Abstract
Objectives:
Increasing interests have been focused on using artificial intelligence (AI) to extend prognostic value of medical imaging. Feature extraction is a critical step for successful application of AI. The aim of this study was to explore several metabolic parameters measured by 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) as potential AI features in predicting the effectiveness of chemotherapy in patients with non-small cell lung cancer (NSCLC).
Methods:
A set of metabolic parameters of PET/CT and clinical characteristics were detected from 137 patients with NSCLC treated with at least 1 cycle of chemotherapy. Survival receiver–operating characteristic (ROC) analysis was used to define the more significant parameters chosen for the following survival analysis. Patient survival was analyzed by Kaplan-Meier method, log-rank test, and Cox regression.
Results:
Survival ROC showed that maximum standardized uptake value (SUVmax), metabolic tumor volume 50% (MTV50), and total lesion glycolysis 50% (TLG50) had larger area under the curve, and the optimal cutoff values were 11.72, 4.04, and 34.55, respectively. Univariate and multivariate analyses synergistically showed that late PET/CT stage and MTV50 >4.04 were independent factors of poor survival in patients with NSCLC who received chemotherapy.
Conclusions:
Several potential prognostic biomarkers of PET/CT imaging have been extracted for predicting survival and selecting patients with NSCLC who are more likely to benefit from chemotherapy. The identification may accelerate the development of AI methods to improve treatment outcome for NSCLC.
Keywords: cellular imaging and trafficking, quantitation in molecular imaging, cancer treatment efficacy, cancer imaging, cellular imaging, biomarker
Introduction
Lung cancer remains the number 1 cause of cancer-related mortality, and its prevalence continues to increase worldwide,1 the majority of which remains to be non-small cell lung cancer (NSCLC). Surgery is the main curative treatment for NSCLC and is generally accepted as the treatment of choice for early-stage patients. However, due to many reasons, a large number of patients with lung cancer have lost the opportunity for surgery or are not suitable for radiotherapy, targeted therapy, and immunotherapy.2–4 Therefore, conventional cytotoxic chemotherapy (CCC) remains the backbone treatment for patients with NSCLC and represents a key element of the therapeutic armamentarium, particularly in the adjuvant setting.5 Predictive biomarkers of CCC able to identify chemosensitive patients and select appropriate drug combinations are crucially lacking. Such biomarkers would also be helpful to limit toxicities, decrease overall costs, and hopefully improve patient outcome. A variety of biomarkers including genomics, proteomics, metabonomics, and image biomarkers have been evaluated either in the preclinical or in clinical setting.6-8 However, despite all efforts to identify predictive biomarkers of sensitivity to CCC in NSCLC, results have been disappointing, and a strikingly high attrition rate between promising preclinical data and negative clinical results has been observed. Several biomarkers are still in the race, but none is ready yet for clinical practice implementation.
The field of “radiomics” is a burgeoning step toward personalized medicine, focusing on the relationship between quantitative biological features and cancer prognosis by noninvasive method, therefore aiding clinicians in selecting the appropriate treatments. Comprehensive phenotypic characteristics with valuable clinical meaning can be extracted from radiological images by postprocessing techniques. Thus, making artificial intelligence (AI)-aided diagnosis and prognosis become possible and attract more and more eyesight. The AI techniques, such as machine/deep learning, can be used to recognize a wide range of patterns within medical imaging, as they can take account of each pixel, and their relationship, as well as associated clinical metadata. Machine learning models can be trained to “learn” what different features in an image represent so that they can be used to identify images, quantify areas of interest or be associated with particular disease patterns.9 By combining clinician interpretation with information derived from machine learning algorithms, there is the opportunity to enhance the accuracy through a reduction in inter- and intra-operator variability as well as providing additional predictive information that may be too subtle to be detected by the human eye.10,11 Comprehensive phenotypic feature is the key point of AI. Evidences has been accumulated in recent years suggesting that quantitative image descriptors may yield additional predictive and prognostic information, which could be potentially served as noninvasive prognostic biomarkers for individual disease prognosis.12,13 It indicates that easily obtainable noninvasive pretherapy imaging prognostic biomarkers that allow assessment of NSCLC are worth to study.14,15
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)imaging is based on tumor glucose metabolism and serves as a marker of tumor metabolic activity, such as cell viability and proliferative activity. Combining 18F-FDG PET with /computed tomography (CT) provides useful functional and anatomic information and enables more accurate evaluation of initial staging, monitoring of treatment response, and post-treatment surveillance of patients with different cancers. The maximum standardized uptake value (SUVmax), as an estimate of tumor metabolic activity, is the most commonly used parameter in 18F-FDG PET/CT. Recently, metabolic tumor volume (MTV) and total lesion glycolysis (TLG), combining the tumor volume and metabolic activity of the entire tumor, have been introduced as prognostic biomarkers for various solid malignancies, including head and neck cancers, gastric cancers as well as lung cancers.16-18 However, little is known about whether metabolic parameters derived from 18F-FDG PET/CT could predict chemosensitivity. So this study was to investigate their roles in predicting the effectiveness of chemotherapy in patients with NSCLC. We tried to extract more accurate quantitative features and answer the question whether metabolic parameters derived from 18F-FDG PET/CT could predict chemosensitivity and select which kind of patients may be suitable and achieve more benefit from chemotherapy rather than suffer from the adverse events only.
