Short abstract
Retinol isotope dilution (RID) is used to estimate total body vitamin A (VA) stores in groups to assess VA status. Metabolic differences during lactation may affect RID calculations as currently applied. We evaluated the time required for isotopic equilibration between serum and liver retinol in piglets, and the utility of milk retinol isotopic enrichment as a proxy for serum in lactating sows. Piglets (n = 24) and sows (n = 6) were fed 1.75 or 20 µmol 13C2-retinyl acetate, respectively. Piglets (n = 5 or 7) were killed on d 0, 4, 7, or 14. Blood and milk were collected at d 0, 0.5, 1, 2, 4, 7, 10, 14, and 21 before the sows were killed to collect liver. Retinol 13C-enrichment was determined by gas chromatography-combustion-isotope ratio mass spectrometry. Equilibration time and RID-predicted liver VA reserves were calculated. In piglets, serum and liver retinol 13C-enrichment differed significantly in individuals at d 4 and 7 (P = 0.008, 0.03) but not d 14 (P = 0.06); however, mean values were not different by d 4 (P = 0.62). Current RID equations accurately predicted VA deficiency (means ≤0.027 µmol/g liver) in the piglets. In sows, milk and serum retinol 13C-enrichment reached equilibrium between 2 and 7 d post-dose. After correcting for dose lost to milk, RID equations predicted higher liver stores than measured values even though the serum to liver atom % was 1.00 ± 0.01 at kill. In VA deficient infants, a shorter period may be accurate in population-level RID studies when using appropriate assumptions. In lactating women, the RID may have decreased accuracy due to variable losses of tracer in milk. Furthermore, assumptions about storage and loss of the dose in milk must be evaluated in lactating women considering the observed discrepancy between predicted and measured stores.
Impact statement
Vitamin A (VA) deficiency and hypervitaminosis A have been reported in groups of people worldwide. Conventional biomarkers of VA deficiency (e.g. serum retinol concentration, dose response tests) are not able to distinguish between sufficiency and hypervitaminosis A. Retinol isotope dilution (RID) predictions of VA status have been validated in humans and animal models from deficiency through toxicity; however, RID during life stages with unique issues related to isotopic tracing, such as infancy and lactation, requires further evaluation. This study investigated RID in piglets and lactating sows as models for human infants and women. In piglets, RID successfully determined VA deficiency (confirmed with liver analysis), and that the tracer mixes quickly. Conversely, in lactating sows, although serum and milk enrichments were similar, traditional RID equations overestimated VA stores, likely due to losses of tracer and higher extrahepatic VA storage than predictions. These data inform researchers about the challenges of using RID during lactation.
Keywords: Infancy, lactation, retinol isotope dilution, stable carbon isotope, swine, vitamin A
Introduction
Vitamin A (VA) deficiency is a major problem worldwide, especially in Southeast Asia and sub-Saharan Africa.1 The World Health Organization (WHO) currently defines a country as having a severe risk of VA deficiency if the prevalence of low serum retinol concentrations (<0.7 μmol/L) in children less than 6 y is greater than 20%2; however, this biomarker suffers from low sensitivity when compared with retinol isotope dilution (RID) in children.3,4 Liver biopsy is considered the gold standard for VA status,3 but collecting liver samples is difficult and unethical in most situations in humans. To avoid this, the RID test has been used to indirectly predict total body VA stores (TBS) and estimate total liver VA reserves (TLR) using appropriate assumptions.5
In the RID test, a dose of isotopically labeled VA (usually retinyl acetate labeled with deuterium or 13C) is consumed and allowed to mix with TBS before a blood sample is taken. The time period for equilibration is less for rats or humans with VA deficiency than VA adequacy,6,7 and efforts have been made to sample at time points as early as 3 d.8,9 Following the mixing period, the ratio of labeled retinol to endogenous retinol (tracer-to-tracee ratio; TTR) can be determined from baseline 13C-natural abundance, enrichment of the dose, and final 13C enrichment of retinol in the tissue as described by Cobelli.10 From this and the initial dose size of labeled compound, which is corrected for absorption efficiency and catabolism over time, the principle of mass balance can be used to determine the size of the turning-over pool of endogenous retinol.11
RID-estimated TLR have been validated against liver samples using rats,6 rhesus monkeys,12 and two human trials in which liver was accessible for other reasons, such as an unrelated surgery7,13; however, these trials examined healthy, non-lactating adults. Lactation increases demand for VA in women because they supply VA to their infant through breast milk.14 Whether from breast milk or elsewhere, maintaining adequate VA status during infancy is especially important for satisfactory growth and immune function.3 Therefore, it should be considered a priority to verify the accuracy of VA status measurement in infants and women. During lactation, RID is affected by irreversible loss of the tracer to milk and likely altered storage and mobilization of the vitamin. The RID is also affected by differences in VA metabolism in infants and children with different VA status; for example, the half-life of VA was determined to be 32 d in Peruvian children with low VA stores15 compared with 136 d in Zambian children with high TBS16 and 135–140 d in healthy adults.17 Researchers have used the 32-d-estimated half-life in Ghanaian infants with adequate VA status18 and Thai children with VA deficiency.19
Lactating sows and weanling piglets were chosen for this study because of the anatomical and physiological similarity of their gastrointestinal tracts to humans, similar VA metabolism, and access to liver samples.20 In addition, this model was previously used in VA studies.20–23 In particular, piglets are a good model for human infants because of their similar body weights and low VA stores at birth.24 In these experiments, we evaluated the 13C-RID test in weanling piglets as a model for infants, and in lactating sows as a model for nursing mothers, to gain insight into VA metabolism during these life stages.
