Abstract
Background:
Lower reproductive tract infections in women are important causes of morbidity but can also lead to complications and sequelae. This study aimed to establish the prevalence and risk factors of lower genital tract infections among women of reproductive age in Dakar (Senegal).
Methods:
This was a prospective study conducted in 6 maternity hospitals from July to November 2015. Participants ranged in age from 18 to 49 years and presented at health facilities with signs and symptoms of genital infection. Consenting individuals who met the inclusion criteria were recruited for the study.
Results:
During the reporting period, 276 patients were enrolled. According to the laboratory results, the prevalence of any genital infection was 69.6% (192 of 276). The most common vaginal infections were bacterial vaginosis (39.5%) and vaginal candidiasis (29%), with the third most common cause, trichomoniasis, trailing behind in terms of prevalence (2.5%). Among the microorganisms responsible for cervical infections, Ureaplasma urealyticum was the most frequent (27.5%), followed by Mycoplasma hominis (14.5%), Chlamydia trachomatis (4.7%), and Neisseria gonorrhoeae (1.1%). Multivariate analysis showed that young women and women with low levels of education were at increased risk for vaginal/cervical infections.
Conclusions:
This study revealed a high prevalence of bacterial vaginosis and vaginal candidiasis and suggests that health care providers should increase awareness and communication to improve vaginal hygiene practices. If infection with Trichomonas vaginalis, C trachomatis or N gonorrhoeae is suspected, we also recommend systematically performing laboratory diagnostic confirmation.
Keywords: Sexually transmitted infections, vaginal infection, cervical infection, bacterial vaginosis, candidiasis
Introduction
Lower reproductive tract infections (RTIs) in women are common in clinical medicine and are one of the most significant causes of morbidity. These infections are caused by microorganisms and are divided into the following 2 main groups: sexually transmitted infections (STIs) and the overgrowth of vaginal flora.1 Most new cases of the 3 main STIs (Chlamydia trachomatis [CT], Neisseria gonorrhoeae [NG], and Trichomonas vaginalis [TV]) – globally estimated as 250 million cases annually among women of reproductive age – occur in low- or middle-income countries (LMIC).2 However, endogenous infections such as vulvovaginal candidiasis and bacterial vaginosis (BV) remain the most frequent RTIs.3
RTIs are not only a cause of acute morbidity but can also lead to complications and sequelae, including pelvic inflammatory diseases (PIDs), chronic pelvic pain, spontaneous abortion, stillbirth, low birth weight, neonatal infections, and infertility.4 The prevalence of lower RTIs varies across the world and is influenced by the population’s sexual behaviours and age as well as other socioeconomic factors.1 Thus, obtaining data at the local level, including the prevalence of RTIs and associated risk factors, is an important step in improving the prevention and management of these infections and is further useful in evaluating the effectiveness of interventions.4 A couple of studies were recently conducted in the city of Dakar to determine the prevalence of lower genital tract infections among women.5,6 However, both studies were retrospective analyses of pre-existing laboratory data, which limits the representativeness of the population and the accuracy of the estimated parameters, given that syndromic management of genital infections is encouraged and widely used in this city.
Therefore, the main objective of this study was to establish the laboratory-based prevalence of symptomatic lower genital tract infections among women and to identify the associated risk factors.
Materials and Methods
Study design and sample size
This observational prospective cross-sectional study was conducted at the gynaecological outpatient services of health care facilities in Dakar, Senegal. A precision-based sample size calculation with 95% confidence interval (CI) was used in this study.7 A table of the sample size needed for different prevalence levels indicated that a sample of 260 women was adequate as a minimum number to ensure an acceptable precision to describe the observed prevalence between 10% and 90% (the precision of the estimation varied from 3.6% to 6.1%). However, giving the possible voluntary withdrawal, the number of patients to be recruited was set at 300.