Materials and Methods
Patient Selection
Included in this study were 137 patients who received whole-body 18F-FDG PET/CT scan and were historically approved to be NSCLC through surgery or aspiration biopsy at our hospital between January 2012 and December 2013. All patients were PET/CT staged according to the guidelines of the National Comprehensive Cancer Network and treated with at least 1 cycle of platinum-based chemotherapy, including chemotherapy after surgery and first-line or second-line chemotherapy without operation. Tumor and morphological classifications were performed according to the World Health Organization recommendations. Patients with squamous cell carcinoma, adenocarcinoma, adenosquamous, large cell carcinoma, and poorly differentiated NSCLC were eligible for inclusion, while small cell lung cancer, sarcoma, neuroendocrine carcinoma and those patients who had undergone neoadjuvant therapy before PET/CT examination, died from other diseases, or lost follow-up were excluded. Clinical data and survival information were obtained from hospital medical records, outpatient visits, and telephone follow-ups. Clinical characters included gender, age, smoking status, histology type and differentiation degree, surgery status, chemotherapy regimen, cycles and side reaction of chemotherapy, and level of serum tumor markers, which included squamous cell carcinoma antigen (SCC), carcinoembryonic antigen (CEA), and neuron-specific enolase (NSE).
The median duration of clinical follow-up was 22.9 months (ranging from 2.3 to 67.9 months). During the follow-up period, patients were clinically assessed every 3 months the first year after the initial treatment (surgery or chemotherapy) by means of imaging protocols such as CT, magnetic resonance imaging and X-ray, and laboratory examinations including associating serum tumor markers, then every 6 months the following years. If the clinical assessment or follow-up studies showed an abnormal finding, additional diagnostic studies and biopsy with histopathologic confirmation were performed to evaluate for recurrence. As the objective of this study was to evaluate correlations between metabolic parameters measured by 18F-FDG PET/CT and the effectiveness of chemotherapy, progression-free survival (PFS) and overall survival (OS) were therefore used as the outcome measures in this study. Progression-free survival was defined as the time between patients receiving initial treatment and discovery of progression, recurrence, or occurrence of death of any cause. Overall survival was referred to the time from patients receiving initial treatment to death due to any cause.
The study design and procedure were approved by the ethics committee of our hospital. All patients had provided oral consent forms for the use of their medical data. We could not obtain written informed consent from all participants, as this was a retrospective study and the majority of the patients had been discharged from hospital at the time of analysis. The oral informed consent was documented in the electronic or paper patient file and approved by the local ethics committee. We collected and analyzed the data anonymously, and no results were ever connected to their identities.
Acquisition of PET/CT
All patients fasted for 4 to 6 hours. Blood glucose levels were checked in the peripheral blood (<150 mg/dL was considered normal) before PET/CT examination. The PET/CT images were obtained using an integrated PET/CT scanner (Discovery ST: GE Medical systems, Milwaukee, Wisconsin) for 60 minutes after intravenous administration of 18F-FDG (5.55-7.40 MBq/kg). The scan range started at the mid-thighs and proceeded to the head. A whole-body unenhanced CT scan was performed using the following parameters: 140 kV, 150 mA, 0.8 s/rotation, 22.5 mm/s table speed, and slice thickness of 3.75 mm. Data from the CT scans were reconstructed from a 512 × 512 matrix to a 128 × 128 matrix to satisfactorily match the PET data and allow image fusion. The PET scan was carried out in the same position for all patients and using the 2-dimensional imaging mode. The PET image data sets were reconstructed using an iterative algorithm (the ordered subsets expectation maximization). The emission scan was obtained at 3 minutes per bed position, and 6 to 7 bed positions were generally performed for all patients.
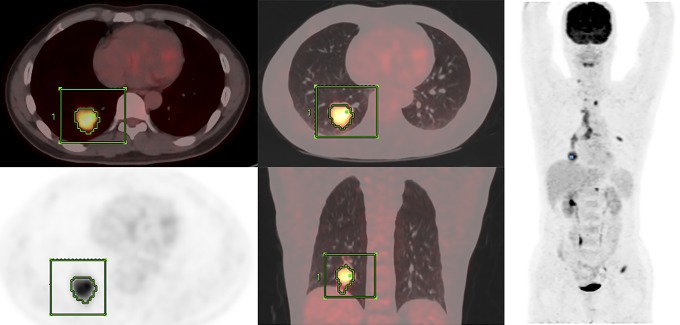
The 18F-FDG PET/CT images were assessed by 2 experienced nuclear medicine physicians with PET/CT imaging experience as well as familiarity with PET-VCAR software and our picture archiving and communication system. All images were displayed and analyzed on a workstation (Xeleris; GE Medical Systems). After identification of the tumors, parameters were measured from the attenuation-corrected torso 18F-FDG PET/CT images and calculated semiautomatically using PET-VCAR. Each tumor was examined with a spheric-shaped volume of interest (VOI) that included the entire lesion in the axial, sagittal, and coronal planes. The SUV of the VOI was calculated as (decay-corrected activity/tissue volume)/(injected dose/body weight). Metabolic tumor volume was defined as total tumor volume with a threshold of SUV, and TLG was calculated as (mean SUV) × (MTV). As for the thresholds, we used 30%, 40%, and 50% of the tumoral SUVmax and SUV2.5. In our study, the output results included SUVmax, SUVmean, SUVpeak, MTV of the above-mentioned thresholds, and corresponding TLG of the tumor (Figure 1).
Figure 1.
