Abstract
After curative treatment of esophageal squamous cell cancer (ESCC), patients are at high risk for recurrence. The objective of this study was to develop an index with a high sensitivity and specificity to predict ESCC patients’ recurrence and prognosis. A retrospective analysis was conducted on consecutive patients with EC who underwent esophagectomy. In total, 1417 patients were included in the current investigation. In total, 770 patients were included in the current study’s exploratory group. Alcohol consumption, TNM classification, number of lymph node station metastases, and number of lymph node metastases were significantly correlated with recurrence. Multivariate logistical regression analysis resulted in the development of an equation for predicting recurrence and prognosis (REEC). When using the REEC value to predict recurrence, the cutoff value was 1.095, the area under the curve (AUC) values of the REEC were 0.68 (p < 0.001) in the Exploratory Group and 0.65 (p < 0.001) in the Validation Group, and the sensitivity and specificity were 76.68% and 51.18%, respectively. When using the REEC value to predict prognosis, the cutoff value was 1.215, the AUC values of the REEC were 0.65 (p < 0.001) in the Exploratory Group and 0.64 (p < 0.001) in the Validation Group, and the sensitivity and specificity were 73.12% and 50.67%, respectively. In the Exploratory Group, when the REEC value was >1.095, patients had a longer median overall survival (OS) and median disease-free survival (DFS) than those whose REEC value was < 1.095 (70.01±2.01 months versus 50.92±2.85 months and 75.66±1.35 months versus 53.68±2.81 months, respectively, p < 0.001). The differences were confirmed to still exist in the Validation Group (48.12±1.47 vs 32.68±2.53 months and 55.61±1.32 vs 35.68±2.73 months respectively, p < 0.001).This study reported an index that can predict esophageal cancer recurrence and prognosis, and its use can benefit patients.
Keywords: esophageal cancer, recurrence, prognosis, model, alcohol consumption, CEA
Introduction
Esophageal cancer (EC), which has a dismal prognosis, is the eighth most common cancer worldwide and the sixth most common cause of death from cancer.1,2 The 5-year survival rate for all patients with EC is only 17%, with better survival for local (33.7%) and regional (16.9%) ECs compared to those with distant (2.9%) disease at presentation.3 However, despite recent advances in diagnosis and staging, improvements in surgical techniques, and the introduction of multimodal therapies that have led to significant improvement in survival, the rate of 5-year overall survival (OS) for patients with EC remains poor. It is widely believed that locoregional recurrence and distant metastasis are the primary causes of poor prognosis for patients with EC. An accurate method to forecast locoregional recurrence and distant metastasis is lacking. Moreover, the majority of patients have developed locally advanced or metastatic disease by the time they present with symptoms, and this limits their survival from any treatment. Although the ability to detect early-stage EC has improved, most tumors are found when regional metastasis (in 30% cases) or distant metastasis (in 40% of cases) has already occurred.4 Lack of an accurate method to forecast early metastases also leads to poor 5-year OS. Moreover, recurrent and early metastases must be diagnosed when they are still minimal or clinically occult to improve the prognosis of patients with esophageal squamous cell carcinoma (ESCC).
Although previous studies have reported methods or indices to elevate and predict recurrence or metastasis in EC, such as an elevated preoperative neutrophil to lymphocyte ratio, the sensitive and specificity are not sufficient, and no significant survival benefits have been found.5 Conventional serum tumor markers, such as carcinoembryonic antigen (CEA) and SCC antigen, have been used in monitoring ESCC tumor dynamics. These serum tumor markers, however, lack sufficient sensitivity and specificity. Thus, development of novel methods using less invasive technology is necessary and could allow clinicians to monitor tumor dynamics. The 2016 European Society for Medical Oncology ESCC clinical guidelines recommended that 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (PET-CT) is particularly helpful to identify otherwise undetected distant metastases. The 18F-FDG-PET should therefore be carried out in patients who are candidates for esophagectomy, as the finding of otherwise unknown distant metastases may prevent patients from futile surgery. However, the availability of PET-CT differs among countries and centers. In addition, the screening of ESCC distant metastases using PET-CT was recommended preoperation. For curatively resected patients with ESCC, using PET-CT for tumor dynamics screening is not cost-effective. Until now, how to screen tumor dynamics among patients with ESCC after curatively resected operation has unknown. Better predictors of recurrence in ESCC remains to be determined.
Therefore, exploration of a prognostic index with a high sensitivity and specificity that can accurately predict prognosis is urgently needed. We performed an analysis to assess the roles of some preoperative clinical indicator to elucidate which indicator or combination of indicators is associated with an increased risk of disease recurrence and distant metastasis.
Patients and Methods
Patient Selection and Data Collection
A retrospective analysis was conducted on consecutive patients with ESCC who underwent esophagectomy at Sun Yat-sen University Cancer Center between January 2005 and December 2010. The study was approved by the Medical Ethics Committee and Clinical Trial Review Committee of this cancer center.
We reviewed 1699 consecutive patients, and patients were enrolled in this study according to the following eligibility criteria: (1) all patients had pathologically confirmed ESCC; (2) all patients received radical esophagectomy, confirming R0 resection, with 2- or 3-lymphadenectomy; (3) no evidence of distant metastasis was detected by preoperative medical imaging examinations, including CT, PET, and endoscopic ultrasonography; and (4) no patients received neoadjuvant therapy, including chemotherapy, radiotherapy, or chemoradiotherapy. The exclusion criteria were as follows: (1) patients who had an additional carcinoma, (2) patients who underwent palliative esophagectomy, and (3) patients whose clinical data were not complete.
Finally, 1417 patients were included in the current investigation. Data were collected from medical records, and survival data were obtained from the cancer center’s follow-up registry. The pathologic staging of tumors for patients was based on the 8th edition of the American Joint Committee on Cancer tumor–node–metastasis (TNM) classification.6 The clinical data obtained were allocated to 2 phases (exploratory group and validation group) in sequential chronological order (Figure 1).
Figure 1.
Flowchart of 2 groups in this study.
