Short abstract
Background
The western medical arsenal for treating stroke is rather limited, and the only treatments shown to improve outcomes are not accessible to most in the third world. Even in the developed world, many patients present too late to receive thrombolysis or thrombectomy. Stroke patients in India commonly use Ayurvedic therapies, but there are no published data regarding the efficacy or safety of these therapies, the latter being of particular concern in acute ischemic stroke (AIS).
Objective
To obtain preliminary data on the safety and efficacy of stand-alone whole-system Ayurvedic treatment in AIS.
Methods
We present here an observational study prospectively comparing outcomes in 2 cohorts of AIS patients treated with whole-system classical Ayurveda (n = 13) or conservative (nonthrombolytic, noninterventional) western biomedicine (n = 20).
Results
Pooled analysis of outcomes did not show statistically significant differences in mortality (15.38% vs 15%, P = 1.00), nonfatal adverse event rates (15.38% vs 30%, P = .4), or functional disability measures. A paired analysis performed using a matching algorithm to reduce baseline disparities between the cohorts also showed no statistically significant differences in outcomes.
Conclusions
The safety profiles of classical Ayurveda and conservative western biomedicine in AIS are similar. This is the first ever report of stand-alone Ayurvedic therapy in AIS. Our results support the conduct of a randomized controlled trial to study the efficacy of Ayurvedic treatment of AIS.
Keywords: Ayurveda, complementary and alternative medicine, integrative medicine, integrative neurology, stroke
Introduction
Stroke is the number 1 cause of severe and complex disability in the human population worldwide,1,2 but treatment options for stroke in western biomedicine are limited. The only proven treatments for ischemic stroke are intravenous thrombolysis or angiointervention within a limited time window. Even in developed societies like the United States, only a small minority of stroke patients receive thrombolytic therapy.3 In addition, thrombolysis or thrombectomy are often ineffective because they either fail to recanalize the occluded artery4–6 or because the brain is already irreversibly injured.7 It is estimated that only 12% to 25% of patients benefit from thrombolysis.8 Thus, we need treatments that are available to, and beneficial in, a greater number of stroke patients.
Ayurveda is a traditional system of medicine developed in the Indian subcontinent and codified in written form over 5000 years ago. Ayurveda and other closely related indigenous systems of medicine are very widely used in the subcontinent.9–11 Ayurveda describes disease as a derangement or imbalance of 1 or more doshas, and treatment consists of measures to correct the derangement and restore balance. Pakshaghaata or hemiparesis has been well described in classical texts of Ayurveda,12–14 with specific treatments prescribed.15–17 While Ayurvedic literature does not describe any strict time limit for these treatments, the treatment is believed to be more effective the earlier it is begun.
Over one-third of stroke patients surveyed at 2 major tertiary hospitals in India reported using complementary and alternative medicine (CAM), of which Ayurvedic manual therapies and herbal medications were the commonest.18 Note that this estimate completely fails to capture those who receive exclusively Ayurvedic care. In spite of the popularity of indigenous medical systems among stroke patients,19 there are only 3 reported studies of Ayurveda in stroke. One is a pilot on adjunct marma therapy, a specialized branch of Ayurveda akin to acupressure therapy.20 The second study focused on Ayurveda-induced improvement in cardiac autonomic dysfunction in stroke when used as an adjunctive treatment.21 The third is a randomized trial comparing 2 different Ayurvedic muscle-nourishing procedures in chronic stroke patients with hemiplegia.22 Thus, no published literature exists on the safety or efficacy of stand-alone whole-system Ayurvedic treatment in ischemic stroke.
As acute ischemic stroke (AIS) has the potential for life-threatening complications and deterioration, questions regarding the safety of Ayurvedic therapy in AIS need to be addressed prior to studying its efficacy using randomized controlled trials. We present here the results of a pilot observational study designed to assess the safety and feasibility of a future larger trial of Ayurveda in AIS.
Methods
We used a 2-arm prospective observational study design with a whole-systems approach to Ayurvedic therapy. The study was approved by the Institutional Ethics Committee of the Institute for Ayurvedic and Integrative Medicine (No. 12-13/PR 002).