A 47-year-old male patient with adenocarcinoma in right lung whose PET/CT stage was IV. The PFS was 6.97 months and the OS was 11.77 months. A volume of interest around the primary lung tumor lesion in 18F-FDG PET/CT images is drawn with an isocontour standard uptake value threshold method to measure MTV and TLG. CT indicates computed tomography; MTV, metabolic tumor volume; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; TLG, total lesion glycolysis; 18F-FDG, 18F-fluorodeoxyglucose.
Statistical Analysis
The results of 18F-PET/CT and survival time were displayed as continuous variables and clinical data as disperse variables. Survival receiver–operating characteristic curve analysis (survival ROC) was used to achieve the maximal area under the curve (AUC) and the optimal cutoff value (refers to the maximal sum of sensitivity and specificity) of each PET/CT parameter. Both PFS and OS were analyzed as end point of survival, and the more significant one was chosen for the following analysis. The PET/CT parameters were divided into high-risk group and low-risk group according to the cutoff value derived from the survival ROC. Univariate analysis of prognostic factors for PFS and OS was achieved using the Kaplan-Meier method, and the log-rank test was used to evaluate the significance of the differences between the survival curves, the Cox proportional hazards model that included significant univariate variables was used to determine independent prognostic factors for PFS and OS in multivariate survival analyses. Risk of death was estimated on the basis of hazard ratios and the 95% confidence interval and was recorded. Age, type of histology, differentiate degree, PET/CT staging, serum SCC, CEA and NSE level, and 18F-FDG PET/CT-derived parameters were used for univariate and multivariate prediction of OS and PFS. Variables with a P value <.05 on univariate analysis were selected for multivariate analysis. To evaluate multicollinearity between PET/CT parameters and the relationship between clinicopathological variables, Spearman rank correlation coefficient was calculated.
Two-sided P <.05 was considered statistically significant. All analyses were performed using R X64 (version 3.4.3).
Results
Clinical Characteristics of the Patients
Enrolled in this study were 85 males and 52 females with an average age of 58.58 ± 9.67 years (ranging from 31 to 79 years). The main histological subtypes were squamous cell carcinoma and adenocarcinoma. Of the 137 patients, 51 patients (37.2%) received kinds of lung surgeries or metastatic lesions surgeries; 7 patients (5.1%) were staged as I, 18 patients (13.1%) as II, 40 patients (29.2%) as III, and 72 patients as IV. The 7 patients staged as I all received lobectomy plus mediastinal lymph node dissection. Postoperative pathology indicated that 3 patients had vascular invasion and 4 patients had visceral pleura involvement. All these 7 patients received chemotherapy because of high-risk factors. Ninety-six (70.1%) patients finished at least 4 cycles of chemotherapy, and 58 (42.3%) patients experienced grades 2 to 4 adverse events during the therapy processes. The main chemotherapy regimen included pemetrexed (500 mg/m2, every 21 days), paclitaxel (175 mg/m2, day 1), gemcitabine (1250 mg/m2, days 1 and 8), and docetaxel (75 mg/m2, day 1), combined with or without cisplatin (75 mg/m2, day 1)/carboplatin (area under the plasma concentration time curve 5, day 1). Of the 137 patients, 78 (56.9%) patients experienced regimen alteration, 70 (51.1%) patients experienced adjuvant radiotherapy, and 37 (27.0%) patients underwent molecule-targeted therapy. A full description of the patient characteristics is provided in Table 1.
Table 1.
Clinicopathological Characteristics of the Studied Patients.
| N = 137 | ||
|---|---|---|
| Characteristics | n | % |
| Gender | ||
| Male | 85 | 62.0 |
| Female | 52 | 38.0 |
| Age | ||
| ≤58 | 66 | 48.2 |
| >58 | 71 | 51.8 |
| Smoking status | ||
| No | 76 | 55.5 |
| Yes | 61 | 44.5 |
| PET/CT stage | ||
| I and II | 25 | 18.2 |
| III | 40 | 29.2 |
| IV | 72 | 52.6 |
| Histology | ||
| Adenocarcinoma | 90 | 65.7 |
| Squamous cell carcinoma | 29 | 21.2 |
| Poorly differentiated NSCLC | 15 | 10.9 |
| Others | 3 | 2.2 |
| Differentiation degree | ||
| Well-differentiated | 4 | 2.9 |
| Moderately differentiated | 10 | 7.3 |
| Poorly differentiated | 37 | 27.0 |
| Not checked | 86 | 62.8 |
| Type of surgery | ||
| Lobectomy | 28 | 20.4 |
| Pneumonectomy | 3 | 2.2 |
| Others | 6 | 4.4 |
| No surgery | 100 | 73.0 |
| Chemotherapy regimen | ||
| PMX-based | 58 | 42.3 |
| PTX-based | 35 | 25.5 |
| GEMZ-based | 19 | 13.9 |
| TXT-based | 16 | 11.7 |
| Others | 9 | 6.6 |
| Adjuvant radiotherapy | ||
| No | 67 | 48.9 |
| Yes | 70 | 51.1 |
| Targeted therapy | ||
| No | 100 | 73.0 |
| Yes | 37 | 27.0 |
Abbreviations: CT, computed tomography; GEMZ, gemcitabine; NSCLC, non-small cell lung cancer; PET, positron emission tomography; PMX, pemetrexed; PTX, paclitaxel; TXT, docetaxel.