Study End Points
In this study, the primary end point was disease-free survival (DFS), and the secondary end point was OS. Disease-free survival was defined from the date of surgery to the date of disease locoregional relapse or distant metastasis or death from any other cause. Overall survival was defined as the interval from the date of surgery to the date of death from any cause.
Laboratory Tests
Serum CEA and SCC levels were measured using a commercially available electrochemiluminescence immunoassay (Cobas E602-2; Hoffmann-La Roche Ltd, Pleasanton, California). The normal values of CEA and SCC are <5 and <1.5 ng/mL, respectively. Serum albumin (ALB) and globulin (GLB) levels were determined using automated techniques (LABOSPECT 008; Hitachi-Hitec Globe Ltd, Tokyo, Japan). The normal ALB, GLB, and glucose levels are 40 to 55, 20 to 30, and 20 to 40 g/L, respectively.
The tumor size, volume, pathological type, differentiation, and numbers of lymph node (LN) metastasis were reported by at least 3 experienced pathologists who were not informed about the patients’ preoperative conditions, such as alcohol consumption or tumor marker level.
Sociodemographic Information
Sociodemographic details, such as gender, age, duration of alcohol exposure, daily alcohol intake, and family history, were collected from all participating patients by a questionnaire. All participants finished the questionnaire surveys in a quiet room without any interference or disruptions. Professional staff members were available to answer questions if any problems occurred with understanding the survey questions.
Statistical Analysis
Categorical variables were calculated using Fisher exact tests and χ2 tests, while continuous variables were analyzed using Student t tests. Multivariable logistical regression was performed to assess patient and tumor characteristics. All end points were estimated by the Kaplan-Meier method and compared using the log-rank test. Multivariable survival analyses were performed using the Cox proportional hazards model to identify important prognostic factors for OS and DFS.
Two-sided P values of <.05 were considered statistically significant. All analyses were performed using SPSS20.0 software (SPSS Inc, Chicago, Illinois).
Baseline Demographics and Clinical Characteristics in the Exploratory Group
A total of 770 patients were included in the current study. Of these, 199 patients with ESCC suffered recurrences (recurrence group, average age: 56.5 ± 8.8 years) and 571 patients did not (nonrecurrence group average age: 59.7 ± 29.2 years). There were 41 (20.6%) and 147 (25.7%) females in the recurrence group and nonrecurrence group, respectively. The alcohol consumption, TNM classification, number of LN station metastases, and number of LN metastases of the recurrence group were significantly different from those of the nonrecurrence group: alcohol consumption: 2.1 ± 3.6 versus 1.3 ± 3.0; number of LN station metastases: 1.2 ± 1.7 versus 0.8 ± 1.2; number of LN metastases: 2.1 ± 4.2 versus 1.3 ± 2.4 (P < .05), as shown in Table 1.
Table 1.
Demographics and Clinical Characteristics of the 2 Groups.
| Characteristic | Recurrence Group | Nonrecurrence Group | P Value |
|---|---|---|---|
| Gender, F/M | 41/158 | 147/424 | .152 |
| Age, year | 56.5 ± 8.8 | 59.7 ± 29.2 | .127 |
| Duration of exposure, year | 18.2 ± 15.7 | 17.9 ± 16.0 | .779 |
| Alcohol consumption | 2.1 ± 3.6 | 1.3 ± 3.0 | .005 |
| Family history, n | 41 | 116 | .424 |
| ALB, g/L | 43.5 ± 4.8 | 43.6 ± 4.8 | .830 |
| GLB, g/L | 26.4 ± 5.0 | 27.1 ± 10.4 | .334 |
| Fasting glucose, mmol/L | 5.2 ± 1.1 | 5.2 ± 1.1 | .663 |
| CEA, ng/mL | 3.6 ± 4.1 | 3.0 ± 2.3 | .009 |
| SCC, ng/mL | 0.7 ± 1.3 | 0.8 ± 1.8 | .633 |
| Tumor site, n | .224 | ||
| Upper | 20 | 47 | |
| Middle | 136 | 359 | |
| Distal | 43 | 165 | |
| Tumor size, cm | 4.5 ± 1.8 | 4.6 ± 2.1 | .480 |
| Tumor volume, cm | 1.5 ± 0.6 | 1.4 ± 0.7 | .599 |
| Differentiation | .541 | ||
| High | 54 | 130 | |
| Mid | 89 | 281 | |
| Low | 56 | 158 | |
| None | 1 | 1 | |
| T staging, n | .780 | ||
| Stage 0 | 2 | 10 | |
| Stage 1 | 15 | 57 | |
| Stage 2 | 44 | 118 | |
| Stage 3 | 135 | 376 | |
| Stage 4 | 3 | 10 | |
| TNM, n | .019 | ||
| Stage 0 | 1 | 9 | |
| Stage 1 | 13 | 73 | |
| Stage 2 | 92 | 270 | |
| Stage 3 | 92 | 218 | |
| Stage 4 | 1 | 1 | |
| Number of lymph node station metastasis | 1.2 ± 1.7 | 0.8 ± 1.2 | <.001 |
| Number of lymph node metastasis | 2.1 ± 4.2 | 1.3 ± 2.4 | <.001 |
Abbreviations: ALB, albumin; CEA, carcinoembryonic antigen; F, female; GLB, globulin; M, male; SCC, squamous cell carcinoma antigen; TNM, tumor–node–metastasis.
Model Used to Predict Postoperative Recurrence or Prognosis
Univariate analysis for recurrence indicated that patient age (odds ratio [OR] = 1.03, P = .006), alcohol consumption (OR = 0.94, P = .004), CEA level (OR = 0.92, P = .013), number of LN station metastases (OR = 0.81, P < .001), and number of LN metastases (OR = 0.90, P = .001) are related to recurrence. Multivariable analysis revealed that alcohol consumption (OR = 0.91; 95% confidence interval [CI], 0.86-0.97, P = .002), CEA level (OR = 0.92; 95% CI, 0.86-1.00, P = .042), and number of LN station metastases (OR = 0.86; 95% CI, 0.74-1.00, P = .05) were independent factors (Table 2). Hence, we developed an index to evaluate, grade, and predict recurrence, which we called “REEC.” The computational formula of the index was as follows: REEC = 1.493 – (0.215 × number of LN station metastases) – (0.025 × CEA) – (0.067 × alcohol consumption).
