Short abstract
The aim of this study is to assess whether overexpressing neuregulin 4 (Nrg4), a growth factor known to attenuate hepatic lipogenesis, in mesenchymal stem cells (MSCs) could enhance their ability to ameliorate insulin resistance (IR) and improve lipid metabolism in high-fat diet (HFD)-fed mice. Six-week-old C57BL/6 mice were fed a HFD for 12 weeks and then were given intravenous transplantation of adipose tissue-derived MSCs (ADSCs) or ADSCs overexpressing Nrg4 (Nrg4-ADSCs). Assessment of body weight and blood glucose and insulin levels as well as glucose tolerance test and insulin tolerance test was performed four and eight weeks after cell injection. Triglyceride (TG) and total cholesterol (TC) levels in the plasma and liver were also measured. The mRNA levels of glucose transporter 4 (GLUT4), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) in muscle and adipose tissues were assessed by Real-time Polymerase Chain Reaction (RT-PCR) analysis. Expression of genes related to lipid metabolism, including sterol regulatory element binding protein-1c (SREBP-1c) and fatty acid synthase, was evaluated at the mRNA and protein levels by RT-PCR and western blotting, respectively. The HFD-fed mice receiving ADSCs or Nrg4-ADSCs showed reduced blood glucose levels and enhanced insulin sensitivity, with the Nrg4-ADSC group exhibiting increased improvement in these aspects. HFD-induced changes in the expression of GLUT4 and IL-6 and TNF-α in skeletal muscle and adipose tissues were partially reversed by ADSC or Nrg4-ADSC infusion; however, no difference was observed between these two groups. Nrg4-ADSC-treated mice showed less fat cell deposition and lower TG and TC levels in the serum and liver with decreased expression of SREBP-1c and fatty acid synthase compared with the ADSC group. ADSC transplantation can reduce blood glucose level and ameliorate IR induced by HFD. The protective effects of ADSC can be attributed to suppression of inflammation and augmentation of glucose uptake in skeletal muscle and adipose tissues. More importantly, Nrg4 overexpression in ADSCs could strengthen this efficacy by attenuating hepatic lipogenesis.
Impact statement
Due to high-fat and high-sugar diets accompanied by sedentary lifestyles, diabetes has become a global epidemic. Literature findings suggest a potential therapeutic effect of Nrg4 on treating obesity-related metabolic disorders including type 2 diabetes (T2D). Adipose tissue-derived MSCs (ADSCs) were used in our study as they are abundant and can be harvested with minimally invasive procedures. In the end, our study reveals that ADSC transplantation improves glucose tolerance and metabolic balance in HFD-fed mice by multiple mechanisms, including upregulating GLUT4 expression and suppressing inflammation. More importantly, our study shows that Nrg4 overexpression could improve the efficacy of ADSCs in ameliorating insulin resistance (IR) and other obesity-related metabolic disorders, given the function of Nrg4 in attenuating hepatic lipogenesis. It would provide a new therapeutic strategy for the treatment of obesity, IR, and T2D.
Keywords: Adipose-derived stem cells, neuregulin 4, obesity, insulin resistance, hepatic lipogenesis, hepatic steatosis
Introduction
According to the World Health Organization, 347 million people worldwide have diabetes, of which the type 2 diabetes (T2D) accounts for around 90%.1,2 Due to high-fat and high-sugar diets accompanied by sedentary lifestyles, diabetes has become a global epidemic.3 Numerous epidemiological studies have demonstrated that obesity is a leading cause of insulin resistance (IR) and T2D.4–6 It has also been demonstrated that obesity management can delay progression from prediabetes to T2D and may be beneficial in the treatment of T2D.7
Neuregulin 4 (Nrg4) is a member of a small family of epidermal growth factor-like (EGFL) domain-containing proteins that are synthesized as transmembrane precursors with an extracellular EGFL domain at the N-terminus.8 These precursors then undergo proteolytic cleavage to release the extracellular fragments, which act on target cells via activation of the ErbB receptors, particularly ErbB4.9,10 Nrg4 expression is restricted to adipose tissues with a high enrichment in brown adipose tissue (BAT).11,12 However, Nrg4 does not directly engage in BAT thermogenesis.12 Instead, Nrg4 secreted from BAT specifically targets the liver, where it attenuates the lipogenesis in hepatocytes via activating the ErbB3/ErbB4 signaling in these cells.13 Indeed, gain- and loss-of-function studies in mice revealed that Nrg4 protects against high-fat diet (HFD)-induced IR and hepatic steatosis. Interestingly, Nrg4 expression in adipose tissues is reduced in murine and human obesity.14,15 These literature findings suggest a potential therapeutic effect of Nrg4 on treating obesity-related metabolic disorders including T2D.
Mesenchymal stem cells (MSCs) are a population of self-renewable cells with the potential to differentiate into various cell types.16 It has been identified in a number of tissues, such as the bone marrow, umbilical cord, adipose tissue, and dental pulp.17,18 Previous research focused on their ability to self-renew and their capacity to differentiate into adipocytes, osteocytes, myocytes, and many other kinds of tissues. But current evidence suggests that MSCs can secrete a myriad of cytokines which may play a significant role in immunomodulatory or anti-inflammatory responses.19–21
MSCs have been tested in the treatment of diabetes. In animal studies, it has been shown that MSC transplantation could reduce apoptosis of injured pancreatic β-cells and enhance the differentiation of endogenous progenitor cells through paracrine mechanisms.22–25 In clinical studies, it has been shown that autologous MSC treatment for new-onset type 1 diabetes (T1D) may be a safe and practicable strategy to preserve β-cell function.23 In addition, a Randomized Controlled Open-Label Clinical Study involving 42 patients with T1D showed that combined umbilical cord MSC and autologous bone marrow mononuclear cell transplantation was safe and may lead to measurable improvements of metabolic function.26 Besides the potential for MSCs to reverse T1D, MSC transplantation has been evaluated for treatment of T2D.27 Andrea et al.28 proved that systemic MSC transplantation improves glucose tolerance and metabolic balance in T2D through multisystemic regulation with tissue-specific mechanisms. A preliminary clinical study involving 22 patients with T2D showed that Wharton’s jelly-derived MSCs are able to significantly improve β-cell function without adverse effects.29
Here we assess the effect of MSC transplantation in HFD-fed mice. Adipose tissue-derived MSCs (ADSCs) were used in our study as they are abundant and can be harvested with minimally invasive procedures. We further explored the potential strategy to potentiate the efficacy of ADSC transplantation in HFD-fed mice. Considering the effects of Nrg4 against IR,15 we investigate whether Nrg4 overexpression in ADSCs can improve the efficacy of ADSCs in ameliorating IR and other obesity-related metabolic disorders in HFD-fed mice.
Materials and methods
Animals
Five-week-old male C57BL/6 mice were purchased from Shanghai Jiao Tong University School of Medicine and all experiments were performed in compliance with the Guide for the Care and Use of Medical Laboratory Animals (Ministry of Health, People’s Republic of China, 1998). All experimental procedures were ethically approved by the independent ethics committee of Shanghai Ninth People’s Hospital (No. HKD27). All animals were maintained in a temperature-controlled facility with a 12:12 h light–dark cycle. At six weeks of age, mice were fed with either a HFD (60% of calories from fat) or a standard chow diet (10% of calories from fat).