Methods
Animals and diets
Approval for these studies was obtained from the University of Wisconsin-Madison Research Animal Resources Center. A total of 15 sows (crossbreeds of Large White and Landrace) were housed at the Swine Research and Teaching Center in Arlington, WI. Sows were fed standard feed until the time of their first or second pregnancy, and then maintained on a maize-based, preformed VA-free feed for multiple gestation-lactation cycles for the purpose of depleting their liver VA stores.20 Weanling piglets from the second parity after the sows were switched to the preformed VA-free feed (n = 24, 2 from each of 12 sows, Table 1) were placed on a maize-based feed,25 for the duration of the study, including a 10-d weaning period prior to dosing (Figure 1(a)). The type of yellow field maize available for feed mixing typically contains a small amount of the provitamin A carotenoid β-cryptoxanthin but was otherwise devoid of VA activity. After two parities consuming the maize-based VA-free feed, 6 sows (Table 1) were chosen for the lactation study and placed on a wheat-based VA-free feed20 for their third or fourth gestation-lactation cycle (this study, Figure 1(b)). They consumed this feed until the end of the study when they were killed. All sows were given 2 kg feed/d during gestation and allowed ad libitum access during lactation.
Table 1.
Characteristics of weanling piglets and lactating sows dosed with 13C-retinyl acetate.a
| Characteristic | Weanling piglets (n = 24) | Lactating sows (n = 6) |
|---|---|---|
| Age at dosing | 17 d | 3 y |
| Prior parities of sows, n | 2 | 3.3 ± 0.5 |
| Body weight at time of death, kg | 7.1 ± 2.6 | 271 ± 21 |
| Liver weight, kg | 0.22 ± 0.07 | 2.7 ± 0.2 |
| Baseline serum retinol, µmol/L | 0.83 ± 0.41 | 1.28 ± 0.38 |
aValues are means ± SD.
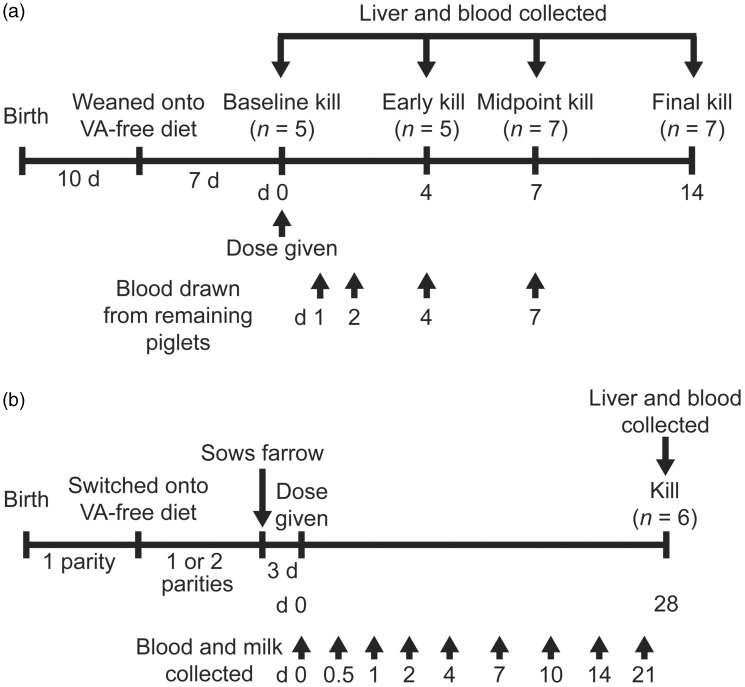
Figure 1.
Timeline of work performed in (a) weanling piglets and (b) lactating sows.
Dose preparation, administration, and sample collection
[14, 15]-13C2-retinyl acetate was synthesized as previously described.26 After an overnight fast, piglets were orally dosed with 1.75 μmol 13C2-retinyl acetate in corn oil. Blood samples (7 mL) were collected on d 1, 2, and 4 by cervical venipuncture. Piglets were killed on d 0 (n = 5, predose), d 4 (n = 5), d 7 (n = 7) and d 14 (n = 7) by electrical stunning and exsanguination, and blood and whole liver were collected (Figure 1(a)).
Sows were fasted overnight 1–2 d after farrowing and orally dosed with 20 μmol 13C2-retinyl acetate dissolved in 4.7 mL corn oil by syringe. The sows were observed to ascertain that the entire dose was consumed. Sows were not anesthetized during dose administration. Blood (10 mL, as described above) and milk (40 mL) samples were collected at d 0 (predose), 0.5, 1, 2, 4, 7, 10, 14, and 21 (Figure 1(b)). Before milk collection, piglets were removed to a heated creep to maximize available milk. Sows were injected with 40 IU oxytoxin and fore- and hindmilk were collected from 1 or more teats to obtain the desired volume. On d 28, sows were killed and blood and whole liver were collected. All samples were stored on dry ice before transfer to a −80°C freezer.
Serum, milk, and liver retinol analysis
Serum, milk, and liver retinol were determined by HPLC as previously described.25 Serum (1 to 1.5 mL) was treated with ethanol to precipitate proteins, and retinol was extracted with three hexanes, pooled, and evaporated under argon.27 Milk (1 mL) was mixed with ethanol (1.5 mL) and saponified at 45°C for 2 h using 800 μL 50:50 potassium hydroxide:water (mass:vol). Retinol was extracted with 3 × 1 mL hexanes. Liver samples (∼1.5 g using at least three sampling sites) were weighed, mixed with 4–5 g NaSO4, ground with a mortar and pestle, and extracted with 25 mL dichloromethane, from which 5 mL was dried, saponified, and extracted. The organic extract was evaporated under argon and resuspended in 100 μL 50:50 methanol:dichloroethane (vol:vol) and 10–100 μL (depending on concentration) was injected on the first of two HPLC systems as published.27 Retinol concentration was determined at 325 nm using an external standard curve; C23-β-apo-carotenol28 was used as the internal standard for all analyses.
Analysis of 13C-enrichment of retinol
Determination of 13C atom percent of retinol, calculated as 13C/(12C + 13C) X 100, was performed by gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS) as previously described.6,27 The purified retinol was dried by centrifugation in a ThermoSavant SpeedVac system (Thermo Scientific, Waltham, MA), resuspended in hexanes, and injected on the GC. As retinol elutes from the GC column, it is completely combusted to CO2 and H2O and the ratio of produced 13CO2 to 12CO2 was determined by a magnetic-sector mass spectrometer.