Table 2.
Relationships Between Demographic Characteristics and EC Recurrence.
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Gender, F/M | 1.34 | 0.90-1.98 | .15 | |||
| Age | 1.03 | 1.08-1.05 | .006 | |||
| Duration of exposure | 0.992 | 0.98-1.01 | .147 | |||
| Alcohol consumption | 0.94 | 0.90-0.98 | .004 | 0.91 | 0.86-0.97 | .002 |
| Family history | 0.97 | 0.66-1.42 | .871 | |||
| ALB | 1 | 0.97-1.04 | .830 | |||
| GLB | 1.01 | 0.99-1.04 | .328 | |||
| Fasting glucose | 0.97 | 0.84-1.12 | .663 | |||
| CEA | 0.92 | 0.86-0.99 | .013 | 0.92 | 0.86-1.00 | .042 |
| SCC | 1.03 | 0.91-1.17 | .634 | |||
| Tumor site | 1.33 | 0.99-1.77 | .059 | |||
| Tumor size | 1.03 | 0.95-1.12 | .480 | |||
| Tumor volume | 0.94 | 0.40-1.19 | .599 | |||
| Differentiation | 1.1 | 0.87-1.36 | .474 | |||
| Number of lymph node station metastasis | 0.81 | 0.71-0.90 | <.001 | 0.86 | 0.74-1.00 | .05 |
| Number of lymph node metastasis | 0.90 | 0.85-0.95 | <.001 | |||
Abbreviations: ALB, albumin; CEA, carcinoembryonic antigen; CI, confidence interval; F, female; GLB, globulin; M, male; OR, odds ratio; SCC, squamous cell carcinoma antigen.
From our calculation and patient data analysis, we drew a receiver operating characteristic (ROC) curve. A cutoff REEC value of 1.095 was used in the present study to predict tumor recurrence. When using the REEC value to predict prognosis, the cutoff value was chosen as 1.215.
Results
Relationship Between REEC Value and Prognosis in the Exploratory Group
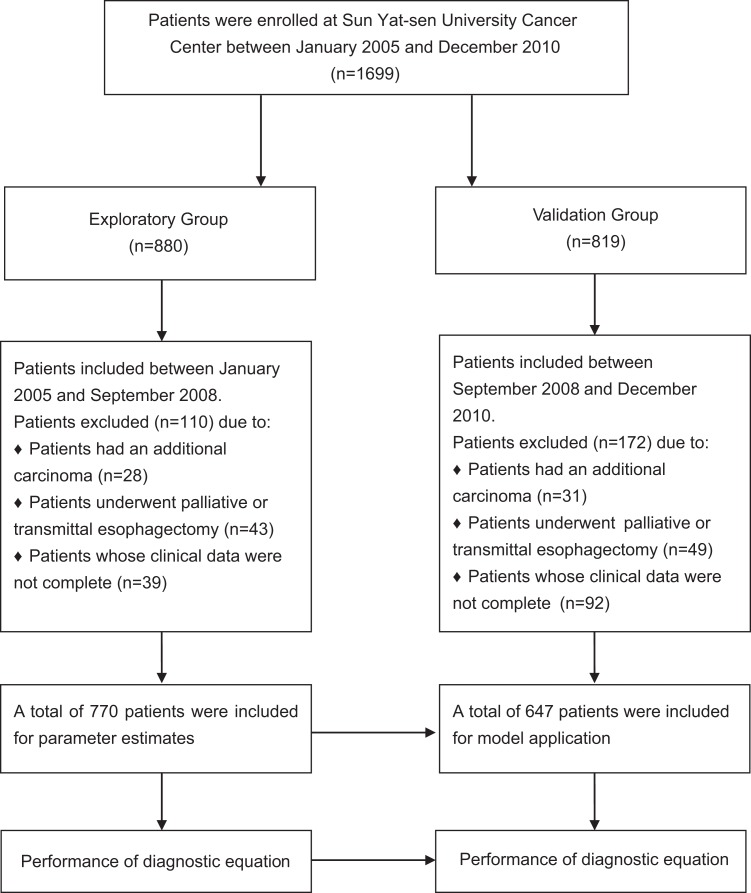
The REEC value was significantly correlated with the incidence of recurrence (P < .001); the sensitivity and specificity using the cutoff REEC value of 1.095 were 76.68% and 51.18%, respectively. In addition, the area under the curve (AUC) was 0.679 (95% CI, 0.64-0.72; Figure 2A). Using this cutoff of 1.215, the REEC value was highly related to the prognosis of the patients. The sensitivity and specificity were 73.12% and 50.67%, respectively, and the AUC was 0.65 (95% CI, 0.61-0.68; Figure 2B).
Figure 2.
The ROC curve when using the REEC value to predict recurrence and prognosis of patients with EC in the exploratory group. A, Using the REEC value for predict recurrence, we used the AUC to examine the use of the REEC value as an indicator to predict the recurrence of patients with EC. The AUC was 0.679 (95% CI, 0.64-0.72, P < .001) when the REEC value was >1.095; the sensitivity and specificity were 76.68% and 51.18%, respectively. B, Using the REEC value to predict prognosis, we used the AUC to examine the use of the REEC value as an indicator to predict the prognosis of patients with EC. The AUC was 0.65 (95% CI, 0.61-0.68, P < .001). When the REEC value was >1.215, the sensitivity and specificity were 73.12% and 50.67%, respectively. AUC indicates area under the curve; CI, confidence interval; EC, esophageal cancer; ROC, receiver operating characteristic.