ADSCs isolation and culture
ADSCs were isolated and cultured as described previously.30 Briefly, mice were anesthetized via intraperitoneal injection of chloral hydrate (200 mg/kg). Inguinal adipose tissues were excised, washed three times with phosphate-buffered saline (PBS), and minced. These tissues were then digested with 1 mg/mL type II collagenase (Sigma-Aldrich, USA) at 37°C for 40 min and centrifuged at 500 g for 5 min. The pellets were re-suspended, plated at a density of 105 cells/cm2 in DMEM containing 10% FBS and incubated at 37°C with 5% CO2. After 48 h, non-adherent cells were removed and fresh media were added. When adherent cells were confluent, they were trypsinized, harvested, and expanded.
Isolated ADSCs were characterized for cell surface markers by flow cytometry. After the cell concentration was adjusted to 107 per mL, the cells were stained with rat anti-mouse CD105, CD34, CD90, and CD11b (1:100; eBioscience, San Diego, CA). After a 30 min incubation at 4°C, the cells were washed with PBS and incubated by phycoerythrin (PE)-conjugated or allophycocyanin (APC)-conjugated secondary antibody (eBioscience). The control group were stained with isotype-matched control antibodies. The fluorescence intensity was tested with a flow cytometer (FACSan Becton Dickinson, NJ, USA).
Osteogenic and adipogenic induction
For osteogenic induction, cells were dissociated and seeded onto a 6-well plate at a density of 3 × 103 cells/cm2, maintained in the ADSC osteogenic differentiation medium (Cyagen, China) for 21 days and then stained with Alizarin red. The osteogenic induction medium was purchased from Cyagen and the content including DMEM, 10% FBS, 10 mmol/L sodium β-glycerophosphate + 0.1 mol/L dexamethasone + 50 mg/L Vit C. For adipogenic induction, cells were dissociated and seeded onto a 6-well plate at a density of 2 × 104 cells/cm2, maintained in the ADSC adipogenic differentiation medium (Cyagen, China) for 21 days and stained with Oil Red O. The adipogenic induction medium was purchased from Cyagen and the content including DMEM, 10% FBS, 1% penicillin/streptomycin, 1 µmol/L dexamethasone, 10 µmol/L insulin, 0.5 mmol/L isobutylmethylxanthine, and 200 µmol/L indomethacin.
Plasmid constructs, lentivirus production, and transduction of ADSCs
Coding sequences of mice Nrg 4 (NCBI RefSeq: NM_032002.2) were synthesized by Cyagen (Guang Zhou, China). The Nrg4-expressing lentiviral vectors termed pLV-EGFP/Neo-CMV>mNrg4 were produced by transfecting HEK293T cells according to manufacturer’s instructions. Briefly, plasmid DNA was transfected into HEK293T cells via the method of standard calcium phosphate method. The cells were incubated for 12 h and were then washed with PBS twice. After 24 of incubation, the virus particles were collected and concentrated by centrifuging at 20,000 r/min (4°C) for 2 h. The concentrated virus particles were re-suspended in PBS and stored at −80°C.
Transduction was performed in 24-well plates. ADSCs were digested, plated on a 24-well culture plate with a cell density of 1.0 × 105 and cultured for 24 h. These cells were then cultured with 1 mL of serum-free DMEM containing pLV-EGFP/Neo-CMV>mNrg4 viral particles or lentiviral vector particles with the multiplicity of infection ranging from 10 to 200 in the presence of 5 µg/mL polybrene (Sigma-Aldrich, USA) for 8 h at 37°C with 5% CO2. The virus-containing medium was then removed and replaced with 1 mL of fresh culture medium per well. The infection efficiency was assessed by fluorescent microscopy. ADSCs overexpressing Nrg4 were referred to as Nrg4-tADSCs.
Experimental design
Forty mice were randomly divided into four groups (n = 10), including the normal diet (ND), HFD, ADSCs (HFD + ADSCs transplantation), and Nrg4-tADSCs group (HFD + Nrg4-tADSCs transplantation) groups. ADSCs or Nrg4-ADSCs cells were cultured to the third generation to perform the transplantation. And cell transplantation was conducted via injection of ADSCs or Nrg4-ADSCs through tail vein 12 weeks after the HFD feeding. 2 × 106 cells suspended in 0.2 mL PBS were injected into each mouse. Mice in the HFD group were infused with 0.2 mL PBS via tail vein.
Body weight, blood glucose, intraperitoneal glucose tolerance test (IPGTT), and intraperitoneal insulin tolerance test (ITT)
Body weight was measured every two weeks. The blood glucose level was measured after a mouse was starved for 8 h. To assess glucose tolerance and insulin sensitivity the IPGTT and ITT were performed.31 IPGTT was performed at four and eight weeks after transplantation. Glucose solution (2 g/kg) was intraperitoneally injected into the overnight food fasted mice, and the blood glucose level was monitored at the time points 0, 30, 60, 90, 120 min after injection. Two days after IPGTT, we performed ITT. The 0.75 U/kg insulin (Sigma-Aldrich) was intraperitoneally injected and the blood glucose level was measured using a OneTouch Ultra (Johnson & Johnson) at 0, 30, 60, 90, 120 min after injection. We let the mice recover for three days after ITT and then collect specimens of liver tissues, quadriceps muscle, and inguen adipose tissues.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of Nrg4 in the cell culture medium were measured using the commercially available ELISA kits (Elabscience Biotechnology Co.), according to manufacturer’s instructions. These experiments were performed at least three times independently. The collected sera were separated by centrifugation at 1000 g at 4°C for 20 min, and then stored at −80°C for further use. The serum levels of TC, TG, and insulin were measured using TC, TG, insulin ELISA kits, respectively, according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, China).
Liver TG level assessment
Liver TG levels were determined as described previously with slight modifications.32 Hepatic TG levels were measured using the GPO-triglyceride kit (Sigma, Deisenhofen, Germany) as standards.
Histological and immunohistochemical (IHC) staining
For HE staining, the samples were excised, fixed in 4% paraformaldehyde for 24 h, dehydrated through xylenes and alcohols and embedded in paraffin. Sections were routinely cut at 5 µm and stained with hematoxylin and eosin. For IHC staining, liver tissue sections were fixed with 4% paraformaldehyde and embedded with paraffin. Sections of the specimens were incubated overnight at 4°C with anti-Nrg4 antibodies (Abcam, UK) at a dilution of 1:100. After three washes with PBS, the sections were incubated with peroxidase-conjugated goat anti-rabbit antibody for 1 h, followed by incubation with 3,3′-diaminobenzidine substrate for 3 min.
qRT-PCR analysis
Total RNA was extracted from liver tissues or cells using TRIzol reagent according to the manufacturer’s instructions. cDNA was synthesized from 1 µg of total RNA in 20 µL final volume using a PrimeScript™ RT reagent kit (Takara, Japan). qRT-PCR was performed using the ABI 7500 Real Time PCR Detection System (Life Technologies, USA). The PCR conditions were as follows: 10 min at 95°C, followed by 40 cycles of 5 s at 95°C and 60 s at 60°C. Relative gene expression was determined by the 2-ΔΔCT method (where Ct = threshold cycle) and was calculated using β-actin as the reference gene. Sequences of PCR primer pairs were given in Table 1.
Table 1.