Patient Recruitment
We recruited our patient cohorts separately from an Ayurvedic and western medical hospital, respectively, as hospitals that provide AIS treatment generally provide only one modality of treatment: western medicine or stand-alone Ayurveda. All stroke patients who presented to these hospitals during the study period (April–November 2014 and October 2014–August 2015, respectively) were screened for enrollment. Our goal was to enroll 20 patients into each cohort. Patients satisfying the following eligibility criteria were enrolled after obtaining written informed consent.
Inclusion criteria
(1) Diagnosis of anterior circulation ischemic stroke, with confirmation by radiological imaging, (2) time from stroke onset to start of in-hospital treatment ≤72 hours, (3) age 18 to 80 years, (4) National Institutes of Health stroke scale score (NIHSS) 6-25, and (5) able to have magnetic resonance imaging (MRI) scan within 10 days after stroke.
Exclusion criteria
(1) Patients treated with thrombolysis, angiointerventional therapies, or surgical decompression, (2) patients planning to receive treatment from a different system of medicine, (3) febrile illness/sepsis within 4 days of admission, (4) pregnant women, (5) contraindications to MR imaging such as implanted electronic devices, (6) receiving investigational drug therapies or procedures, (7) severe coexisting or terminal systemic disease that limits life expectancy or may interfere with the conduct of the study, and (8) unable or unwilling to complete the course of treatment and follow-ups planned.
Diagnostic Workup and Intervention
Both cohorts received the standard therapeutic regimen for AIS per the system of medicine practiced in the respective hospital.
The Ayurvedic arm received classical Ayurvedic management, that is, both diagnosis and treatment per Ayurvedic protocol based on the Sushruta Samhita15 and Bhavaprakasha.17 In brief, it consisted of a sequence of interventions meant to achieve pitta-control, followed by treatments to detoxify accumulated ama, and finally, calm vata with nourishing treatments to support regeneration of depleted tissues. The types of interventions used are listed in Table 1. The complete treatment protocol is provided in Supplemental Figure 1.
Table 1.
Interventions Used in Ayurvedic Treatment Protocol.
| Diet modification (pathya) |
| External medicament application/massage |
| Intranasal administration of herbal medications (nasya) |
| Oral administration of herbal medications |
| Dripping therapy (dripping liquid medications on the head—shirodhara) |
| Purgation (virechana) |
| Enema with liquid medications (basti) |
Treatment was individualized within this broad framework and adjusted day-to-day on the basis of symptomatic response, for example, by varying the combination of herbs used to prepare medicaments or by varying the number of days spent on each step of this protocol. Some interventions in the Ayurvedic protocol are contraindicated in a febrile condition, hence they were withheld in any patient who developed a fever. This was why an early poststroke fever was one of our exclusion criteria. The patient remained in the hospital for the duration of this protocol, typically 2 weeks. Rehabilitative therapy (barring Ayurvedic massages that were part of the protocol) was not provided in the hospital, but patients were advised to undergo rehabilitative therapy after discharge.
The western medicine arm received standard conservative, supportive care—that is, control of stroke risk factors for secondary prevention, measures to prevent and treat complications, and rehabilitative therapy. Following completion of investigations and clinical stabilization, patients were discharged and asked to continue rehabilitative therapy at home.
Although Ayurvedic cohort patients were not routinely prescribed western medications, the treating physician was free to do so in the patient’s interest—for example, antibiotics for aspiration pneumonia or urinary infection. Similarly, western medical drugs that the patient was on prior to the stroke for chronic comorbid conditions (eg, antihypertensives) were continued unchanged.
Patients in both arms underwent standard diagnostic workup for stroke: this included MRI and magnetic resonance angiography of the head, carotid Doppler, transthoracic echocardiography, glycosylated hemoglobin level, and fasting lipid profile. Based on this workup, strokes were classified by TOAST criteria into small vessel, cardioembolic, and others. AP reviewed all radiological images from the Ayurvedic arm, and those from the western medicine arm where the radiologists’ report did not allow for a clear classification. Clinical parameters including BP and NIHSS were recorded through the course of the hospital stay and at follow-up.
