Abstract
Case summary
A 3-year-7-month-old female neutered domestic shorthair cat was presented for further investigation of acute-onset neurological abnormalities, including marked decreased mentation, ataxia and abnormal cranial nerve responses, with concurrent marked pyrexia (40ºC). Initial blood testing was non-specific with mild-to-moderate increases in alanine aminotransferase (ALT) (194 IU/l; reference interval [RI] 17–62 IU/l), aspartate aminotransferase (AST; 150 IU/l [RI 0–51 IU/l]) and total bilirubin (20 µmol/l; RI 0–11 µmol/l), and neutropenia (1.17 ×109/l; RI 2.5–12.5 ×109/l). Brain MRI and cerebrospinal fluid analysis were unremarkable and Toxoplasma serology was negative. Worsening of hepatic biochemical parameters (ALT 265 IU/l, AST 205 IU/l, total bilirubin 42.9 µmol/l) led to further investigations for liver disease, including ultrasound, fine-needle aspirate cytology, histology, fluorescent in situ hybridisation and culture of liver tissue and bile, resulting in a diagnosis of Yersinia pseudotuberculosis hepatitis. The cat was treated with a combination of potentiated amoxicillin (62.5 mg PO q12h), marbofloxacin (5mg PO q24h) and combined s-adenosyl methionine (SAMe)/silybin (90 mg PO q24h), and made a full recovery. Follow-up over 14 months identified a persistent mild increase in ALT, despite no apparent ongoing disease.
Relevance and novel information
Yersinia pseudotuberculosis hepatitis should be considered as a differential diagnosis in cats presenting with acute-onset neurological signs, and, when diagnosed, can be successfully treated with a combination of marbofloxacin, potentiated amoxicillin and SAMe/silybin. This is the first such case treated successfully with licensed veterinary antimicrobials and the first instance where Y pseudotuberculosis hepatitis has presented with primarily neurological clinical signs.
Keywords: Yersiniosis, Yersinia pseudotuberculosis, hepatitis
Case description
A 3-year-7-month old female neutered domestic shorthair cat presented to the Queen’s Veterinary School Hospital with a 2 day history of acute onset progressive ataxia, dullness and reduced appetite. Prior to presentation it had no medical history, had never been outside of the UK, was vaccinated against feline panleukopenia virus, feline herpes virus, feline calicivirus and feline leukaemia virus (FeLV), and received regular preventive antiparasitic medications (praziquantel and moxidectin/imidacloprid). No toxin exposure was reported; however, it was a mixed indoor/outdoor cat with a known history of hunting. No other clinical signs were reported by the owner at the time of presentation or in the preceding period.
On clinical examination the cat was depressed and poorly responsive. It weighed 2.97 kg, with a body condition score of 3/9. It was pyrexic (rectal temperature 40.0ºC) with a normal heart rate (184 beats per min), with a normal respiratory rate (24 breaths per min). Oral examination, thoracic auscultation and abdominal palpation were unremarkable. No detectable peripheral lymphadenopathy was present, and no detectable changes in mucous membrane moistness or skin elasticity were noted to indicate significant dehydration. Direct ophthalmoscopic examination was unremarkable. Neurological examination was performed, which identified reduced mentation, absent menace response bilaterally, absent response to nasal planum stimulation and ataxia on all four limbs. The cat appeared visual, with normal pupillary light reflexes and response to moving objects. It had a wide-based stance with truncal sway. The remainder of the cranial nerve reflexes and the spinal reflexes and proprioceptive responses were all within normal limits.
Biochemistry revealed moderate increases in alanine aminotransferase (ALT) (194 IU/l; reference interval [RI] 17–62 IU/l), aspartate aminotransferase (AST; 150 IU/l [RI 0–51 IU/l]) and total bilirubin (20 µmol/l [RI 0–11µmol/l]). Alkaline phosphatase and gamma-glutamyl transferase were below the detectable limit on the assay. A mild hyperglycaemia (9.8 mmol/l [RI 3.9–5.8 mmol/l]) was reported; however, the remainder of the biochemistry, including urea, albumin and globulin, was unremarkable. Haematology identified a marked leukopenia (1.83 ×109 /l [RI 5.5–19.5 ×109 /l]) with neutropenia (1.17 ×109 /l [RI 2.5–12.5]) with left shift and toxic change and lymphopenia (0.4 ×109 /l [RI 1.5–7.0×109 /l]). All other white cell, red blood cell (RBC) and platelet parameters were within the RIs.