The primers for qRT-PCR analysis.
| Gene | Primer sequence 5′–3′ |
|---|---|
| GLUT4 | F-GGACCGGATTCCATCCCACR-TCCCAACCATTGAGAAATGATGC |
| TNF-α | F-CCCTCACACTCAGATCATCTTCTR-GCTACGACGTGGGCTACAG |
| IL-6 | F-TCTATACCACTTCACAAGTCGGAR-GAATTGCCATTGCACAACTCTTT |
| SREBP-1 | F-GATGTGCGAACTGGACACAGR-CATAGGGGGCGTCAAACAG |
| FASN | F-GGAGGTGGTGATAGCCGGTATR-TGGGTAATCCATAGAGCCCAG |
| β-actin | F-CCTGGCACCCAGCACAATR-GGGCCGGACTCGTCATACT |
GLUT4: ; IL-6: ; FASN: ; qRT-PCR: ; SREBP-1: sterol regulatory element binding protein-1; TNF-α: .
Western blot
The total cell lysates were centrifuged and the supernatants were used for western blotting. Equal amounts of protein samples for each analysis were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were transferred to polyvinylidene difluoride membranes and then blocked with 5% non-fat milk for 1 h at room temperature. The membranes were washed with TBST (10 mM Tris [pH 8.0], 150 mM NaCl, and 0.05% Tween-20) and incubated with the appropriate primary antibody (1:1000) at 4°C overnight, followed by incubation with HRP-conjugated secondary antibody (1:5000) Cell Signaling Technology (USA) at room temperature for 1 h. Labeled proteins were visualized using ECL regents (Merck Millipore, USA).
Statistical analysis
The results are presented as the means ± S.E.M. The Student’s t-test was used for between-group comparisons. Results involving more than two groups were assessed by one-way ANOVA followed by the Bonferroni test. A value of p < 0.05 was considered statistically significant.
Results
Characterization of ADSCs
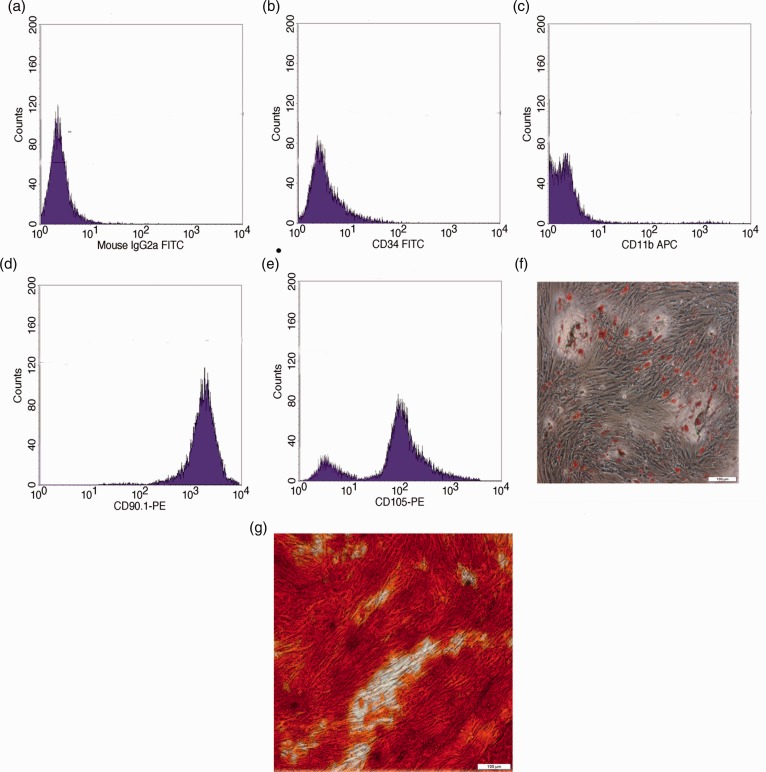
ADSCs were isolated from mouse inguinal adipose tissues and cultured according to a standardized protocol reported previously.30 These ADSCs exhibited a fibroblast-like morphology and were positive for the surface expression of ADSC marker, CD105 (79.26%) and CD90 (85.77%) (Figure 1(d) and (e)). Consistent with the immunophenotype of ADSCs reported previously,33 the expression of hematopoietic markers, CD34 and CD11b, is absent in these cells (Figure 1(b) and (c)). Furthermore, the in vitro differentiation assays demonstrated that these ADSCs can be induced into adipocytes (Figure 1(f)) or osteocytes (Figure 1(g)) under appropriate culture conditions. And that indicated that ADSCs cells have pluripotency.
Figure 1.
Characterization of ADSCs. (a–e) Flow cytometry analysis of the surface expression of the indicated markers on ADSCs. The cells were incubated by PE- or APC-conjugated secondary antibody, (f) detection of lipid droplet accumulation with Oil Red O staining in ADSCs that have been subjected to adipogenic induction for 21 days, and (g) detection of calcium mineralization with Alizarin Red staining in ADSCs that have been subjected to osteogenic induction for 21 days. APC: allophycocyanin; FITC: fluorescein isothiocyanate. (A color version of this figure is available in the online journal.)
Establishment of ADSCs overexpressing Nrg4
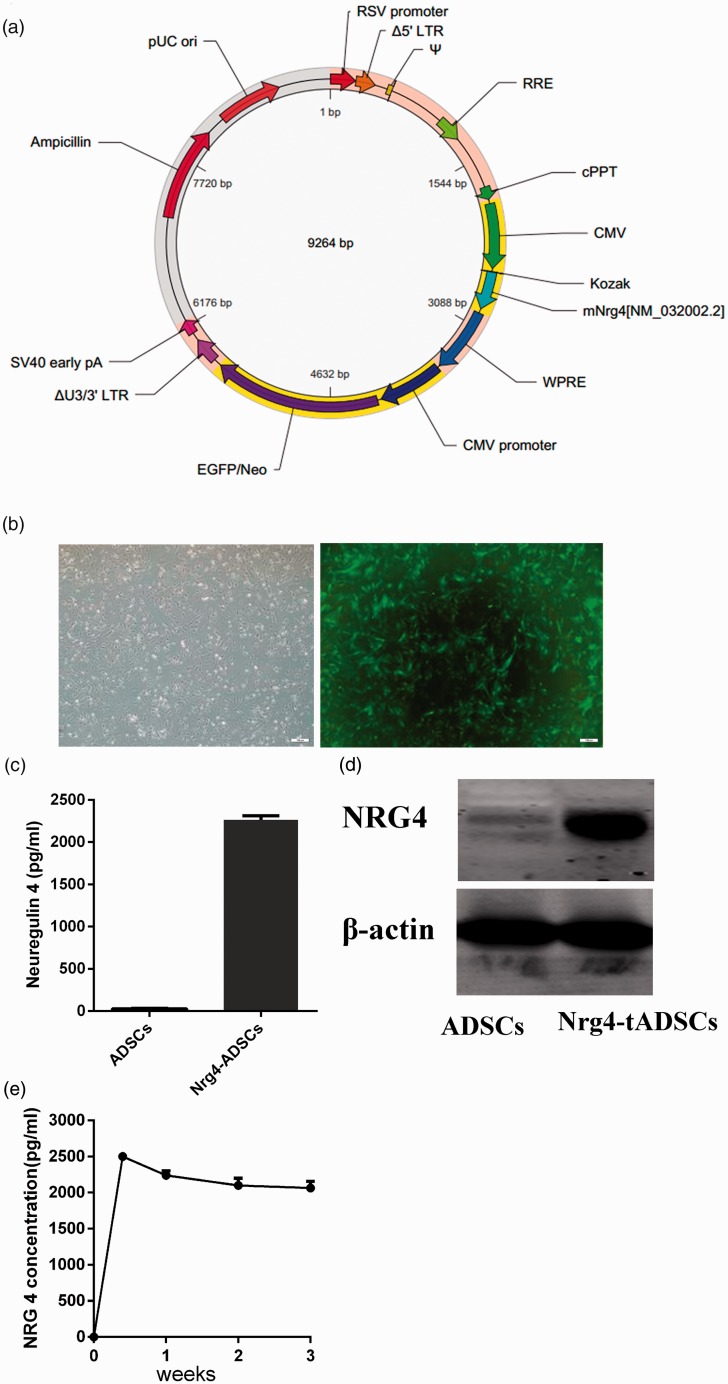
We next established Nrg4-overexpressing ADSCs using lentivirus-mediated infection. Seventy-two hours after the infection, EGFP can be easily seen through a fluorescence microscope. The infection efficiency is > 90% (Figure 2(b)). Western blot analysis revealed that Nrg4 expression is almost absent in ADSCs, while a large amount of Nrg4 proteins can be detected in Nrg4-ADSCs (Figure 2(d)), further confirming the infection efficiency. Nrg4 is known to undergo proteolytic cleavage, leading to the releasing of its extracellular fragments. We thus performed ELISA analysis to examine whether Nrg4-ADSCs can secrete Nrg4. Indeed, a high level of Nrg4 (∼2500 pg/mL) can be detected in Nrg4-ADSC culture media. ELISA failed to detect the presence of Nrg4 in ADSC culture media (Figure 2(c)). We next tracked the content of Nrg4 proteins in Nrg4-ADSC culture media for three weeks. The percentage of GFP-positive cells was largely stable throughout the 21 days of culture. ELISA revealed a relatively stable presence of Nrg4 in the media (∼2100 pg/mL on day 21; Figure 2(e)), suggesting that Nrg4-ADSCs constantly secreted Nrg4 during the three-week culture.
