Short abstract
To investigate the effect of Irbesartan on the changes of myocardial advanced glycation end products and their receptor (AGEs-RAGE), and matrix metalloproteinases (MMPs) systems in rat type 2 diabetes myocardial fibrosis model. All male Sprague-Dwaley rats were randomly divided into four groups: control (CON), high glucose and high-caloric diet (HC), type 2 diabetes (T2DM) and Irbesartan + T2DM (Ir+T2DM) groups. At 12th week, the fasting blood glucose (FBG) and fasting serum insulin (FINS) levels, insulin resistance index (IRI), insulin sensitivity index (ISI), body weight (BW), the ratio of heart weight/body weight (H/B), left ventricular weight index (LVWI), and cardiac col I, col III contents, plasma MMP-2, MMP-9 levels were evaluated. The protein expressions of col I, AGE, RAGE, MMP-2, MMP-14, and TIMP-2 were analyzed by Western blot. In the T2DM group, FBG, H/B, LVWI, IRI were increased (P < 0.01), while FINS, BW, ISI were decreased in contrast to the CON and HC groups (P < 0.05–0.01). In the Ir+ T2DM group, BW was higher, IRI, H/B, LVWI were lower than in the T2DM group. Compared with the CON and HC groups, the contents of col I and col III, the protein expressions of col I, AGE, RAGE, TIMP-2 and MMP-14 were increased, MMP-2 protein expression, the ratios of MMP-2/TIMP-2 and MMP-14/TIMP-2, MMP-2, and MMP-9 levels were decreased in the T2DM group (P < 0.01). After Irbesartan treatment, all parameters were reversed. Irbesartan can ameliorate myocardial fibrosis in type 2 diabetes rat model, the likely mechanisms may be related to the down-regulation of AGEs-RAGE system and changes of MMPs pathway.
Impact statement
There are about 425 million diabetes patients (20–79 years) in the world according to the International Diabetes Federation Diabetes Atlas – 8th Edition. The cardiovascular complication is one of the major causes of death in diabetes patients. Myocardial fibrosis is one of the serious pathological changes, so investigating the pathogenesis of myocardial fibrosis has the significant value. Our study aims to investigate the effect of Irbesartan (the angiotensin II receptor antagonist) on the changes of AGE-RAGE system and MMP family components, and analyzes the potential mechanisms in type 2 diabetes-induced myocardial fibrosis. Our results provide the theoretical base for better understanding the pathogenesis in type 2 diabetes-induced myocardial complication. It is useful for clinicians to select the effective therapeutic measures for treatment of type 2 diabetes-induced organ fibrosis.
Keywords: Type 2 diabetes mellitus, myocardial fibrosis, Irbesartan, advanced glycation end products and their receptor system, matrix metalloproteinases pathway
Introduction
The incidence of diabetes is increasing year by year. According to the International Diabetes Federation (IDF) data, the epidemiological survey results have indicated that there were more than 425 million diabetes patients, and it is estimated that there would be nearly 700 million diabetics by 2045.1 About 50% to 80% of diabetes patients died from cardiovascular complications.2–4 Therefore, how to effectively prevent the occurrence of or to delay diabetes induced cardiovascular complications is of great interest to researchers. Diabetic cardiomyopathy (DCM) is characterized by cardiac diastolic and/or systolic dysfunction, finally inducing heart failure. The pathogenesis of DCM is complicated, including metabolic abnormalities, renin-angiotensin-aldosterone system (RAAS) activation, myocardial fibrosis, and so on.5 It is well known that myocardial fibrosis progresses with increasing myofibroblast activity and excessive extracellular matrix (ECM) deposition, these are the important pathological changes affecting myocardial function. The occurrence of myocardial fibrosis involves many mechanisms. Our preliminary researches had found that enhanced aldehyde dehydrogenase 2 (ALDH2) expression could antagonize the occurrence of myocardial fibrosis in diabetic rats, which may be related to the downregulation of the c-Jun N-terminal kinase (JNK) pathway.6 Activation of ALDH2 could decrease high glucose- induced apoptosis and fibrosis through inhibition of oxidative stress in neonate rat cardiac fibroblasts.7 RAAS is considered as an important signal link in the process of myocardial fibrosis, and angiotensin II (Ang II) is one of the most important factors. Ang II can combine with angiotensin receptors (ATs) to stimulate and mediate the synthesis and secretion of collagen, eventually leading to the formation of myocardial fibrosis. Glucagon-like peptide 1 limits angiotensin II-induced cardiac fibrosis by modulating the expressions of AT1 and AT2 receptors, as well as the activity of angiotensin converting enzyme 2.8,9 There had been reported that Irbesartan had the beneficial effects against diabetes-induced myocardial damage, but the mechanisms were unclear.10 Irbesartan is one of the most common Ang II type 1 receptor (AT1R) antagonist. Compared with other AT1R antagonist such as Losartan and Olmesartan, Irbesartan has a long-lasting effect (the half-life is between 11 to 15 h), the potent and strongly selective AT1R blocking effect, and has the superior cardio-protective and renoprotective effects.11,12 So in the study, we want to observe intensively the likely protective mechanisms of Irbesartan on Type 2 diabetes-induced myocardial fibrosis.
It has been reported that the matrix metalloproteinases (MMPs) family, advanced glycation end products (AGEs), and their receptor (RAGE) systems both participate in myocardial fibrosis.13–15 The different enzymes in MMPs and the natural tissue inhibitors of metalloproteinases (TIMPs) contribute to normal and pathological tissue remodeling, especially the synthesis and decomposition of collagen. In the MMPs family, the different components play the different roles.16 Among the MMPs family members, MMP-2 is expressed by cardiomyocytes, endothelial cells, vascular smooth muscle cells, macrophages, and fibroblasts. It has the high constitutive activity, and is considered as a MMP housekeeping gene to regulate normal tissue turnover. MMP-9 regulates tissue remodeling by directly degrading ECM and activating cytokines and chemokines. MMP-14 is expressed in cardiomyocytes, macrophages, and fibroblasts, and it can degrade collagen, fibronectin, and gelatin, leading to the structure and support loss of ECMs. The MMPs activity is regulated by TIMPs.17,18 AGEs-RAGE systems also play a central role in the pathogenesis of organ fibrosis occurrence. AGEs initiate myocardial complications through regulating the structural and functional alteration of ECM proteins, as well as regulating the intracellular signaling molecules.19 Type 2 diabetes mellitus (T2DM) is a long-term metabolic disorder which is characterized by hyperglycemia, insulin resistance, and relative lack of insulin, the insulin-resistance state is closely related to myocardial fibrosis. About 90% diabetes cases belong to T2DM.20,21 What happens of MMPs and AGE-RAGE systems in T2DM-induced myocardial fibrosis? Whether MMPs and AGE-RAGE systems participate in Irbesartan’s anti-myocardial fibrosis role? It is worth further investigation.