Survival ROC Analysis
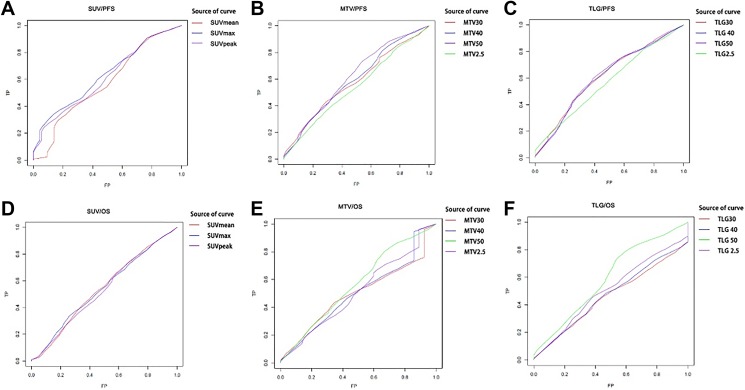
The AUC of each metabolic parameter was observed to define which one was better related to PFS and OS. The optimal cutoff value was determined using the value representing the maximal AUC and maximal sum of sensitivity and specificity. All results are shown in Table 2 and Figure 2. As the results shown, the AUC of each parameter was significantly greater when PFS was used as the end point than that of OS; therefore, the corresponding optimal cutoff value of each parameter was chosen for next analyses. As for the groups of SUVs, MTVs, and TLGs, SUVmax, MTV50, and TLG50 had the highest AUC (0.634, 0.617, and 0.616 respectively); therefore, we used the 50% of SUVmax showing the lowest P value and the highest AUC as the optimal fixed threshold for MTV.
Table 2.
AUC and Optimal Cutoff Value of Each Metabolic Parameter.
| OS | PFS | |||||
|---|---|---|---|---|---|---|
| Survival Endpoint | Predict Time | AUC | Optimal Cutoff Value | Predict Time | AUC | Optimal Cutoff Value |
| SUVmax | 67.93 | 0.540 | 11.72 | 54.33 | 0.634 | 11.72 |
| SUVmean | 67.93 | 0.537 | 3.9 | 54.33 | 0.575 | 7.92 |
| SUVpeak | 67.93 | 0.530 | 6.62 | 54.33 | 0.615 | 17.13 |
| MTV30 | 67.93 | 0.498 | 21.38 | 54.33 | 0.577 | 21.38 |
| MTV40 | 67.93 | 0.503 | 2.48 | 54.33 | 0.591 | 4.56 |
| MTV50 | 67.93 | 0.566 | 1.13 | 54.33 | 0.617 | 4.04 |
| MTV2.5 | 67.93 | 0.510 | 7.87 | 54.33 | 0.543 | 31.29 |
| TLG30 | 67.93 | 0.459 | 319.22 | 54.33 | 0.612 | 82.28 |
| TLG40 | 67.93 | 0.470 | 96.03 | 54.33 | 0.608 | 58.58 |
| TLG50 | 67.93 | 0.594 | 62.94 | 54.33 | 0.616 | 34.55 |
| TLG2.5 | 67.93 | 0.506 | 41.22 | 54.33 | 0.569 | 24.06 |
Abbreviations: AUC, area under the curve; MTV, metabolic tumor volume; OS, overall survival; PFS, progression-free survival; SUVs, standardized uptake values; TLG, total lesion glycolysis.
Figure 2.
Survival curves according to different parameters of PET/CT. PFS curve according to (A) SUVs, (B) MTVs, and (C) TLGs, and OS curve according to (D) SUVs, (E) MTVs, and (F) TLGs. CT indicates computed tomography; MTV, metabolic tumor volume; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; SUVs, standardized uptake values; TLG, total lesion glycolysis.
Kaplan-Meier Survival Analysis
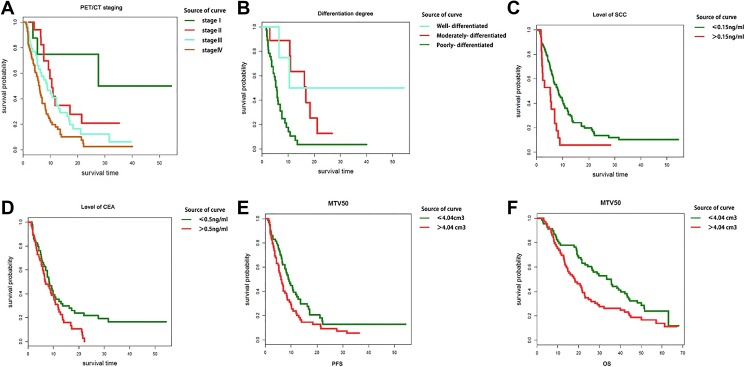
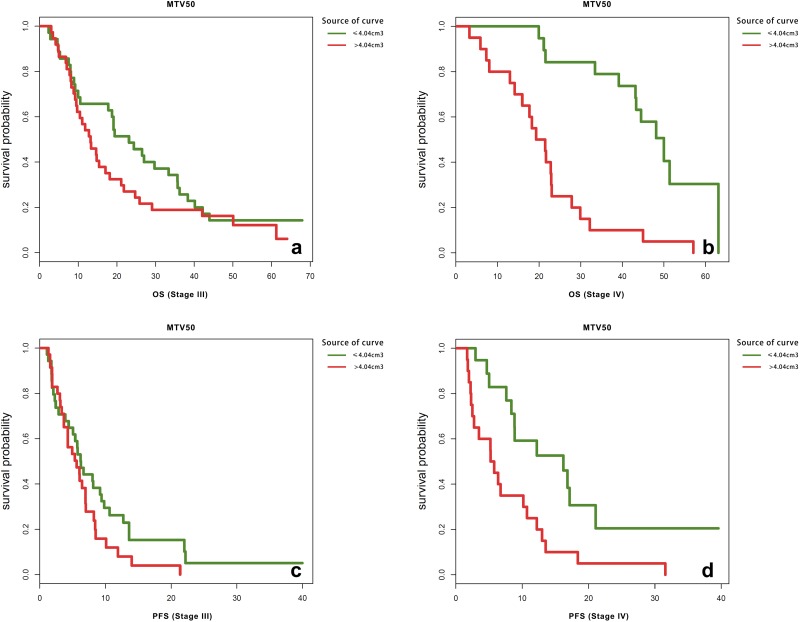
Kaplan-Meier method and log-rank test were employed to evaluate the correlations between PET/CT parameters as well as clinicopathological factors and patient outcomes. After a median follow-up of 22.9 months (ranging from 2.3 to 67.9 months), 135 patients (98.5%) presented with metastatic or recurrent tumors as confirmed by imaging examination or pathologic diagnosis and 110 patients (80.3%) died. The median PFS was 6.6 months, and median OS was 22.93 months. As shown in Table 3 and Figure 3, besides those widely accepted prognostic factors including gender, age, smoking status, histological subtype, PET/CT stage (P ≤ .001, Figure 3A) and differentiation degree (P ≤ .001, Figure 3B), type of surgery, chemotherapy regimen, level of SCC (P = .005, Figure 3C) and CEA (P = .038, Figure 3D), and MTV50 exhibited a significant value in predicting PFS (P = .034, Figure 3E), whereas adjuvant radiotherapy, targeted therapy, level of NSE, SUVmax, and TLG50 were of no significant predictive value related to