Figure 2.
Characterization of Nrg4-ADSCs. (a) An overview of the Nrg4 lentivirus construct, (b) photomicrographs of ADSCs and transfected cells. At 72 h after the infection with the Nrg4 lentivirus detected by phase-contrast microscopy and fluorescence microscopy. The infection efficiency is >90%, (c) concentrations of Nrg4 in the cell culture medium 72 h after the infection, (d) expression of Nrg4 in ADSCs and Nrg4-ADSCs analyzed by western blotting, and (e) the persistent presence of Nrg4 in the culture medium. ELISA was performed to determine Nrg4 levels in the culture medium at the indicated time points. ADSC: adipose tissue-derived MSC; CMV: cytomegalovirus; cPPT: central polypurine tract; EGFP: enhanced green fluorescent protein; LTR: long terminal repeat; Nrg4: neuregulin 4; RRE: Rev responsive element; RSV: Respiratory Syncytial Virus; WPRE: woodchuck hepatitis virus posttranscriptional regulatory element. (A color version of this figure is available in the online journal.)
Effects of transplantation of ADSCs or Nrg4-ADSCs on HFD-induced hyperglycemia
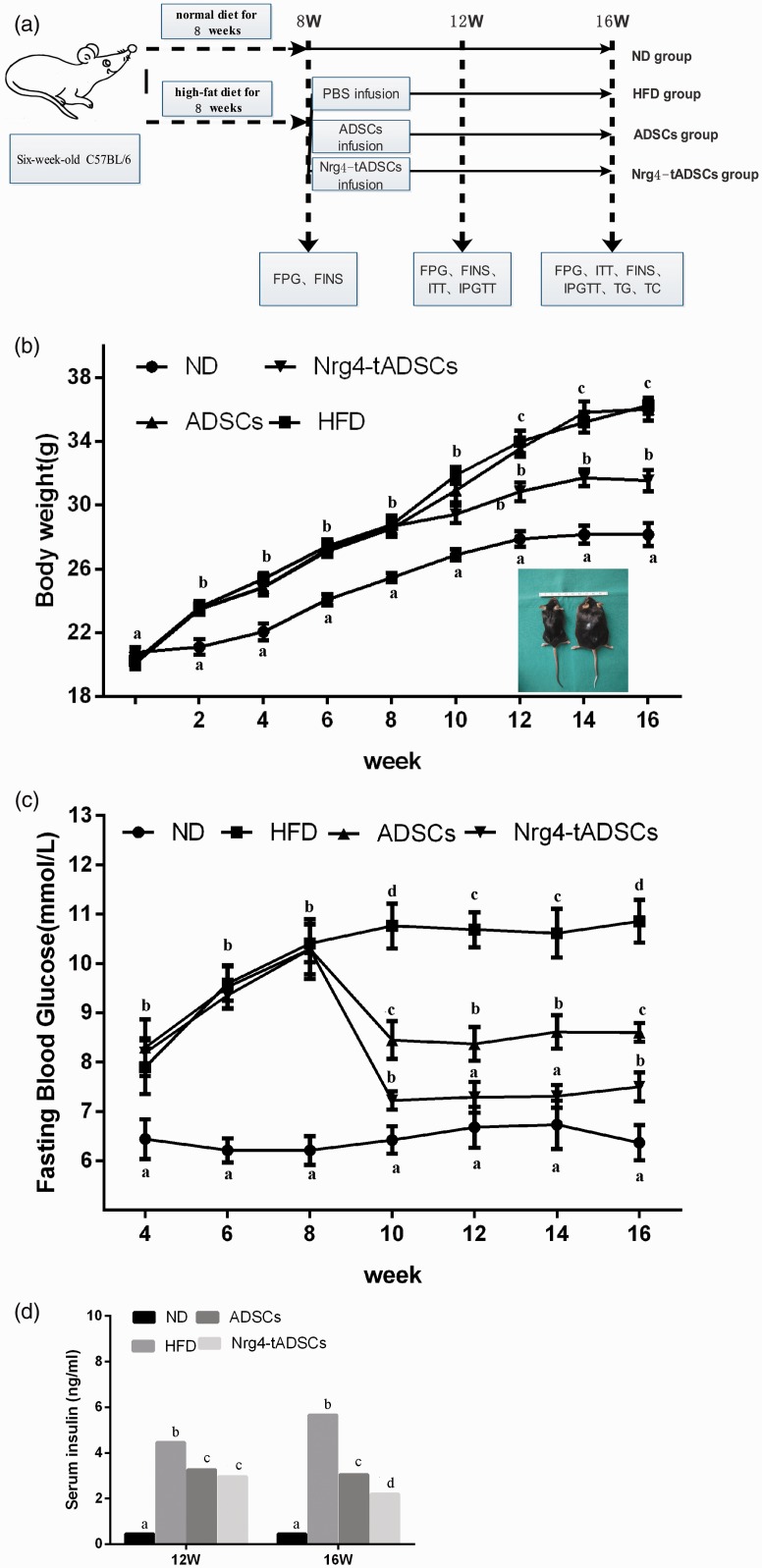
We next assessed the effects of transplantation of ADSCs or Nrg4-ADSCs on HFD-induced hyperglycemia. Mice were fed HFD for eight weeks before the cell transplantation (Figure 3(a)). These HFD-fed mice showed increased body weight compared with ND-fed control mice. ADSCs or Nrg4-ADSCs were then injected via the tail vein. As shown in Figure 3(b), Nrg4-ADSC transplantation, but not ADSC transplantation, reduced HFD-induced weight gain. Of note, no significant difference in food intake was observed in mice with or without the cell transplantation.