Therefore, in this paper, we selected the combination of high glucose and high-caloric diet with a low dose of streptozotocin to duplicate T2DM-mimic rat model22 and induce the cardiovascular complications, then to analyze the roles of MMP family and AGEs-RAGE system in Irbesartan’s cardioprotection. It will be beneficial for Irbesartan’s clinical application against DCM.
Materials and methods
Animals
Healthy male Sprague-Dawley (SD) rats weighing 160–180 g were obtained from Ai Er Mai Te Technology Co. Ltd of Suzhou Industrial company (Suzhou, China) [Reg. no. SCXK (su2009-001)].
The rats were allocated into two dietary regimens by feeding either basic diet (6% fat, 23% protein and 64% carbohydrate) or high glucose and high-caloric diet (67% basic diet, 20% sugar, 10% fat, 2.5% cholesterol, 0.5% sodium cholate). The animal experiments were conducted in accordance with the guidelines established by the Guide for the Care and Use of Laboratory Animals of Bengbu Medical College.
Chemicals and drugs
Streptozotocin (STZ) was purchased from Sigma-Aldrich (St. Louis, MO, USA), Irbesartan tablets were purchased from Hengrui Pharmaceutical Co., Ltd (Jiangsu, China). ImmobilonTM Western chemiluminescent HRP substrate was purchased from Millipore (USA). Bicinchoninic acid (BCA) protein assay kit was purchased from Beyotime Institute of Biotechnology (Shanghai, China). Rabbit anti-AGE, RAGE, MMP-14, col I polyclonal antibodies were purchased from Abcam (USA). Rabbit anti-MMP-2, TIMP-2 polyclonal antibodies were purchased from Beijing Biosynthesis Biotechnology Co., Ltd (Bioss, Bejing, China). Secondary anti-rabbit antibody was purchased from Boster (Wuhan, China). ELISA kits for rat cardiac col I, col III, plasma insulin, MMP-2, and MMP-9 detections were purchased from Calvin biological science and Technology Co., Ltd (Suzhou, China).
Experimental groups
After one week of acclimation to laboratory conditions, 24 male SD rats were randomly divided into four groups (n = 6): (1) control (CON) group, the rats were fed with the basic diet for 16 weeks; (2) high glucose and high-caloric diet (HC) group, the rats were fed with high glucose and high-caloric diet for 16 weeks; (3) T2DM-mimic (T2DM) group, for duplicating the T2DM-mimic model, based on four weeks high glucose and high-caloric diet feeding, the rats were injected with 30 mg/kg STZ intraperitoneally after fasting for 12 h. Rats with fasting blood glucose (FBG) >300.86 mg/dl (16.7 mmol/L) in three consecutive days following polydipsia, polyphagia, and polyuria symptoms were considered as diabetic23; and (4) Irbesartan (Ir) + T2DM group, the T2DM rats were administered with Irbesartan at 50 mg/kg/d intragastrically for 12 weeks.24,25 After T2DM rats modeled successfully, the rats in the latter two groups were fed with high glucose and high-caloric diet continuously.
FBG, FINS, IRI, ISI, BW, H/B, and LVWI measurement
At the 16th week, the FBG level of all rats was measured. The blood was collected in EDTA anticoagulant tube and centrifuged for 30 min at 1100g at 4°C to collect serum, and the fasting serum insulin (FINS) level was measured at 450 nm by ELISA using a commercial assay kit according to the manufacturer’s instruction. Insulin resistance index (IRI) was calculated as FBG (mmol/L) × FINS (mIU/L)/22.5. Insulin sensitivity index (ISI) was calculated as 1/[FBG (mmol/L) × FINS (mIU/L)].26
After the body weight (BW) was measured, rats were anesthetized with a single intraperitoneal injection of 4% chloral hydrate (1 mL/100 g). The chests were opened, hearts were rapidly removed, and washed with pre-cooled normal saline. Thereafter, heart weight (HW) and left ventricular weight were measured. The ratio of HW to BW (H/B, mg/g) was used as the cardiac index, and the ratio of left ventricular weight to body weight was measured and taken to be the left ventricular weight index (LVWI, mg/g).
Plasma TG and TC levels measurement
Plasma total cholesterol (TG) and triglyceride (TC) levels in different groups were measured by the clinical laboratory of the first affiliated hospital of Bengbu medical college.
Myocardial interstitial and perivascular fibrosis observations by Masson-trichrome staining method
For observing the changes of myocardial interstitial and perivascular fibrosis, the ventricles were carefully dissected, weighed, fixed in 10% neutral formalin at room temperature for 48–72 h. The myocardial tissues were dehydrated in an increasing alcohol series, immersed in benzene for 20 min, and embedded in paraffin for Masson-trichrome staining.
Cardiac and plasma parameters measurement
Myocardial tissue samples were homogenized, then centrifuged at 1100g at 4°C for 10 min, then the supernatants were collected. The levels of cardiac col I, col III were measured, using ELISA kits according to the manufacturer’s instructions, along with plasma MMP-2 and MMP-9 level measurements.
Western blot analysis
Myocardial tissues (100 mg) were homogenized, and centrifuged at 12,000g for 30 min at 4°C. The supernatants were normalized for equal amounts of total protein as determined by BCA protein assay kit. Protein of each sample was separated on SDS-PAGE. Thereafter, proteins in SDS-PAGE were transferred to a PVDF membrane (Millipore, Bedford, MA) at 380 mA for 90 min. Then membranes were blocked with 5% nonfat milk buffer in TBS-T for 120 min at room temperature and incubated with primary antibodies – AGE, RAGE, col I, MMP-14, MMP-2, and TIMP-2 at 37°C for 30 min and overnight at 4°C. After four washings with TBS-T, membranes were incubated with the corresponding horseradish peroxidase (HRP)-linked anti-mouse immunoglobulin (IgG) or HRP-linked anti-rabbit IgG secondary antibody for 60 min. Protein bands were analyzed by an ECL chemiluminescence system.
Statistical analysis
The results were expressed as mean ± SEM. Statistical analyses were performed using Prism 6. Differences among different groups were evaluated by one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls post hoc test, P < 0.05 was considered as statistically significant.