Follow-up
Follow-up assessments were done at 1 and 3 months poststroke. Besides the clinical evaluation, patients’ NIHSS, modified Rankin scale (mRS) scores, and Barthel Index (BI) were recorded during these visits. When patients were unable to return to the hospital, the final follow-up assessment was done by a home visit, and if that was not feasible, the mRS and BI were obtained by interviewing the caregiver telephonically. All study related assessments and evaluations were carried out by study personnel who were not part of the treating team.
Outcomes
The safety outcomes studied were mortality and incidence of stroke-related complications or major nonfatal adverse events at 3 months. The following clinical and functional disability outcomes were also studied: improvement in NIHSS, mRS (dichotmomized to 0–2 and > 2), and BI (dichotomized to 90–100 and < 90), all at 3 months.
Data Analysis
MATLAB (http://www.mathworks.com) and R (https://cran.r-project.org/) were used for the analysis. Initially a pooled, intention-to-treat analysis was carried out. Missing data points were imputed using the Last Observation Carried Forward method, and where this was not applicable, the worst possible outcome was assumed. Patients who died were assigned the maximum scorable NIHSS and lowest BI. Wilcoxon rank-sum, Fisher’s exact, and χ2 tests were used to compare the baseline variables and outcomes of the 2 cohorts.
As our sample size was small, we then performed a weighted Euclidean matching between the cohorts to correct for baseline imbalances, specifically with respect to initial NIHSS, age, and time-to-treatment.23 Matching was performed to minimize the overall distance between matched pairs using the Kuhn-Munkres algorithm.24 After generating matches between Ayurvedic and western medicine cohort subjects, matched pairs with distances greater than a predetermined threshold were considered outliers and eliminated from further consideration.23,25 Matching was done by PM without knowledge of the outcomes. The matched-pair outcomes were then compared using a Wilcoxon signed rank test for continuous outcomes and tail probabilities of the binomial distribution for dichotomized outcomes.
Results
We enrolled 13 patients (of 66 screened) into the Ayurvedic arm and 20 patients (of 355 screened) into our western medicine arm. The commonest reasons patients were excluded from enrollment were presenting beyond the 72-hour window, and scoring too low or too high on the NIHSS (45/66 and 137/355, respectively). In the western medicine hospital, posterior circulation strokes (70/355), age over 80 years (31/355), thrombolysis (16/355), and fever (28/355) were other major reasons for nonenrollment. Recruitment for the Ayurvedic arm had to be terminated prior to reaching our goal of 20, as a government-sponsored insurance scheme that covered Ayurvedic care was terminated at that point, leading to a sudden marked decrease in census of AIS patients presenting to the Ayurvedic hospital.
The baseline characteristics of patients in each cohort are summarized in Table 2. The major differences between the cohorts that reached statistical significance were prestroke disability, time to treatment, and severity of stroke as measured by NIHSS. The former 2 characteristics favored the western medicine cohort, while the lattermost favored the Ayurvedic group. Although not statistically significant, the Ayurvedic cohort had a greater incidence of vascular risk factors—diabetes, hypertension, elevated cholesterol, and smoking.
Table 2.
Baseline Characteristics.