Figure 3.
Effects of ADSC transplantation on HFD-induced disorders in weight and glucose levels. (a) Experimental design, (b) changes in body weight after the cell transplantation and (c, d) changes in fasting blood glucose levels (c) and serum insulin levels (d) at the indicated time points. Different letters indicate statistical significance according to Bonferroni test (P < 0.05). ADSCs – HFD-fed mice with ADSC infusion; HFD – HFD-fed mice without cell infusion; ND – normal diet-fed mice; Nrg4-ADSC – HFD-fed mice with Nrg4-ADSC infusion. ADSC: adipose tissue-derived MSC; FINS: fasting insulin; FPG: fasting blood-glucose; HFD: high-fat diet; IPGTT: intraperitoneal glucose tolerance test; ITT: insulin tolerance test; ND: normal diet; PBS: phosphate-buffered saline; TC: total cholesterol; TG: triglyceride. (A color version of this figure is available in the online journal.)
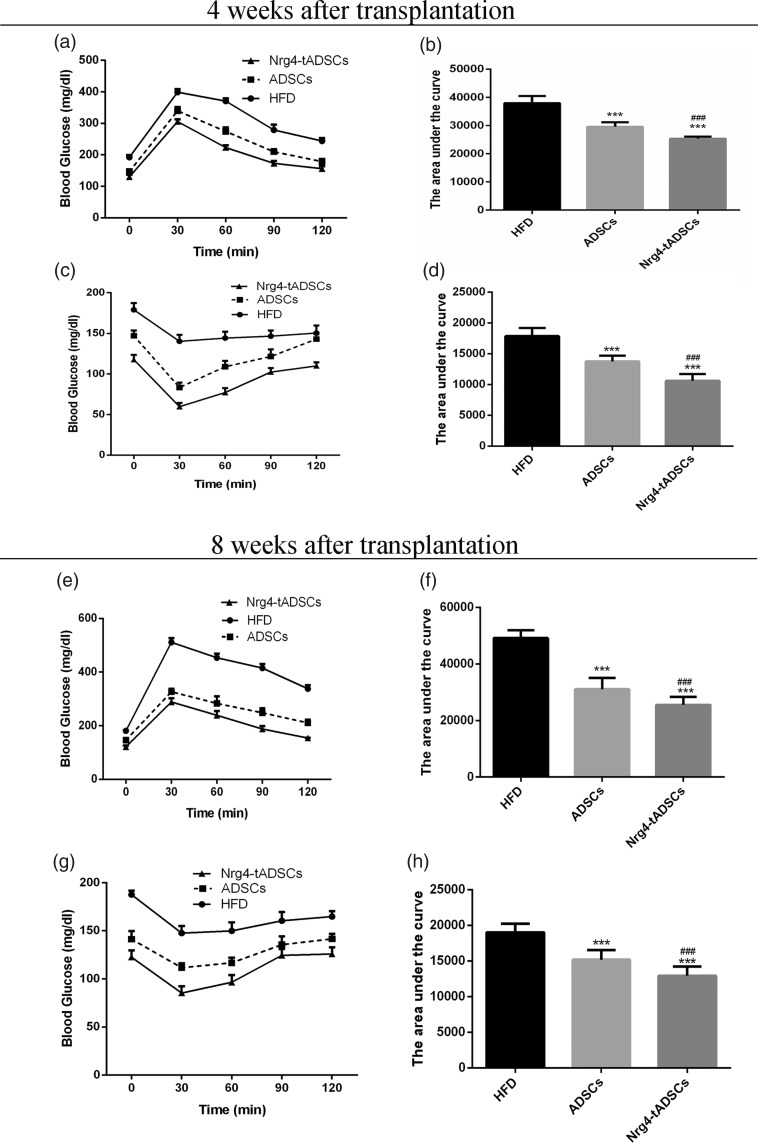
We next measured the blood glucose levels after the cell infusion. While both ADSC and Nrg4-tADSC transplantations lowered the glucose levels in HFD-fed mice at each measurement point, the reduction in blood glucose level was more evident in the Nrg4-ADSC group compared with the ADSC group (Figure 3(c)). We also measured serum levels of insulin. The ADSC and Nrg4-tADSC groups showed significant decrease in the concentration of serum insulin compared with the HFD group and such decrease was greater in the Nrg4-ADSC group (Figure 3(d)). To further analyze insulin sensitivity and glucose metabolism, we performed glucose tolerance test (GTT) and ITT four weeks and eight weeks after infection. Both ADSC and Nrg4-ADSC infusions ameliorated glucose intolerance (Figure 4(a) and (e)) and IR (Figure 4(c) and (g)) in HFD-fed mice. Interestingly, Nrg4-ADSCs were more effective in mitigating glucose intolerance and IR compared with ADSCs (Figure 4(a) to (h)). Taken together, these results suggest that ADSC transplantation can ameliorate HFD-induced glucose metabolic disorders and this beneficial effect can be enhanced by overexpression of Nrg4 in ADSCs.
Figure 4.
Effects of ADSC transplantation on HFD-induced disorders in glucose metabolism. Intraperitoneal GTT (a) and ITT (c) results obtained four weeks after the transplantation, (e, g) intraperitoneal GTT (e) and ITT (g) results obtained eight weeks after the transplantation. The area under the curve (AUC) for the IPGTT results was calculated using the blood glucose levels at 0 (BG0), 30 (BG30), 60 (BG60), 90 (BG90), and 120 (BG120) minutes by the following equation: AUC (min mg/dL) =30 min × (1/2 × [BG0 + BG120] + 1 × [BG30 + BG60 + BG90]); *: compare with HFD, ***P < 0.001; #: compare with ADSCs, ###P < 0.01. ADSCs – HFD-fed mice with ADSC infusion; HFD – HFD-fed mice without cell infusion; Nrg4-ADSC – HFD-fed mice with Nrg4-ADSC infusion. ADSC: adipose tissue-derived MSC; HFD: high-fat diet; Nrg4: neuregulin 4.
ADSC infusion improved insulin sensitivity in insulin target tissues
To characterize the effects of Nrg4-ADSC or ADSCs transplantation on insulin-targeting tissues, skeletal muscle and adipose tissues were excised for HE staining. Neither muscles nor the adipose tissue showed significant histopathological changes (Figure 5(a)). However, the size of adipocytes in inguen adipose tissues was slightly reduced in the group receiving Nrg4-ADSC transplantation. We next examined the expression of GLUT4 in skeletal muscle and adipose tissues. As shown in Figure 5(b) and (c), the levels of GLUT4 in skeletal muscle and adipose tissues were dramatically elevated after ADSC or Nrg4-ADSC infusion (P < 0.01), while there is no difference between the ADSC and Nrg4-tADSC groups.
Figure 5.
Effects of ADSC or Nrg4-ADSC transplantation on expression of Glu4 and inflammation factors in skeletal muscle and adipose tissues. (a) HE staining of skeletal muscle and adipose tissues, (b, c) GLUT4 expression in the skeletal muscle and adipose tissues, and (d) expression of IL-6 and TNF-α in skeletal muscle and adipose tissues. ADSC: adipose tissue-derived MSC; HFD: high-fat diet; IL-6: interleukin-6; ND: normal diet; Nrg4: neuregulin 4; TNF-α: tumor necrosis factor-alpha. (A color version of this figure is available in the online journal.)