Results
Changes of FBG, FINS, IRI, ISI, BW, H/B, LVWI in different groups
The rat’s BW in the HC group was increased compared to that in the CON group (P < 0.01), whereas there were no significant changes in FBG, FINS, IRI, ISI, H/B, and LVWI between the two groups. Compared to the rats in the CON and HC groups, in the T2DM group, FINS, ISI, and BW were decreased, However, FBG, IRI, H/B, and LVWI were increased (P < 0.01), which suggested insulin resistance was happened in the T2DM rats, and the ventricular volume was enlarged. In the Ir+ T2DM group, FBG was higher than in the CON and HC groups, while Irbesartan increased BW, and decreased IRI, H/B, and LVWI, respectively (P < 0.01); however, no significant difference was shown in FBG and FINS in contrast to the T2DM group. Irbesartan had the trend to increase ISI, but no statistical difference compared with the T2DM group (Table 1).
Table 1.
Changes of FBG, FINS, IRI, ISI, BW, H/B, and LVWI in different groups.
| Group | FBG (mmol/L) | FINS (mIU/L) | IRI | ISI (×104) | BW (g) | H/B (mg/g) | LVWI (mg/g) |
|---|---|---|---|---|---|---|---|
| CON | 5.27 ± 0.35 | 36.39 ± 3.61 | 8.53 ± 1.22 | 52.75 ± 7.01 | 289.22 ± 9.77 | 2.79 ± 0.06 | 1.53 ± 0.05 |
| HC | 6.03 ± 0.75 | 36.05 ± 4.81 | 9.64 ± 1.50 | 46.87 ± 7.54 | 321.33 ± 8.17** | 2.84 ± 0.04 | 1.58 ± 0.05 |
| T2DM | 25.60 ± 2.46**^^ | 29.11 ± 4.03*^ | 32.83 ± 1.75**^^ | 13.56 ± 0.74**^^ | 233.56 ± 10.73**^^ | 4.30 ± 0.06**^^ | 2.21 ± 0.07**^^ |
| Ir+T2DM | 23.95 ± 1.54**^^ | 27.93 ± 3.75 | 29.54 ± 2.17## | 15.11 ± 1.11**^^ | 284.89 ± 12.23## | 3.42 ± 0.08## | 1.88 ± 0.05## |
CON: control group; HC: high glucose and high-caloric diet group; T2DM: Type 2 diabetes mellitus mimic model group; Ir+T2DM: Irbesartan + Type 2 diabetes mellitus mimic model group; FBG: fasting blood glucose concentration; Fins: fasting serum insulin concentration; IRI: insulin resistance index; ISI: insulin sensitivity index; BW: body weight; H/B: the ratio of heart weight to body weight; LVWI: left ventricular weight index (the ratio of left ventricular weight to body weight).
*P < 0.05, **P < 0.01 vs. CON; ^P < 0.05, ^^P < 0.01 vs. HC; ##P < 0.01 vs. T2DM.
Changes of plasma TC and TG concentrations in different groups
Compared with the CON group, plasma TG and TC concentrations were increased in the HC, T2DM and Ir+ T2DM groups, respectively (P < 0.01). Compared with the T2DM group, there were no obvious changes of plasma TG and TC levels in the Ir+ T2DM group (Table 2).
Table 2.
Changes of TG and TC concentrations in different groups.
| Group | TG (mmol/L) | TC (mmol/L) |
|---|---|---|
| CON | 0.64 ± 0.19 | 1.38 ± 0.11 |
| HC | 9.32 ± 0.89** | 10.98 ± 0.56** |
| T2DM | 9.31 ± 2.46** | 10.96 ± 1.70** |
| Ir+T2DM | 9.38 ± 3.90** | 10.74 ± 1.27** |
CON: control group; HC: high glucose and high-caloric diet group; T2DM: Type 2 diabetes mellitus mimic model group; Ir+T2DM: Irbesartan + Type 2 diabetes mellitus mimic model group; TG: total cholesterol; TC: triglyceride.
**P < 0.01 vs. CON.
Changes of myocardial morphology and fibrosis in different groups
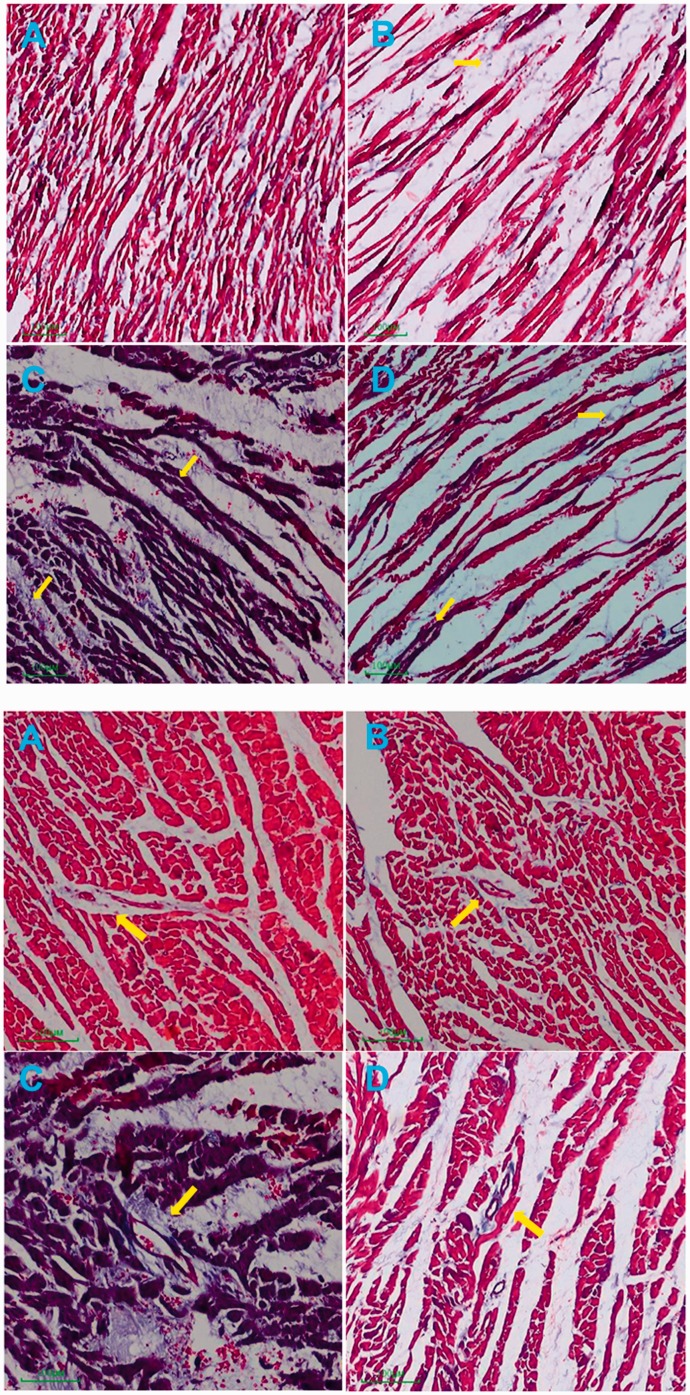
After Masson-trichrome staining, the myocardial cells presented red and collagen fibers presented blue. The myocardium in the CON group was orderly arranged and evenly distributed, the number of collagen fibers was few and scattered. In the HC group, the myocardial interstitial and perivascular collagen fibers were slightly increased, and partial myocardial fibers were ruptured. In the T2DM group, the myocardial fibers were ruptured and disordered, collagen fibers in myocardial interstitium and around the vessel were increased. In the Ir+ T2DM group, myocardial morphology was improved and the number of collagen fibers was decreased (Figure 1).