Determination of distribution of labeled and endogenous retinol
The TTR, TBS, and TLR were calculated as previously described11
| (1) |
where Fa is the proportion of 13C in the isotopic tracer (experimentally determined to be 0.11), Fb and Fc are the GC-C-IRMS-determined 13C-enrichment of serum retinol at baseline and post-dose, respectively.
| (2) |
where a is the amount of isotopic dose given (in μmol). Absorption is typically assumed to be 75–100% in RID16,29 based on studies in rats,30 non-human primates,31 and humans;32 75% was selected here due to the larger dose sizes used in comparison with other studies using 13C-labeled retinol and GC-C-IRMS analysis. Irreversible degradation of the remaining dose was assumed to occur according to a half-life of 140 d in adults for sows13,17,33 or 32 d in children with low VA stores for piglets.15,19 Specific activity between serum and liver was assumed to be 1 by d 14 because they were receiving VA-deficient feed11 (an assumption that was examined in the piglets).
| (3) |
where liver mass was measured directly. Fifty percent of VA stores were assumed to be in the liver due to the low TLR expected in these swine.11
Further correction for dose lost to milk in lactating sows by day x was performed by the following formula using a trapezoidal approximation of daily tracer in milk
| (4) |
where t is the timepoint in days, and d is the number of days since the preceding timepoint. TTRt is the GC-C-IRMS-determined tracer-to-tracee ratio in milk at time t, therefore “TTRt/(1+TTRt)” yields the proportion of retinol that is tracer at time t, milk total Rt is the concentration of retinol (tracer + tracee) in milk at time t determined by HPLC (in μmol/L), the initial dose was 20 μmol and milk production was assumed to be 10 L/d.20
Dose lost to milk reduces retention in addition to the non-lactating human-adult dose degradation assumed by a half-life of 140 d; therefore, the retention factor in TBS (2) at any given timepoint x that is d days since the last timepoint is estimated by
| (5) |
where retention at d 0 is 1.
Statistical analysis
Statistical analyses were performed in Microsoft Excel (Microsoft, Redmond, WA). Group values are mean ± SD. Comparisons between serum and liver retinol 13C-enrichment in piglets were performed using Student’s t-test, paired and unpaired as noted, while liver and serum retinol concentrations were compared using least-squares linear regression. Individual sow enrichment data and predicted/measured TLR values are presented and compared by paired Student’s t-test, and group means are used when appropriate. Significance was defined as P ≤ 0.05.
Results
Isotopic equilibration of labeled retinol, and predicted and actual liver stores in piglets
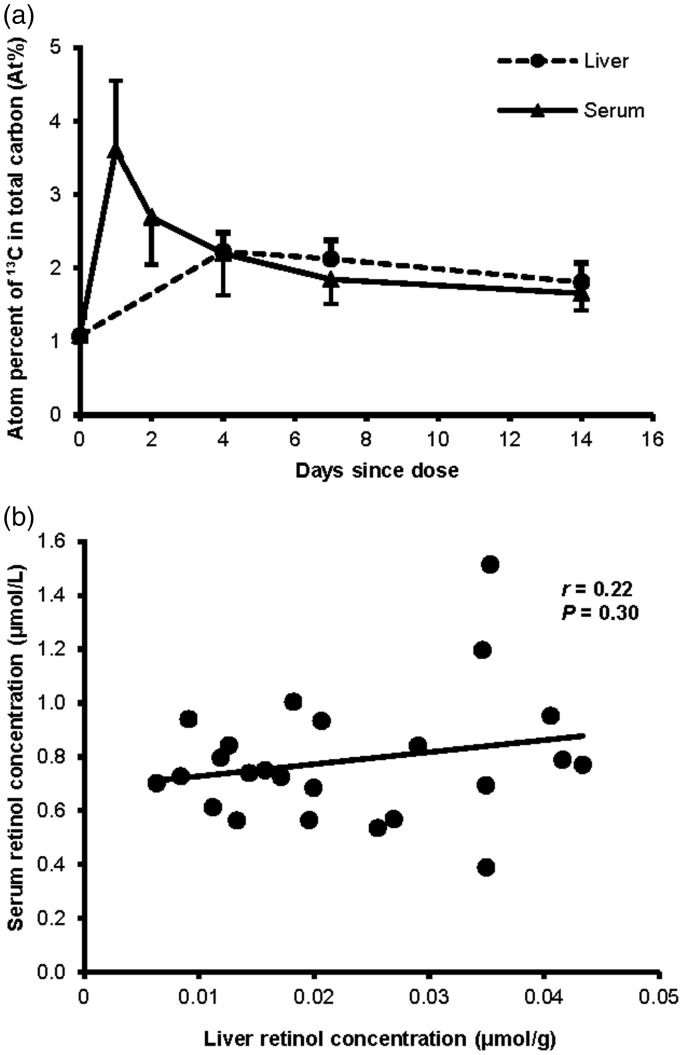
The ratio of 13C-enrichment in serum and liver in the weanling piglets was measured at d 4, 7, and 14 after dosing with 1.75 μmol 13C2-retinyl acetate using GC-C-IRMS (Table 2, Supplemental Table 1). When comparing paired liver and serum samples from each piglet, the difference in 13C-enrichment between the two VA pools was significant at d 4 (P = 0.008) and 7 (P = 0.03), and a trend occurred at d 14 (P = 0.062). Comparing mean enrichment of the two pools at each time point (Figure 2(a)), similar to determining the mean VA status in a large group, showed no difference at any time point after dosing. Serum retinol concentrations did not correlate with liver retinol concentration in these piglets (Figure 2(b)).
Table 2.