ELISA testing for feline immunodeficiency virus/FeLV was negative. Serology for toxoplasmosis (IgM and IgG) was performed on a single sample and was negative at 1:20 and 1:200, respectively. Urinalysis by cystocentesis identified an increase in urine protein:creatinine ratio (0.94 [RI 0.0–0.2]) and mild increase in RBC numbers 7/high-power field (RI 0–5). On urine dipstick, 3+ bilirubin was present. Urine specific gravity by refractometry was 1.023. Urine culture was negative. MRI of the brain was undertaken and identified no abnormalities. Cerebrospinal fluid (CSF) tap found an increased specific gravity 1.008 (RI 1.004–1.006) but was otherwise unremarkable, with no increased cellularity or protein. Toxoplasma PCR was performed on the CSF sample and was negative. MRI and CSF tap were performed under general anaesthetic, during which intravenous fluid therapy was provided and monitoring by a diploma-holding anaesthetist was undertaken.
The cat initially recovered well from anaesthesia; however, it showed a marked deterioration over the subsequent 24 h, after which time the biochemistry and haematology were repeated. This identified further moderate increases in ALT (265 IU/l), AST (205 IU/l) and total bilirubin (42.9 µmol/l). Abdominal ultrasound was performed under sedation with dexmedetomidine and methadone, and identified no abnormalities, with normal size and echotexture of the liver and no abdominal lymphadenomegaly. The pancreas and biliary tree were reported to be unremarkable. Cytology of the liver was performed by fine-needle aspiration and showed a marked, mixed, predominantly neutrophilic bacterial inflammation with intra- and extracellular rod-shaped bacteria. Smaller numbers of macrophages were also reported, potentially suggestive of a more chronic course.
Minimally invasive laparotomy using a small incision and ring retractor (Figure 1) was subsequently performed under general anaesthetic to acquire hepatic biopsies and bile aspirate. Three liver samples from different areas were taken into formalin and two samples were taken for fresh submission. The cat was placed on intravenous fluid therapy for the duration of the procedure and for the subsequent 3 days until adequate voluntary food intake was achieved and hydration status considered to be normal. During the postoperative period the cat received opioid analgesia (initially methadone 0.3 mg/kg IV q4h, reducing to buprenorphine 0.02 mg/kg q6h after 24 h). Coagulation testing prior to surgery would have been desirable; however, this was overlooked in the face of the marked deterioration in the cat’s clinical status and the rapid progression to biopsy.
Figure 1.
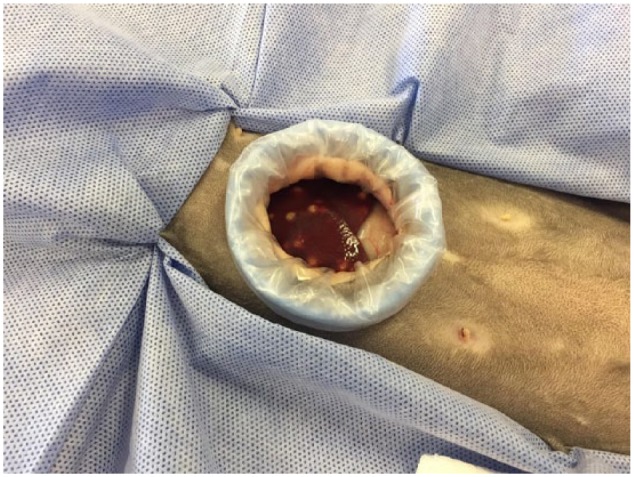
Minimally invasive midline approach. Multiple suspected abscesses present on the visible surface of the liver and palpable throughout the liver parenchyma
The gross appearance of the liver was suggestive of multifocal abscessation (Figure 1.) After sample acquisition the cat was placed on potentiated amoxicillin (20 mg/kg IV q8h) as broad-spectrum empiric treatment for a suspected bacterial hepatitis. Histology identified macroscopic focal lesions roughly 1–2 mm in diameter on the sample and cut surfaces. Microscopic examination identified severe (60% of assessed area) necrotising and pyogranulomatous hepatitis (Figure 2) with intralesional gram-negative bacterial microcolonies (rods). Toxoplasma PCR on liver tissue was negative. Samples were submitted to the reference laboratory for culture of the bile and fresh hepatic tissue. These were both positive for Yersinia pseudotuberculosis. Fluorescent in situ hybridisation for eubacteria, and including Salmonella, Campylobacter, Helicobacter and Leptospira species, was negative for all other bacteria.