Figure 1.
Changes of myocardial interstitial and perivascular fibrosis in different groups (Original magnification: × 100). (a) control group (CON); (b) high glucose and high-caloric diet group (HC); (c) Type 2 diabetes mellitus mimic model group (T2DM); (d) Irbesartan + Type 2 diabetes mellitus mimic model group (Ir+ T2DM).
Changes of cardiac col I, col III concentrations in different groups
At the end of the 12th week, when compared with the CON and HC groups, in the T2DM group, the cardiac col I, col III concentrations were increased, as well as the ratio of col I/col III. In the Ir+ T2DM group, cardiac col I, col III concentrations, and the ratio of col I/col III were lower (P < 0.01) (Table 3).
Table 3.
Changes of cardiac col I, col III concentrations and the ratio of col I/col III in different groups.
| Group | col I(ng/mL) | col III (ng/mL) | col I/col III |
|---|---|---|---|
| CON | 13.19 ± 0.89 | 3.36 ± 0.26 | 3.92 ± 0.04 |
| HC | 13.67 ± 0.89 | 3.49 ± 0.01 | 3.90 ± 0.24 |
| T2DM | 40.69 ± 1.15**^^ | 7.92 ± 0.26**^^ | 5.13 ± 0.28**^^ |
| Ir+T2DM | 18.95 ± 0.78## | 4.68 ± 0.30## | 4.06 ± 0.35## |
CON: control group; HC: high glucose and high-caloric diet group; T2DM: Type 2 diabetes mellitus mimic model group; Ir+T2DM: Irbesartan + Type 2 diabetes mellitus mimic model group.
**P < 0.01 vs. CON; ##P < 0.01 vs. T2DM; ^^P < 0.01 vs. HC.
Changes of plasma MMP-2 and MMP-9 levels in different groups
Plasma MMP-2 and MMP-9 levels were decreased in the T2DM group when compared with the CON and HC groups (P < 0.01). In the Ir+ T2DM group, plasma MMP-2 and MMP-9 levels were higher than those in the T2DM group (P < 0.01) (Table 4).
Table 4.
Changes of plasma MMP-2 and MMP-9 levels in different groups.
| Group | MMP-2 (ng/mL) | MMP-9 (ng/mL) |
|---|---|---|
| Con | 232.08 ± 17.89 | 72.99 ± 3.21 |
| HC | 239.44 ± 18.14 | 72.87 ± 1.53 |
| T2DM | 91.26 ± 10.48**^^ | 31.95 ± 2.06**^^ |
| Ir+T2DM | 185.02 ± 7.72## | 55.99 ± 2.23## |
CON: control group; HC: high glucose and high-caloric diet group; T2DM: Type 2 diabetes mellitus mimic model group; Ir+T2DM: Irbesartan + Type 2 diabetes mellitus mimic model group.
**P < 0.01 vs. CON; ##P < 0.01 vs. T2DM; ^^P < 0.01 vs. HC.
Changes of cardiac col I, AGE, RAGE, MMP-2, MMP-14 and TIMP-2 proteins, the ratio of MMP-2/TIMP-2 and the ratio of MMP-14/TIMP-2 in different groups
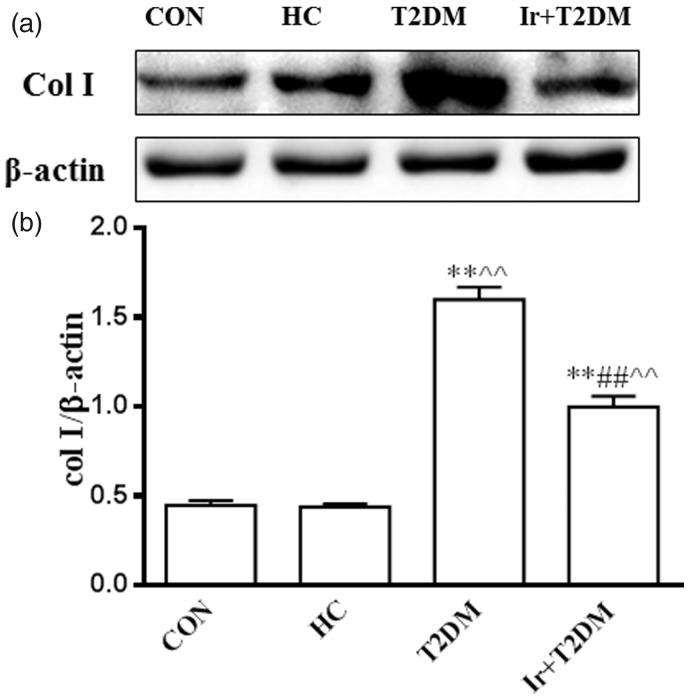
Compared with the CON and HC groups, the col I protein expression was increased in the T2DM group (P < 0.01), and decreased after Irbesartan administration in the T2DM rats (P < 0.01) (Figure 2).
Figure 2.
Changes of cardiac col I protein expression in different groups (a) and the ratio of col I/β-actin (b). CON: control group; HC: high glucose and high caloric diet group; T2DM: Type 2 Diabetes mellitus mimic model group; Ir+ T2DM: Irbesartan + Type 2 Diabetes mellitus mimic model group. **P < 0.01 vs CON; ##P < 0.01 vs T2DM; ^^P < 0.01 vs HC.