Site and patient selection
In Dakar, midwives are the first point of contact for women presenting with vaginal symptoms, and therefore, they ensure examination and treatment of patients. They were the only eligible study’s investigators. Thus, site selection was based on the role of midwives as the main providers of health services to women with genital symptoms. Six public health care facilities were selected including 2 hospitals and 4 primary health centres.
Among the 5 existing hospitals in Dakar, 2 declined to participate because the health care providers were not willing to send samples outside their own laboratory. Another hospital was excluded as midwives were not the main health providers for women presenting with genital symptoms. Of the 9 primary health centres located in Dakar, 4 were selected based on the high proportion of patients with gynaecologic symptoms who were managed by midwives in 2015.
From July to November 2015, all new patients presenting at the study centres complaining of genital symptoms were screened for their eligibility. Consecutive patients at each study site who met the inclusion criteria were enrolled. A patient was eligible if she met the following 3 criteria: being between 18 and 49 years of age; having symptoms of vaginal discharge, irritation/itching, burning, dyspareunia, or bleeding between menses/during sexual intercourse; and having consented voluntarily to participating in the study. Patients were excluded if they were pregnant or menstruating, reported using antibiotics or vaginal douches within the last 15 days, were commercial sex workers, had gynaecological surgery indications, or had symptoms indicating the development of PID, including spontaneous pelvic pain or adnexal pain/pain in the uterine mobilization.
Data collection and procedures
A training session was organized for all investigators on the study procedures including the consent process and data collection. All women attending the study site received usual standard care provided by investigator midwife including patient interview and physical and gynaecological examination using a sterile bivalve speculum. Clinical information collected during physical and gynaecological examination were reported into the standard questionnaire.
Patients were referred to the laboratory of Hôpital Aristide Le Dantec for specimen collection. Following routine laboratory examination, samples were collected from the lateral and posterior vaginal fornix and endocervical canal according to standard laboratory procedures.
Vaginal swabs were used for the diagnosis of trichomoniasis, candidiasis, and BV. Wet mount microscopy examination at 40× power was used for the detection of TV. The test was considered positive if motile trichomonads were visualized. Candida species were detected by culture. The vaginal specimen was inoculated onto a medium containing Sabouraud agar with chloramphenicol and incubated for 24 hours at 37°C. The diagnosis of candidiasis was made if vaginal colonization by Candida spp. was associated with vaginal symptoms suggestive of vaginal candidiasis (itching or erythema). If the diagnosis was positive, Candida albicans (CA) was then identified using the serum filamentation test. Gram staining and scoring using Nugent criteria was performed for the diagnosis of BV. The swab specimens were placed on a clean glass slide and then heat fixed by passing the glass slide over the flame. The scoring system counts the individual morphotype frequency, and a Gram stain score of 7 to 10 was considered positive for BV.
The endocervical swab was used for the diagnosis of CT and NG. The diagnosis was performed using nucleic acid amplification tests (NAATs) with the Cepheid Xpert CT/NG Assay.8 The assay detects the DNA of CT and NG, and the test is performed using a modular cartridge-based platform to testing each specimen by nucleic acid amplification. Chlamydia trachomatis and NG were diagnosed by a positive result on the test. The endocervical swab was also used to investigate the presence of Mycoplasma hominis (MH) and Ureaplasma urealyticum (UU) by culturing in liquid medium using the Mycoview kits (Zeakon Diagnostics, France)9 according to manufacturer’s recommendations.
Vaginal infection was defined according to the laboratory findings if at least one of the 3 following pathogens was reported: TV, Gardnerella vaginalis, or CA. Cervical infection was defined as the presence of at least one of the following pathogens based on laboratory results: CT, NG, or genital Mycoplasma spp. (MH and UU).
Statistical analysis
Data analysis was performed using SPSS. A descriptive analysis of patient characteristics was conducted with the participant sociodemographic data. The prevalence of infection with each aetiology was calculated with its 95% CI based on the women with laboratory-based diagnoses of infections and excluding missing data. Risk factors were analysed through sociodemographic, obstetrical, and behavioural data and evaluated using bivariate and multivariate analyses, including odds ratios (ORs), 95% CIs, and P value.