Impact of the REEC Value on Survival After Surgery in the Exploratory Group
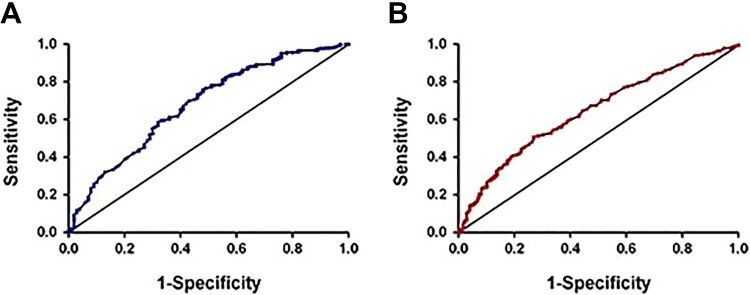
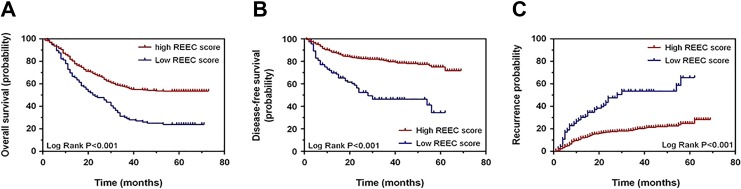
Using the cutoff value of 1.095, we divided all of the patients in the exploratory group into 2 groups. The patients whose REEC values were higher than 1.095 were included in group 1 (high REEC value group, HRG). Those with REEC values lower than 1.095 were incorporated into group 2 (low REEC value group, LRG). Overall survival was better among patients in group 1, whose median OS was 70.01 ± 2.01 months, compared with that of patients in group 2, who had a median OS of 50.92 ± 2.85 months (P < .001; Figure 3A).
Figure 3.
Overall survival, disease-free survival, and recurrence analysis regarding the REEC score in the exploratory group. A, Overall survival between patients with high and low REEC scores. B, Disease-free survival between patients with high and low REEC scores. C, Recurrence between patients with high and low REEC scores. When we used the cutoff score of 1.095, the median OS and DFS in patients whose REEC scores were higher than 1.095 were 70.01 ± 2.01 months and 75.66 ± 1.35 months, respectively. The median OS and DFS in patients whose REEC scores were lower than 1.095 were 50.92 ± 2.85 months and 53.68 ± 2.81 months, respectively. This difference is of statistical significance (P < .001). DFS indicates disease-free survival; OS, overall survival.
Similar to the OS, the DFS of the 770 patients was significantly different between the 2 groups. Group 1 had a median DFS duration of 75.66 ± 1.35 months, while group 2 had a median DFS duration of 53.68 ± 2.81 months (P < .001; Figure 3B). The recurrence possibility also showed a similar difference between the 2 groups (P < .001; Figure 3C).
Relationship Between the REEC Value and Recurrence in the Validation Group
In the validation group, recurrent disease was found in 156 (24.1%) of 647 cases. The patients who suffered a recurrent disease had a lower REEC score than those who did not undergo a recurrence (1.16 ± 0.03 vs 1.36 ± 0.01), and the difference was of statistical significance (P < .001).
Ability of REEC Values to Predict Recurrence or Prognosis in the Validation Group
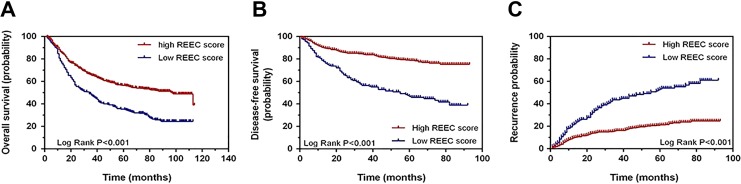
Using the cutoff of 1.095 to predict the recurrence of patients in the validation group and draw an ROC curve, the AUC was 0.65 (95% CI, 0.60-0.71; P < .001; Figure 4A). This result indicated that the REEC value was applicable when it used in the validation group for the purpose of predicting recurrence. In the same way, using the previous cutoff REEC score to predict prognosis in these patients, the AUC was 0.643 (95% CI, 0.60-0.69; P < .001; Figure 4B). This finding indicates that the REEC score can also play a predictive role in prognosis in the validation group.
Figure 4.
The ROC curve when using the REEC value for predicting the recurrence and prognosis of patient with ECs in the validation group. A, Using the REEC value to predict recurrence, the AUC was 0.65 (95% CI, 0.60-0.71, P < .001) when the REEC cutoff value was 1.095. B, Using the REEC value to predict prognosis, the AUC was 0.643 (95% CI, 0.60-0.69, P < .001) when the REEC cutoff value was 1.215. AUC indicates area under the curve; EC, esophageal cancer; ROC, receiver operating characteristic.
Impact of the REEC Value on Survival After Surgery in the Validation Group
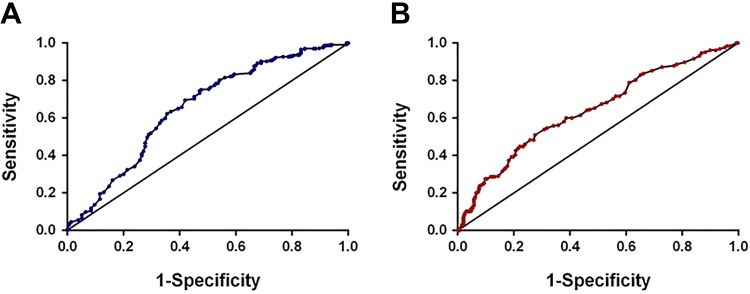
We used an identical method to divide the 647 patients into 2 groups according to the same cutoff value mentioned previously. Patients in the high REEC value group (group 1, HRG) had a longer OS than those in the low REEC value group (group 2, LRG); the median survival durations were 48.12 ± 1.47 months versus 32.68 ± 2.53 months, respectively (P < .001; Figure 5A). The DFS showed a similar pattern. The DFS was much better in group 1 patients than in group 2 patients (55.61 ± 1.32 months vs 35.68 ± 2.73 months, respectively; P < .001; Figure 5B). The recurrence possibility also showed a similar difference between the 2 groups (P < .001; Figure 5C).
