited power levels would be used. Without pre-ablation hydrodisplacement, 145 of the 151 tumours would have been amenable to percutaneous treatment (96.0%). Thus, the use of hydrodisplacement expanded the potential case pool by 4%.
Primary effectiveness and local tumour progression
Primary technical success rate was 100% with no residual tumour visible in any case on the final, immediate post-procedure CT. The overall rate of local tumour progression in this study was 7.9% (12/151). This included two cases of microscopic foci of ‘viable’ tumour discovered on explant specimens using haematoxylin and eosin (H&E) staining. Vital staining was not performed, and histopathological findings were discordant with a lack of imaging evidence for local tumour progression. Tumour progression was higher in patients receiving intraperitoneal fluid than in the control group (17.2% vs. 4.9% respectively, p = 0.02). The local tumour progression rate was also higher in the hydrodisplacement subgroup of the study population compared to the control group (30% vs. 4.9% respectively, p = 0.001). The artificial ascites subgroup had a higher rate of local tumour progression than controls, but this was not statistically significant (10.5 vs. 4.9% respectively, p = 0.4) (Table 2). No cases of peritoneal seeding were identified in either the control or study groups.
Non-target structures and complications
Intraperitoneal fluid was used for protection of various non-target organs in the fluid administration group – body wall (11), diaphragm (9), stomach (6), duodenum (1) and colon (4). An average fluid thickness of 0.74 cm was obtained (0.84 cm for hydrodisplacement and 0.58 cm for artificial ascites) (Table 3). The fluid volume instilled was recorded for 28 of the 29 lesions treated utilising intraperitoneal fluid. The average volume of fluid instilled was 814 mL ± 599 mL. Though there were fewer complications in the study group (4.0% minor, 0% major vs. 8.1% minor, 2.7% major), this did not meet statistical significance. No major complications occurred during any ablation utilising fluid administration. Two major complications occurred in the control group – postprocedural portal venous thrombus (HCC) and thermal injury of the diaphragm resulting in a pleural effusion requiring two thoracenteses (metastatic lesion). There was a single minor complication in the artificial ascites group (body wall pain) and six minor complications in the control group (five body wall pain, one prolonged nausea) (Table 4). No symptomatic bleeding was seen in either group.
Table 3.
Distances to non-target organ.
| Distance (cm) | Fluid administration all (n = 29) | Hydrodisplacement (n = 10) | Artificial ascites (n = 19) |
|---|---|---|---|
| Prior to fluid administration | 0.29 ± 0.46 (0–1.7) | 0.63 ± 0.59 (0–1.7) | 0.14 ± 0.24 (0–0.4) |
| After fluid administration | 1.1 ± 0.84 (0–3.3) | 1.65 ± 1.10 (0–3.3) | 0.73 ± 039 (0–1.4) |
| Fluid thickness | 0.74 ± 0.61 (0–2.8) | 1.02 ± 0.87 (0–2.8) | 0.57 ± 0.35 (0–0.9) |
Table 4.
Complications by patient.
| Fluid administration (n = 25 patients) | Control (n = 74 patients) | p | |
|---|---|---|---|
| Minor complications | 1/5 (4.0%) | 6/74 (8.1%) | 0.675 |
| Major complications | 0/25 (0%) | 2/74 (2.7%) | 1.000 |
Power and time
The average power utilised for cases in which intraperitoneal fluid was administered was significantly lower than the power utilised in cases where no fluid was administered (69.6 vs. 81.3 W respectively, p = 0.024). In cases requiring hydro-displacement of vulnerable structures, powers were even lower: 63.9 vs. 81.3 W, p = 0.044. In cases where generalised artificial ascites was used, power settings were not signifi-cantly different from controls (Table 5). Conversely, the total ablation time for lesions where fluid was administered was significantly longer than when no fluid was administered (6.7 vs. 5.2 min respectively, p = 0.010). This difference was also more marked in patients requiring hydrodisplacement (8.0 vs. 5.2 min, p = 0.002). The artificial ascites ablation time was not significantly different from controls (Table 5).
Table 5.