PFS. Among all the significant factors in predicting PFS, smoking status, histological subtype, and level of CEA were of no significant predict value for OS. Univariate analysis indicated that patients whose MTV50 ≤4.04 had at least a PFS benefit of 1.8 months, and an OS benefit of 16.4 months, compared to those whose MTV50 >4.04. Because tumor stage played a rather important role in prognosis, and the managements were also different for stages I to IV patients, further stratified analysis was conducted to evaluate whether MTV50 was a significant predictor of OS and PFS in stages I to IV patients, respectively. The results suggested that MTV50 exhibited a significant value in predicting PFS (P = .005) and OS (P = .000) in patients staged IV (shown in Figure 4). While for patients staged I, II, and III, the survival differences were not significant. Small sample size and uneven distribution might be the cause of this result. In addition, further stratified analysis of the correlation between these PET/CT parameters and the effectiveness of the chemotherapy showed that there was no significant difference in PFS and OS between patients who received different chemotherapy regimens.
Table 3.
Univariate Analysis for Clinical Factors and PET/CT Parameters Related to PFS and OS.
| Clinical Factors and Parameters | Median PFS, Months | P | Median OS, Months | P | |
|---|---|---|---|---|---|
| Gender | Male | 8.4 | .004 | 26.6 | .04 |
| Female | 6.2 | 21.5 | |||
| Age | ≤58 | 7.4 | .032 | 31.7 | .018 |
| >58 | 5.8 | 20.7 | |||
| Smoking status | No | 8.2 | .025 | 24.6 | .382 |
| Yes | 5.4 | 19.9 | |||
| PET/CT stage | I | 9.6 | .0 | 55.5 | .0 |
| II | 8.4 | 37.8 | |||
| III | 7.6 | 29.9 | |||
| Ⅳ | 5.7 | 17.4 | |||
| Histology | Adenocarcinoma | 6.7 | .002 | 25.5 | .051 |
| Squamous cell carcinoma | 8.1 | 21.1 | |||
| Poorly differentiated NSCLC | 4.8 | 21.5 | |||
| Others | 5.2 | 32.9 | |||
| Differentiation degree | Well-differentiated | 23.4 | .0 | 49.1 | .014 |
| Moderately differentiated | 13.6 | 48.3 | |||
| Poorly differentiated | 5.2 | 21.1 | |||
| Type of surgery | Lobectomy | 12.7 | .0 | 48.6 | .0 |
| Pneumonectomy | 10.5 | 35.6 | |||
| Others | 8 | 19.3 | |||
| No surgery | 6 | 19.3 | |||
| Chemotherapy regimen | PMX-based | 7 | .013 | 29.4 | .001 |
| PTX-based | 5.2 | 20.7 | |||
| GEMZ-based | 6.3 | 23.1 | |||
| TXT-based | 7.7 | 19.4 | |||
| Adjuvant radiotherapy | No | 6.3 | .932 | 21.5 | .725 |
| Yes | 7 | 26.6 | |||
| Targeted therapy | No | 6.4 | .782 | 23 | .812 |
| Yes | 7.2 | 21.8 | |||
| SCC, ng/mL | ≤1.5 | 7 | .005 | 27.4 | .001 |
| >1.5 | 5.2 | 13.3 | |||
| CEA, ng/mL | ≤5.0 | 7.6 | .038 | 31 | .155 |
| >5.0 | 6.5 | 21.6 | |||
| NSE, ng/mL | ≤15.2 | 6.5 | .071 | 29.1 | .1 |
| >15.2 | 6.6 | 20.7 | |||
| SUVmax | ≤11.72 | 7 | .798 | 24.7 | .886 |
| >11.72 | 6.5 | 21.7 | |||
| MTV50 | ≤4.04 | 8.2 | .034 | 34.5 | .021 |
| >4.04 | 6 | 18.1 | |||
| TLG50 | ≤34.55 | 7.6 | .094 | 29.3 | .052 |
| >34.55 | 6.3 | 19.3 | |||
Abbreviations: CEA, carcinoembryonic antigen; CT, computed tomography; GEMZ, gemcitabine; MTV, metabolic tumor volume; NSCLC, non-small cell lung cancer; NSE, neuron-specific enolase; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; PMX, pemetrexed; PTX, paclitaxel; SCC, squamous cell carcinoma antigen; SUVs, standardized uptake values; TLG, total lesion glycolysis; TXT, docetaxel.