Figure 5.
Overall survival, disease-free survival, and recurrence analysis regarding the REEC score in the exploratory group. A, Overall survival for patients with high and low REEC scores. B, Disease-free for patients with high and low REEC scores. C, Recurrence between patients with high and low REEC scores. When we used the cutoff score of 1.095, the mean OS and DFS in patients whose REEC scores were higher than 1.095 were 48.12 ± 1.47 months and 55.61 ± 1.32 months, respectively. The mean OS and DFS in patients whose REEC scores were lower than 1.095 were 32.68 ± 2.53 months and 35.68 ± 2.73 months, respectively. This difference is of statistical significance (P < .001). DFS indicates disease-free survival; OS, overall survival.
Relationship Between the REEC Score and Other Clinicopathological Characteristics
Overall, 196 (18.2%) of 1079 patients with high REEC scores had recurrence and 159 (47.0%) of 338 patients with low REEC scores suffered recurrent disease; the difference was of statistical significance (P < .001).
We analyzed the relationship between the REEC value and patients’ clinical characteristics. We found that high REEC scores were significantly correlated with gender (P < .001), duration of tobacco exposure (P < .001), alcohol consumption (P < .001), CEA level (P < .001), tumor size (P < .001), tumor volume (P = .001), T staging (P < .001), TNM stage (P < .001), number of LN station metastases (P < .001), and number of LN metastases (P < .001; Table 3).
Table 3.
Relationship Between the REEC Score and Characteristics.
| Characteristic | REEC Group | P Value | |
|---|---|---|---|
| High Score | Low Score | ||
| Recurrence, n | 196 | 159 | <.001 |
| Gender, F/M | 281/798 | 31/307 | <.001 |
| Age, year | 58.9 ± 9.3 | 59.1 ± 35.9 | .875 |
| Duration of exposure, year | 17.0 ± 16.2 | 23.5 ± 14.5 | <.001 |
| Alcohol consumption | 0.3 ± 1.6 | 3.0 ± 4.1 | <.001 |
| Family history, n | 265 | 68 | .113 |
| ALB, g/L | 43.2 ± 4.3 | 42.7 ± 4.6 | .081 |
| GLB, g/L | 27.5 ± 8.0 | 26.8 ± 5.1 | .129 |
| Fasting glucose, mmol/L | 5.3 ± 1.0 | 5.2 ± 1.3 | .242 |
| CEA, ng/mL | 2.4 ± 1.3 | 5.7 ± 8.4 | <.001 |
| SCC, ng/mL | 0.9 ± 1.7 | 1.1 ± 1.8 | .09 |
| Tumor site, n | 0.146 | ||
| Upper | 106 | 38 | |
| Middle | 696 | 201 | |
| Lower | 277 | 99 | |
| Tumor size, cm | 4.4 ± 2.0 | 4.8 ± 1.9 | <.001 |
| Tumor volume, cm | 1.4 ± 0.6 | 1.5 ± 0.6 | .001 |
| Differentiation | 0.171 | ||
| High | 245 | 69 | |
| Mid | 534 | 158 | |
| Low | 294 | 107 | |
| None | 6 | 4 | |
| T staging, n | <0.001 | ||
| Stage 0 | 36 | 0 | |
| Stage 1 | 125 | 23 | |
| Stage 2 | 207 | 55 | |
| Stage 3 | 670 | 246 | |
| Stage 4 | 41 | 14 | |
| TNM, n | <.001 | ||
| Stage 0 | 32 | 0 | |
| Stage 1 | 609 | 120 | |
| Stage 2 | 362 | 199 | |
| Stage 3 | 75 | 17 | |
| Stage 4 | 1 | 2 | |
| Number of lymph node station metastasis | 0.7 ± 1.0 | 1.9 ± 1.9 | <.001 |
| Number of lymph node metastasis | 1.1 ± 2.2 | 3.4 ± 4.6 | <.001 |
Abbreviations: ALB, albumin; CEA, carcinoembryonic antigen; F, female; GLB, globulin; M, male; SCC, squamous cell carcinoma antigen; TNM, tumor–node–metastasis.
Discussion
Esophageal cancer is one of the most fatal cancers and is the sixth leading cause of cancer-related mortality worldwide.7 It affects more than 450 000 people worldwide, and its incidence is increasing sharply.3,8-11 Esophageal squamous cell carcinoma is the histopathological form of the majority of cases worldwide. Approximately 70% of all EC cases worldwide occur in China, and ESCC is the predominant form of these cases (>90%).12 The etiology of ESCC remains unclear, and epidemiological studies suggest that tobacco smoking, heavy alcohol drinking, micronutrient deficiency, and dietary carcinogen exposure may cause this malignancy.13 Despite increasingly radical surgery for ESCC, chemotherapy and radiotherapy are frequently used to treat ESCC; the prognosis for patients with ESCC is poor, as the 5-year OS ranges from 15% to 25%. The poor outcomes in these patients with ESCC are significantly related to the propensity for recurrence soon after operation. In our study, we discovered a new method to easily predict recurrence and metastasis of patients with ESCC, and it will assist clinicians to rapidly evaluate the occurrence of recurrence and distant metastasis. This will be conducive to clinical governance and timely clinical intervention for patients with ESCC, ultimately improving their prognosis.
A previous study14 that focused on EC recurrence patterns reported that 75% of all recurrences occurred within the first 2 years after surgery. The median time to recurrence was 5.5 years. The overall recurrence rate was 27 per 100 person-years in postoperative year 1, which rapidly decreased to 4 per 100 person-years by postoperative year 6. Median postrecurrence survival among patients who experienced recurrence was 11 months. Studies that evaluated recurrence patterns in ESCC15-17 showed that distant, locoregional, and mixed recurrences represented 55%, 28%, and 17% of all new events, respectively. They also pointed out that patients with locoregional disease did the best and those with both distant and locoregional disease did the worst. The treatment of advanced disease such as multirecurrence and distant metastasis is disappointing, although timely intervention for inchoate locoregional recurrence could improve the curative effect significantly.18 Therefore, early detection of recurrence is of great importance and can probably prolong survival. For all these reasons, accurately identifying patients at high risk of recurrence is important. Analysis of our data shows that we can accurately predict the occurrence of recurrence and distant metastasis in patients with ESCC using serum CEA, alcohol consumption, and number of LN station metastases. Although our index could not improve prognosis itself, it could actually define patients at high risk of recurrence. As a result, use of this model in the clinic would ensure that patients get treatment and intervention in time, which will lead to a better prognosis in patients with ESCC.
