Abstract
Background
Studies have shown increased intestinal permeability in irritable bowel syndrome. Validating serum biomarkers for altered intestinal permeability in irritable bowel syndrome will facilitate research and pathophysiology-based therapy.
Objective
To measure serum zonulin and intestinal fatty acid binding protein levels in diarrhea-predominant irritable bowel syndrome and constipation-predominant irritable bowel syndrome and compare with healthy controls and celiac disease.
Methods
Serum zonulin and intestinal fatty acid binding protein levels were measured using enzyme-linked immunosorbent assays in constipation-predominant irritable bowel syndrome (n = 50), diarrhea-predominant irritable bowel syndrome (n = 50), celiac disease (n = 53) and healthy controls (n = 42). Irritable bowel syndrome symptom severity was measured using the irritable bowel syndrome-symptom severity scale.
Results
Patients with constipation-predominant irritable bowel syndrome and diarrhea-predominant irritable bowel syndrome had higher zonulin levels compared with healthy controls (p = 0.006 and 0.009 respectively), which was comparable to those with active celiac disease. Although zonulin levels did not correlate with the overall irritable bowel syndrome symptom severity scale, it positively correlated with stool frequency per week (p = 0.03) and dissatisfaction with bowel habits (p = 0.007) in diarrhea-predominant irritable bowel syndrome. Patients with diarrhea-predominant irritable bowel syndrome and constipation-predominant irritable bowel syndrome had lower intestinal fatty acid binding protein levels compared with celiac patients (p = 0.005 and p = 0.047 respectively).
Conclusion
Serum zonulin is upregulated in irritable bowel syndrome and the levels are comparable to those in celiac disease. Zonulin levels correlated with severity of bowel habits in diarrhea-predominant irritable bowel syndrome. Intestinal fatty acid binding protein levels in irritable bowel syndrome patients were not increased suggesting no significant increase in enterocyte death.
Keywords: Intestinal permeability, tight junction, gut barrier, serine protease
Key summary
Summarize the established knowledge on this subject
Several studies have shown increased intestinal permeability in patients with irritable bowel syndrome.
Validating serum biomarkers for altered increased permeability in irritable bowel syndrome is needed as current techniques are invasive, unreliable and/or cumbersome.
What are the significant and/or new findings of this study
We showed that serum zonulin, a biomarker of impaired increased permeability, is increased in patients with diarrhea-predominant irritable bowel syndrome and constipation-predominant irritable bowel syndrome compared to healthy controls and the levels are comparable to celiac disease.
In our study, increased permeability in patients with irritable bowel syndrome was not associated with increase in enterocyte turnover as levels of intestinal fatty acid binding protein were not increased.
In our patients with diarrhea-predominant irritable bowel syndrome, the increase in permeability was clinically relevant as serum zonulin levels correlated with diarrhea severity.
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by abdominal pain and altered bowel habits.1 It affects approximately 11% of adults around the world and is associated with impaired quality of life and increased use of healthcare services.2–5 There is increasing evidence that the pathophysiology of IBS is multifaceted involving mucosal inflammation, visceral hypersensitivity, microbial dysbiosis, dietary factors and altered intestinal permeability (IP).6
Several studies have investigated IP in patients with IBS.6–9 Increased IP has been noted in both adult and pediatric patients, primarily with diarrhea-predominant IBS (IBS-D).6,10–13 Others have shown that increased small intestinal and/or colonic permeability in IBS is not restricted to IBS-D and could also be seen in constipation-predominant IBS (IBS-C).14–16 However, commonly used techniques to measure IP have significant limitations, making them cumbersome in clinical practice. Although widely used, the lactulose mannitol assay (LMA) is time consuming and unreliable.17 Ex-vivo techniques, such as Ussing chamber and western blotting/immune-fluorescence of tight junction (TJ) proteins, are invasive as they require intestinal biopsies.17 Identifying serological biomarkers of altered IP in patients with IBS would facilitate further research as well as personalized medicine using future pharmacotherapies targeted to the underlying disease mechanism.17
Zonulin and intestinal fatty acid binding protein (I-FABP) are two serum markers of IP. Zonulin is a human analogue of Vibrio Cholerae zonula occludens toxin, which is an endogenous modulator of the intercellular TJ. Serum zonulin levels are increased in several diseases in which IP dysfunction is central, including celiac disease (CeD), and type 1 diabetes (T1DM).18–20 The importance of zonulin in modulating IP is also demonstrated by larazotide acetate, a synthetic peptide that inhibits zonulin, which has been shown to prevent intestinal TJ opening and improve symptoms in non-responsive CeD.21 I-FABP is a small cytosolic protein, present in mature enterocytes, primarily jejunum.17 Serum I-FABP is a marker of enterocyte turnover and elevated levels indicate intestinal epithelial cell damage.22 I-FABP has been shown to be elevated in diseases with enterocyte damage such as CeD.22,23
The role of zonulin and I-FABP in IBS has not been well studied. Preliminary data from 15 patients with IBS-D showed that serum zonulin levels were similar to healthy controls (HC).24 Undseth et al. found a significantly lower level of I-FABP in patients with IBS compared with HC, but the study was again limited by small sample size (67 subjects).25
To address these gaps in our knowledge of the role of altered IP in IBS, we compared serum zonulin and I-FABP levels in patients with IBS-D and IBS-C with that in HC and patients with increased permeability due to active celiac disease.