Ethical considerations
Eligible patients were informed by investigators about the study objective and assessment process and were invited to participate in this study. Before enrolment of the patient, a written informed consent form was signed by both the investigator and the patient. A standard anonymous standardized questionnaire was then administered to participant.
This study was approved by the National Ethics Committee for Research in Health of the Ministry of Health and Social Action, Senegal (No. 00000070 MSAS/DPRS/CNERS).
Results
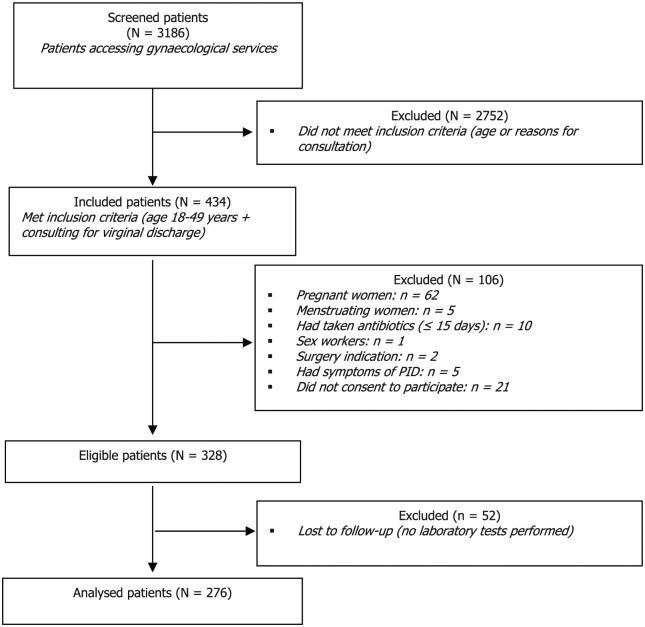
Over the course of 3 months, 3186 consecutive women attending outpatient gynaecological services were screened. Of these, 434 met the inclusion criteria; 328 women were enrolled, out of whom 52 were excluded because of missing laboratory tests (Figure 1).
Figure 1.
Flowchart of patient inclusion.
The distribution of characteristics among the women is shown in Table 1. The mean age of the participants was 31.5 ± 7.4 years; 227 (82.2%) were older than 24 years, and the predominant age group was 25 to 34 years (47.5%). Many participants (60.9%) had fewer than 7 years of education; most of them (84.4%) were married, with 70.6% in a monogamous relationship. Slightly more than 30% of women reported not having had a sexual partner in the past 3 months, while 60 women (21.7%) reported having new sexual partner in the same period.
Table 1.
Sociodemographic characteristics, obstetric history, and sexual behaviour of 276 participating women.
| Characteristics | Number (%) |
|---|---|
| Age (years) | |
| Mean (±SD) | 31.5 ± 7.47 |
| Age (years) | |
| ⩽24 | 49 (17.8%) |
| 25-34 | 131 (47.5%) |
| ⩾35 | 96 (34.8%) |
| Education | |
| None/Islamic/basic literacy | 74 (26.8%) |
| Primary school (1-6 years) | 94 (34.1%) |
| Secondary school (7-13 years) | 75 (27.2%) |
| University/professional (>13 years) | 33 (12.0%) |
| Current employment status | |
| Salaried (public/private sector) | 38 (13.8%) |
| Entrepreneur (restaurant/shop/sewing shop/hairdressing salon owner) | 27 (9.8%) |
| Unemployed | 14 (5.1%) |
| Housewife (unpaid) | 89 (32.2%) |
| Housewife (paid) | 40 (14.5%) |
| Student | 22 (8.0%) |
| Trainee (sewing, hairdressing) | 10 (3.6%) |
| Others | 36 (13.0%) |
| Marital status | |
| Never married | 21 (7.6%) |
| Currently married | 233 (84.4%) |
| Widowed/divorced | 21 (8.0%) |
| Missing value | 1 (NA) |
| Marital relationship | |
| Monogamy | 156 (70.6%) |
| Polygamy | 65 (29.4%) |
| Missing value | 12 (NA) |
| Gestity | |
| Median (range) | 2.0 (0; 11) |
| Parity | |
| Median (range) | 2.0 (0; 10) |
| Abortion/stillbirth (<12 months) | |
| Yes | 21 (7.6%) |
| No | 255 (92.4%) |
| Sexual partners in the past 3 months | |
| None | 86 (31.2%) |
| 1 | 175 (63.4%) |
| >1 | 15 (5.4%) |
| New sexual partners in the past 3 months | |