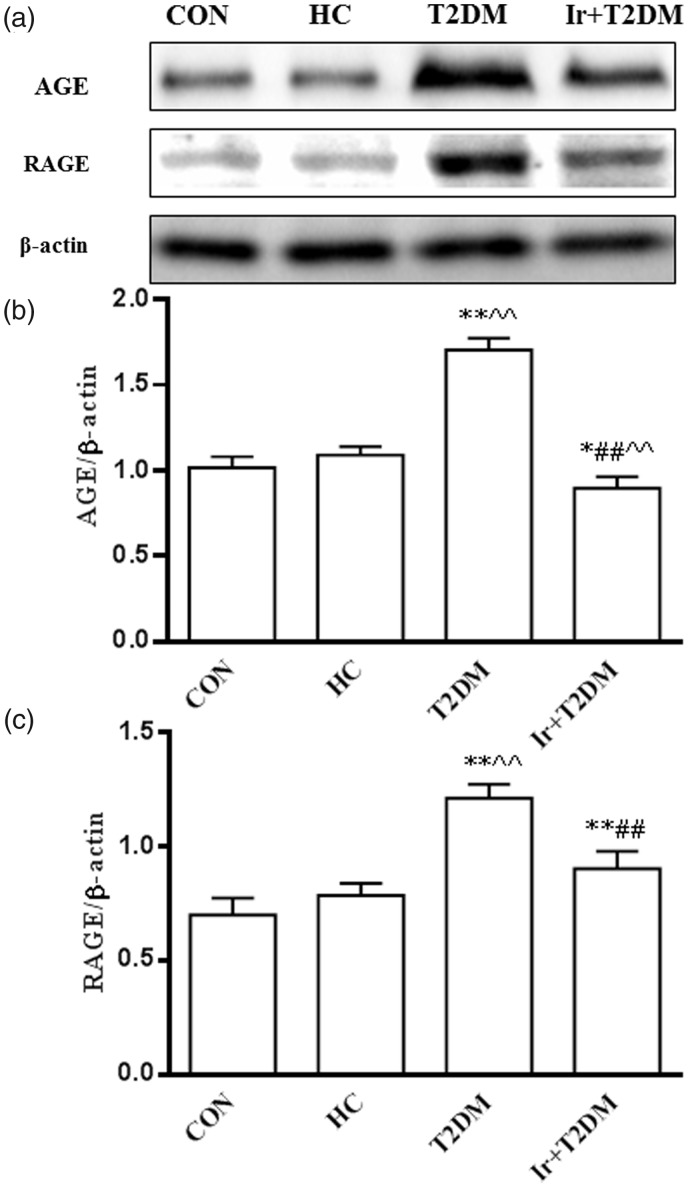
The expressions of AGE and RAGE proteins were increased in the T2DM rats compared with the HC group (P < 0.01). Irbesartan decreased AGE and RAGE protein expressions in the T2DM rats (P < 0.01) (Figure 3).
Figure 3.
Changes of cardiac AGE and RAGE protein expressions in different groups (a), the ratio of AGE/β-actin (b), and the ratio of RAGE/β-actin (c). CON: control group; HC: high glucose and high caloric diet group; T2DM: Type 2 Diabetes mellitus mimic model group; Ir+ T2DM: Irbesartan + Type 2 Diabetes mellitus mimic model group. *P < 0.05, **P < 0.01 vs CON; ##P < 0.01 vs T2DM; ^^P < 0.01 vs HC.
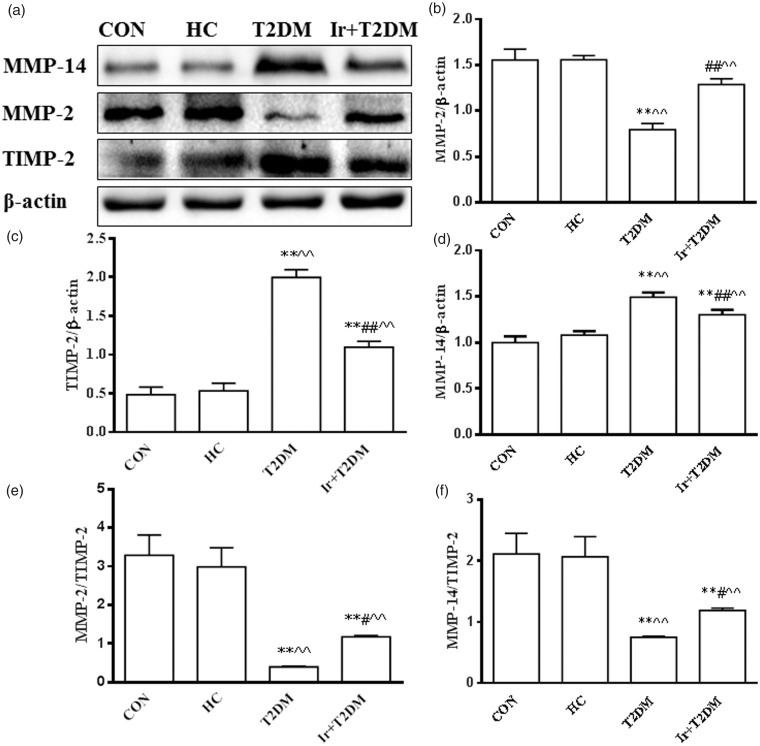
In the T2DM rats, cardiac MMP-2 protein expression and the ratio of MMP-2/TIMP-2 were decreased, while MMP-14 and TIMP-2 protein expressions were increased when compared with the HC group (P < 0.01), but the ratio of MMP-14/TIMP-2 was decreased. Irbesartan increased MMP-2 protein expression, the ratio of MMP-2/TIMP-2 and the ratio of MMP-14/TIMP-2, while it decreased TIMP-2, MMP-14 protein expressions in the T2DM rats (P < 0.01) (Figure 4).
Figure 4.
Changes of cardiac MMP-14, MMP-2 and TIMP-2 protein expressions in different groups (a), the ratio of MMP-2/β-actin (b), the ratio of TIMP-2/β-actin (c), the ratio of MMP-14/β-actin (d), the ratio of MMP-2/TIMP-2 (e) and the ratio of MMP-14/TIMP-2 (f) in different groups by western blot. CON: control group; HC: high glucose and high caloric diet group group; T2DM: Type 2 Diabetes mellitus mimic model group; Ir+ T2DM: Irbesartan + Type 2 Diabetes mellitus mimic model group. *P < 0.05, **P < 0.01 vs CON; #P < 0.05 and ##P < 0.01 vs T2DM; ^^P < 0.01 vs HC.