Power and time.
| Fluid administration (n = 29 | Hydrodisplacement (n = 10) | Artificial ascites (n = 19) | Control (n = 122) | |
|---|---|---|---|---|
| Power (W) | 69.6 ± 14.3 | 63.9 ± 17.1 | 72.6 ± 11.9 | 81.3 ± 26.6 |
| p | 0.024 | 0.044 | 0.164 | |
| Time (min) | 6.7 ± 3.8 | 8 ± 4.1 | 6.1 ± 3.5 | 5.2 ± 2.5 |
| p | 0.010 | 0.002 | 0.171 |
Discussion
The results of this study demonstrate that hydrodisplacement can increase the number of tumours eligible for treatment by microwave ablation by displacing and protecting vulnerable structures adjacent to the ablation zone. Eight of ten tumours adjacent to bowel in this study would not have been treated with ablation prior to the advent of fluid administration or other thermoprotective technique. The use of hydrodisplacement increased our ablation population by 4%. However, the local tumour progression rate in the fluid instillation study group (17.2%) was also higher than in controls (4.9%). A possible reason for this increase is that tumours in fluid instillation cases were treated with significantly lower power due to operator caution prompted by high risk locations. The trend towards larger tumour size in the fluid instillation group may also contribute to these results, as larger tumours have been shown to be associated with increased local tumour progression [24–26]. While Zhang et al. did not report results from a control group, their results of fluid instillation with MW ablation of liver tumours are similar (16.1% local tumour progression) [21].
Heat sink effect seems an unlikely cause of the increased local tumour progression, given that previous studies have demonstrated MW energy to be less susceptible to heat sink effects than RF and no RF studies have ever shown worse local tumour progression with fluid instillation [2]. However, more studies will be needed to evaluate the interaction of MW ablation with fluid instillation given that this series and the series from Zhang et al. both show higher rates of local tumour progression than expected.
Although the complication rate might be expected to be lower in cases utilising fluid instillation, there was no significant difference in the complication rate between the study and control groups. This likely reflects the overall small number of complications in the entire series as well as the protective properties of hydrodisplacement. Importantly, there were no instances of bowel injury in spite of the close proximity of the ablation zone and bowel in several cases utilising hydrodisplacement. These findings suggest that the patient population eligible for MW ablation treatment of liver lesions can be safely expanded if an adequate fluid buffer can be placed between the ablation zone and bowel. While not a primary end point, it is notable that there were no cases of peritoneal tumour seeding or intraperitoneal bleeding.
There is minimal technical complexity associated with fluid instillation techniques. To create artificial ascites, a needle is advanced under imaging guidance between the liver and body wall or diaphragm and fluid is then instilled. In cases where hydrodisplacement is used, the technical difficulty is slightly higher, as the fluid must be instilled in a manner that results in physical displacement of the non-target organ. Occasionally the instilled fluid will disperse from the desired displacement area, but adequate displacement can almost always be achieved with needle and patient positioning (i.e. the fluid will track dependently). Although we did not calculate the added time required to perform fluid instillation, other studies have demonstrated fluid instillation to add a mean of less than 10 min per case [13]. Because there were no bowel injuries in this series across a variety of fluid volumes and displacement distances, the required volume of fluid or displacement distance to prevent injury cannot be elucidated.
For RF ablation, D5W is considered the fluid medium of choice due to the lack of electrical current propagation related to its nonionic nature. Given its ionic composition, saline can conduct electrical current, thus potentially leading to non-target tissue heating [12,27]. MW ablation relies on an electromagnetic field effect not associated with the conduction of electrical current and MW heating is more proportional to the water content and less influenced by ionic content in tissue [28]. Because the water content of D5W and normal saline is virtually identical, either fluid can be safely used in conjunction with MW, as reported by Zhang et al. [21].
Intraperitoneal gas instillation, intraperitoneal catheter balloon displacement, and blunt needle displacement can be used as alternative thermoprotective techniques. No direct comparison of these methods has been performed. Fluid instillation has a theoretical advantage in that it protects non-target structures by two methods. The first is simply increasing the distance from the heat source, the same as balloon or needle displacement. The second method of protection is related to the instilled fluid acting as heat sump, moving heat away from the heat source and non-target organ via convective heat transfer. That is, as the instilled fluid circulates, either due to natural circulation or the continued instillation of additional fluid, heat is drawn away from the non-target organ and the heat source conferring additional protection. Though no clinical studies have evaluated the interplay between these two protective properties, we feel that this convective heat sump effect adds an important element of protection to the physical displacement distance. Therefore, we instil more fluid than is necessary for simple displacement and often continue to instil fluid during the ablation cycle. This convective heat sump effect is minimised when using gas displacement and is lost entirely when using needle or balloon displacement techniques, also leading us to choose hydrodissection over alternative displacement techniques [29].