| Yes | 60 (21.7%) |
| No | 216 (78.3%) |
| Sexual partner has other partners | |
| Yes | 65 (23.6%) |
| No | 153 (55.4%) |
| Do not know | 58 (21.0%) |
Aetiology of lower genital infections
Laboratory investigations of the vaginal and cervical infections revealed that overall, 69.6% of women had some aetiological diagnosis (Table 2). The most common vaginal infections were BV (39.5%) and vaginal candidiasis (29%). Mycoplasma spp. were by far the most common pathogens causing cervical infections, with UU as the predominant agent (27.5%). Chlamydia trachomatis cervicitis was diagnosed in 13 (4.7%) of the 276 women, and NG infection was diagnosed in 3 (1.1%) women. The overall prevalence of vaginal infections was 56.9% (157 women), and cervical infections were found in 99 (35.9%) participants.
Table 2.
Aetiology and prevalence of lower genital tract infections among symptomatic women of reproductive age.
| Infections | N (%) | 95% CI |
|---|---|---|
| Bacterial vaginosis (BV) | 109 (39.5) | 33.68-45.53 |
| Candida albicans (CA) | 80 (29.0) | 23.70-34.73 |
| Trichomonas vaginalis (TV) | 7 (2.5) | 1.02-5.16 |
| Ureaplasma urealyticum | 76 (27.5) | 22.35-33.21 |
| Mycoplasma hominis | 41 (14.9) | 10.88-19.61 |
| Chlamydia trachomatis (CT) | 13 (4.7) | 2.53-7.92 |
| Neisseria gonorrhoeae (NG) | 3 (1.1) | 0.22-3.14 |
| Other infections | 23 (8.3) | 5.36-12.24 |
| Vaginal infection (TV, BV, and CA) | 157 (56.9) | 50.81-62.81 |
| Cervical infections (CT, NG, Mycoplasma spp.) | 99 (35.9) | 30.21-41.84 |
| Lower genital tract infections | 192 (69.6) | 63.77-74.94 |
Abbreviations: CI, confidence interval.
Risk factors of vaginal and cervical infections
Sociodemographic, obstetric, and sexual behaviour data were used to determine the risk factors. A bivariate analysis was performed to investigate the association between these factors and vaginal and cervical infections. The results are displayed in Tables 3 and 4. No significant differences were observed in the prevalence of vaginal infections for any potential risk factor except recent sexual intercourse. Women who had sexual intercourse in the past 3 months were nearly 2 times more likely to have vaginal infections (TV, CA, or BV) compared with those who did not (OR = 1.8, P = .021). Age, education level, marital status, multiple sexual partners, and the presence of an infection in the past 12 months were not statistically associated with current vaginal infections. Among the potential risk factors associated with cervical infections (age, education level, recent genital infection, multiple partners), marital status was the only confirmed risk factor. The risk of a single woman having a cervical infection caused by CT, NG, or Mycoplasma spp. was significantly higher than the risk of a similar infection in a married woman (OR = 2.3, P = .014).
Table 3.