13C-enrichment in matched serum and liver retinol and total liver reserves (TLR) of vitamin A in piglets determined by retinol isotope dilution (RID) or liver sample, by day since dosing with 13C2-retinyl acetate.a
| Time since dose (d) | n | Serum retinol 13C-enrichment (At%) | Liver retinol 13C-enrichment (At%) | P b | Serum-to-liver ratio of retinol 13C-enrichment | Predicted TLR (µmol/g) | Measured TLR (µmol/g) |
|---|---|---|---|---|---|---|---|
| 4 | 5 | 2.43 ± 0.65 | 2.22 ± 0.59 | 0.008 | 1.09 ± 0.04 | 0.016 ± 0.010 | 0.020 ± 0.011c |
| 7 | 7 | 1.97 ± 0.16 | 2.12 ± 0.26 | 0.03 | 0.93 ± 0.05 | 0.026 ± 0.029 | 0.027 ± 0.008c |
| 14 | 7 | 1.64 ± 0.28 | 1.81 ± 0.36 | 0.06 | 0.91 ± 0.08 | 0.021 ± 0.021 | 0.013 ± 0.005c |
At%: atom percent; TLR: total liver reserves of vitamin A.
aValues are means ± SD. 13C enrichment values were determined by gas chromatography-combustion-isotope ratio mass spectrometry as previously described.6 Predicted total liver reserves were calculated using serum retinol 13C enrichment by retinol isotope dilution calculations (see text). Measured total liver reserves were determined by high pressure liquid chromatography.
bPaired-sample Student’s t-test.
cPredicted and measured TLR values were not significantly different, determined by paired-sample Student’s t-test.
Figure 2.
(a) 13C-enrichment in weanling piglet serum retinol and liver (mean ± SD) expressed as At% by days since dose, determined by GC-C-IRMS. For the four timepoints with both liver and serum data (d 0, liver n = 5, serum n = 19; d 4, liver n = 5, serum n = 19; d 7, liver n = 7, serum n = 10; d 14, n = 7), mean values are not significantly different by two-sample student’s t-test. Liver samples were all collected from different piglets at death, while serum was taken at death and from blood draws in remaining piglets. At%, atom percent of 13C in total carbon. (b) Liver and serum retinol concentrations of piglets. Retinol concentrations were determined by HPLC. Liver total retinol was determined by summing retinol and retinyl esters. The slope of the line is not significantly different from zero using Least Squares Regression.
Although the main focus of the piglet study was isotopic equilibration, TLR were estimated by RID at each time point and compared to HPLC-determined liver VA concentration (Table 2). Calculated values were not different from measured liver VA reserves at any time point (paired Student’s t-test), and RID accurately determined the piglets to be VA deficient [<0.1 μmol VA/g liver3] in all cases.
Isotopic equilibration of labeled retinol, and predicted and actual liver stores in sows
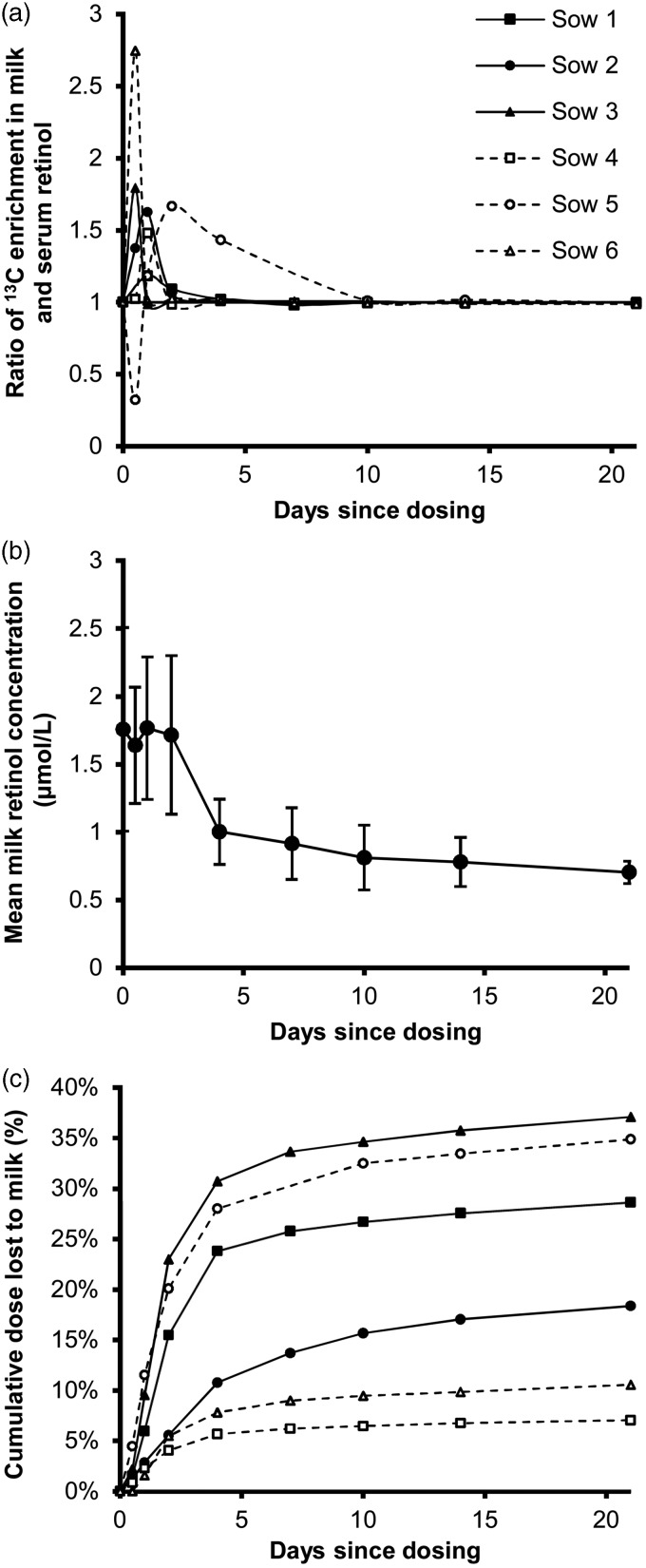
13C-enrichment of serum and milk retinol from the sows was determined at each time point (d 0, 0.5, 1, 2, 4, 7, 10, 14, 21, and 28) and the ratio of the two was plotted over time (Figure 3(a)). Retinol concentrations in sow milk were initially high (1.76 ± 0.75 μmol/L) but decreased after d 2 (1.00 ± 0.24 μmol/L) (Figure 3(b)). Because the quantity of 13C-labeled dose present in milk was determined to be high before equilibrating with whole body stores, and milk is produced at a rate of approximately 10 L/d in lactating sows, the cumulative proportion of the dose lost to milk over time was determined and plotted (Figure 3(c)). Final serum and liver 13C-enrichments were compared after kill at d 28, showing that complete equilibrium had occurred with a final ratio of 13C-enrichment of serum to that in liver of 1.00 ± 0.01 (Table 3).
