Abstract
The ability to measure the intrinsic functional architecture of the brain has grown exponentially over the last 2 decades. Measures of intrinsic connectivity within the brain, typically measured using resting-state functional magnetic resonance imaging (MRI), have evolved from primarily “static” approaches, to include dynamic measures of functional connectivity. Measures of dynamic functional connectivity expand the assumptions to allow brain regions to have temporally different patterns of communication between different regions. That is, connections within the brain can differentially fire between different regions at different times, and these differences can be quantified. Applying approaches that measure the dynamic characteristics of functional brain connectivity have been fruitful in identifying differences during brain development and psychopathology. We provide a brief overview of static and dynamic measures of functional connectivity and illustrate the synergy in applying these approaches to identify both age-related differences in children and differences between typically developing children and children with autistic symptoms.
Keywords: Resting-state functional magnetic resonance imaging, autism spectrum disorders, development, neurodevelopment, children
Comment on: Rashid B, Blanken LME, Muetzel RL, et al. Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Hum Brain Mapp. 2018;39:3127-3142. doi:10.1002/hbm.24064. PubMed PMID: 29602272; PubMed Central PMCID: PMC6045960. https://www.ncbi.nlm.nih.gov/pubmed/29602272
Commentary
Brain function involves the orchestration of neuronal signals within a complex array of distributed neural networks. Using functional neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), a number of different measures of brain connectivity can be obtained. Functional connectivity is one commonly derived metric from fMRI, which is defined as the pair-wise correlation of blood-oxygenation-level-dependent (BOLD) time series between different brain regions. The phrase “what is wired together, fires together” is used to capture the essence of functional connectivity. Many papers equate a higher covariance between brain regions to reflect greater connectivity. While this is likely true, there are more factors that can influence measurements of functional connectivity.
For example, say that I plug my computer charger into the wall socket and watch as the green light comes on, indicating that a connection is made and my computer is charging. Using the mathematical models for functional connectivity, there would be strong “connectivity” between the voltage at the outlet and the transformer, as a result of the 50 Hz (60 Hz in the United States) alternating current (AC) signal. However, between the transformer and my computer, there would be essentially no “connectivity,” as the signal is converted to direct current (DC), which has a stable voltage. Certainly, if there were voltage spikes or drifts that propagated between the transformer and the computer, then the correlations would be higher. This example, when applied to measurements of brain connectivity implies that if regions of the brain were to have constant communication with little fluctuation, similar to the DC example, brain regions would show low or no connectivity. This is especially true given that fMRI is relatively noisy. Fortunately, the “resting” brain is never static, but has fluctuations (often measured between the range of 0.1-0.01 Hz) between brain regions that derive consistent and robust functional connectivity networks.1,2 Furthermore, these resting-state networks model very well with known connections derived from task-based functional MRI studies.3,4
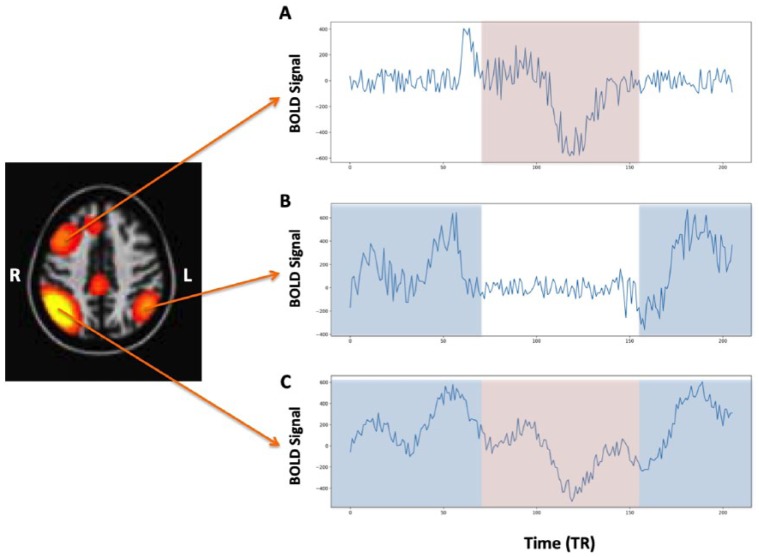
Within the past decade, a second form of functional connectivity, labeled “dynamic functional connectivity” (dFC) has emerged.5,6 This type of functional connectivity could best be portrayed by the phrase “what is wired together, fires together … unless of course at that time it’s firing somewhere else.” While a single neuron has spatially constrained connections, there are millions of neurons within a voxel of the dimensions that are typically used for functional MRI. While clusters of neurons within a single voxel may be firing with one brain region, other clusters may be firing with other brain regions (Figure 1). Furthermore, there may be some periodicity of the firing between the different regions, with one cluster of connections showing greater dominance within a specific window of time and a different set of connections more dominant at another time. Thus, by applying, for example, a “sliding window” analyses to paired BOLD time series, the dynamic characteristics of brain connectivity can be elucidated.