We further investigated the expressions of IL-6 and TNF-α in muscle and adipose tissues (Figure 5(d)). ADSC or Nrg4-ADSC infusion significantly down-regulated the expression of IL-6 and TNF-α. However, no significant difference in the expression of these genes was observed between the ADSC and Nrg4-ADSC groups. Taken together, these data suggest that ADSC transplantation could improve insulin sensitivity of target tissues via enhancing glucose uptake and regulating inflammatory reaction. However, these two beneficial effects were not further amplified in mice transplanted with Nrg4-ADSCs. Considering the stronger effects of Nrg4-ADSC transplantation in mitigating glucose intolerance (GTT) and IR (ITT) compared with ADSC transplantation, we hypothesized that some other mechanisms may contribute to the improved effects of Nrg4-ADSC transplantation.
Nrg4-ADSCs showed increased ability to decrease hepatic steatosis compared with ADSCs
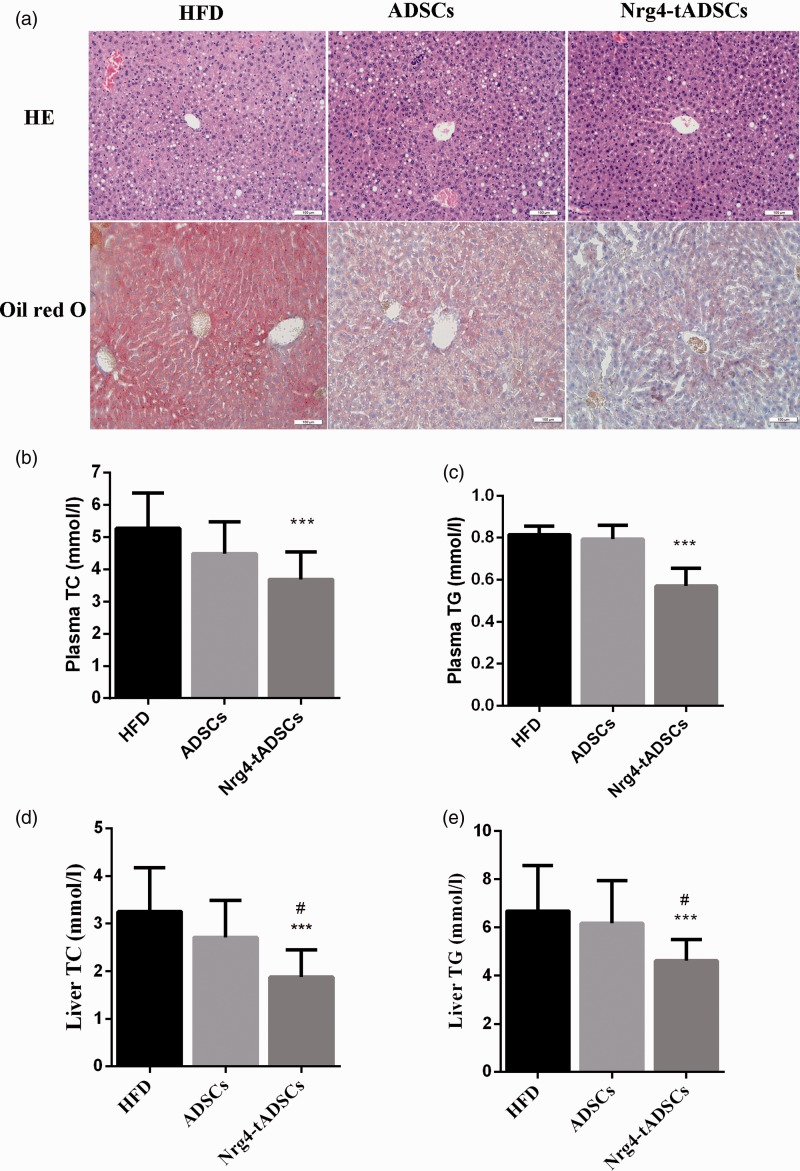
Considering the ability of Nrg4 to inhibit hepatic adipogenesis,12 we assessed whether transplantation of Nrg4-ADSCs was more efficient in reducing hepatic steatosis compared with ADSC injection. Oil Red O staining revealed significant decrease in lipid droplet accumulation after Nrg4-ADSC transplantation, whereas a moderate decrease was observed in the ADSC transplantation group (Figure 6(a)). The decreased accumulation of lipid droplets in the liver upon Nrg4-ADSC transplantation encouraged us to further analyze levels of TC and TG in the plasma and liver. As shown in Figure 6(b) to (e), the levels of TC and TG in the plasma and liver tissues were significantly decreased in Nrg4-tADSC-treated HFD-fed mice compared with those without the transplantation; however, such reduction was not observed in the group receiving the ADSC transplantation.
Figure 6.
Effects of ADSC or Nrg4-ADSC transplantation on hepatic steatosis. (a) Representative micrographs of HE and Oil Red O staining of the liver tissue sections, (b, c) levels of total cholesterol (TC, B) and TG (C) and in the plasma of the indicated mouse groups, and (d, e) the TC (d) and TG (e) levels in the liver tissues of the indicated mouse groups. *: compare with HFD, ***P < 0.001; #: compare with ADSCs, #P < 0.05. ADSCs – HFD-fed mice with ADSC infusion; HFD – HFD-fed mice without cell infusion; Nrg4-ADSC – HFD-fed mice with Nrg4-ADSC infusion. ADSC: adipose tissue-derived MSC; HFD: high-fat diet; Nrg4: neuregulin 4; TC: total cholesterol; TG: triglyceride. (A color version of this figure is available in the online journal.)
Nrg4-tADSCs transplantation inhibits hepatic lipogenesis
We next determined the effect of Nrg4-ADSC transplantation on hepatic lipogenesis. IHC staining revealed the presence of Nrg4 in the liver, but not in the skeletal muscle and adipose tissues (Figure 7(a)). This finding is consistent with the previous report that Nrg4 secreted specifically targets the liver.12 We further investigated the mRNA expression profiles of key genes involved in lipid metabolism by real-time PCR. The expression of sterol regulatory element binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) was significantly down-regulated in the Nrg4-ADSC group (Figure 7(b) and (c)). Consistently, western blot analysis of the liver tissue extracts revealed that the expression level of SREBP-1c was significantly decreased in the Nrg4-ADSC group compared with the HFD and ADSC groups (Figure 7(d)). Taken together, these data suggest that Nrg4-ADSCs transplanted could secrete Nrg4 proteins; these secreted Nrg4 could reach the liver and act on hepatocytes, where Nrg4 inhibits lipogenesis by suppressing the expression of adipogenesis-related genes such as SREBP-1c.
Figure 7.
Nrg4-tADSC transplantation mitigated hepatic adipogenesis. (a) IHC staining with anti-Nrg4 of the adipose, muscle, and liver tissues of HFD-fed mice transplanted with ADSCs or Nrg4-ADSCs, (b, c) expression of SREBP-1c and FAS at the mRNA level. Total RNA was extracted from liver tissue extracts collected from the indicated groups. RT-PCR was then performed. The mRNA level shown was normalized by β-actin level and (d) the protein levels of SREBP-1c and FAS in the liver tissue extracts obtained from the indicated groups. Western blot analysis was performed to examine the protein levels. β-actin served as a loading control. ADSCs – HFD-fed mice with ADSC infusion; HFD – HFD-fed mice without cell infusion; Nrg4-ADSC – HFD-fed mice with Nrg4-ADSC infusion. ADSC: adipose tissue-derived MSC; FAS: fatty acid synthase; HFD: high-fat diet; Nrg4: neuregulin 4; SREBP-1: sterol regulatory element binding protein-1. (A color version of this figure is available in the online journal.)