In the present study, we analyzed the clinicopathological data from a cohort of patients with ESCC to develop an index, called “REEC,” as a predictor of recurrent disease. To our knowledge, this is the first study that combined important clinicopathological characteristics to generate an index for the purpose of predicting recurrence of ESCC and to monitor patients with ESCC at various stages of the disease.
In our research, we found that alcohol consumption is an independent risk factor associated with distant metastasis and tumor relapse, which has not been reported to our knowledge. Alcohol abuse is a major risk factor for the development of ESCC.19-21 Drinking and smoking have a synergistic effect in increasing the risk of ESCC,22 but drinking has an even greater effect.23,24 The OR for oral cavity cancers of chronic alcohol drinkers is high relative to that of nondrinkers.19 Therefore, heavy alcohol consumption is a risk factor for the incidence of EC. A reasonable explanation for the link between alcohol consumption and ESCC incidence is inactive aldehyde dehydrogenase-2 (ALDH2). Aldehyde dehydrogenase-2 is a major enzyme in the metabolism of acetaldehyde, an established human carcinogen for ESCC, after alcohol consumption. A mutant allele (ALDH2_2; rs671) encodes an inactive subunit, and alcohol consumption by East Asians who are inactive ALDH2_2 carriers markedly increases their risk of ESCC.25-28 Thus, we devised a simple alcohol questionnaire for patients with ESCC after operation to predict the risk of tumor progression. For patients with ESCC with heavy alcohol consumption, follow-up for screening after operation may need to be more frequent.
Additionally, our index also includes serum CEA and number of LN station metastases. Lymph node metastasis is an independent risk factor for recurrence.14 Node ratio is an independent prognostic factor after esophagectomy regardless of the number of retrieved LNs and has more potential for predicting patient outcomes.15 Moreover, CEA was significantly increased in patients with ESCC.29 Various tumor markers have been used in attempts to detect EC at an early stage; CEA is a tumor marker commonly used in the management of patients with EC.30,31 Another study also reported that CEA messenger RNA (mRNA) expression in blood can predict recurrence.32 All this evidence confirms the reasonability and scientificity of our index. When the cutoff value was considered to be 1.095, the sensitivity and specificity of this REEC value were 76.68% and 51.18%, respectively.
Studies have reported the relationship between biomarker detection and recurrence. Duzgun and Sarici firstly describe CA125 associated with peritoneal spreading and Glisson capsule involvement in patients with peritoneal carcinamatosis.33 Koike et al showed that ΔNp63 is potentially useful for the monitoring of patients with recurrent ESCC, with a sensitivity of 60%.34 Reports on CYFRA21-1 indicated that the serum CYFRA 21-1 level served as a predictive factor for patients’ recurrence with EC after surgery, and its sensitivity was 43.9%.35,36 The predictive functions of other biomarkers, such as serum CEA, CA 19-9, CA 125, and SCC, were also investigated. However, individual sensitivities for CEA, CA 19-9, CA 125, and SCC were only 28%, 34%, 10%, and 32%, respectively.29 Postoperative mRNA expression was also considered a predictor of recurrence. A Japanese study revealed that examination of CEA mRNA in peripheral blood is useful for the early detection of occult recurrence with a higher sensitivity, specificity, positive predictive value, and negative predictive value than those for serum CEA or SCC.32 In another report, the detection of SCCA mRNA could detect EC cells in peripheral blood, which is a useful predictor of recurrent disease.37 Nevertheless, all these results indicate that the power of these biomarkers for predicting recurrence is not sufficient for ESCC screening and has poor prognostic significance in those undergoing treatment. In addition, they focused on only one factor rather than considering all the related clinicopathological characteristics as a whole. Moreover, the cutoff values of these biomarkers are not definite and still need further investigation.
In the present study, the incidence of recurrence was significantly higher in patients with low REEC values than in those with high REEC levels. In addition, the sensitivity and specificity of REEC were higher than those of serum biomarkers. Interestingly, the relationship between the elevation of preoperative CEA and clinical outcome remains controversial.29,38-43 However, detecting recurrence at an early stage using these markers before the recurrent disease is diagnosed by imaging or symptom onset is difficult. Our study confirmed that the serum CEA level was a predictive factor related to recurrence and was included in the equation. Moreover, the REEC level was an independent factor for recurrence. In this series, a low REEC level was useful to predict recurrence even before operation; therefore, it may be a new index for the prediction of ESCC recurrence.
Moreover, in addition to the function of predicting recurrence similar to the tumor markers mentioned before, the REEC value can play a prognostic role in postoperative patients with ESCC. Patients in the high REEC group had a significantly longer DFS and OS than those with low REEC levels. Previous studies have shown that neoadjuvant chemotherapy is useful to decrease the size and stage of ESCC and can prolong OS and DFS.44,45 REEC, measured before neoadjuvant chemoradiotherapy (nCRT), is an independent predictor of final outcome in patients with ESCC, which may be useful in selecting patients suitable for nCRT to increase the outcome and decrease the recurrence rate.46
The treatment response of advanced recurrent ESCC is disappointing. Patients at high risk of developing recurrence need to be identified, and early diagnosis is essential to improve the curability and resectability of this cancer. Strategies aimed at decreasing the recurrence rate and improving survival must focus on patients with low REEC levels, such as adjuvant chemotherapy should be considered to be performed to these patients. A more meticulous lymphadenectomy and surgery with an extended field should be used when operating these patients, for the purpose of delay or prevention of recurrence. Computed tomography scans were effective at identifying subclinical recurrences; thus, for patients with low REEC values, follow-up should be more frequent during the first 2 years,14 and appropriate consideration of using endoscopic ultrasonography to monitor recurrence, which is better than upper endoscopy and CT scan for the evaluation of recurrence, should occur.47 Follow-up surveillance scans after the sixth year are likely unnecessary, but for people with low REEC values, this point should be prolonged to some extent.