Figure 3.
Kaplan-Meier curves of PFS (A-E) and OS (F) according to clinicopathological factors and PET/CT parameters. CT indicates computed tomography; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival.
Figure 4.
Kaplan-Meier curves of OS and PFS according to MTV50 for patients staged III and IV, respectively. MTV indicates metabolic tumor volume; OS, overall survival; PFS, progression-free survival.
Cox Univariate and Multivariate Analyses
Cox univariate analysis showed that the age, gender, PET/CT stage, smoking status, level of SCC and CEA, differentiation degree, and MTV50 all exhibited a significant value in predicting PFS. Subsequent multivariate analysis was conducted by incorporating all the above variables as covariates. The results from the Cox proportional hazard model using forward stepwise method suggested that PET/CT stage, level of SCC and CEA, differentiation degree, and MTV50 retained their significant value in predicting PFS after taking age, gender, smoking status, surgery type, chemotherapy regimen, adjuvant radiotherapy, and targeted therapy into consideration. Spearman correlation analysis showed that there were significant correlations between MTV50 and differentiation degree (P = .031), MTV50, and the level of SCC (P = .002), while no significant association was found between MTV50 and PET/CT stage and the level of CEA (P >.05). Same procedure was conducted as to OS, and the results showed that PET/CT stage, MTV50, and level of SCC and CEA were independent prognostic factors of OS in patients with NSCLC who underwent chemotherapy (Table 4). These models suggested that MTV50 was independent prognostic factor for both PFS and OS; in other words, MTV50 ≤4.04 was a prognostic marker of a longer PFS and OS (hazard ratio 1.001, 95% confidence interval [CI], 0.999-1.002, P = .003 and hazard ratio 1.000, 95% CI, 1.000-1.001, P = .022) in those patients with NSCLC who received chemotherapy.
Table 4.
Multivariate Analysis for Factors Related to PFS and OS using the Cox Proportional Model.
| PFS | OS | |||||
|---|---|---|---|---|---|---|
| Factor | Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P |
| PET/CT stage | 1.884 | 1.416-2.507 | .000 | 1.913 | 1.445-2.531 | .000 |
| Differentiation degree | 0.574 | 0.227-1.447 | .004 | 0.781 | 0.342-1.781 | .129 |
| SCC | 1.465 | 0.768-2.794 | .012 | 2.032 | 1.070-3.861 | .018 |
| CEA | 2.102 | 1.334-3.311 | .000 | 1.445 | 0.962-2.171 | .026 |
| MTV50 | 1.001 | 0.999-1.002 | .003 | 1.000 | 1.000-1.001 | .022 |
Abbreviations: CEA, carcinoembryonic antigen; CI, confidence interval; CT, computed tomography; GEMZ, gemcitabine; MTV, metabolic tumor volume; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; SCC, squamous cell carcinoma antigen; TLG, total lesion glycolysis.
Discussion
The 18F-FDG PET/CT reflecting tumor metabolic activity, such as cell viability and proliferative activity, is playing a more and more important role in the studies of initial diagnosis, accurate staging, evaluation of treatment response, and prognosis of lung cancer as an advanced noninvasive examination method. The application of AI in medical imaging is one of the most promising areas of health innovation. Indeed, AI may find multiple applications, from image acquisition and processing to aided reporting, follow-up planning, data storage, data mining, and many others. Due to this wide range of applications, AI is expected to massively impact the radiologist’s daily life. We hypothesized that the metabolic parameters derived from 18F-FDG PET/CT might be of value in predicting the effectiveness of chemotherapy and be used as a new biological factor to screen which patients are more likely to benefit from chemotherapy in patients with NSCLC, in order to provide more accurate imaging features for AI prognosis qualitatively and quantitatively. The experimental design of this study has a strict entry standard of patient: All patients had undergone standardized chemotherapy and PET/CT examination before tumor-related treatment, and then survival analysis was carried out comprehensively considering the interference of all other clinical factors. Therefore, if any of the metabolic parameters of PET/CT were significantly related to survival of patients, we can say that this parameter could serve as an independent prognostic biomarker for predicting chemosensitivity and efficacy of patients with NSCLC. Several previous basic medical studies had drawn conclusions using similar method.7
The SUV is the most important semi-quantitative index in PET/CT imaging. However, SUV is affected by a variety of factors, including the physique of the subjects, the level of blood glucose, the time of imaging after the injection, the condition of image acquisition and reconstruction, the size of the lesion, and so on. Previous study has suggested that there was some false-positive or overstaging effect of PET in NSCLC, especially in tuberculosis endemic area in Asia. This study also showed that semi-quantitative SUV method does not result in better diagnostic accuracy than visual analysis of PET images.19 At present, SUVmax, SUVmean, and SUVpeak representing the maximum, average, and peak SUV of region of interest, respectively, are the most widely used SUV parameters in the application of PET. In order to choose a most valuable one, we measured and analyzed all these 3 parameters. However, SUVs cannot reflect the metabolic activity of the whole tumor, whereas MTV and TLG measure not only tumor volume but also tumor metabolism. Accumulating evidence from a variety of tumor types indicates that MTV and TLG are better predictors of prognosis than SUV.20-23 In previous studies, a variety of isocontour threshold methods have been used to accurately delineate tumor volume in 18F-FDG PET/CT,24 including a fixed percentage of SUVmax, a fixed SUV value, or mediastinal/liver background activity. However, no consensus has been established as to the standard method for delineating the tumor boundaries.25 Soret et al found that tumor volume delineation based on a fixed percentage of SUVmax (41%-70%) might be affected by the variability and noise inherent in SUVmax itself.26 Krak et al consider that delineation based on a fixed percentage of SUVmax or a fixed SUV value of 2.5 might erroneously include a significant proportion of the background in the tumor volume.27 We therefore measured a series of isocontour thresholds ranging from 30% to 50% of SUVmax and the fixed value of SUV = 2.5 for MTV and TLG delineation. Survival ROC analysis showed that SUVmax, MTV50, and TLG50 had the highest AUC when both OS and PFS were taken as the end point of the study, thus suggesting that SUVmax, MTV50, and TLG50 are better related to survival in patients with NSCLC.