Methods
Study participants
Patients with IBS were identified by reviewing an existing gastrointestinal sera repository maintained at the Division of Gastroenterology, Beth Israel Deaconess Medical Center. Only patients in whom diagnosis of IBS-D and IBS-C were made by gastroenterologists with expertise in functional bowel disorders (AL, JN) utilizing Rome III criteria were included in the study. For IBS-D patients, only those who had upper endoscopy with histologically normal duodenal mucosa as part of their evaluation were included. Sera were collected from patients with IBS-D and IBS-C at the time of their clinic visit as part of gastrointestinal sera repository maintained at our institute. Patients with a diagnosis of IBS completed the IBS severity scale (IBS-SSS) at the time of sera collection.26
Patients with CeD were selected from participants in the Mantioba Celiac Disease Cohort. Diagnosis of CeD was based on positive celiac serology and villous atrophy on duodenal biopsies. Sera from celiac patients were obtained within 6 weeks of initiation of a gluten-free diet (GFD). HC were individuals without known gastrointestinal disorders or symptoms who were seen in a primary care clinic for annual health examinations and underwent routine laboratory work for health maintenance.
Analyses of blood samples
Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to measure serum zonulin (Catalog number 30-ZONSHU-E01; ALPCO, Salem, NH) and I-FABP levels (HK406; Hycult Biotech, Uden, The Netherlands), according to the manufacturers' protocol.
Ethics approval
Ethics approval was obtained from Institutional Review Board (IRB) (Protocol # 2018P000009, 16 January 2018). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee. The need for informed consent was waived by the IRB for HC and IBS patients in gastrointestinal sera repository as the repository used discarded sera. Written informed consent was obtained from all patients with CeD.
Statistical analyses
Data were analyzed using STATA 13.0 (StataCorp, College Station, TX, USA) and Prism 6 (GraphPad) software. Mean age and gender were compared among the groups using analysis of variance and chi square testing. Serum zonulin and FABP were not normally distributed and are reported as median (interquartile range, IQR). Group differences in the level of biomarkers were analyzed using Kruskal-Wallis one-way analysis of variance, with post hoc testing and correction for multiple comparisons. Correlation analysis was performed using Pearson's correlation coefficient. A p value < 0.05 was considered statistically significant.
Results
Study participants
A total of 50 patients with IBS-D, 50 patients with IBS-C, 53 patients with CeD and 42 HC were included in the study. The mean age in all four groups ranged from 31.6 years to 41.2 years (Table 1). Although the majority of patients with CeD and IBS-C were females, IBS-D was more common in male patients; a finding reported in prior studies.27
Table 1.
Demographic characteristics of study population.
| Celiac disease (n = 53) | IBS-D (n = 50) | IBS-C (n = 50) | Healthy controls (n = 42) | p value | |
|---|---|---|---|---|---|
| Age (years) | 41.2 ± 16.6 | 32.1 ± 12 | 31.6 ± 10.5 | 35.2 ± 10.5 | <0.001 |
| Female gender | 35 (66%) | 12 (24.0%) | 48 (96%) | 33 (78.6%) | <0.001 |
IBS-C: constipation-predominant irritable bowel syndrome; IBS-D: diarrhea-predominant IBS.
Serum zonulin levels
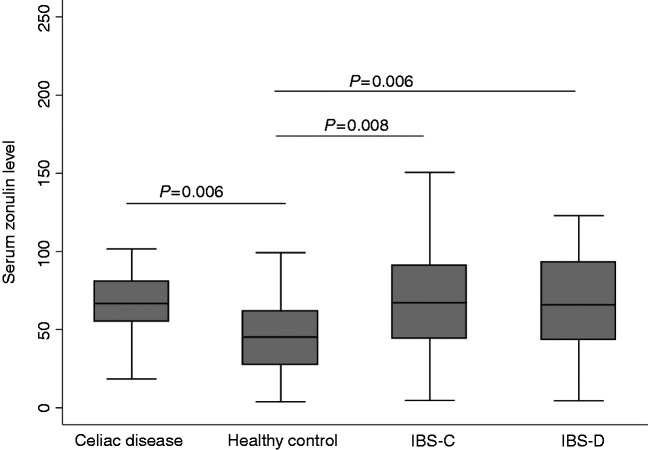
The median zonulin levels were lowest in HC (45.2 ng/ml) and highest in patients with CeD (66.7 ng/ml) (p = 0.006) (Table 2). Patients with IBS-C and IBS-D also had significantly higher zonulin levels compared with HC (p = 0.006 and 0.009 respectively) (Figure 1). Serum zonulin levels in patients with IBS-D and IBS-C were comparable to those with active CeD (p > 0.9 for each).