Figure 2.
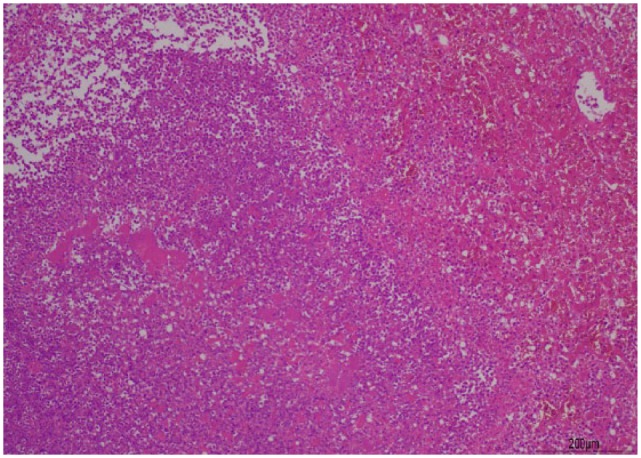
Hepatic parenchyma exhibits severe pyogranulomatous and necrotising hepatitis. Haematoxylin and eosin stain. Scale bar indicates 200 µm. Courtesy of Dr Kate Hughes
The cultured Yersinia pseudotuberculosis was tested for sensitivity by disc diffusion and was found to be sensitive to amoxicillin, marbofloxacin and oxytetracycline (resistant to cephalexin, metronidazole and clindamycin). On the basis that Yersinia species are not typically sensitive to amoxicillin, and that previous reported cases had responded poorly to therapy, additional treatment with marbofloxacin was instituted (1.7 mg/kg IV q24h) alongside the potentiated amoxicillin. The cat was also started on combined s-adenosyl methionine (SAMe) and silybin (Denamarin; Protexin, 30 mg/kg PO q24h). The cat remained hospitalised for a further 6 days during which time it showed rapid improvement in all clinical signs, with return to partial voluntary calorie intake within 24 h, and subsequent return to full voluntary food intake within 3 days. Fluid therapy was continued at maintenance rates throughout this period. Pyrexia resolved within 48 h of institution of antimicrobial treatment. Intravenous medications were transitioned to orally administered enteral formulations 1 day prior to discharge.
Biochemistry was repeated at the time of discharge, at which point all clinical signs had resolved. This revealed a reduced ALT (201 IU/l), AST (50 IU/l) and total bilirubin (11.1 µmol/l). Haematology identified a moderate, left-shifted neutrophilia (22.03 ×109 /l [RI 2.5–12.5 ×109 /l]) that was considered likely due to postoperative inflammation. The remaining haematological parameters were unremarkable. Clinical and neurological examinations were repeated daily during hospitalisation and showed gradual improvement, and at the time of discharge the neurological abnormalities present at presentation had resolved in their entirety.
The cat remained on treatment with both potentiated amoxicillin (21 mg/kg PO q12h) and marbofloxacin (1.66 mg/kg PO q24h) for an initial 4 week course. At the time of the 4 week recheck, the ALT level was found to have increased mildly; therefore, these medication courses were extended for a further 4 weeks to a total of 8 weeks as a precaution. This decision was driven heavily by the reported poor prognosis in previous cases of hepatic yersiniosis. At the time of the recheck the cat was otherwise well and no other biochemical or haematological changes were found. After this extension, the marbofloxacin was withdrawn and the potentiated amoxicillin maintained for a further 4 weeks before this and the SAMe/silybin were withdrawn. The cat remained clinically well, with no changes in biochemical parameters and therefore it was considered that no further treatment was required.
The cat was seen for multiple rechecks over the following 14 months. At all appointments it was reported to be clinically normal, and clinical and neurological examinations identified no abnormalities. Biochemistry was monitored on each occasion (Table 1). All haematological parameters remained within normal limits from the time of discharge. The cat remains alive and free of clinical signs at 23 months post-diagnosis.