Figure 1.
Simulated example of a network where the (C) right parietal region communicates differentially between the (B) left parietal region and the (A) right prefrontal region. The light blue rectangles reflect the regions in the time series in which the correlations are highest between (A) and (B) and the light red rectangles reflect the regions of highest correlation between (A) and (C).
Both techniques of measuring “static” and dFC have been used to explore the underlying functional architecture in typical development and psychopathology.7 While we use the term “static” functional connectivity (sFC), we point out that we are not referring to the BOLD time series being “static” in the same way as the DC example; but rather, we define sFC as using the entire time BOLD time series to derive the average connectivity between regions. However, we agree that the distinction between sFC and dFC can be somewhat arbitrary. Say that we collect a 5-minute 20-second resting-state fMRI (rs-fMRI) sequence, which we did in the first neuroimaging wave of the Generation R Study8 and defined a series of sFC networks.2 Subsequently, in a study of autism spectrum disorder (ASD), we used a 50-second sliding window to assess dFC.9 However, had we collected rs-fMRI resting-state data continuously for an hour and used a sliding window analysis of 5-minute 20-seconds (the same duration as the total dFC sequence that we used in our study),8 then the dFC window would yield many different versions of what we labeled sFC. While there is some debate regarding optimum time for sliding window analyses,5,10 most brain networks reach stable11 and replicable2 characteristics of functional connectivity within 5 minutes.
The underlying neurobiology of psychopathology is often attributed to aberrant connectivity between brain regions.7 But actually, at its core, brain function occurs through distributed neural networks and thus any disorder that affects behavior, emotion, or cognition is ultimately due to disrupted connectivity between brain regions. What is not known, however, is the nature of the disrupted connectivity or the brain regions involved in and between different disorders. Furthermore, it is not clear when during development, beginning in fetal life, that trajectories deviate from typical brain functional connectivity. Thus, it is crucial to embed studies of psychopathology within the context of typical brain development so as to better understand when during the development of functional connectivity that deviations take place.
One approach to define brain connectivity within the pediatric population at large is to nest neuroimaging studies within large population-based studies of child development.8 The advantage of population-based studies, and especially birth cohorts, is that the results, by definition, are more generalizable to the population.12 Birth cohorts, dependent on the level of attrition, provide less bias compared with case/control studies that compare children with specific disorders to “super-controls” or extremely healthy and high functioning controls who are often much easier to recruit. In addition, birth cohorts offer the ability to study psychopathology across the continuum, as non-help-seeking children with subclinical symptoms are present in the general population.13
We recently explored both static and dynamic connectivity in a large population-based study of child development, which included a group of children with ASD.9 To embed the study within typical development, we first assessed age- and sex-related differences in both sFC and dFC in the general population. Reassuringly, we found that the sFC and dFC analyses revealed network patterns similar to those seen in adults. Furthermore, the older children spent a greater amount of time in dynamic network states that have been shown to be associated with greater developmental maturity.14 Compared with typically developing children, however, children with autistic traits showed a mixed pattern of both higher and lower sFC in different regions of the brain, which has also been described in the literature.15 In the dFC analysis, we found that children with ASD had a greater dwell time in a hyperconnected state, meaning connectivity patterns in these children with autistic symptoms tended to “dwell” longer in states with high levels of connectivity both between and within networks. Thus, we address the question as to how to make sense of these findings in children with autistic traits within the context of static and dynamic network connectivity and typical development?
First, what does it mean if some regions show higher sFC, whereas others show lower dFC? One possibility is that children with autistic symptoms use different networks than typically developing children, possibly due to specific nodes that are less efficient and thus other areas take over. An overall different network pattern would result in a mixed pattern of higher and lower sFC between typically developing children and children with ASD. But are the sFC patterns consistent across studies? While previous studies point to the default mode network (DMN) as a network most implicated in ASD, even this network shows both hyper- and hypo-connectivity in ASD.15 We did not find abnormalities in the DMN, but rather decreased connectivity between the insula and supramarginal gyrus, consistent with task-based fMRI studies.16
Findings from our dFC analysis showed that children with autistic symptoms spent more time in what we labeled as a “globally disconnected state,” which includes a globally disconnected DMN. Greater time in a globally disconnected state could be a result of a number of different reasons. Younger children spend greater time in a globally disconnected state, and thus, children with higher autistic symptoms may show less brain maturity. Alternatively, there may be differences in the intrinsic timing of the brain such that periods exist with less synchronicity between brain regions. A globally disconnected state should not be equated to a nonfunction brain, but rather the connectivity between different regions becomes either highly stable with little change or noisier.