Table 2.
Zonulin and I-FABP levels in patients with IBS compared with healthy controls and celiac disease.
| Biomarker | Celiac disease (n = 53) | IBS-D (n = 50) | IBS-C (n = 50) | Healthy controls (n = 42) |
|---|---|---|---|---|
| Zonulin levels* (ng/ml) | 66.7 (55.2, 80.5) | 65.8 (43.3, 93.4) | 67.2 (44.2, 91.2) | 45.2 (27.3, 61.5) |
| I-FABP levels* (pg/ml) | 391.4 (217.1, 768.9) | 224.1 (121.8, 384.9) | 205.5 (99.4, 333.3) | 338.2 (204.9, 479.0) |
Values expressed as median (interquartile range).
IBS-C: constipation-predominant irritable bowel syndrome; IBS-D: diarrhea-predominant IBS; I-FABP: intestinal fatty acid binding protein.
Figure 1.
Serum zonulin levels in patients with irritable bowel syndrome compared with healthy controls and celiac disease.
Zonulin levels and symptom correlation
Symptom severity was available for 64 IBS patients (27 IBS-C, 37 IBS-D) with a mean IBS-SSS score of 238.4 ( ± 61.8). IBS-SSS was not available for the remaining IBS patients as they were still undergoing evaluation and had not yet received a formal diagnosis of IBS at the time of serum collection. Overall IBS-SSS did not correlate with zonulin in patients with IBS-C and/or IBS-D (Table 3). In the IBS-D group, self-reported dissatisfaction with bowel habits positively correlated with serum zonulin levels (p = 0.007). None of the other IBS-SSS subdomains correlated with zonulin levels in patients with IBS-D and/or IBS-C (Table 3).
Table 3.
Correlation between IBS symptom severity and serum zonulin levels.
| IBS-D and IBS-C (n = 64) |
IBS-D (n = 37) |
IBS-C (n = 27) |
||||
|---|---|---|---|---|---|---|
| Correlation coefficient | p value | Correlation coefficient | p value | Correlation coefficient | p value | |
| Overall IBS severity* | 0.14 | 0.25 | 0.24 | 0.15 | 0.02 | 0.91 |
| Subscales | −0.03 | 0.82 | −0.11 | 0.50 | 0.02 | 0.91 |
| Pain severity | 0.01 | 0.93 | 0.13 | 0.45 | −0.13 | 0.51 |
| Pain frequency | −0.01 | 0.94 | 0.12 | 0.47 | −0.21 | 0.30 |
| Bloating severity | 0.29 | 0.02 | 0.43 | 0.007 | 0.15 | 0.46 |
| Bowel dissatisfaction | 0.16 | 0.20 | 0.19 | 0.25 | 0.16 | 0.42 |
| Interference | ||||||
| IBS-D and IBS-C (n = 50) |
IBS-D (n = 25) | IBS-C (n = 25) | ||||
| Stool frequency | 0.27 | 0.06 | 0.45 | 0.03 | −0.18 | 0.41 |
Measured using IBS-SSS. Bold values refer to P value less than < 0.05 which is statistically significant.
IBS: irritable bowel syndrome; IBS-C: constipation-predominant IBS; IBS-D: diarrhea-predominant IBS; IBS-SSS: IBS severity scale score.
Of these 64 patients, 50 patients (25 IBS-C and 25 IBS-D) also reported usual stool frequency per week. Zonulin levels correlated with stool frequency in patients with IBS-D (p = 0.03).