Association of vaginal infections (Trichomanas vaginalis, bacterial vaginosis, or Candida albicans) with participant characteristics.
| Characteristics | Total number (prevalence) | OR | 95% CI | P value |
|---|---|---|---|---|
| Age (years) | ||||
| ⩽24 | 49 (67.3) | 1.7 | 0.9-3.3 | .103 |
| >24 | 227 (54.6) | |||
| Education (years) | ||||
| ⩽7 | 168 (60.1) | 1.4 | 0.9-2.3 | .176 |
| >7 | 108 (51.9) | |||
| Marital status | ||||
| Single | 42 (50.0) | 0.7 | 0.4-1.4 | .339 |
| Married/concubine | 233 (57.9) | |||
| Marital relationship | ||||
| Monogamy | 156 (59.6) | 1.4 | 0.8-2.5 | .225 |
| Polygamy | 65 (50.8) | |||
| Abortion | ||||
| ⩽12 months | 20 (50.0) | 0.74 | 0.3-1.8 | .519 |
| None or >12 months | 256 (57.4) | |||
| Sexual intercourse in the past 3 months | ||||
| Yes | 181 (61.9) | 1.8 | 1.1-2.9 | .021 |
| No | 95 (47.4) | |||
| Sexual partner in the past 3 months | ||||
| >1 | 17 (47.1) | 0.6 | 0.3-0.7 | .398 |
| None or 1 | 259 (57.5) | |||
| STI diagnosed by HCP | ||||
| ⩽12 months | 167 (58.2) | 1.1 | 0.7-1.8 | .320 |
| None or >12 months | 109 (55.8) | |||
Abbreviations: CI, confidence interval; HCP, health care professional; OR, odds ratio; STI, sexually transmitted infection.
Table 4.
Association of cervical infections (Chlamydia trachomatis, Neisseria gonorrhoeae, or Mycoplasma spp.) with participant characteristics.
| Characteristics | Total number (prevalence) | OR | 95% CI | P value |
|---|---|---|---|---|
| Age (years) | ||||
| ⩽24 | 49 (32.7) | 0.8 | 0.4-1.6 | .103 |
| >24 | 227 (36.6) | |||
| Education (years) | ||||
| ⩽7 | 168 (36.9) | 1.1 | 0.7-1.9 | .176 |
| >7 | 108 (34.3) | |||
| Marital status | ||||
| Single | 42 (52.4) | 2.3 | 1.2-4.4 | .014 |
| Married/concubine | 233 (32.6) | |||
| Marital relationship | ||||
| Monogamy | 156 (32.7) | 1.1 | 0.6-2.0 | .780 |
| Polygamy | 65 (30.8) | |||
| Abortion | ||||
| ⩽12 months | 20 (40.0) | 1.2 | 0.5-3.1 | .689 |
| None or >12 months | 156 (35.5) | |||
| Sexual intercourse in the past 3 months | ||||
| Yes | 181 (36.5) | 1.0 | 0.6-1.7 | .776 |
| No | 95 (34.7) | |||
| Sexual partner in the past 3 months | ||||
| >1 | 17 (35.3) | 0.9 | 0.3-2.7 | .959 |
| None or 1 | 259 (35.9) | |||
| STI diagnosed by HCP | ||||
| ⩽12 months | 167 (35.2) | 0.9 | 0.6-1.6 | .456 |
| None or >12 months | 109 (36.4) | |||
| Infected sexual partner | ||||
| Yes | 13 (34.0) | 0.7 | 0.1-5.3 | 1.000 |
| No | 201 (57.1) | |||
Abbreviations: CI, confidence interval; HCP, health care professional; OR, odds ratio; STI, sexually transmitted infection.
A multivariate analysis was then performed to identify the predictors of lower genital tract infections, using a stepwise logistic regression with the backward selection approach. All variables that were associated with cervical/vaginal infection in the bivariate analysis at P ⩽ .20 were included. After controlling for marital status and marital relationship (monogamy or polygamy), age, education, and sexual intercourse were factors associated with female genital infections (Table 5). For example, women aged <25 years were 2 times more likely to have lower genital infections than those aged 25 years or older. Compared with women who were more educated and who had not had recent sexual intercourse, those with low education levels (<7 years education) and who had recent sexual intercourse 1.7-fold and 2.1-fold higher risk of female genital infection, respectively.