Table 1.
Serial measurement of hepatic biochemical parameters
| Day (post-treatment) | 0 | 6 | 16 | 30 | 63 | 88 | 105 | 182 | 425 | RI |
|---|---|---|---|---|---|---|---|---|---|---|
| ALT (IU/l) | 265 | 201 | 130 | 158 | 86 | 102 | 68 | 80 | 64 | 17–62 |
| AST (IU/l) | 205 | 50 | 34 | – | 18 | – | – | 32 | 23 | 0–51 |
| Total bilirubin (µmol/l) | 42.9 | 11.1 | 6.0 | – | 1.6 | – | – | 2.4 | 1.9 | 0–11 |
Day 0 represents day on which antibiotic treatment was initiated
RI = reference interval; ALT = alanine aminotransferase; AST = aspartate aminotransferase
Discussion
Yersinia pseudotuberculosis is a gram-negative, motile coccobacillus that is most commonly considered to be a cause of enteritis in people and many species of domestic animal. Spread of the bacteria is thought to be by the faecal–oral route, either as a consequence of faecally contaminated food or as a result of ingestion of other infected small mammals, such as mice and rodents, which have been suggested as possible reservoir hosts.1,2 Yersinia species are atypical in that they can continue to replicate at refrigerated temperatures but are quickly killed by heating at temperatures of ⩾60ºC.3
Yersiniosis in cats has, in the past, been a relatively common disease. Between 1920 and 1955, over 70 cases were documented of feline infections in France, Germany, former Yugoslavia and Hungary.4 The first reported case in the UK was reported in 1967, after which there have been only nine cases published anywhere within the veterinary literature. Of these, six were reported to have had liver involvement, with only one cat surviving treatment. This particular case was reported in 1979 after presenting only with non-specific signs of vomiting and malaise, and was treated with hetacillin, an antibiotic no longer available for use.5 The most recent report is of a Persian cat with hepatic yersiniosis, published in 1991, which did not survive despite antibiotic therapy.2
Reported presenting signs in previous cases of hepatic Y pseudotuberculosis have included lethargy (n = 4), reduced appetite (n = 3), abdominal pain (n = 3), pyrexia (n = 2), jaundice (n = 2), vomiting (n = 1) and diarrhoea (n = 1).2,4–8
In contrast, this cat was presented with primarily neurological signs, including reduced-to-absent menace responses and nasal sensation, marked reduction in mentation and marked ataxia of all four limbs. These signs were considered consistent predominantly with a diffuse forebrain localisation; however, the presence of a truncal sway may have indicated multifocal disease within the brain. Initial blood testing also revealed only mild-to-moderate increases in hepatic parameters and therefore differential diagnoses at the time of presentation were considered to be most likely intracranial. The presence of the marked pyrexia led to primary differential diagnoses being infectious (feline infectious peritonitis, toxoplasmosis, cryptococcosis) or an inflammatory/immune-mediated brain disease, and therefore subsequent investigations by MRI scanning and CSF analysis were prioritised in the first instance. Only after negative findings on these tests and a progressive worsening in hepatic parameters on biochemistry was liver disease elevated to the primary differential diagnosis. No abnormalities were noted on abdominal ultrasound, even after re-examination of the initial imaging after diagnosis. This may be due to a combination of the small nature of the lesions and the inherent lack of ultrasound sensitivity for lesions of this size.
Based on the absence of other findings on diagnostic testing, it is considered possible that the neurological signs were as a result of hepatic encephalopathy and pyrexia; however, bile acid stimulation testing was not indicated owing to the presence of icterus, and accurate measurement of ammonia levels was not locally available. No evidence of haematogenous spread of the bacteria to the brain could be identified on CSF cytology; however, this possibility cannot be entirely excluded. Bacterial culture was not performed at the time of sampling owing to the absence of visible bacteria or nucleated cells; however, in retrospect this would have provided further certainty. Other possible causes of the neurological signs may have included undetected intra-cranial infectious or inflammatory diseases that were not detected on the MRI scan, CSF analysis or other testing.