Discussion
Myocardial fibrosis is an important process of cardiac remodeling which leads to heart failure and death.27–29 With the development of diabetes, myocardial fibrosis occurs accompanied with the increase of cardiac collagen fibers.30 How to reverse myocardial fibrosis is of particular interest to researchers. Despite the presence of many therapies to decrease the degree of myocardial fibrosis, the effective measures are still lacking. It is widely recognized that Irbesartan has a protective effect against myocardial fibrosis.25 Some groups have reported that Irbesartan can reduce the degrees of cardiomyopathy injury and fibrosis in the diabetic rat model,10 but the mechanisms are unclear. It is reported Irbesartan attenuates AGEs-mediated damage in diabetes-associated bone damage and kidney injury,31,32 some members of MMPs family such as MMP-2 participate in the Irbesartan’s kidney-protection33; however, the changes involved in the MMPs family and the AGE-RAGEs system are unclear in the mechanism of Irbesartan’s cardio-protection. Since MMPs family and AGE-RAGEs system both regulate organ remodel and fibrosis, so in our study, we observed the changes of MMPs family and the AGE-RAGEs system in Irbesartan’s protection against T2DM-induced myocardial injury. Our results showed Irbesartan improved the diabetes-induced myocardial fibrosis through inhibiting the AGE-RAGE pathway, increasing MMP-2, MMP-9 expression, and decreasing TIMP-2, MMP-14 expressions.
In our study, the results showed that when the type 2 diabetes rats were fed for 12 weeks, in addition to the pathological changes, the myocardial interstitial and perivascular collagen fibers were increased, the cardiac col I and col III levels, ratio of col I/col III and col I protein expression were also increased. These results suggested that myocardial fibrosis is a significant process in type 2 diabetic rats. RAAS is an important signal pathway in the process of myocardial fibrosis. Ang II is one of the main members of RAAS. In cardiac fibroblasts, Ang II can combine with the receptor to mediate interstitial collagen’s synthesis, induce some inflammation factors release such as TGF-1, and eventually lead to ventricular hypertrophy, fibrosis, and myocardial remodeling.34,35 Irbesartan, as an antagonist of Ang II receptor, can block the biological effect of Ang II at the receptor level.36 In contrast to the T2DM rats, after the T2DM rats were treated with Irbesartan, the body weight increased, IRI, H/B, and LVWI decreased, ISI had the increasing trend, and these results suggested Irbesartan could improve T2DM-induced weight loss and myocardial hypertrophy. We speculated in Ir + T2DM group, the target tissues such as liver, skeletal muscle, and adipocyte increased the sensitivity to insulin and promoted the material synthesis, then increased the T2DM rat’s body weight. Moreover, cardiac col I, col III levels, and the ratio of col I/col III levels were decreased, it suggested Irbesartan could alleviate T2DM-induced myocardial fibrosis. This result was in keeping with other’s reports.10
AGEs refer to the amino, nucleic acids, or lipids spontaneously reacting with glucose or other reducing sugars without enzyme to produce a stable complex. Long-term hyperglycemia levels in the diabetic condition can lead to the excessive accumulation of AGEs.37 Hyperglycemia increases the glycosylation of collagen, increases the accumulation of AGEs cross-linking, and AGEs combine with their receptor RAGEs, and then promote collagen accumulation and fibroblast proliferation, decrease collagen degradation, and eventually lead to myocardial fibrosis.15 AGEs can also decrease the elasticity of collagen, increase the stiffness of the ventricular wall, and reduce ventricular compliance. So decreasing the expressions of AGE and RAGE protein can reduce the occurrence of myocardial fibrosis. In our study, the results showed that with the increase of cardiac col I, the levels of myocardial AGE and RAGE protein expressions were increased in the 12 week T2DM rats, while they were decreased when the T2DM rats were treated with Irbesartan, suggesting that Irbesartan may decrease myocardial fibrosis through inhibiting the AGE-RAGE pathway.
Another system—MMPs and the tissue inhibitor TIMPs both participate in collagen degradation and regulate the dynamic balance of ECM in vivo. Different MMPs family members play the different roles. MMPs are generally expressed at low levels in normal conditions, and the members will up-regulate or down-regulate during tissue remodeling, inflammation, wound healing.38 The changes of MMPs are a hallmark of myocardial fibrosis in diabetes.39,40 MMP-2 and MMP-9 had been widely reported in the pathological changes of diabetes,41 but other MMPs role in diabetes had undefined. In our study, besides observing the changes of MMP-2, MMP-9, we also observed the changes of MMP-14 and TIMPs, because these members are closely related to each other. MMP-2 was assayed as an indicator of ECM degradation. MMP-2 activation is mediated by MMP-14 and inhibited by TIMP-2. On the other hand, MMP-9 activation is mediated by MMP-2 and other MMPs members.42 MMP-2 and MMP-14 can degrade col I, col II, and col III. Active MMP-14 is inhibited by TIMP-2.43 In our study, we observed that in the T2DM s rats, the plasma MMP-2 and MMP-9 levels and cardiac MMP-2 protein expression were decreased with the increase of TIMP-2 protein expression. The ratio of MMP-2/TIMP-2 was also decreased, and the results suggested that in the T2DM rat model, hyperglycemia could increase TIMP-2 protein expression, then induced the decrease of MMP-2 and MMP-9, the occurrence of myocardial fibrosis was related with the weakening of ECM degradation, the ability to degrade collagen was reduced in T2DM condition. When the T2DM rats were administered with Irbesartan, along with a decrease of TIMP-2, plasma MMP-2, and MMP-9 levels, the ratio of MMP-2/TIMP-2 was increased, suggesting the inhibiting role of TIMP-2 was alleviated, and the increases of MMP-2 and MMP-9 accelerated the degradation of ECM, so that the occurence of cardiac fibrosis was attenuated.
In our experiment, a controversial finding is that in the T2DM rats, the cardiac MMP-14 protein expression was increased, but the ratio of MMP-14/TIMP-2 was decreased compared to the rats in the CON and HC groups. Irbesartan decreased MMP-14 protein expression and increased the ratio of MMP-14/TIMP-2. Hiden et al.44 had also reported that in the first trimester of type 1 diabetes, MMP-14 expression was increased. We speculated that in 12 weeks of T2DM rats, myocardial fibrosis had occurred with the accumulation of collagen, despite the increase of MMP-14 could induce collagen degradation, but the ratio of MMP-14/TIMP-2 was decreased in the T2DM rats, suggesting to some extent, TIMP-2 could also inhibit the role of MMP-14, maybe the synthesis, degradation, and reconstruction of myocardial ECM were simultaneously occurring, which lead to the complexity of myocardial fibrosis process. Irbesartan improves ventricular remodeling in the diabetic condition through decreasing MMP-14 expression and increasing the ratio of MMP-14/TIMP-2, thus finally protecting the heart.