Discussion
The aim of the present study is to assess the effect of ADSCs or Nrg4-tADSCs transplantation on glucose and lipid metabolism in HFD-fed mice. The results demonstrate that ADSCs or Nrg4-tADSCs transplantation could reverse hyperglycemia and ameliorate IR induced by HFD. In addition, ADSCs overexpressing Nrg4 could improve the efficacy of ADSCs in ameliorating hyperglycemia and IR. The possible mechanism is that Nrg4 could mitigate hepatic adipogenesis by down-regulating SREBP-1c and FAS expression in the liver.
Obesity is a worldwide epidemic that is characterized not only by excessive accumulation of fat tissue but also by systemic micro-inflammation and high oxidative stress. Long-term feeding on a HFD can result in hyperglycemia, hyperinsulinemia, hyperlipidemia, and impaired glucose tolerance, which are typically linked with human obesity. Consistent with the previous reports,21 our study revealed that HFD increased body weight, and led to glucose metabolic disorders, including increase in fasting blood levels of glucose and insulin and defect in glucose tolerance and insulin sensitivity. ADSC transplantation showed effectiveness in reversing these glucose metabolic disorders, but did not affect HFD-induced weight gain. Compared with ADSCs, Nrg4-ADSCs possessed enhanced ability to normalize fasting blood glucose and insulin levels and to improve glucose tolerance and insulin sensitivity. Nrg4-ADSC transplantation also reduced body weight in HFD mice, which was not observed in mice with the ADSC infusion.
IR is a serious state characterized by reduced insulin sensitivity in peripheral organs like the liver, adipose tissue, and skeletal muscle.34 IR is a characteristic feature of most patients with obesity and is one of the defining clinical features in the Metabolic Syndrome.35 The exact mechanisms and causes of IR are still unclear; however, it has become increasingly evident that obesity and the accompanying inflammation are the principal reasons of IR. In the last decade, it has been reported that the chronic activation of pro-inflammatory signaling pathways was increased IR.36 It has been reported that the secretions of IL-6 and TNF-α in the liver, which are considered to be major inflammatory mediators of steatosis, IR, and associated inflammatory disorders, were significantly inhibited following ADSC transplantation.28 In this study, we investigated the expressions of IL-6 and TNF-α in muscles and adipose tissue. The results showed that both ADSCs and Nrg4-ADSCs infusions significantly down-regulated the expression of IL-6 and TNF-α in insulin-targeting tissues, suggesting both transplantations are effective in ameliorating HFD-induced inflammation. However, Nrg4-ADSCs were not superior to ADSCs in terms of mitigating inflammation, implying the existence of some other mechanisms underlying the improved efficacy of Nrg4-ADSCs in promoting insulin sensitivity in obese mice.
GLUT4, a glucose transporter family protein, is mostly expressed in adipocytes, skeletal muscle, and cardiomyocytes.37 GLUT4 plays a critical role in glucose-sensing. In addition, GLUT4 can regulate insulin sensitivity, which makes it become a unique isoform among glucose transporters.38 It has been shown that GLUT4 levels are significantly decreased in the skeletal muscle of T2D patients as well as in severe obesity patients.39,40 It has been proved that potent promotes of GLUT4 expression can improve insulin sensitivity in transgenic mice.41 In this study, HFD decreased the GLUT4 expression in the adipocytes and skeletal muscle, which could be reversed by ADSC infusion. However, Nrg4-ADSCs showed similar effects in upregulating GLUT4 expression, compared with ADSCs. This result suggests while promoting glucose utilization by upregulating GLUT4 expression contributes to ADSC infusion-induced improvement in glucose homeostasis; this mechanism still cannot explain the enhanced ability of Nrg4-ADSCs to correct the glucose metabolic disorder.
Considering the effect of Nrg4 in preventing against adiposeness in liver,15 we attempted to explore whether such mechanism accounts for the superior effects of Nrg4-ADSC transplantation. Nrg4-ADSC transplantation successfully led to the increased levels of Nrg4 in the liver, which was not observed after ADSC transplantation. Consistent with a previous report,22 ADSC transplantation attenuated the lipid accumulation in the liver. Interestingly, the Nrg4-ADSC group showed much less fat cell deposition in the liver as well as decreased levels of TG and TC in the liver and plasma compared with the ADSC group. Furthermore, consistent with the function of Nrg4 in attenuating lipogenesis in hepatocytes, Nrg4-ADSC transplantation, but not ADSC transplantation, reduced the expression of SREBP-1c and its target gene FAS, both of which are critical for lipogenesis and lipid metabolism.42–44 It is known that activated SREBP-1 can induce hepatic lipogenic gene transcription, thus facilitating hepatic steatosis.45 Enhancing the expression of FAS may increase fatty acid synthesis and lead to the body fat ectopic accumulation.46 It has been proved that the proteins of SREBP-1c, and FAS were increased impressively in the liver of HFD-fed mice. These results demonstrate that ectopic expression of Nrg4 in ADSCs enabled these cells to effectively attenuate hepatic lipogenesis, and consequently improve their ability to ameliorate diet-induced fatty liver disease and IR. The effect of Nrg4-ADSC in reducing hepatic lipogenesis also provides a possible explanation for the decreased body weight in mice receiving Nrg4-ADSC infusion.
Taken together, our study reveals that ADSC transplantation improves glucose tolerance and metabolic balance in HFD-fed mice by multiple mechanisms, including upregulating GLUT4 expression and suppressing inflammation. More importantly, our study shows that Nrg4 overexpression could improve the efficacy of ADSCs in ameliorating IR and other obesity-related metabolic disorders, given the function of Nrg4 in attenuating hepatic lipogenesis. It would provide a new therapeutic strategy for the treatment of obesity, IR, and T2D.
Authors’ contributions
WW and YZ contributed equally to this paper. All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; WW, YZ, CY, YW, JS, and MS conducted the experiments, WW supplied critical C57BL/6 mice and ADSCs, WW and YZ wrote the manuscript, and BW contributed to the experimental instruction and final revision.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by the Clinical Research Plan of SHDC (No.16CR2005A) and Clinical Research Program of 9th People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine (JYLJ0130).