Figure 3.
(a) Ratio of 13C-enrichment in milk and serum retinol, determined by gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS), of lactating sows (n = 6) after a 20 μmol dose of 13C2-retinyl acetate. At%, atom percent of 13C in total carbon. (b) Milk concentration at each sampling timepoint following dosing. Error bars represent SD. (c) Estimated cumulative percent of the 13C2-retinol dose lost through the milk of lactating sows, determined by GC-C-IRMS, which was calculated as the area under the curve, determined by trapezoidal approximation, of the product of the retinol concentration and tracer-tracee ratio of 13C2 enrichment of milk retinol over time, multiplied by average milk production of a sow [10 L/d]20.
Table 3.
Predicted and measured total liver vitamin A reserves (TLR), and 13C enrichment of lactating sow serum and liver retinol after 21 and 28 days of isotopic equilibration of a 20 μmol 13C2-retinyl acetate dose.
|
21-d timepoint |
28-d timepoint (measured at death) |
||||||
|---|---|---|---|---|---|---|---|
| Sow | Serum-predicted TLR (µmol/g) | Milk-predicted TLR (µmol/g) | Measured TLRa (µmol/g) | Serum At%b | Liver At%b | Ratio | |
| 1 | 0.50 | 0.41 | 0.14 | 1.110 | 1.103 | 0.99 | |
| 2c | 0.67 | 0.52 | – | 1.114 | – | – | |
| 3 | 0.49 | 0.46 | 0.34 | 1.112 | 1.111 | 1.00 | |
| 4 | 3.81 | 2.99 | 0.18 | 1.087 | 1.083 | 1.00 | |
| 5 | 0.25 | 0.23 | 0.10 | 1.124 | 1.116 | 0.99 | |
| 6 | 1.89 | 0.83 | 0.37 | 1.093 | 1.088 | 1.00 | |
| Meand | 1.27 ± 1.37e | 0.91 ± 1.04e | 0.23 ± 0.12 | 1.105 ± 0.015f | 1.100 ± 0.014f | 1.00 ± 0.01 | |
At%: atom percent; TLR: total liver reserves of vitamin A.
aDetermined by high pressure liquid chromatography.
bDetermined by gas chromatography-combustion-isotope ratio mass spectrometry.
cThe liver from sow 2 was missing at the point of supply and could not be analyzed.
dValues are mean ± SD.
eValues were not significantly different by paired sample t-test.
fValues were not significantly different by paired sample t-test. Neither mean includes sow 2.
Total liver VA stores were determined by RID with a 21-d equilibration period using either milk or serum values as the accessible pool and compared with actual liver values (Table 3). No difference was noted in the calculated TLR using milk or serum retinol 13C enrichment. In this case, the retention of dose used in the RID calculations incorporated loss of dose to milk and irreversible degradation from one timepoint to the next. Sows 4 and 6 had particularly large discrepancies between calculated and measured TLR (5–20 times more predicted liver VA than measured and likely reflects loss of tracer during dose administration).
Discussion
In this study, we examined the equilibration of a tracer dose of 13C2-retinyl acetate in weanling pigs as a model for infants. Similar to adult rats,6 these piglets had a significantly different isotopic enrichment in liver and serum VA before 14 d as the tracer enters serum from absorption and is then taken up by storage organs. In this case, however, there was a crossover point at approximately 4 d (Figure 2(a)), after which the serum retinol 13C-enrichment was maintained below the liver value, although it is no longer significantly different at d 14. This pseudo-equilibrium, in which the dose is preferentially retained in the liver due to new, incoming unlabeled VA in the diet and/or sequestration of a large dose, has been seen in earlier RID studies, leading to the inclusion of a specific activity factor of 0.65–0.80 in RID equations when these factors are present.7,11,34 In the absence of either of those two factors, it has been predicted11,35 and shown in rats and monkeys6,31 that complete equilibrium (specific activity of 1) can be achieved. In this study, feed was used that contained a small amount of β-cryptoxanthin but was otherwise VA-deficient, which resulted in deficiency (liver VA stores ≤0.043 μmol/g) in these piglets. Furthermore, the dose used was relatively larger than other studies in children (1.75 versus 1.0 μmol currently being used in children), potentially leading to its preferential storage in the liver, and equilibrium specific activities below 1 at 0.93 and 0.91 on d 7 and 14, respectively. However, this discrepancy did not affect the calculated versus actual measured TLR values.