To summarize, Irbesartan, acting as an Ang II receptor antagonist can weaken the synthesis of myocardial collagen, and the mechanisms may include down-regulation of AGEs and the RAGE family; down-regulation of TIMP-2, MMP-14 protein expression, up-regulation of MMP-2 and MMP-9 protein expressions to reduce collagen synthesis, finally affecting collagen metabolism and promoting collagen degradation, then protecting the rats against type 2 diabetes-induced myocardial fibrosis.
Limitations
While we had observed the mechanisms about Irbesartan’s cardioprotection against type 2 diabetes, but there had some limitations in the study. MMPs family is a large family, we just observe some components not all MMPs components, and not analyze the complex relationships in detail. In the future, we will select some interventions to change MMPs expression or AGEs-RAGE expression, to do more intensive investigation on the relationship of MMPs family and AGEs-RAGE system. The mechanism investigation may be beneficial for Irbesartan’s clinical application.
Authors’ contributions
Conceived, designed the experiments, and wrote the manuscript: Ye Hongwei, Hu Junfeng, Zhang Heng. Performed the experiments: Ye Hongwei, Cao Ruiping, Fang Yingyan, Zhang Guanjun, Hu Jie, Liu Xingyu, Tang Jie. Analyzed the data: Li Zhenghong, Gao Qin. Reviewed and approved the manuscript: All authors.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This study was supported by research grants from the Natural Science Foundation of China (grant no. 81770297), Anhui Province University Top-Notch Talent Project (grant no. gxbjZD18), Anhui Province Natural Science Foundation (1808085MH280) and Anhui Province Education Key Project (grant no. KJ2017A221).
References
- 1.New IDF figures show continued increase in diabetes across the globe, reiterating the need for urgent action. International Diabetes Federation (IDF) 14 November 2017. https://www.idf.org/news/94:new-idf-figures-show-continued-increase-indiabetes-across-the-globe,-reiterating-the-need-for-urgentaction.html
- 2.Coccheri S. Approaches to prevention of cardiovascular complications and events in diabetes mellitus. Drugs 2007; 67:997–1026 [DOI] [PubMed] [Google Scholar]
- 3.Kovacic JC, Castellano JM, Farkouh ME, Fuster V. The relationships between cardiovascular disease and diabetes: focus on pathogenesis. Endocrinol Metab Clin North Am 2014; 43:41–57 [DOI] [PubMed] [Google Scholar]
- 4.Alvarez CA, Lingvay I, Vuylsteke V, Koffarnus RL, McGuire DK. Cardiovascular risk in diabetes mellitus: complication of the disease or of antihyperglycemic medications. Clin Pharmacol Ther 2015; 98:145–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 2004; 25:543–67 [DOI] [PubMed] [Google Scholar]
- 6.Yu Y, Jia XJ, Zhang WP, Fang TT, Hu J, Ma SF, Gao Q. The Protective effect of low-dose ethanol on myocardial fibrosis through downregulating the JNK signaling pathway in diabetic rats. J Diabetes Res 2016; 2016:3834283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu X, Fang T, Kang P, Hu J, Yu Y, Li Z, Cheng X, Gao Q. Effect of ALDH2 on high glucose-induced cardiac fibroblast oxidative stress, apoptosis, and fibrosis. Oxid Med Cell Longev 2017; 2017:9257967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang LH, Pang XF, Bai F, Wang NP, Shah AI, McKallip RJ, Li XW, Wang X, Zhao ZQ. Preservation of glucagon-like peptide-1 level attenuates angiotensin II-induced tissue fibrosis by altering AT1/AT 2 receptor expression and angiotensin-converting enzyme 2 activity in rat heart. Cardiovasc Drugs Ther 2015; 29:243–55 [DOI] [PubMed] [Google Scholar]
- 9.Patel VB, Bodiga S, Basu R, Das SK, Wang W, Wang Z, Lo J, Grant MB, Zhong J, Kassiri Z, Oudit GY. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ Res 2012; 110:1322–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Xu Q, Wang X, Zhao Z, Zhang L, Zhong L, Li L, Kang W, Zhang Y, Ge Z. Irbesartan ameliorates diabetic cardiomyopathy by regulating protein kinase D and ER stress activation in a type 2 diabetes rat model. Pharmacol Res 2015; 93:43–51 [DOI] [PubMed] [Google Scholar]
- 11.Watanabe R, Suzuki J, Wakayama K, Kumagai H, Ikeda Y, Akazawa H, Komuro I, Isobe M. Angiotensin II receptor blocker irbesartan attenuates cardiac dysfunction induced by myocardial infarction in the presence of renal failure. Hypertens Res 2016; 39:237–44 [DOI] [PubMed] [Google Scholar]
- 12.Vignier N, Le Corvoisier P, Blard C, Sambin L, Azibani F, Schlossarek S, Delcayre C, Carrier L, Hittinger L, Su JB. AT1 blockade abolishes left ventricular hypertrophy in heterozygous cMyBP-C null mice: role of FHL1. Fundam Clin Pharmacol 2014; 28:249–56 [DOI] [PubMed] [Google Scholar]
- 13.Münch J, Avanesov M, Bannas P, Säring D, Krämer E, Mearini G, Carrier L, Suling A, Lund G, Patten M. Serum matrix metalloproteinases as quantitative biomarkers for myocardial fibrosis and sudden cardiac death risk stratification in patients with hypertrophic cardiomyopathy. J Card Fail 2016; 22:845–50 [DOI] [PubMed] [Google Scholar]
- 14.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 2016; 90:84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Randive R, Stewart JA. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J Diab 2014; 5:860–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinale FG, Janicki JS, Zile MR. Membrane-associated matrix proteolysis and heart failure. Circ Res 2013; 112:195–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afratis NA, Selman M, Pardo A, Sagi I. Emerging insights into the role of matrix metalloproteases as therapeutic targets in fibrosis. Matrix Biol 2018; 68-69:167–79 [DOI] [PubMed] [Google Scholar]
- 18.DeLeon-Pennell KY, Meschiari CA, Jung M, Lindsey ML. Matrix metalloproteinases in myocardial infarction and heart failure. Prog Mol Biol Transl Sci 2017; 147:75–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukami K, Yamagishi S-I, Okuda S. Role of AGEs-RAGE system in cardiovascular disease. Curr Pharm Des 2014; 20:2395–402 [DOI] [PubMed] [Google Scholar]