| Characteristic |
Full Cohort Analysis |
Matched Analysis |
|||||
|---|---|---|---|---|---|---|---|
| Ayurveda (n = 13) | Western Medicine (n = 20) | P | Ayurveda (n = 11) | Western Medicine (n = 11) | P | ||
| Female | 4 (30.77%) | 6 (30.00%) | 1.00 | 3 (27.27%) | 4 (36.36%) | 1.00 | |
| Age | 62.92 ±9.75 | 58.35 ±12.35 | .44 | 61.2 ±9.55 | 59.54 ±10.53 | .87 | |
| Completed follow-up | 11 (84.62%) | 16 (80.00%) | 1.00 | 11 (100%) | 11 (100%) | 1.00 | |
| Diabetes | 7 (53.85%) | 9 (45.00%) | .73 | 7 (63.64%) | 6 (54.55%) | 1.00 | |
| HbA1c | 7.05 ±1.89 | 6.41 ±1.53 | .13 | 7.28 ±1.97 | 6.45 ±1.19 | .21 | |
| Hypertension | 10 (76.92%) | 11 (55.00%) | .28 | 9 (81.82%) | 6 (54.55%) | .38 | |
| Hypercholesterolemia | 3 (23.08%) | 2 (10.00%) | .36 | 3 (27.27%) | 0 (0.00%) | .25 | |
| Prior strokes | 1 (7.69%) | 4 (20.00%) | .62 | 1 (9.09%) | 1 (9.09%) | 1.00 | |
| Smoking | 8 (61.54%) | 7 (35.00%) | .17 | 7 (63.64%) | 4 (36.36%) | .45 | |
| On antithrombotics | 0 (0.00%) | 5 (25.00%) | .13 | 0 (0.00%) | 1 (9.09%) | 1.00 | |
| Prestroke mRS | 1 (0–1) | 0 (0–0) | .018* | 1 (0–1) | 0 (0–0) | .13 | |
| Initial NIHSS | 8 (7–12) | 13 (8–17) | .046* | 8 (7–12) | 10 (8–17) | .13 | |
| Time to treatment (mins) | 1264.6 ±1062.5 | 695.4 ±924.0 | .046* | 1193.6 ±989.0 | 898 ±1050.4 | .31 | |
| Small vessel infarct | 7 (53.85%) | 4 (20.00%) | .064 | 7 (63.64%) | 3 (27.27%) | .22 | |
| Left hemispheric | 8 (61.54%) | 9 (45.00%) | .48 | 6 (54.55%) | 4 (36.36%) | .69 | |
Abbreviations: mRS, modified Rankin scale; NIHSS, National Institutes of Health stroke scale score.
Outcomes
Two of thirteen subjects in the Ayurvedic arm did not return for follow-up, while in the western medicine arm, 4 of 20 did not. Of these, we were able to obtain telephonic follow-up of all but 1 subject in the Ayurvedic arm. For the latter, we could only obtain mortality status. Two Ayurvedic and 3 western medicine subjects switched to treatment with the other system of medicine after discharge. One subject each in the Ayurvedic (unknown reason) and western medicine (unable to afford) arms discontinued prescribed medications before the end of the follow-up period. At discharge, all patients in the western medicine arm were prescribed antithrombotics; only 2 of the Ayurvedic arm patients were prescribed antithrombotics at discharge for concern for recurrence/noncompliance with Ayurvedic medications.
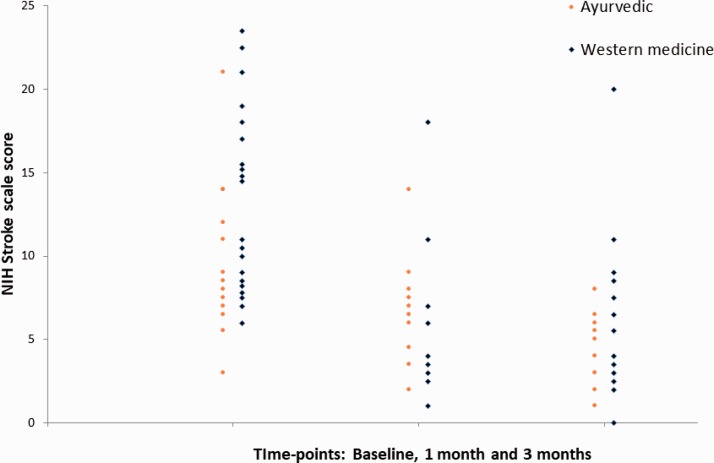
The outcomes are summarized in Table 3. Two patients of the Ayurvedic and 3 of the western medicine cohort died within the follow-up period. In the former, one died shortly after complaining of respiratory difficulty on day 11 poststroke, while the other died at 2½ months after a reported gradual, progressive decline. In the latter, 1 died of an acute myocardial infarction on day 19, and the other 2 of unknown causes in the sixth week and third month, respectively. Nonfatal adverse events included a hypotensive episode to 80/50 mm Hg on day 2 and an episode of aspiration pneumonia on day 6 in the Ayurvedic arm; the latter was treated with an antibiotic. Three cases of aspiration pneumonia and 1 of hematuria occurred in the western medicine cohort. One patient developed poststroke seizures, while one had a recurrence of stroke in the second month, both in the western medicine cohort. Overall, none of the safety or efficacy outcomes showed any statistically significant differences between the 2 groups. The NIHSS scores obtained at baseline, 1 month, and 3 months for all surviving patients with follow-up are shown in Figure 1.