At the same time, survival ROC analysis suggested that the AUC of each metabolic parameter was significantly greater when PFS was used as the endpoint than that of OS; therefore, the corresponding optimal cutoff value was chosen for next analyses. The PFS had a better performance than OS for indirect display of the efficacy of treatment. This may be because the objective of this study was to evaluate correlations between metabolic biomarkers and the effectiveness of chemotherapy, whereas OS was often affected by other subsequent treatments after tumor recurrence or metastasis. On the other hand, ROC analysis showed that the optimal cutoff for sensitivity and specificity for SUVmax, MTV50, and TLG50 were 11.72, 4.04, and 34.55, respectively. These were considered clinically meaningful and were included in further analysis. Using these cutoffs, Kaplan-Meier and Cox survival analysis separated the study population into 2 distinct prognostic groups.
In our study, univariate analysis showed that SUVmax was neither the predicting factor for PFS nor the independent prognostic factor for OS, coincided with the previous researches results.20,22,23 But we cannot identify that SUVmax was of no value totally and further large sample and multicenter studies are needed to confirm this conclusion. MTV, the volume of the tumor displaying 18F-FDG uptake and indicating the distribution of metabolic activity, has proved to be a better prognostic guide than SUVmax in kinds of solid malignancies.28,29 Total lesion glycolysis represents metabolic activity throughout the entire tumor above a minimum threshold designed to exclude background activity. Hence, a large TLG may reflect a small volume with high metabolic activity or a large volume with lower metabolic activity. Therefore, volume-based parameters such as MTV and TLG may reflect the metabolic burden of the active tumor more accurately and provide a potentially more sensitive method than SUVmax or tumor diameter. But in the current study, univariate analysis suggested that TLG50 was not a predictive factor for PFS or OS, whereas univariate and multivariate analysis together showed that MTV50 was an independent prognostic factor for both PFS and OS. The risk of disease progression and death were 7.84 times and 6.9 times higher, respectively, for patients with an MTV50 greater than 4.04 than for those with an MTV50 of 4.04 or less.
Histologic differentiation degree, initial tumor stage, and level of serum tumor markers are known to be important prognostic factors for clinical outcome. So it is rational that they were prognostic factors of disease progression in the current study. However, histologic differentiation degree was not a significant factor for OS as shown in the Cox multivariate analysis. The lack of prognostic power of differentiation degree in OS may be explained in that OS was often affected by many other factors such as subsequent treatments after tumor recurrence or metastasis. Moreover, for more than half of the patients (62.8%), the histological differentiation of the tumor was not checked in this study. Thus, we were dealing with only 51 patients whose differentiation degree is known, and among these patients, only 4 were well differentiated and 10 moderately differentiated. This seems to be a rather small sample size, so the statistical analysis result might have bias. Interestingly, correlation analysis revealed a significant association between increased MTV50 and histologic differentiation degree (P = .031) and the level of SCC (P = .002). This finding underlines the association between the metabolic parameters, histologic grades, and serum tumor markers. No significant association was found between MTV50 and PET/CT stage, which may be due to the disequilibrium of stage of enrolled patients, most of which were III and IV.
A major limitation of the current study was the retrospective nature of data collection from a single center. However, the relatively large sample size using detailed electronic 18F-FDG PET/ CT imaging data and a uniform institutional lung cancer data system for medical records and follow-up strengthen the findings of the study, yet a prospective multicenter validation study using standardized protocols across different 18F-FDG PET/CT scanners is urgently needed. Another limitation of the study was that the statistical analysis of the relationship between histologic differentiation degree and prognosis may be biased due to the small sample basis as mentioned in the previous paragraph. Although MTV and TLG might be affected by many confounding factors, such as the partial volume effect, image resolution, reconstruction method, noise, and the time between tracer injection and imaging,28 our results indicate that MTV50 could be valuable in the assessment of tumor burden and prognosis of patients with NSCLC undergoing chemotherapy.
Conclusions
This study demonstrated that MTV50 derived from 18F-FDG PET/CT is an independent prognostic biomarker for predicting PFS and OS in patients with NSCLC treated with chemotherapy and that MTV50 had a distinctive opposing value in predicting PFS and OS. These findings suggest that the patients with MTV50 ≤4.04 may achieve more benefit from chemotherapy than those with MTV50 >4.04. This biomarker could help select appropriate patients with NSCLC for chemotherapy or predict the efficacy of chemotherapy, which may accelerate the development of AI methods to improve treatment outcome for NSCLC.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (81671771) and the Hainan Natural Science Fund (2018CXTD347).