- 20.Li L, Sheng X, Zhao S, Zou L, Han X, Gong Y, Yuan H, Shi L, Guo L, Jia T, Liu S, Wu B, Yi Z, Liu H, Gao Y, Li G, Li G, Zhang C, Xu H, Liang S. Nanoparticle-encapsulated emodin decreases diabetic neuropathic pain probably via a mechanism involving P2X3 receptor in the dorsal root ganglia. Purinergic Signal 2017; 13:559–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol 2008; 51:93–102 [DOI] [PubMed] [Google Scholar]
- 22.Fuentes-Antrás J, Picatoste B, Gómez-Hernández A, Egido J, Tuñón J, Lorenzo Ó. Updating experimental models of diabetic cardiomyopathy. J Diabetes Res 2015; 2015:656795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding L, Qu Z, Chi J, Shi R, Wang L, Hou L, Wang Y, Pang S. Effects of preventative application of metformin on bile acid metabolism in high fat-fed/streptozotocin-diabetic rats. Int J Clin Exp Pathol 2015; 8:5450–6 [PMC free article] [PubMed] [Google Scholar]
- 24.Dai HY, Zheng M, Tang RN, Ni J, Ma KL, Li Q, Liu BC. Effects of angiotensin receptor blocker on phenotypic alterations of podocytes in early diabetic nephropathy. Am J Med Sci 2011; 341:207–14 [DOI] [PubMed] [Google Scholar]
- 25.Tang RN, Lv LL, Zhang JD, Dai HY, Li Q, Zheng M, Ni J, Ma KL, Liu BC. Effects of angiotensin II receptor blocker on myocardial endothelial-to-mesenchymal transition in diabetic rats. Int J Cardiol 2013; 162:92–9 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Gao Y, Duan L, Wei S, Liu J, Tian L, Quan J, Zhang Q, Liu J, Yang J. Metformin ameliorates skeletal muscle insulin resistance by inhibiting miR-21 expression in a high-fat dietary rat model. Oncotarget 2017; 8:98029–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Gao L, Zhao XY, Guo S, Liu YZ, Li R, Liang C, Li L, Dong JZ, Li LN, Yang HB. Saikosaponin a protects from pressure overload-induced cardiac fibrosis via inhibiting fibroblast activation or endothelial cell EndMT. Int J Biol Sci 2018; 14:1923–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci 2017; 38:448–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, Lin J, Bagchi RA. Novel molecular therapeutic targets in cardiac fibrosis: a brief overview. Can J Physiol Pharmacol 2018; 2019;97:246–256 [DOI] [PubMed] [Google Scholar]
- 30.Benedicto HG, Bombonato PP, Macchiarelli G, Stifano G, Prado IM. Structural arrangement of the cardiac collagen fibers of healthy and diabetic dogs. Microsc Res Tech 2011; 74:1018–23 [DOI] [PubMed] [Google Scholar]
- 31.Cheng YZ, Yang SL, Wang JY, Ye M, Zhuo XY, Wang LT, Chen H, Zhang H, Yang L. Irbesartan attenuates advanced glycation end products-mediated damage in diabetes-associated osteoporosis through the AGEs/RAGE pathway. Life Sci 2018; 205:184–92 [DOI] [PubMed] [Google Scholar]
- 32.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Beneficial effects of metformin and irbesartan on advanced glycation end products (AGEs)-RAGE-induced proximal tubular cell injury. Pharmacol Res 2012; 65:297–302 [DOI] [PubMed] [Google Scholar]
- 33.Liu BC, Xu Y, Ma KL, Huang HQ, Yin LF, Liu DG. Effects of irbesartan on the expression of matrix metalloproteinase-2/tissue inhibitor of metalloproteinase-2 in streptozotocin-induced diabetic rat kidney. Chin Med J 2005; 118:1040–4 [PubMed] [Google Scholar]
- 34.Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, Sánchez-López E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant 2006; 21:16–20 [DOI] [PubMed] [Google Scholar]
- 35.Al-Badri A, Hashmath Z, Oldland GH, Miller R, Javaid K, Syed AA, Ansari B, Gaddam S, Witschey WR, Akers SR, Chirinos JA. Poor glycemic control is associated with increased extracellular volume fraction in diabetes. Diabetes Care 2018; 41:2019–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie JY, Chen N, Ren H, Wang WM. Angiotensin II-mediated activation of fibrotic pathways through ERK1/2 in rat peritoneal mesothelial cells. Ren Fail 2010; 32:871–9 [DOI] [PubMed] [Google Scholar]
- 37.Lin X, Chen X, Ye J, Li Q, Zhou J, Wu X, Huang Y, Li X, Shi Y, Li S, Li L, Cai H. Association between endogenous secretory receptor for advanced glycation-end products (esRAGE) and carotid intima-media thickness in type 2 diabetes. Exp Clin Endocrinol Diabetes 2014; 122:277–80 [DOI] [PubMed] [Google Scholar]
- 38.Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J 2011; 278:28–45 [DOI] [PubMed] [Google Scholar]
- 39.Abreu BJ, de Brito Vieira WH. Metalloproteinase changes in diabetes. Adv Exp Med Biol 2016; 920:185–90 [DOI] [PubMed] [Google Scholar]
- 40.Berry E, Bosonea AM, Wang X, Fernandez-Patron C. Insights into the activity, differential expression, mutual regulation, and functions of matrix metalloproteinases and a disintegrin and metalloproteinases in hypertension and cardiac disease. J Vasc Res 2013; 50:52–68 [DOI] [PubMed] [Google Scholar]
- 41.Silva FS, Bortolin RH, Araújo DN, Marques DES, Lima Jpms Rezende AA, Vieira WHB, Silva NB4, Medeiros KCP, Ackermann PW, Abreu BJ, Dias F. Exercise training ameliorates matrix metalloproteinases 2 and 9 messenger RNA expression and mitigates adverse left ventricular remodeling in streptozotocin-induced diabetic rats. Cardiovasc Pathol 2017; 29:37–44 [DOI] [PubMed] [Google Scholar]
- 42.Cerofolini L, Fragai M, Luchinat C. Mechanism and inhibition of matrix metalloproteinases. Curr Med Chem 2018; doi: 10.2174/0929867325666180326163523 [DOI] [PubMed] [Google Scholar]
- 43.Xu J, Liu XJ, Li L, Zhang SH, Li Y, Gao RJ, Zhen YS. An engineered TIMP2-based and enediyne-integrated fusion protein for targeting MMP-14 shows potent antitumor efficacy. Oncotarget 2015; 6:26322–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiden U, Glitzner E, Ivanisevic M, Djelmis J, Wadsack C, Lang U, Desoye G. MT1-MMP expression in first-trimester placental tissue is upregulated in type 1 diabetes as a result of elevated insulin and tumor necrosis factor-alpha levels. Diabetes 2008; 57:150–7 [DOI] [PubMed] [Google Scholar]