Differences in liver and serum retinol 13C-enrichment values indicate that a specific activity factor is still needed in studies with large doses, but the inaccessibility of liver in infants and children means that determination of this factor is unlikely to progress in humans due to ethical issues. The natural conclusion is therefore to use small doses that are not sequestered in the liver whenever possible; however, these smaller doses can push the limits of detection of most current mass spectrometers used with RID, e.g., GCMS and liquid chromatography-MS/MS.36 Data are needed comparing dose equilibration and sequestration using different doses of 13C-retinol before undertaking large-scale studies. Regardless, assuming a specific activity factor of 1, the mean RID-predicted TLRs in the piglets did not differ from measured TLR at any time point. Given that serum and liver retinol 13C-enrichment crossover had occurred by d 4 and were not different when using the unpaired mean 13C-enrichment in each pool, it follows that mean TBS and therefore TLR would be accurate. This finding gives support to the use of the RID in population-level studies with many subjects, where the impact of random error and the inter-individual variation in VA kinetics, would be minimized.37,38
Adequate VA status of lactating women is vital to providing infants with VA-adequate breast milk. Two aspects of lactation physiology are important to the RID. First, if isotopic equilibration between serum and milk retinol occurs in a similar timeframe as that between liver and serum retinol, milk could be used as an accessible pool to sample 13C-enrichment of retinol before and after the dose, increasing the acceptability of the test, because blood draws can be a significant deterrent to participation in studies.39 In this study, serum and milk achieved this equilibrium between d 10 and d 21, yielding 21-d milk- and serum-derived TLR estimates that did not differ, and was likely maintained because a VA-deficient feed was consumed.11
Second, we discovered during analysis that the studied sows lost significant amounts of the labeled retinol dose to milk, which impacts the mass balance calculations. Previous studies have shown that chylomicron delivery of retinol to milk could account for loss of 25% of a newly ingested dose within 3 d23 in addition to the redistribution of body stores to milk as needed from retinol bound to retinol-binding protein. This effect is compounded by the high concentration of retinol in milk (>1 μmol/L) early in the lactation cycle, which did not decrease until 5–6 d after farrowing (2 d after dosing), an effect consistent with previous observations.40 Using α-retinol, a positional isomer of retinol that cannot bind retinol-binding protein and therefore represents only lipoprotein delivery, it was demonstrated that dietary retinol delivery to milk peaks around 8 h,23 which was before the first post-dose milk retinol 13C-enrichment measured in this study (0.5 d). This could result in significant quantities of dose lost before correction was possible. While we estimated the fraction of dose lost at any given point that data were collected, and corrected the TBS calculations, these values varied substantially between individual sows. Monitoring breast-milk tracer concentrations more frequently in the initial period after isotope administration could result in more accurate loss calculations. In a population survey setting, it may be inaccurate to correct by a single factor for loss of dose to breast milk. On the other hand, milk production of sows [7.5–13.6 L/d20] is much higher than that for humans [0.5–0.9 L/d41] due to the size of the litter. The loss of dose, therefore, should be quantified in human studies before conclusions can be drawn about the RID in lactation, given that losing 7–37% of the dose results in changes in predicted TBS by the same percent. In this regard, non-human primates that usually have singletons would be a more applicable model to use for lactation studies than multiparous mammals, such as swine. Furthermore, waiting a short period of time for milk retinol to decrease following initiation of lactation, could reduce the quantity of dose lost in the initial period following dosing.
Despite correcting for this loss, a large discrepancy remained between calculated and measured stores. In all cases, the differences are attributable either to uncaptured dose loss (spit out/not swallowed, not absorbed, not seen due to time between milk samples) or an inaccurate assumption of the proportion of VA stores in the liver to be 50%. For individual sows with especially large discrepancies (4 and 6), the first cause seems more likely, while the difference between predicted and measured liver stores across all five animals with data points to VA stores that were traced but incorrectly predicted to be in the liver. As noted, the sows weighed 271 ± 21 kg and might have ∼25% body fat,42 indicating significant total adipose mass. Future studies in swine, should consider collecting adipose tissue to assist in data interpretation. In rats for example, despite an approximately 50-times lower concentration of retinol in adipose than liver (20–24 nmol/g tissue), adipose has been predicted to contain a proportion as high as one-fifth the total VA found in the liver due to relative mass of the tissues.43 In obese humans, 50% of TBS could be in adipose.11 In these sows, if we assume similar adipose retinol concentrations as determined in the rats (22 nmol VA/g tissue) given that liver concentrations are comparable, and assume Sow 1 to have 25% body fat of 303 kg total weight, we would expect approximately 1667 μmol of VA, with liver accounting for another 440 μmol if retinol concentrations are uniform throughout the liver, for a total of 2107 μmol of that sow’s RID-predicted 3700 μmol (i.e., 57% explained by liver and adipose). It is not, therefore, implausible that a significant store of retinol could be found in the adipose tissue; however, this still leaves 43% of the predicted retinol unaccounted for, indicating further research is needed in lactating women.
Furthermore, during VA deficiency, kidney may contain a similar retinol concentration to liver.44 We did not anticipate that the sows would have this degree of VA deficiency and did not collect the kidneys. Future studies could investigate carcass and other organ concentrations of retinol, as well as unaccounted-for dose loss (e.g. more frequent milk collections for milk loss, feces analysis for unabsorbed dose or intramuscular injection as a ∼100% absorption control) to determine whether these stores exist or are artifacts of the RID method in lactating sows.
Newborn infants are typically born with low VA stores,45 and VA comes from colostrum and breast milk in the early stages of life. Due to its function in growth and the immune system, VA deficiency can be devastating in countries with poor maternal VA status. Thus, the data presented above supports the utility of the RID in large-scale infant and child surveys, adjusting for their shorter VA half-life15,19 and low initial stores. The RID has been applied in Ghanaian infants, as a method of comparing liver stores between a group receiving high-dose VA supplements and controls.18 Our study provides support for the accuracy of the data showing that the infants in both groups were VA sufficient, which was corroborated by the baseline measure of VA sufficiency in both groups by the modified relative-dose-response test, another biomarker of VA liver status.3 A similar equation was used in that study to these piglets with an absorption factor of 80% for a 1.0 μmol dose, 0.8 serum to liver enrichment because diet was not controlled, and 80% storage in the liver because of adequate status.18
The RID in lactating women, however, needs to be further investigated in humans to correct for dose losses to milk as well as potential extrahepatic storage. It is important to note that extrahepatic storage that is turning over with the plasma pool (i.e., being traced), likely represents usable VA, and so TBS may prove to be a more relevant metric than TLR. If the estimations with RID in lactation prove to be more qualitative than quantitative, dose response tests may be more appropriate than RID during lactation. Studies are currently underway in Zambian and Thai lactating women to evaluate the utility of the RID test during this life stage.
Supplemental Material
Supplemental Material for Retinol isotope dilution accurately predicts liver reserves in piglets but overestimates reserves in lactating sows by Jesse Sheftel, Rebecca L Surles and Sherry A Tanumihardjo in Experimental Biology and Medicine
ACKNOWLEDGMENTS
The authors thank Dr Tom Crenshaw (Animal Sciences, University of Wisconsin, Madison) and the UW-Madison Swine Research and Teaching Center (Arlington, WI) for their help with the animal work, and Christopher Davis for analyzing the sow samples.