Table 3.
Outcomes.
|
Full Cohort Analysis |
Matched Analysis |
||||||
|---|---|---|---|---|---|---|---|
| Outcomes | Ayurveda (n = 13) | Western Medicine (n = 20) | P | Ayurveda (n = 11) | Western Medicine (n = 11) | P | |
| Safety | |||||||
| Mortality | 2 (15.38%) | 3 (15.00%) | 1.00 | 2 (18.18%) | 0 (0.00%) | .5 | |
| Nonfatal adverse events | 2 (15.38%) | 6 (30.00%) | .43 | 1 (9.09%) | 2 (18.18%) | 1.00 | |
| Recurrent stroke | 0 (0.00%) | 1 (5.00%) | 1.00 | 0 (0.00%) | 1 (9.09%) | 1.00 | |
| Poststroke seizures | 0 (0.00%) | 1 (5.00%) | 1.00 | 0 (0.00%) | 1 (9.09%) | 1.00 | |
| Clinical/disability score | |||||||
| Improvement in NIHSS | 3 (2–6) | 4 (0–6) | 1.00 | 4 (2–6) | 4 (1–6) | .79 | |
| mRS 0-2 | 2 (15.38%) | 7 (35.00%) | .26 | 2 (18.18%) | 4 (36.36%) | .62 | |
| BI 90-100 | 3 (23.08%) | 7 (35.00%) | .70 | 3 (27.27%) | 4 (36.36%) | 1.00 | |
Abbreviations: mRS, modified Rankin scale; NIHSS, National Institutes of Health stroke scale score.
Figure 1.
NIHSS scores obtained at baseline, 1 month and 3 months for all surviving patients with follow-up. Red circles: Ayurvedic cohort; Blue diamonds: Western medicine.
To reduce the baseline imbalances in our data, we performed a reanalysis after weighted Euclidean matching as described above. The single subject for whom telephonic follow-up could not be obtained was excluded from this matching. This resulted in 11 matched pairs of subjects after 1 outlier pair was eliminated. Post-match, the baseline imbalances were reduced and dropped below the threshold of significance (Table 2). The safety analysis continued to show small nonsignificant differences, while the efficacy measures remained very similar (Table 3).
We closely monitored the blood pressure and pulse rate in our patients to determine if Ayurvedic treatment affected those parameters. The Ayurvedic arm patients started with higher blood pressures, and although the pressures decreased over time, they continued to remain higher than those of the western medicine arm throughout the 3 months of monitoring (Supplemental Figure 2). Similarly, no significant effects of Ayurvedic treatment were seen on heart rate.
A full-cost analysis was not performed, but the cost of hospital stay was available for all but 5 subjects of the western medicine cohort. The average hospital bill (excluding investigations such as MRI, Echocardiography, and Doppler) amounted to USD 325 ± 63 in the Ayurvedic, while it was USD 646 ± 523 in the western medicine cohort (P < .05). This was in spite of subsidies several patients in the western medicine cohort received. On the other hand, the length of stay was significantly longer in the Ayurvedic cohort owing to the multistage treatment protocol (12.69 ± 1.44 vs 6.40 ± 4.50 days, P < .001).