Table 5.
Predictors of lower genital tract infection among symptomatic adult women of reproductive age.
| Characteristicsa | OR | 95% CI | P value |
|---|---|---|---|
| Age <25 years | 2.1 | 1.073-4.185 | .031 |
| Education < 7 years | 1.6 | 1.000-2.775 | .050 |
| Sexual intercourse in the past 3 months | 2.1 | 1.249-3.569 | .005 |
Abbreviations: CI, confidence interval; OR, odds ratio.
After controlling for marital status and marital relationship.
Discussion
The present prospective cross-sectional study of 276 women complaining of vaginal discharge conducted in the city of Dakar found that lower genital tract infections affected more than two-thirds of the participants (69.6%). Younger age (less than 25 years), fewer years of formal education (fewer than 7 years), and recent sexual intercourse (less than 3 months prior) were independently associated with an increased likelihood of lower genital tract infections among women with vaginal discharge. The most common vaginal infection among symptomatic women was BV (39.5%). The prevalence of BV varies considerably among studies. One of the major concerns for epidemiological studies is the imprecise method of diagnosing BV (use of clinical criteria, Gram staining, molecular diagnostic methods, etc.). In one review of BV using data compiled from studies performed in multiple countries where the definition of BV was based on Nugent criteria, as in this study, the prevalence was as high as 30% in most of the studies from sub-Saharan Africa, ranging from 6.4% in Burkina Faso to 58.3% in South Africa.10 In contrast, one previous study in Dakar found a very low BV prevalence of 1.4%.6 However, it was a retrospective study based on laboratory data, while an aetiological diagnosis is made mainly in the case of presumptive treatment failure, which can reduce the vaginal colonization with bacteria that is strongly associated with BV.11
Vulvovaginal candidiasis is a very common condition that affects up to 4 out of 5 women at least once in their lifetime.12 This observation is supported by our finding that vaginal candidiasis caused by CA was the second most frequent infection, affecting 29% of the participating women; similar results were reported in previous studies in Senegal and other neighbouring countries.5,13,14
Interestingly, STIs diagnosed by the syndromic management of women presenting with vaginal discharge were relatively rare in our study. We found a prevalence of 2.5% for TV, 4.7% for CA, and 1.1% for NG. The low prevalence of STIs in our study may result from the fact that most of the participants were married, in a monogamous relationship, and had no sexual partner or a single sexual partner. Sexually transmitted infections are more prevalent among younger women with multiple sexual partners.1 In addition, wide use of the syndromic management approach, in addition to behaviour changes, can reduce the prevalence of symptomatic STIs,15 which may be the case in our study population.
Mycoplasmas are bacterial agents that are found in the saprophyte state in humans; however, some of these species are associated with various health problems.16 Ureaplasma urealyticum (27.5%) and M hominis (14.9%) were the most common pathogens causing cervical infections in this study; this finding is similar to those reported elsewhere.17,18 While the role of Mycoplasmas in adverse pregnancy outcomes, such as chorioamnionitis, spontaneous preterm labour, and preterm premature rupture of membranes, is increasingly being recognized, there is controversy concerning the need to treat these organisms based solely on their presence in the vaginal flora.16 This current uncertainty may be the reason why these infections are not part of the World Health Organization (WHO) syndromic management guidelines for STIs. This also implies that local surveillance is needed to facilitate a better understanding of the role of Mycoplasma spp. in female genital infections.
The risk factor analysis identified the women at greatest risk for lower genital tract infections in this population and those who may benefit from particular attention from health care providers. Young women and those with lower levels of education were at increased risk for genital infections. These results are consistent with those observed by Francis et al19 in Northwestern Tanzania. In contrast to other observations,19,20 our study was not able to identify positive associations between the presence of infection, multiple partners, and symptoms of urinary tract infections in partners. This is not surprising as these factors are mainly associated with STIs, and the prevalence of STIs was very low in our study.