ORCID iD: Xueyan Li  https://orcid.org/0000-0002-7753-8429
https://orcid.org/0000-0002-7753-8429
References
- 1. Malvezzi M, Bertuccio P, Rosso T, et al. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women?. Ann Oncol. 2015;26(4):779–786. [DOI] [PubMed] [Google Scholar]
- 2. Shea M, Costa DB, Rangachari D. Management of advanced non-small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches. Ther Adv Respir Dis. 2016;10(2):113–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sundar R, Cho BC, Brahmer JR, et al. Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7(2):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non small cell lung cancer. Cochrane Database Syst Rev. 2015;(3):CD011430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calikusu Z, Yildirim Y, Akcali Z, et al. The effect of HER2 expression on cisplatin-based chemotherapy in advanced non-small cell lung cancer patients. J Exp Clin Cancer Res. 2009;28:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi Y, Chen L, Li J, et al. Prognostic and predictive values of pERK1/2 and pAkt-1 expression in non-small cell lung cancer patients treated with adjuvant chemotherapy. Tumor Biol. 2011;32(2):381–390. [DOI] [PubMed] [Google Scholar]
- 8. Grabauskiene S, Bergeron EJ, Chen G, et al. CHK1 levels correlate with sensitization to pemetrexed by CHK1 inhibitors in non-small cell lung cancer cells. Lung Cancer. 2013;82(3):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee JG, Jun S, Cho YW, et al. Deep learning in medical imaging: general overview. Korean J Radiol. 2017;18(4):570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Narula S, Shameer K, SalemOmar AM, et al. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol. 2016;68(21):2287–2295. [DOI] [PubMed] [Google Scholar]
- 11. Jeganathan J, Knio Z, Amador Y, et al. Artificial intelligence in mitral valve analysis. Ann Card Anaesth. 2017;20(2):129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumor heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40(3):133–140. [DOI] [PubMed] [Google Scholar]
- 13. Aerts HJWL, Velazquez ER, Leijenaar RTH, et al. Decoding tumor phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang H, Graham C, Elci O, et al. Advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated. Radiology. 2013;269(4):801–809. [DOI] [PubMed] [Google Scholar]
- 15. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multi-region sequencing. N Engl J Med. 2012;366(12):883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryu IS, Kim JS, Roh JL, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis measured by 18F-FDG PET/CT in salivary gland carcinomas. J Nucl Med. 2013;54(7):1032–1038. [DOI] [PubMed] [Google Scholar]
- 17. Krystal GW, Alesi E, Tatum JL. Early FDG/PET scanning as a harmacodynamic marker of anti-EGFR antibody activity in colorectal cancer. Mol Cancer Ther. 2012;11(7):1385–1388. [DOI] [PubMed] [Google Scholar]
- 18. Ryogo M, Jamali M, Gevaert O, et al. Prediction of EGFR and KRAS mutation in non-small cell lung cancer using quantitative 18F FDG-PET/CT metrics. Oncotarget. Advance Publications 2017;8(32):52792–52801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yen RF, Chen KC, Lee JM, et al. 18F-FDG PET for the lymph node staging of non-small cell lung cancer in a tuberculosis-endemic country: is dual time point imaging worth the effort? Eur J Nucl Med Mol Imaging. 2008;35(7):1305–1315. [DOI] [PubMed] [Google Scholar]
- 20. Hyun SH, Choi JY, Kim K, et al. Volume based parameters of 18F FDG-PET/CT improve outcome prediction in early-stage non-small cell lung cancer after surgical resection. Ann Surg. 2013;257(2):364–370. [DOI] [PubMed] [Google Scholar]
- 21. Hatt M, Visvikis D, Albarghach NM, Tixier F, Pradier O, Cheze-le Rest C. Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumor delineation methodology. Eur J Nucl Med Mol Imaging. 2011;38(7):1191–1202. [DOI] [PubMed] [Google Scholar]
- 22. Hyun SH, Ahn HK, Kim H, et al. Volume based assessment by 18F-FDG PET/CT predicts survival in patients with stage III non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):50–58. doi:10.1007/s00259-013-2530-8. [DOI] [PubMed] [Google Scholar]
- 23. Liao S, Penney BC, Wroblewski K, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39(1):27–38. [DOI] [PubMed] [Google Scholar]
- 24. VandeWiele C, Kruse V, Smeets P, et al. Predictive and prognostic value of metabolic tumor volume and total lesion glycolysis in solid tumors. Eur J Nucl Med Mol Imaging. 2013;40(2):290–301. [DOI] [PubMed] [Google Scholar]
- 25. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48(6):932–945. [DOI] [PubMed] [Google Scholar]
- 27. Krak NC, Boellaard R, Hoekstra OS, Twisk JW, Hoekstra CJ, Lammertsma AA. Effects of ROI definition and reconstruction method on quantitative outcome and applicability in a response monitoring trial. Eur J Nucl Med Mol Imaging. 2005;32(3):294–301. [DOI] [PubMed] [Google Scholar]
- 28. Romesser PB, Qureshi MM, Shah BA, et al. Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity modulated radiotherapy. Ann Nucl Med. 2012;26(7):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang D, Zhang M, Gao X, et al. Prognostic value of baseline 18F-FDG PET/CT functional parameters in patients with advanced lung adenocarcinoma stratified by EGFR mutation status. PLoS One. 11(6):e0158307 doi:10.1371/ journal.pone.0158307. [DOI] [PMC free article] [PubMed] [Google Scholar]