Authors’ contributions
JS interpreted data, reanalyzed a subset of samples, and wrote the first draft of the manuscript. RLS conducted research with the swine. SAT designed the studies, analyzed the piglet samples, revised the paper, and had primary responsibility for final content. All authors read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This research was supported by NIHNIDDK 61973 and the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number USDA NRI 2007–35200-17729.
References
- 1.Wirth JP, Petry N, Tanumihardjo SA, Rogers LM, McLean E, Greig A, Garrett GS, Klemm RDW, Rohner F. Vitamin A supplementation programs and country-level evidence of vitamin A deficiency. Nutrients 2017; 9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva, Switzerland, www.who.int/vmnis/indicators/retinol.pdf (accessed 19 February 2018).
- 3.Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of nutrition for development (BOND) – vitamin A review. J Nutr 2016; 146:1816S–48S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suri DJ, Tanumihardjo JP, Gannon BM, Pinkaew S, Kaliwile C, Chileshe J, Tanumihardjo SA. Serum retinol concentrations demonstrate high specificity after correcting for inflammation but questionable sensitivity compared with liver stores calculated from isotope dilution in determining vitamin A deficiency in Thai and Zambian children. Am J Clin Nutr 2015; 102:1259–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cifelli CJ, Green JB, Green MH. Use of model-based compartmental analysis to study vitamin A kinetics and metabolism In: Litwack G. (ed) Vitamins and hormones. Amsterdam: Elsevier, 2007, pp. 161–95. [DOI] [PubMed] [Google Scholar]
- 6.Tanumihardjo SA. Vitamin A status assessment in rats with 13C4-retinyl acetate and gas chromatography/combustion/isotope ratio mass spectrometry. J Nutr 2000; 130:2844–9 [DOI] [PubMed] [Google Scholar]
- 7.Haskell MJ, Handelman GJ, Peerson JM, Jones AD, Rabbi MA, Awal MA. Assessment of vitamin A status by the deuterated-retinol-dilution technique and comparison with hepatic vitamin A concentration in Bangladeshi surgical patients. Am J Clin Nutr 1997; 66:67–74 [DOI] [PubMed] [Google Scholar]
- 8.Green MH. Evaluation of the “Olson equation”, an isotope dilution method for estimating vitamin A stores. Int J Vitam Nutr Res 2014; 84:9–15 [DOI] [PubMed] [Google Scholar]
- 9.Ribaya-Mercado JD, Solon FS, Dallal GE, Solomons NW, Fermin LS, Mazariegos M, Dolnikowski GG, Russell RM. Quantitative assessment of total body stores of vitamin A in adults with the use of a 3-d deuterated-retinol-dilution procedure. Am J Clin Nutr 2003; 77:694–9 [DOI] [PubMed] [Google Scholar]
- 10.Cobelli C, Toffolo G, Foster DM. Tracer-to-tracee ratio for analysis of stable isotope tracer data: link with radioactive kinetic formalism. Am J Physiol 1992; 262:E968–75 [DOI] [PubMed] [Google Scholar]
- 11.Gannon BM, Tanumihardjo SA. Comparisons among equations used for retinol isotope dilution in the assessment of total body stores and total liver reserves. J Nutr 2015; 145:847–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escaron AL, Tanumihardjo SA. Orally ingested 13C2-retinol is incorporated into hepatic retinyl esters in a nonhuman primate (Macaca mulatta) model of hypervitaminosis A. Comp Med 2010; 60:71–6 [PMC free article] [PubMed] [Google Scholar]
- 13.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen IIH, Jones AD, Anderson DP, Olson JA. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr 1989; 49:713–6 [DOI] [PubMed] [Google Scholar]
- 14.Tanumihardjo SA, Muherdiyantiningsih PD, Komala M, Karyadi D, Olson JA. Daily supplements of vitamin A (8.4 µmol, 8000 IU) improve the vitamin A status of lactating Indonesian women. Am J Clin Nutr 1996; 63:32–5 [DOI] [PubMed] [Google Scholar]
- 15.Haskell MJ, Lembcke JL, Salazar M, Green MH, Peerson JM, Brown KH. Population-based plasma kinetics of an oral dose of [2H4] retinyl acetate among preschool-aged, Peruvian children. Am J Clin Nutr 2003; 77:681–6 [DOI] [PubMed] [Google Scholar]
- 16.Gannon BM, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalungwana N, Mosonda M, Pixley K, Masi C, Tanumihardjo SA. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial. Am J Clin Nutr 2014; 100:1541–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauberlich HE, Hodges RE, Wallace DL, Kolder H, Canham JE, Hood J, Raica N, Jr, Lowry LK. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm 1974; 32:251–75 [DOI] [PubMed] [Google Scholar]
- 18.Newton S, Owusu-Agyei S, Asante KP, Amoaful E, Mahama E, Tchum SK, Ali M, Adjei K, Davis CR, Tanumihardjo SA. Vitamin A status and body pool size of infants before and after consuming fortified home-based complementary foods. Arch Public Health 2016; 74:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinkaew S, Wegmuller R, Wasantwisut E, Winichagoon P, Hurrell RF, Tanumihardjo SA. Triple-fortified rice containing vitamin A reduced marginal vitamin A deficiency and increased vitamin A liver stores in school-aged Thai children. J Nutr 2014; 144:519–24 [DOI] [PubMed] [Google Scholar]
- 20.Surles RL, Hutson PR, Valentine AR, Mills JP, Tanumihardjo SA. 3, 4-Didehydroretinol kinetics differ during lactation in sows on a retinol depletion regimen and the serum:milk 3, 4-didehydroretinol:retinol ratios are correlated. J Nutr 2011; 141:554–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun T, Surles RL, Tanumihardjo SA. Vitamin A concentrations in piglet extrahepatic tissues respond differently ten days after vitamin A treatment. J Nutr 2008; 138:1101–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surles RL, Li J, Tanumihardjo SA. The modified-relative-dose-response values in serum and milk are positively correlated over time in lactating sows with adequate vitamin A status. J Nutr 2006; 136:939–45 [DOI] [PubMed] [Google Scholar]