The disease reservoir for Y pseudotuberculosis is considered most likely to be birds and small rodents.1 Although cats have been suggested as another possible enteric reservoir, this cat was a known hunter and therefore it is considered most likely that the disease was acquired this way. Spread of the pathogen from the gastrointestinal tract to the liver may have occurred as an ascending biliary infection (bacteria were also successfully cultured from the bile) or by translocation across the intestinal epithelium followed by haematogenous spread. The locations of the microcolonies seen on histology are reported to be random and therefore no inference regarding route of transmission can be made.
Of the six previously reported cats with Y pseudotuberculosis hepatitis, only one cat is reported to have been successfully treated.5 This cat was treated with hetacillin (110 mg PO q12h) for 20 days and is reported to have made a full recovery at the time of final follow-up after 3 months; however, no longer term follow-up was provided and no repeat blood sampling undertaken. Hetacillin is, however, not a licensed veterinary product in the UK and therefore not available for use. In view of this, in this cat, initial empiric treatment was instituted with an alternative penicillin derivative, to which, after testing, the bacteria was found to be sensitive. Marbofloxacin was added on the basis that culture and sensitivity identified this as a potentially effective treatment, and in view of the severity of the cat’s presentation, the speed of progression and particularly the apparent poor prognosis for this condition in the literature in some cases treated with beta-lactams, further antibiosis beyond that of this class was desirable.2,4,8 Discontinuation of the potentiated amoxicillin at the time of institution of the marbofloxacin was considered; however, in view of the initial apparent response to amoxicillin it was felt that its removal constituted an unnecessary risk to the patient. This was further supported by the absence of any prior confirmed efficacy of marbofloxacin in this condition in the available veterinary literature.
Conclusions
This case report represents the only described case of feline Y pseudotuberculosis hepatitis presenting initially with neurological clinical signs, alongside the first published long-term follow-up of such a case and the first record of serial biochemistry during and after treatment. It also represents the only reported case of successful treatment of Y pseudotuberculosis hepatitis in a cat with currently available licensed veterinary antimicrobials based on culture and sensitivity testing. Y pseudotuberculosis hepatitis should be considered as a differential diagnosis in cats presenting with acute-onset neurological signs, and, when diagnosed, can be successfully treated with a combination of marbofloxacin, potentiated amoxicillin and SAMe/silybin.
Footnotes
Accepted: 8 May 2019
Conflict of interest: The author declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This study involved the use of client-owned animal(s) only, and followed internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was not therefore needed.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal guardian of all animal(s) described in this study for the procedure(s) undertaken. For any animals or humans individually identifiable within this publication, informed consent for their use in the publication (verbal or written) was obtained from the people involved.
References
- 1. Hirsh DC. Enterobacteriaceae: Yersinia. In: Hirsh DC, Maclachlan NJ, Walker RL. (eds). Veterinary microbiology. 2nd ed. Oxford: Blackwell Publishing, 2004, pp 77–79. [Google Scholar]
- 2. Iannbelli F, Caruso A, Castellucio A, et al. Yersinia pseudotuberculosis in a Persian cat. Vet Rec 1991; 129: 103–104. [DOI] [PubMed] [Google Scholar]
- 3. Greene CE. Yersiniosis. In Green CE. (ed). Infectious diseases of the dog and cat. 4th ed. St Louis, MO: Elsevier Saunders, 2012, p 390. [Google Scholar]
- 4. Mair NS, Harbourne JF, Greenwood MT, et al. Pasteurella pseudotuberculosis infection in the cat: two cases. Vet Rec 1967; 81: pp 461–462. [Google Scholar]
- 5. Spearman JG, Hunt P, Nayar PSG. Yersinia pseudotuberculosis infection in a cat. Can Vet J 1979; 20: 361–364. [PMC free article] [PubMed] [Google Scholar]
- 6. Obwolo MJ, Gruffydd-Jones TJ. Yersinia pseudotuberculosis in the cat. Vet Rec 1977; 100: 424–425. [DOI] [PubMed] [Google Scholar]
- 7. Allard AW. Yersinia pseudotuberculosis in a cat. J Am Vet Med Assoc 1979; 174: 91–92. [PubMed] [Google Scholar]
- 8. Robinson M. Pasteurella pseudotuberculosis infection in the cat. Vet Rec 1972; 91: 676–677. [DOI] [PubMed] [Google Scholar]