Discussion
We report for the first time a study comparing stand-alone whole-system Ayurvedic therapy with conservative western medical care in AIS, and show no significant difference in safety outcomes between the two at 3 months. The most effective window for Ayurvedic therapy is said to be the first month after onset—hence, Ayurveda offers us a potential therapeutic intervention that can be used in the vast majority of stroke patients around the world who are unable to receive the benefit of thrombolysis or thrombectomy.
The Ayurveda cohort was managed using classical Ayurveda—that is, both diagnosis and treatment were based on classical Ayurvedic texts. It is becoming commonplace for Ayurvedic herbs and formulations to be used in the treatment of conditions diagnosed per western medicine, without venturing into Ayurvedic diagnosis. However, in our study, Ayurvedic diagnostic methodology was employed to identify the Ayurvedic etiopathogenesis of stroke in each patient, and thus, individualize the treatment regimen.
The Ayurvedic therapeutic regimen consists of multiple sequential steps. Of note, the first internal medication in this series is a freshly prepared aqueous extract of certain herbs, administered intranasally (typically within the first hour of admission). The intranasal route has become favored in recent times in western biomedicine as the most direct noninvasive route to get medications into the brain, entirely bypassing the blood–brain barrier26,27; it is intriguing that medication administration by this very route is a key early step in the treatment of stroke outlined in centuries-old Ayurvedic textbooks.
Our results should be interpreted in light of the two major shortcomings of this study. The number of patients enrolled, especially in the Ayurvedic cohort, was small; this limited our ability to detect differences between the cohorts. Small numbers also resulted in significant baseline differences between the groups, especially in crucial parameters such as NIHSS score, time to treatment, and prestroke mRS. We reduced the impact of these baseline differences by using a matching algorithm to find the most similar pairs of subjects between the 2 cohorts and repeated our analysis using this paired data. Following this, we continued to find no significant differences in the safety outcomes of classical Ayurvedic and conservative western medical treatment.
The second major shortcoming was the recruitment of each cohort from a different hospital. Very few Ayurvedic hospitals in modern times can boast of prior experience and capability of treating stroke in the acute phase with classical, stand-alone Ayurveda. Therefore, we do not believe that there is any medical facility currently able to provide stand-alone Ayurvedic as well as western medical treatment for AIS. This dual-site recruitment led to systematic biases in our study: The patient-base for our Ayurveda group tended to be more rural, had lesser access to western medicine, had more vascular risk factors, had had fewer past strokes, but had greater disability to begin with. On the other hand, the western medical group had better access to western medicine, presented sooner after onset of the stroke, was more likely to have had a prior stroke and be on antithrombotic medications, but presented with more severe strokes. The acuity of care was greater in the tertiary-level western medical hospital, where full-fledged intensive care units, resuscitation teams and equipment, and on-site computed tomography and MRI scanners were available. This could well have affected outcomes—although nonsignificant, our matched analysis showed greater mortality in the Ayurveda group, but greater nonfatal complications/adverse effects in the western medicine group.
As this was a pilot with a small sample size, we attempted to enroll a cohort of early strokes of moderate severity so as to maximize possible therapeutic benefit, and reduce imbalances between our cohorts using these inclusion and exclusion criteria. This resulted in exclusion of a large number of stroke patients who arrived outside the time window or had strokes of lesser or greater severity. In addition, there were a large number of strokes involving the posterior circulation at our western medical site, possibly as it is a tertiary care and referral hospital.
Ayurvedic treatment of AIS should be investigated for a few different roles against the backdrop of current western biomedicine. First, it could provide a stand-alone therapeutic alternative with a much longer time window as discussed above. Second, in those who undergo thrombolysis/thrombectomy, it could be a useful adjunctive treatment that may further improve outcomes. We set out to investigate its potential in the first scenario—that is, for patients who could not receive thrombolysis/thrombectomy. Therefore, we excluded all patients who received these interventions from the study.