References
- 1.Mendis S, Armstrong T, Bettcher D, Branca F, Lauer J, Mace C, Poznyak V, Riley L, Silva VD, Stevens G. Global status report on noncommunicable diseases 2014. Geneva: World Health Organization, 2014
- 2.World Health Organization. Global health estimates: deaths by cause, age, sex and country, 2000–2012. Geneva: WHO, 2014 [Google Scholar]
- 3.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015; 314:1021. [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW, Smith RJ, Smith SR. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab 2011; 96:1654–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Stefanetti R, Trenell M, Welsh P, Kean S, Ford I, McConnachie A, Sattar N, Taylor R. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018; 391:541–51 [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017; 317:2515–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA 2012; 308:581–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harari D, Tzahar E, Romano J, Shelly M, Pierce JH, Andrews GC, Yarden Y. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene 1999; 18:2681–9 [DOI] [PubMed] [Google Scholar]
- 9.Bernard JK, McCann SP, Bhardwaj V, Washington MK, Frey MR. Neuregulin-4 is a survival factor for colon epithelial cells both in culture and in vivo. J Biol Chem 2012; 287:39850–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McElroy SJ, Castle SL, Bernard JK, Almohazey D, Hunter CJ, Bell BA, Al Alam D, Wang L, Ford HR, Frey MR. The ErbB4 ligand neuregulin-4 protects against experimental necrotizing enterocolitis. Am J Pathol 2014; 184:2768–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E, Millership S, Fenech ME, MacIntyre D, Turner JO, Moore JD, Blackburn E, Gullick WJ, Cinti S, Montana G, Parker MG, Christian M. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab 2014; 306:E945–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, Li S, Bluher M, Lin JD. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med 2014; 20:1436–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nugroho DB, Ikeda K, Kajimoto K, Hirata KI, Emoto N. Activation of neuregulin-4 in adipocytes improves metabolic health by enhancing adipose tissue angiogenesis. Biochem Biophys Res Commun 2018; 504:427–33 [DOI] [PubMed] [Google Scholar]
- 14.Cai C, Lin M, Xu Y, Li X, Yang S, Zhang H. Association of circulating neuregulin 4 with metabolic syndrome in obese adults: a cross-sectional study. BMC Med 2016; 14:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Gao M, Liu D. Preventing high fat diet-induced obesity and improving insulin sensitivity through neuregulin 4 gene transfer. Sci Rep 2016; 6:26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplan AI. Mesenchymal stem cells. J Orthop Res 1991; 9:641–50 [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143–7 [DOI] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315–7 [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol 2012; 33:136–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 2014; 54:1418–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong SP, Rowley JE, Redpath AN, Tilman JD, Fellous TG, Johnson JR. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther 2015; 151:107–20 [DOI] [PubMed] [Google Scholar]
- 22.Cao M, Pan Q, Dong H, Yuan X, Li Y, Sun Z, Dong X, Wang H. Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem Cell Res Ther 2015; 6:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015; 64:587–92 [DOI] [PubMed] [Google Scholar]
- 24.Zhao K, Hao H, Liu J, Tong C, Cheng Y, Xie Z, Zang L, Mu Y, Han W. Bone marrow-derived mesenchymal stem cells ameliorate chronic high glucose-induced beta-cell injury through modulation of autophagy. Cell Death Dis 2015; 6:e1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahavi H, Hashemi SM, Soleimani M, Mohammadi J, Tajik N. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res 2015; 2015:878535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai J, Wu Z, Xu X, Liao L, Chen J, Huang L, Wu W, Luo F, Wu C, Pugliese A, Pileggi A, Ricordi C, Tan J. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care 2016; 39:149–57 [DOI] [PubMed] [Google Scholar]
- 27.Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y, Shen J, Cheng Y, Fu X, Han W. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes 2012; 61:1616–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji AT, Chang YC, Fu YJ, Lee OK, Ho JH. Niche-dependent regulations of metabolic balance in high-fat diet-induced diabetic mice by mesenchymal stromal cells. Diabetes 2015; 64:926–36 [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Zheng P, Wang X, Dai G, Cheng H, Zhang Z, Hua R, Niu X, Shi J, An Y. A preliminary evaluation of efficacy and safety of Wharton’s jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther 2014; 5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, Chun JM, Lee SK, Kim SJ. Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc 2008; 40:2649–54 [DOI] [PubMed] [Google Scholar]
- 31.Peng Y, Wen D, Lin F, Mahato RI. Co-delivery of siAlox15 and sunitinib for reversing the new-onset of type 1 diabetes in non-obese diabetic mice. J Control Release 2018; 292:1–12 [DOI] [PubMed] [Google Scholar]
- 32.Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Buttner R, Scholmerich J, Hellerbrand C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res 2009; 19:996–1005 [DOI] [PubMed] [Google Scholar]
- 33.Chen XD, Deng M, Zhou JS, Xiao YZ, Zhou XS, Zhang CC, Wu M, Wang ZD, Chen XT. Bone morphogenetic protein-2 regulates in vitro osteogenic differentiation of mouse adipose derived stem cells. Eur Rev Med Pharmacol Sci 2015; 19:2048–53 [PubMed] [Google Scholar]
- 34.Sharawy MH, El-Awady MS, Megahed N, Gameil NM. Attenuation of insulin resistance in rats by agmatine: role of SREBP-1c, mTOR and GLUT-2. Naunyn-Schmiedeberg’s Arch Pharmacol 2016; 389:45–56 [DOI] [PubMed] [Google Scholar]
- 35.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116:1793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lien GS, Liu JF, Chien MH, Hsu WT, Chang TH, Ku CC, Ji AT, Tan P, Hsieh TL, Lee LM, Ho JH. The ability to suppress macrophage-mediated inflammation in orbital fat stem cells is controlled by miR-671-5p. Stem Cell Res Ther 2014; 5:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos JM, Benite-Ribeiro SA, Queiroz G, Duarte JA. The interrelation between aPKC and glucose uptake in the skeletal muscle during contraction and insulin stimulation. Cell Biochem Funct 2014; 32:621–4 [DOI] [PubMed] [Google Scholar]
- 38.dos Santos JM, Benite-Ribeiro SA, Queiroz G, Duarte JA. The effect of age on glucose uptake and GLUT1 and GLUT4 expression in rat skeletal muscle. Cell Biochem Funct 2012; 30:191–7 [DOI] [PubMed] [Google Scholar]
- 39.Bouzakri K, Koistinen HA, Zierath JR. Molecular mechanisms of skeletal muscle insulin resistance in type 2 diabetes. CDR 2005; 1:167–74 [DOI] [PubMed] [Google Scholar]
- 40.Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep 2006; 6:177–81 [DOI] [PubMed] [Google Scholar]
- 41.Katz EB, Burcelin R, Tsao TS, Stenbit AE, Charron MJ. The metabolic consequences of altered glucose transporter expression in transgenic mice. J Mol Med 1996; 74:639–52 [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyake K, Furukawa K, Hayashi Y, Iguchi H, Matsuki Y, Hiramatsu R, Shimano H, Yamada N, Ohno S, Kasuga M, Noda T. PKClambda in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest 2003; 112:935–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109:1125–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan Y, Ren C, Wang Z, Jia R, Wang D, Zhang Y, Zhang G, Wan Y, Huang M, Wang F. Transgenesis of humanized fat1 promotes n-3 polyunsaturated fatty acid synthesis and expression of genes involved in lipid metabolism in goat cells. Gene 2016; 576:249–55 [DOI] [PubMed] [Google Scholar]
- 45.Qi ZG, Zhao X, Zhong W, Xie ML. Osthole improves glucose and lipid metabolism via modulation of PPARalpha/gamma-mediated target gene expression in liver, adipose tissue, and skeletal muscle in fatty liver rats. Pharm Biol 2016; 54:882–8 [DOI] [PubMed] [Google Scholar]
- 46.Yan D, Lehto M, Rasilainen L, Metso J, Ehnholm C, Yla-Herttuala S, Jauhiainen M, Olkkonen VM. Oxysterol binding protein induces upregulation of SREBP-1c and enhances hepatic lipogenesis. Arterioscler Thromb Vasc Biol 2007; 27:1108–14 [DOI] [PubMed] [Google Scholar]