Our study has several limitations. The most important limitation is an inherent case selection bias limiting the value of the comparison between the study and control groups. In general, physicians performing the procedures chose to perform hydrodisplacement only in cases where a bowel loop was in close proximity to the target tumour. Lower powers were applied in these cases in an attempt to minimise the potential for thermal damage to the bowel. This limits the applicability of the study to the generalised ablation patient population in which identical powers were applied in all cases. There are also cases in the hydrodisplacement subgroup that otherwise would not have been treated without hydrodisplacement, making comparison to the control group even more difficult. However, data from RF ablations have shown the efficacy of fluid instillation techniques. The subsequent lack of equipoise makes a randomised controlled trial impossible as it would knowingly place some patients at significant risk of non-target injury. While the comparison of these two groups is limited, it is not meaningless. It provides actual data to use when discussing the likelihood of long-term success with patients – we now know that cases in which fluid protective techniques are used are associated with higher rates of local tumour progression. It remains unclear whether this is a causal relationship, related to lower applied powers, or simply an artefact of only using fluid in more difficult cases; this will need further study.
Additional limitations of our study include its retrospective nature and the relatively small fluid instillation study group. Also, the short nature of the imaging follow-up may cause us to underestimate the rate of local tumour progression. We also acknowledge that the consensus panel decision on whether it was safe to attempt an ablation procedure without thermoprotective techniques is inherently subjective and could vary amongst different centres and physicians. However, the consensus panel is quite experienced in MW ablation and there is no better method that we are aware of to determine ablation eligibility retrospectively.
A final limitation of our study is the two separate methods of determining local tumour progression – imaging evidence and pathological findings at liver explantation. Imaging findings of local tumour progression are well documented, typically resulting in nodular, enhancing tissue at the ablation zone margin [23,30]. Pathological evidence of viable tumour in the ablation zone is more difficult to define. Standard H&E staining overestimates the presence of residual viable tumour cells due to heat fixation artefact. Our pathology department currently does not perform oxidative staining on liver explant specimens, required to determine whether tumour cells seen on H&E stains are viable [31,32]. Neither of the two cases of local progression seen only at explant had imaging evidence of local tumour progression, suggesting those cases may not represent viable tissue, and our local tumour progression rate may be overestimated.
In conclusion, this study demonstrates that intraperitoneal fluid administration is a safe and effective method of protecting non-target structures during percutaneous hepatic MW ablation. The use of hydrodisplacement allowed microwave ablation to be performed in cases that otherwise would be unsafe due to the risk of damage to vulnerable structures such as bowel. MW ablation cases utilising fluid instillation were associated with higher rates of local tumour progression; further studies will be necessary to determine the exact cause.
Acknowledgments
Declaration of interest
Fred Lee is the founder of, and shareholder in, NeuWave Medical, a company which manufactures the MW technology used clinically in our hospital and in this study population. He is also the inventor and patent holder of the RF switching controller for RF ablation devices. J. Louis Hinshaw is a shareholder in NeuWave Medical and also on its board of medical consultants. Christopher Brace is a shareholder in and consultant for NeuWave Medical. The authors alone are responsible for the content and writing of the paper.
References
- 1.Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Intervent Radiol 2009;20(Suppl7):S342–7. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology 2011;258:351–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awad MM, Devgan L, Kamel IR, Torbensen M, Choti MA. Microwave ablation in a hepatic porcine model: Correlation of CT and histopathologic findings. HPB 2007;9:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study. J Gastroenterology 2005;40:1054–60. [DOI] [PubMed] [Google Scholar]
- 5.Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003; 180:1547–55. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Wang Y, Ni X, Gao Y, Shao Q, Liu L, et al. Comparison of ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: Results in in vivo porcine livers. Am J Roentgenol 2009; 192:511–14. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Choyke LT, Locklin JK, Wood BJ. Use of hydrodissection to prevent nerve and muscular damage during radiofrequency ablation of kidney tumors. J Vasc Intervent Radiol 2006;17:1967–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell MA, Charboneau JW, Callstrom MR, Reading CC, Engen DE, Blute ML. Paranephric water instillation: A technique to prevent bowel injury during percutaneous renal radiofrequency ablation. Am J Roentgenol 2003;181:1315–17. [DOI] [PubMed] [Google Scholar]