- 23.Dever JT, Surles RL, Davis CR, Tanumihardjo SA. α-Retinol is distributed through serum retinol-binding protein-independent mechanisms in the lactating sow-nursing piglet dyad. J Nutr 2011; 141:42–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gannon BM, Davis CR, Nair N, Grahn M, Tanumihardjo SA. Single high-dose vitamin A supplementation to neonatal piglets results in a transient dose response in extrahepatic organs and sustained increases in liver stores. J Nutr 2017; 147:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surles RL, Mills JP, Valentine AR, Tanumihardjo SA. One-time graded doses of vitamin A to weanling piglets enhance hepatic retinol but do not always prevent vitamin A deficiency. Am J Clin Nutr 2007; 86:1045–53 [DOI] [PubMed] [Google Scholar]
- 26.Tanumihardjo SA. Synthesis of 10,11,14,15-13C4-and 14,15-13C2-retinyl acetate. J Labelled Cpd Radiopharm 2001; 44:365–72 [Google Scholar]
- 27.Howe JA, Valentine AR, Hull AK, Tanumihardjo SA. 13C natural abundance in serum retinol acts as a biomarker for increases in dietary provitamin A. Exp Biol Med (Maywood) 2009; 234:140–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanumihardjo SA, Howe JA. Twice the amount of α-carotene isolated from carrots is as effective as β-carotene in maintaining the vitamin A status of Mongolian gerbils. J Nutr 2005; 135:2622–6 [DOI] [PubMed] [Google Scholar]
- 29.Green MH, Ford JL, Oxley A, Green JB, Park H, Berry P, Boddy AV, Lietz G. Plasma retinol kinetics and β-carotene bioefficacy are quantified by model-based compartmental analysis in healthy young adults with low vitamin A stores. J Nutr 2016; 146:2129–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen LE, Green MH, Green JB, et al. Correspondence re: Effects of pharmacological retinoids on several vitamin A-metabolizing enzymes. Cancer Res., 53:2965–2969, 1993. Cancer Res 1994; 54:3319. [PubMed] [Google Scholar]
- 31.Escaron AL, Green MH, Howe JA, Tanumihardjo SA. Mathematical modeling of serum 13C-retinol in captive rhesus monkeys provides new insights on hypervitaminosis A. J Nutr 2009; 139:2000–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed F, Mohiduzzaman M, Jackson AA. Vitamin A absorption in children with ascariasis. Br J Nutr 2007; 69:817–25 [DOI] [PubMed] [Google Scholar]
- 33.Valentine AR, Davis CR, Tanumihardjo SA. Vitamin A isotope dilution predicts liver stores in line with long-term vitamin A intake above the current Recommended Dietary Allowance for young adult women. Am J Clin Nutr 2013; 98:1192–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hicks VA, Gunning DB, Olson JA. Metabolism, plasma transport and biliary excretion of radioactive vitamin A and its metabolites as a function of liver reserves of vitamin A in the rat. J Nutr 1984; 114:1327–33 [DOI] [PubMed] [Google Scholar]
- 35.Green MH, Ford JL, Green JB. Retinol isotope dilution is applied during restriction of vitamin A intake to predict individual subject total body vitamin A stores at isotopic equilibrium. J Nutr 2016; 146:2407–11 [DOI] [PubMed] [Google Scholar]
- 36.Preston T. Existing and emerging technologies for measuring stable isotope labelled retinol in biological samples: isotope dilution analysis of body retinol stores. Int J Vitam Nutr Res 2014; 84:S30–9 [DOI] [PubMed] [Google Scholar]
- 37.Green MH, Ford JL, Green JB, Berry P, Boddy AV, Oxley A, Lietz G. A retinol isotope dilution equation predicts both group and individual total body vitamin A stores in adults based on data from an early postdosing blood sample. J Nutr 2016; 146:2137–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Teros V, Ford JL, Green MH, Tang G, Grusak MA, Quihui-Cota L, Muzhingi T, Paz-Cassini M, Astiazaran-Garcia H. Use of a “super-child” approach to assess the vitamin A equivalence of Moringa oleifera leaves, develop a compartmental model for vitamin A kinetics, and estimate vitamin A total body stores in young Mexican children. J Nutr 2017; 147:2356–63 [DOI] [PubMed] [Google Scholar]
- 39.Kaliwile C, Arscott SA, Gannon BM, Masi C, Tanumihardjo SA. Community mobilization during biofortified orange maize feeding trials in Zambia. Int J Vitam Nutr Res 201. 9; doi:10.1024/0300-9831/a000541 [DOI] [PMC free article] [PubMed]
- 40.Heying EK, Grahn M, Pixley KV, Rocheford T, Tanumihardjo SA. High-provitamin A carotenoid (orange) maize increases hepatic vitamin A reserves of offspring in a vitamin A-depleted sow-piglet model during lactation. J Nutr 2013; 143:1141–6 [DOI] [PubMed] [Google Scholar]
- 41.Brown K, Dewey K, Allen L. Complementary feeding of young children in developing countries: a review of current scientific knowledge. Geneva: World Health Organization, 1998. [Google Scholar]
- 42.Clowes EJ, Aherne FX, Schaefer AL, Foxcroft GR, Baracos VE. Parturition body size and body protein loss during lactation influence performance during lactation and ovarian function at weaning in first-parity sows. J Anim Sci 2003; 81:1517–28 [DOI] [PubMed] [Google Scholar]
- 43.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, Blaner WS. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem 1992; 267:1805–10 [PubMed] [Google Scholar]
- 44.Morita A, Nakano K. Change in vitamin A content in tissues of rats fed on a vitamin A-free diet. J Nutr Sci Vitaminol 1982; 28:343–50 [DOI] [PubMed] [Google Scholar]
- 45.Montreewasuwat N, Olson JA. Serum and liver concentrations of vitamin A in Thai fetuses as a function of gestational age. Am J Clin Nutr 1979; 32:601–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Retinol isotope dilution accurately predicts liver reserves in piglets but overestimates reserves in lactating sows by Jesse Sheftel, Rebecca L Surles and Sherry A Tanumihardjo in Experimental Biology and Medicine