In addition, some common behaviours in Senegalese women such as vaginal douching and the use of vaginal aphrodisiac unguents were not explored in our study. The normal vaginal microbiome is disturbed by feminine hygiene products,21 and a relationship between vaginal douching and vaginal symptoms has been documented by some authors.22,23 These factors should be explored in future investigations.
One of the limitations of this study is that it was only conducted in public health facilities in the city of Dakar; thus, it cannot be considered representative of the general population as the results are likely to overestimate the observed prevalence. Socioeconomic inequality has been found to be strongly correlated with STIs.24 In private health sectors in Dakar, the cost of medical care is so high that the users are mainly people fully insured by private health insurance. Thus, the largest number of patients uses public health facilities, and the selection bias could have had a slight impact on the observed prevalence. Another factor is the selected population that was limited to women presenting with vaginal symptoms, which underestimates the prevalence of infections because most women in developing countries consider vaginal discharge normal.
Nevertheless, this study also had several strengths including the recruitment of participating women across multiple sites covering primary and tertiary health facilities and investigators experienced in the management of genital infections and the use of the recommended methods for laboratory-based diagnosis of lower genital infections. Thus, findings from this study could be applied to nonpregnant women living in urban areas, who present with vaginal symptoms and attend public health facilities. It further provides useful clinical and epidemiological information that could contribute to improving the quality of management of lower genital tract infections.
Conclusions
This study revealed a high prevalence of BV and vaginal candidiasis, which accounted for most of the lower genital tract infections observed, and suggests that health care providers should increase awareness of and communication regarding vaginal hygiene practices. Our findings also indicate that the most common curable STIs (TV, CT, and NG) are rare in this population, and thus, we recommend that in cases of suspected TV, CT, and NG infections, systematic laboratory diagnosis should be performed.
Acknowledgments
The authors would like to thank the investigators and their colleagues for contributing to data collection and Dr Amy K. Bei for her critical reading of the manuscript. The authors are very grateful to the Sanofi study review committee for their valuable support in shaping the methods. The authors thank Habsa Diagne Samb and Aliou Thiam, who performed the laboratory analysis, and the CEFOREP team (Thierno Dieng, Hawa Barry, Amadou Sy, Yaye Madeleine Cisse, and Pape Malick Sene) for logistic support.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The whole study including laboratory services was funded by SANOFI French sub-Saharan Africa.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MSB, SCA, EG, and ANF are full-time Sanofi employees. JCM, Head of Obstetrical and Gynaecology Department of Cheikh Anta Diop University of Dakar (UCAD), was the principal investigator. He is also the Chairman of CEFOREP, a research institution focusing on reproductive health, which conducted this study. All other authors declare that they have no competing interests.
Author Contributions: MSB designed and coordinated the study. ABD, MD and IM reviewed the study protocol and tools. ABD, IM, OG and MDNG monitored data collection and management under the supervision of AGD and JCM. MD drafted the manuscript, and all authors provided inputs for the final draft and agreed to be accountable for all aspects of the work. All authors gave final approval for publication of the manuscript.