In summary, measurements of both sFC and dFC can provide a unique glimpse into brain function in typically developing children and aberrant connectivity patterns in children with psychiatric or neurological disorders. Our dFC results showed that both younger children and those with greater autistic symptoms spend more time in a globally disconnected state.9 As children with greater autistic symptoms also show immaturity in cognitive performance,17 the findings related to autistic symptoms may not necessarily be illness specific, although future longitudinal studies will be extremely beneficial in assessing this relationship. The great challenge for both sFC and dFC is the presence of many variables related to both typical brain development and emerging psychopathology, considerable heterogeneity in both clinical characteristics and functional brain patterns during development, and the relationship of these factors within the context of individual differences between children. These factors will need to be fully explored to better understand psychopathology nested in brain developmental trajectories and within an individual. Large population-based studies of child development can help define both the typical developmental trajectories as well as the level of heterogeneity within the general population so as to better understand brain wiring gone amiss.
Acknowledgments
The authors acknowledge the excellent contribution of Barnaly Rashid, Laura Blanken, and Ryan Muetzel on the original publication of children with autism spectrum disorders.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Netherlands Organization for Health Research and Development (ZonMw) TOP project number 91211021, the Simons Foundation Autism Research Initiative (SFARI-307280), and the National Institutes of Health Grants R01EB020407 and P30GM122734 and NSF grant 1539067.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: The contribution from the authors followed a highly damped sinusoidal waveform; the initial draft was written by TW, then edits by VDC, additional edits by TW, etc. until the final version of the manuscript was attained.
ORCID iD: Vince D. Calhoun  https://orcid.org/0000-0001-9058-0747
https://orcid.org/0000-0001-9058-0747
References
- 1. Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muetzel RL, Blanken LME, Thijssen S, et al. Resting-state networks in 6-to-10 year old children. Hum Brain Mapp. 2016;37:4286–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Preti MG, Bolton TA, van de Ville D. The dynamic functional connectome: state-of-the-art and perspectives. Neuroimage. 2017;160:41–54. doi: 10.1016/j.neuroimage.2016.12.061. [DOI] [PubMed] [Google Scholar]
- 6. Calhoun VD, Miller R, Pearlson G, Adali T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84:262–274. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Martino A, Fair DA, Kelly C, et al. Unraveling the miswired connectome: a developmental perspective. Neuron. 2014;83:1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White T, El Marroun H, Nijs I, et al. Pediatric population-based neuroimaging and the Generation R Study: the intersection of developmental neuroscience and epidemiology. Eur J Epidemiol. 2013;28:99–111. doi: 10.1007/s10654-013-9768-0. [DOI] [PubMed] [Google Scholar]
- 9. Rashid B, Blanken LME, Muetzel RL, et al. Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Hum Brain Mapp. 2018;39:3127–3142. doi: 10.1002/hbm.24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zalesky A, Breakspear M. Towards a statistical test for functional connectivity dynamics. Neuroimage. 2015;114:466–470. doi: 10.1016/j.neuroimage.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 11. White T, Muetzel R, Schmidt M, et al. Time of acquisition and network stability in pediatric resting-state functional magnetic resonance imaging. Brain Connect. 2014;4:417–427. doi: 10.1089/brain.2013.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. White T, Muetzel RL, El Marroun H, et al. Paediatric population neuroimaging and the Generation R Study: the second wave. Eur J Epidemiol. 2018:33:99–125. doi: 10.1007/s10654-017-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blanken LME, Mous SE, Ghassabian A, et al. Cortical morphology in 6- to 10-year old children with autistic traits: a population-based neuroimaging study. Am J Psychiatry. 2015;172:479–486. doi: 10.1176/appi.ajp.2014.14040482. [DOI] [PubMed] [Google Scholar]
- 14. Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hyseni F, Blanken LME, Muetzel R, Verhulst FC, Tiemeier H, White T. Autistic traits and neuropsychological performance in 6- to-10-year-old children: a population-based study. Child Neuropsychol. 2018:25:352–369. doi: 10.1080/09297049.2018.1465543. [DOI] [PubMed] [Google Scholar]