Although the safety and efficacy outcomes were similar between the groups, the average hospital bill was significantly higher in the western medicine group, in spite of the much shorter average length of stay and provision of subsidized care to some patients. This could have been related to the greater acuity of care and more severe strokes in the western medicine group. We suggest that a formal cost analysis would be important to include in any future trials of Ayurveda in AIS.
This study was not designed to show differences in efficacy of treatment given the small sample sizes planned. However, as a first step, we show that stand-alone Ayurvedic treatment of stroke in the acute phase appears safe, and it is feasible to conduct a larger randomized controlled trial of Ayurvedic treatment in AIS.
Conclusion
Stand-alone classical Ayurvedic treatment of AIS is safe. A randomized controlled study of Ayurvedic stroke treatment is feasible to examine the efficacy of Ayurveda in stroke.
Supplemental Material
Supplemental Material for Ayurvedic Treatment of Acute Ischemic Stroke: A Prospective Observational Study by J Aarthi Harini BAMS Avineet Luthra BPT, MSc Shrey Madeka MSc Prasan Shankar BAMS, MD (Ayu.) Pitchaiah Mandava MD, PhD, MSEE Ravishankar Pervaje BAMS, MS (Ayu.) Sanjith Aaron MBBS, MD, DM Archana Purushotham MD, PhD in Global Advances in Health and Medicine
Acknowledgments
The authors thank Dr HN Nagaraja, Professor Emeritus of Biostatistics, The Ohio State University, for discussions regarding statistical methods to be used in the analysis. They also thank Dr Mahadevan Seetharaman, Advisor, School of Health Sciences, The University of Transdisciplinary Health Sciences and Technology for inputs into study design and implementation and Mr Panikar Anbalagan, Stroke Research Coordinator, CMC Vellore, for assistance with enrollment.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by intramural funding from the Institute of Stem Cell Science and Regenerative Medicine (inStem), Bengaluru, and the Institute of Ayurveda and Integrative Medicine, School of Health Sciences, The University of Transdisciplinary Health Sciences and Technology, Bengaluru.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American heart association. Circulation. 2015; 131(4):e29–e322. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global Status Report on Non-Communicable Diseases. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 3.Skolarus LE, Meurer WJ, Shanmugasundaram K, Adelman EE, Scott PA, Burke JF. Marked regional variation in acute stroke treatment among Medicare beneficiaries. Stroke. 2015; 46(7):1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007; 38(3):948–954. [DOI] [PubMed] [Google Scholar]
- 5.Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology. 1992; 42(5):976–982. [DOI] [PubMed] [Google Scholar]
- 6.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992; 32(1):78–86. [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006; 60(5):508–517. [DOI] [PubMed] [Google Scholar]
- 8.Lansberg MG, Schrooten M, Bluhmki E, Thijs VN, Saver JL. Treatment time-specific number needed to treat estimates for tissue plasminogen activator therapy in acute stroke based on shifts over the entire range of the modified Rankin Scale. Stroke. 2009; 40(6):2079–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta M, Shafiq N, Kumari S, Pandhi P. Patterns and perceptions of complementary and alternative medicine (CAM) among leukaemia patients visiting haematology clinic of a north Indian tertiary care hospital. Pharmacoepidemiol Drug Saf. 2002; 11(8):671–676. [DOI] [PubMed] [Google Scholar]
- 10.Roy V, Gupta M, Ghosh RK. Perception, attitude and usage of complementary and alternative medicine among doctors and patients in a tertiary care hospital in India. Indian J Pharmacol. 2015; 47(2):137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tandon M, Prabhakar S, Pandhi P. Pattern of use of complementary/alternative medicine (CAM) in epileptic patients in a tertiary care hospital in India. Pharmacoepidemiol Drug Saf. 2002; 11(6):457–463. [DOI] [PubMed] [Google Scholar]
- 12.Acharya JT. Chapter 24:5-10: Charaka Samhita With Ayurveda Dipika Commentary of Chakrapanidatta. In: Sutrasthana Varanasi, India: Chaukhamba Orientalia; 2007:125.