ORCID iD: Mamadou Saidou Barry  https://orcid.org/0000-0002-9682-1887
https://orcid.org/0000-0002-9682-1887
References
- 1. World Health Organization (WHO). Guidelines for the Management of Sexually Transmitted Infections. Geneva, Switzerland: WHO; 2003. [Google Scholar]
- 2. World Health Organization (WHO). Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- 3. Ezeh A, Bankole A, Cleland J, García-Moreno C, Temmerman M, Ziraba AK. Burden of reproductive ill health. In: Black RE, Laxminarayan R, Temmerman M, Walker N, eds. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities. Vol 2, 3rd ed. Washington, DC: The World Bank; 2016:Chapter 2. [Google Scholar]
- 4. World Health Organization (WHO). Report on Global Sexually Transmitted Infection Surveillance. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 5. Seck MC, Faye B, Ndiaye M, et al. Prévalence de Trichomonas vaginalis et de Candida albicans chez les femmes au laboratoire de l’hôpital militaire de Ouakam, Dakar (Sénégal). Médecine d’Afrique Noire 2015;6201:31–37. [Google Scholar]
- 6. Ndiaye A, Saware R, Diouf M, et al. Algorithm of genital infections in women about a cohort of 626 women at Abass NDAO hospital from 2011 to 2012. Int J Curr Microbiol App Sci. 2014;3:128–144. [Google Scholar]
- 7. Arya R, Antonisamy B, Kumar S. Sample size estimation in prevalence studies. Indian J Pediatr. 2012;79:1482–1488. [DOI] [PubMed] [Google Scholar]
- 8. Gaydos CA, Van Der Pol B, Jett-Goheen M, et al. Performance of the Cepheid CT/NG Xpert Rapid PCR test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2013;51:1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fouzia R, Andreas P, Abderrahmane B, Loubna EY, Douaa B, Naima E. Urogenital mycoplasma in Moroccan population: prevalence and antibiotic susceptibility. Int J Eng Technol. 2014;4:415–421. [Google Scholar]
- 10. Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505–523. [DOI] [PubMed] [Google Scholar]
- 11. Balkus JE, Srinivasan S, Anzala O, et al. Impact of periodic presumptive treatment for bacterial vaginosis on the vaginal microbiome among women participating in the preventing vaginal infections trial. J Infect Dis. 2017;215:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Schalkwyk J, Yudin MH. Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Can. 2015;37:266–274. [DOI] [PubMed] [Google Scholar]
- 13. Konate A, Yavo W, Kassi FK, et al. Aetiologies and contributing factors of vulvovaginal candidiasis in Abidjan (Cote d’Ivoire). J Mycol Med. 2014;24:93–99. [DOI] [PubMed] [Google Scholar]
- 14. Ogouyemi-Hounto A, Adisso S, Djamal J, et al. Place of vulvovaginal candidiasis in the lower genital tract infections and associated risk factors among women in Benin. J Mycol Med. 2014;24:100–105. [DOI] [PubMed] [Google Scholar]
- 15. Johnson LF, Dorrington RE, Bradshaw D, Coetzee DJ. The effect of syndromic management interventions on the prevalence of sexually transmitted infections in South Africa. Sex Reprod Healthc. 2011;2:13–20. [DOI] [PubMed] [Google Scholar]
- 16. Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis. 2013;26:231–240. [DOI] [PubMed] [Google Scholar]
- 17. Sleha R, Boštíková V, Hampl R, et al. Prevalence of Mycoplasma hominis and Ureaplasma urealyticum in women undergoing an initial infertility evaluation. Epidemiol Mikrobiol Imunol. 2016;65:232–237. [PubMed] [Google Scholar]
- 18. Zeng XY, Xin N, Tong XN, Wang JY, Liu ZW. Prevalence and antibiotic susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Xi’an, China. Eur J Clin Microbiol Infect Dis. 2016;35:1941–1947. [DOI] [PubMed] [Google Scholar]
- 19. Francis SC, Ao TT, Vanobberghen FM, et al. Epidemiology of curable sexually transmitted infections among women at increased risk for HIV in northwestern Tanzania: inadequacy of syndromic management. PLoS ONE. 2014;9:e101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ginindza TG, Stefan CD, Tsoka-Gwegweni JM, et al. Prevalence and risk factors associated with sexually transmitted infections (STIs) among women of reproductive age in Swaziland. Infect Agent Cancer. 2017;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fashemi B, Delaney ML, Onderdonk AB, Fichorova RN. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24:19703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yanikkerem E, Yasayan A. Vaginal douching practice: frequency, associated factors and relationship with vulvovaginal symptoms. J Pak Med Assoc. 2016;66:387–392. [PubMed] [Google Scholar]
- 23. Bautista CT, Wurapa E, Sateren WB, Morris S, Hollingsworth B, Sanchez JL. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil Med Res. 2016;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wabiri N, Taffa N. Socio-economic inequality and HIV in South Africa. BMC Public Health. 2013;13:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]