However, there are some limitations in our study. Other authors have indicated32,48 that the pathologic T stage classification appears to be an important prognostic factor of recurrent disease, and the difference in TNM staging between patients who either suffered a recurrent disease or not is of statistical significance. However, the TNM stage was not included as a variate in our equation. However, as we used this equation in an exploratory group and verified it in a validation group, it is still being confirmed as a useful and high-efficiency index for predicting the prognosis of patient with ESCC. On the other hand, because patients with low REEC values had a higher risk of recurrence, meticulous follow-up is essential, and adjuvant therapy for these patients should be considered.
In conclusion, our study confirmed that CEA and LN metastasis are independent risk factors for recurrence. We also found that alcohol consumption is a novel risk factor for ESCC progression. Additionally, combining those factors into the REEC formula could serve as a useful predictive factor for outcome in patients with ESCC who undergo curative resection. We also reported here that a low REEC value may suggest a tendency for recurrent disease. Patients with low REEC values need to be accurately identified so that they can be offered entry into trials of multimodality therapy, which may contribute to decreasing the spreading potential of ESCC and increasing the survival of these patients.
Acknowledgment
The authors thank Shaohang Cai for his helpful assistance in this study.
Authors’ Note: Weidong Wang, Yongqiang Chen, and Lanjun Zhang contributed equally to this work. The study was approved by the Medical Ethics Committee and Clinical Trial Review Committee of Sun Yat-sen University Cancer Center. The approval number: GZR 2018-120. All patients included in this research were signed an informed consent according to the Research Ethics Committee of Sun Yat-Sen University Cancer Center. Data collection procedures in the present study was approved by the Research Ethics Committee of Sun Yat-Sen University Cancer Center.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Weidong Wang  https://orcid.org/0000-0002-1168-8803
https://orcid.org/0000-0002-1168-8803
References
- 1. Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol. 2003;4(8):481–488. [DOI] [PubMed] [Google Scholar]
- 2. Worni M, Martin J, Gloor B, et al. Does surgery improve outcomes for esophageal squamous cell carcinoma? An analysis using the surveillance epidemiology and end results registry from 1998 to 2008. J Am Coll Surg. 2012;215(5):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. [DOI] [PubMed] [Google Scholar]
- 4. RuRustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499–2509. [DOI] [PubMed] [Google Scholar]
- 5. Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18(12):3362–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. [DOI] [PubMed] [Google Scholar]
- 8. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. [DOI] [PubMed] [Google Scholar]
- 9. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252. [DOI] [PubMed] [Google Scholar]
- 10. Lepage C, Rachet B, Jooste V, Faivre J, Coleman MP. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103(11):2694–2699. [DOI] [PubMed] [Google Scholar]
- 11. Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–146. [DOI] [PubMed] [Google Scholar]
- 12. Xu Y, Yu X, Chen Q, Mao W. Neoadjuvant versus adjuvant treatment: which one is better for resectable esophageal squamous cell carcinoma? World J Surg Oncol. 2012;10:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu C, Hu Z, He Z, et al. Genome-wide association study identifies three new susceptibility loci for esophageal squamous-cell carcinoma in Chinese populations. Nat Genet. 2011;43(7):679–684. [DOI] [PubMed] [Google Scholar]
- 14. Lou F, Sima CS, Adusumilli PS, et al. Esophageal cancer recurrence patterns and implications for surveillance. J Thorac Oncol. 2013;8(12):1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan Z, Ma G, Yang H, Zhang L, Rong T, Lin P. Can lymph node ratio replace PN categories in the tumor–node–metastasis classification system for esophageal cancer? J Thorac Oncol. 2014;9(8):1214–1221. [DOI] [PubMed] [Google Scholar]
- 16. Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97(7):1616–1623. [DOI] [PubMed] [Google Scholar]
- 17. Abate E, DeMeester SR, Zehetner J, et al. Recurrence after esophagectomy for adenocarcinoma: defining optimal follow-up intervals and testing. J Am Coll Surg. 2010;210(4):428–435. [DOI] [PubMed] [Google Scholar]
- 18. Raoul JL, Le Prisé E, Meunier B, et al. Combined radiochemotherapy for postoperative recurrence of oesophageal cancer. Gut. 1995;37(2):174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morita M, Saeki H, Mori M, Kuwano H, Sugimachi K. Risk factors for esophageal cancer and the multiple occurrence of carcinoma in the upper aerodigestive tract. Surgery. 2002;131(suppl 1):S1–S6. [DOI] [PubMed] [Google Scholar]
- 20. Islami F, Kamangar F, Nasrollahzadeh D, et al. Socio-economic status and oesophageal cancer: results from a population-based case–control study in a high-risk area. Int J Epidemiol. 2009;38(4):978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee CH, Wu DC, Lee JM, et al. Carcinogenetic impact of alcohol intake on squamous cell carcinoma risk of the oesophagus in relation to tobacco smoking. Eur J Cancer. 2007;43(7):1188–1199. [DOI] [PubMed] [Google Scholar]
- 22. Morita M, Kumashiro R, Kubo N, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. Int J Clin Oncol. 2010;15(2):126–134. [DOI] [PubMed] [Google Scholar]