- 13.Acharya JT. Chapter 28:53: Charaka Samhita With Ayurveda Dipika Commentary of Chakrapanidatta. In: Chikitsasthana Varanasi, India: Chaukhamba Orientalia; 2007:619.
- 14.Murthy KRS. Chapter 22:42-43: Madhava Nidana of Madhavakara With English Translation, Critical Introduction and Appendices. Varanasi, India: Chaukhamba Orientalia; 2009:84.
- 15.Acharya JT. Chapter 5:19: Sushruta Samhita With Nibandha Sangraha commentary of Dalhanacharya. In: Chikitsasthana Varanasi, India: Chaukhamba Orientalia; 2007:427–428. [Google Scholar]
- 16.Sharma S. Chapter 23:27: Ashtanga Samgraha of Vriddha Vagbhata, Sasilekha Sanskrit commentary by Indu. In: Chikitsasthana Varanasi, India: Chaukhamba Orientalia; 2008:568.
- 17.Misra B. Chapter 24:206: Bhavaprakasa of Sri Bhavamisra. In: Vatavyadhi Vikara Vol II. Varanasi, India: Chaukhamba Sanskrit Sansthan; 2005:258.
- 18.Pandian JD, Toor G, Arora R, et al. Complementary and alternative medicine treatments among stroke patients in India. Top Stroke Rehabil. 2012; 19(5):384–394. [DOI] [PubMed] [Google Scholar]
- 19.Pandian JD, Liu M, Misbach J, Venketasubramanian N. Alternative therapies for stroke treatment in Asia. Int J Stroke. 2011; 6(6):541–543. [DOI] [PubMed] [Google Scholar]
- 20.Fox M, Dickens A, Greaves C, Dixon M, James M. Marma therapy for stroke rehabilitation—A pilot study. J Rehabil Med. 2006; 38(4):268–271. [DOI] [PubMed] [Google Scholar]
- 21.Jaideep SS, Nagaraja D, Pal PK, Sudhakara D, Talakad SN. Modulation of cardiac autonomic dysfunction in ischemic stroke following ayurveda (Indian system of medicine) treatment. Evid Based Complement Alternat Med. 2014; 2014:634695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guruprasad Aggithaya M, Narahari SR, Vijaya S, Sushma KV, Kumar NP, Prajeesh P. Navarakizhi and pinda sweda as muscle-nourishing Ayurveda procedures in hemiplegia: Double-blind randomized comparative pilot clinical trial. J Altern Complement Med. 2014; 20(1):57–64. [DOI] [PubMed] [Google Scholar]
- 23.Mandava P, Kalkonde YV, Rochat RH, Kent TA. A matching algorithm to address imbalances in study populations: Application to the National Institute of Neurological Diseases and Stroke Recombinant Tissue Plasminogen Activator acute stroke trial. Stroke. 2010; 41(4):765–770. [DOI] [PubMed] [Google Scholar]
- 24.Munkres J. Algorithms for the assignment and transportation problems. J Soc Ind Appl Math. 1957; 5(1):32–38. [Google Scholar]
- 25.Mcgill R, Tukey JW, Larsen WA. Variations of box plots. Am Stat. 1978; 32(1):12–16. [Google Scholar]
- 26.Badhan RK, Kaur M, Lungare S, Obuobi S. Improving brain drug targeting through exploitation of the nose-to-brain route: A physiological and pharmacokinetic perspective. Curr Drug Deliv. 2014; 11(4):458–471. [DOI] [PubMed] [Google Scholar]
- 27.Mittal D, Ali A, Md S, Baboota S, Sahni JK, Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014; 21(2):75–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Ayurvedic Treatment of Acute Ischemic Stroke: A Prospective Observational Study by J Aarthi Harini BAMS Avineet Luthra BPT, MSc Shrey Madeka MSc Prasan Shankar BAMS, MD (Ayu.) Pitchaiah Mandava MD, PhD, MSEE Ravishankar Pervaje BAMS, MS (Ayu.) Sanjith Aaron MBBS, MD, DM Archana Purushotham MD, PhD in Global Advances in Health and Medicine