- 9.Levit E, Bruners P, Gunther RW, Mahnken AH. Bile aspiration and hydrodissection to prevent complications in hepatic RFA close to the gallbladder. Acta Radiol 2012;53:1045–8. [DOI] [PubMed] [Google Scholar]
- 10.Lee EJ, Rhim H, Lim HK, Choi D, Lee WJ, Min KS. Effect of artificial ascites on thermal injury to the diaphragm and stomach in radiofrequency ablation of the liver: Experimental study with a porcine model. Am J Roentgenol 2008;190:1659–64. [DOI] [PubMed] [Google Scholar]
- 11.Kim YS, Rhim H, Paik SS. Radiofrequency ablation of the liver in a rabbit model: Creation of artificial ascites to minimize collateral thermal injury to the diaphragm and stomach. J Vasc Intervent Radiol 2006;17:541–7. [DOI] [PubMed] [Google Scholar]
- 12.Laeseke PF, Sampson LA, Brace CL, Winter TC III, Fine JP, Lee FT Jr. Unintended thermal injuries from radiofrequency ablation: Protection with 5% dextrose in water. Am J Roentgenol 2006; 186(Suppl5):S249–54. [DOI] [PubMed] [Google Scholar]
- 13.Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: Initial experience. Am J Roentgenol 2008;190:91–8. [DOI] [PubMed] [Google Scholar]
- 14.Hinshaw JL, Laeseke PF, Winter TC III, Kliewer MA, Fine JP, Lee FT Jr. Radiofrequency ablation of peripheral liver tumors: Intraperitoneal 5% dextrose in water decreases postprocedural pain. Am J Roentgenol 2006;186(Suppl5):S306–10. [DOI] [PubMed] [Google Scholar]
- 15.Kariya S, Tanigawa N, Kojima H, Komemushi A, Shomura Y, Ueno Y, et al. Radiofrequency ablation combined with CO2 injection for treatment of retroperitoneal tumor: Protecting surrounding organs against thermal injury. Am J Roentgenol 2005;185:890–3. [DOI] [PubMed] [Google Scholar]
- 16.Akins EW, Hawkins IF Jr, Mladinich C, Tupler R, Siragusa RJ, Pry R. The blunt needle: A new percutaneous access device. Am J Roentgenol 1989;152:181–2. [DOI] [PubMed] [Google Scholar]
- 17.Raman SS, Aziz D, Chang X, Sayre J, Lassman C, Lu D. Minimizing diaphragmatic injury during radiofrequency ablation: Efficacy of intraabdominal carbon dioxide insufflation. Am J Roentgenol 2004;183:197–200. [DOI] [PubMed] [Google Scholar]
- 18.Yamakado K, Nakatsuka A, Akeboshi M, Takeda K. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Intervent Radiol 2003;14:1183–6. [DOI] [PubMed] [Google Scholar]
- 19.Ohmoto K, Tsuzuki M, Yamamoto S. Percutaneous microwave coagulation therapy with intraperitoneal saline infusion for hepatocellular carcinoma in the hepatic dome. Am J Roentgenol 1999;172:65–6. [DOI] [PubMed] [Google Scholar]
- 20.Ohmoto K, Yamamoto S. Percutaneous microwave coagulation therapy for superficial hepatocellular carcinoma using intraperitoneal infusion of local anesthetic. Am J Gastroenterol 2001;96: 1660–2. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Liang P, Cheng ZG, Yu XL, Han ZY, Yu J. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 2014;30:134–41. [DOI] [PubMed] [Google Scholar]
- 22.Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D; Society of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Intervent Radiol 2009; 20(Suppl7):S189–91. [DOI] [PubMed] [Google Scholar]
- 23.Sainani NI, Gervais DA, Mueller PR, Arellano RS. Imaging after percutaneous radiofrequency ablation of hepatic tumors: Part 2, Abnormal findings. Am J Roentgenol 2013;200:194–204. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y-S, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: Analysis of the pattern and risk factors. Eur J Radiol 2006;59:432–41. [DOI] [PubMed] [Google Scholar]
- 25.Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterology 2003;38:977–81. [DOI] [PubMed] [Google Scholar]
- 26.Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer 2003; 97:1253–62. [DOI] [PubMed] [Google Scholar]
- 27.Brace CL, Laeseke PF, Prasad V, Lee FT. Electrical isolation during radiofrequency ablation: 5% dextrose in water provides better protection than saline. Conf Proc IEEE Eng Med Biol 2006; 1:5021–4. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Sato M. Microwave heating of water, ice, and saline solution: Molecular dynamics study. J Chem Phys 2007;126: 034509. [DOI] [PubMed] [Google Scholar]
- 29.Johnson A, Sprangers A, Cassidy P, Heyrman S, Hinshaw JL, Lubner M, et al. Design and validation of a thermoreversible material for percutaneous tissue hydrodissection. J Biomed Mater Res B, Appl Biomater 2013;101:1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dromain C, de Baere T, Elias D, Kuoch V, Ducreux M, Boige V, et al. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology 2002;223: 255–62. [DOI] [PubMed] [Google Scholar]
- 31.Marcovich R, Aldana JPA, Morgenstern N, Jacobson AI, Smith AD, Lee BR. Optimal lesion assessment following acute radio frequency ablation of porcine kidney: Cellular viability or histopathology? J Urol 2003;170:1370–4. [DOI] [PubMed] [Google Scholar]
- 32.Morimoto M, Sugimori K, Shirato K, Kokawa A, Tomita N, Saito T, et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: Radiologic-histologic correlation during follow-up periods. Hepatology 2002;35:1467–75. [DOI] [PubMed] [Google Scholar]