- 23. Cui R, Kamatani Y, Takahashi A, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137(5):1768–1775. [DOI] [PubMed] [Google Scholar]
- 24. Yokoyama T, Yokoyama A, Kumagai Y, et al. Health risk appraisal models for mass screening of esophageal cancer in Japanese men. Cancer Epidemiol Biomark Prev. 2008;17(10):2846–2854. [DOI] [PubMed] [Google Scholar]
- 25. Yokoyama A, Omori T, Yokoyama T, Sato Y, Kawakubo H, Maruyama K. Risk of metachronous squamous cell carcinoma in the upper aerodigestive tract of Japanese alcoholic men with esophageal squamous cell carcinoma: a long-term endoscopic follow-up study. Cancer Sci. 2008;99(6):1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kagemoto K, Urabe Y, Miwata T, et al. ADH1B and ALDH2 are associated with metachronous SCC after endoscopic submucosal dissection of esophageal squamous cell carcinoma. Cancer Med. 2016;5(7):1397–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka F, Yamamoto K, Suzuki S, et al. Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut. 2010;59(11):1457–1464. [DOI] [PubMed] [Google Scholar]
- 28. Yokoyama A, Hirota T, Omori T, et al. Development of squamous neoplasia in esophageal iodine-unstained lesions and the alcohol and aldehyde dehydrogenase genotypes of Japanese alcoholic men. Int J Cancer. 2012;130(12):2949–2960. [DOI] [PubMed] [Google Scholar]
- 29. Mealy K, Feely J, Reid I, McSweeney J, Walsh T, Hennessy TP. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol. 1996;22(5):505–507. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka K, Yano M, Motoori M, et al. CEA-antigen and SCC-antigen mRNA expression in peripheral blood predict hematogenous recurrence after resection in patients with esophageal cancer. Ann Surg Oncol. 2010;17(10):2779–2786. [DOI] [PubMed] [Google Scholar]
- 31. Munck-Wikland E, Kuylenstierna R, Lindholm J, Wahren B. Carcinoembryonic antigen, CA 19-9 and CA 50 in monitoring human squamous cell carcinoma of the esophagus. Anticancer Res. 1990;10(3):703–708. [PubMed] [Google Scholar]
- 32. Setoyama T, Natsugoe S, Okumura H, et al. , Carcinoembryonic antigen messenger RNA expression in blood predicts recurrence in esophageal cancer. Clin Cancer Res. 2006;12(20 pt 1):5972–5977. [DOI] [PubMed] [Google Scholar]
- 33. Duzgun O, Sarici IS. Preoperative CA125 value predicts Glisson capsule involvement in patients with peritoneal carcinomatosis undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Biomark Med. 2019. doi:10.2217/bmm-2019-0009 [DOI] [PubMed] [Google Scholar]
- 34. Koike M, Hibi K, Kasai Y, Ito K, Akiyama S, Nakao A. Molecular detection of circulating esophageal squamous cell cancer cells in the peripheral blood. Clin Cancer Res. 2002;8(9):2879–2882. [PubMed] [Google Scholar]
- 35. Tsuchiya Y, Onda M, Miyashita M, Sasajima K. Serum level of cytokeratin 19 fragment (CYFRA 21-1) indicates tumour stage and prognosis of squamous cell carcinoma of the oesophagus. Med Oncol. 1999;16(1):31–37. [DOI] [PubMed] [Google Scholar]
- 36. Kawaguchi H, Ohno S, Miyazaki M, et al. CYFRA 21-1 determination in patients with esophageal squamous cell carcinoma: clinical utility for detection of recurrences. Cancer. 2000;89(7):1413–1417. [DOI] [PubMed] [Google Scholar]
- 37. Kaganoi J, Shimada Y, Kano M, Okumura T, Watanabe G, Imamura M. Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg. 2004;91(8):1055–1060. [DOI] [PubMed] [Google Scholar]
- 38. Kosugi S, Nishimaki T, Kanda T, Nakagawa S, Ohashi M, Hatakeyama K. Clinical significance of serum carcinoembryonic antigen, carbohydrate antigen 19-9, and squamous cell carcinoma antigen levels in esophageal cancer patients. World J Surg. 2004;28(7):680–685. [DOI] [PubMed] [Google Scholar]
- 39. Nakashima S, Natsugoe S, Matsumoto M, et al. Clinical significance of circulating tumor cells in blood by molecular detection and tumor markers in esophageal cancer. Surgery. 2003;133(2):162–169. [DOI] [PubMed] [Google Scholar]
- 40. Shimada H, Nabeya Y, Okazumi S, et al. Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery. 2003;133(5):486–494. [DOI] [PubMed] [Google Scholar]
- 41. Miyamoto K, Kusumi T, Sato F, et al. Decreased expression of claudin-1 is correlated with recurrence status in esophageal squamous cell carcinoma. Biomed Res. 2008;29(2):71–76. [DOI] [PubMed] [Google Scholar]
- 42. Sung CO, Han SY, Kim SH. Low expression of claudin-4 is associated with poor prognosis in esophageal squamous cell carcinoma. Ann Surg Oncol. 2011;18(1):273–281. [DOI] [PubMed] [Google Scholar]
- 43. Usami Y, Chiba H, Nakayama F, et al. Reduced expression of claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum Pathol. 2006;37(5):569–577. [DOI] [PubMed] [Google Scholar]
- 44. Lee KW, Kim JH, Han S, et al. Twist1 is an independent prognostic factor of esophageal squamous cell carcinoma and associated with its epithelial–mesenchymal transition. Ann Surg Oncol. 2012;19(1):326–335. [DOI] [PubMed] [Google Scholar]
- 45. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 46. Kobayashi T, Teruya M, Kishiki T, et al. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008;144(5):729–735. [DOI] [PubMed] [Google Scholar]
- 47. Catalano MF, Sivak MV, Jr, Rice TW, Van Dam J. Postoperative screening for anastomotic recurrence of esophageal carcinoma by endoscopic ultrasonography. Gastrointest Endosc. 1995;42(6):540–544. [DOI] [PubMed] [Google Scholar]
- 48. Bhansali MS, Fujita H, Kakegawa T, et al. Pattern of recurrence after extended radical esophagectomy with three-field lymph node dissection for squamous cell carcinoma in the thoracic esophagus. World J Surg. 1997;21(3):275–281. [DOI] [PubMed] [Google Scholar]