Serum I-FABP levels
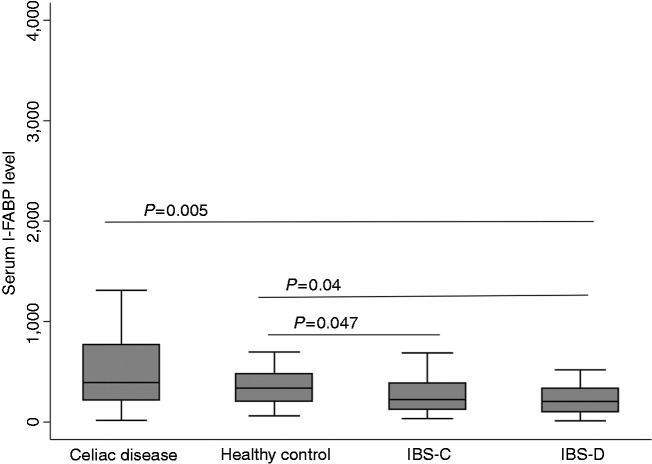
The median levels of I-FABP levels (IQR) were lowest in patients with IBS-D (205.5 pg/ml) and highest in patients with CeD (391.5 pg/ml) (Table 2). The IBS-D group had significantly lower median I-FABP levels compared with the CeD group (p = 0.005) or the HC (p = 0.04) (Figure 2). I-FABP levels were also significantly lower in the IBS-C group compared to the CeD group (p = 0.047). There were no other significant differences among groups.
Figure 2.
Serum intestinal fatty acid binding protein (I-FABP) levels in patients with irritable bowel syndrome compared with celiac disease and healthy controls.
Discussion
This is the first study to show that serum zonulin, a biomarker of impaired IP, is increased in patients with IBS-D and IBS-C compared to HC. We also found that serum levels of zonulin in patients with IBS are comparable to active CeD, a disorder with well-documented TJ dysfunction. Increased IP in patients with IBS was not associated with an increase in enterocyte turnover as levels of I-FABP were not increased. However, this increase in permeability was clinically relevant as zonulin levels correlated with severity of bowel habits and stool frequency in patients with IBS-D, both of which are surrogates of diarrhea severity in these patients.
Zonulin has been shown to regulate IP and elevated levels of zonulin have been well documented in several gastrointestinal as well as non-gastrointestinal disorders associated with alteration in IP including CeD, T1DM, and rheumatoid arthritis.18–20 It alters small intestinal intercellular TJ integrity by activating epidermal growth factor receptor through proteinase-activated receptor 2 (PAR-2).18 It has also been shown to increase colonic permeability in response to exposure to enteric bacteria.28 Although several studies have reported increased small intestinal and colonic permeability in patients with IBS-D using ex-vivo and in-vivo techniques, zonulin has not been systematically studied in IBS.6,10–13
We found that serum zonulin levels in IBS-D patients were significantly higher compared with HC, suggesting increased IP in IBS-D. In a small study, Barbano et al.24 did not find significantly higher levels of zonulin in IBS-D patients (n = 15) compared with controls, which could be a type II error. Interestingly, PAR-2, which is involved in downstream signaling of zonulin, has been suggested to be involved in the increased permeability in IBS-D patients.29 Serine proteases, such as trypsin, are known to activate PAR-2, and elevated levels of serine protease have been reported in fecal samples of IBS-D patients.30 Future studies should investigate the role of zonulin in IP in patients with IBS-D.
Although all patients with IBS-D in our study had normal small bowel histology, zonulin levels in patients with IBS-D were comparable to patients with active CeD. Although our celiac patients were on a GFD, it is unlikely that there would be complete healing of villous atrophy after such a short duration (median 4 weeks). Studies have shown a persistently abnormal small IP in all patients with CeD (15/15) after 8 weeks of a GFD.31 Furthermore, persistently elevated zonulin levels have been noted in >80% of celiac patients after a mean GFD duration of about 10 years (and in 100% of cases with persistent villous atrophy).32 It is possible that untreated celiac patients have higher zonulin levels than IBS patients. However, our data suggest that despite small bowel mucosa appearing normal, there is significant TJ dysfunction in IBS-D patients and degree of dysfunction might be comparable to other gastrointestinal disorders with mucosal abnormalities and increased small IP. We also found that serum zonulin levels correlated with patient reported dissatisfaction with bowel habits as well as stool frequency in patients with IBS-D. Other studies have also shown that IBS-D patients with altered IP have higher stool frequency, visceral hypersensitivity and more severe IBS symptoms.6,10,12
We also found that zonulin levels in patients with IBS-C were significantly higher than HC and comparable to those with IBS-D and CeD. Current studies in patients with IBS-C have focused on colonic permeability and data on small IP are scarce.6 Although some studies have shown increased colonic permeability, others have not.6 Studies based on polyethylene glycol excretion (measuring both small bowel and colonic permeability) have shown increased IP in IBS-C patients, however, a recent study did not report any changes in IP in women with IBS-C.15,16,33
The exact triggers of zonulin release in IBS are unclear. Exposure to enteric pathogens and gluten have been shown as the two most powerful triggers of zonulin release.18 Notably, increased levels of serum zonulin in patients with non-celiac gluten sensitivity (NCGS) has been reported and there is significant overlap between symptoms of patients with IBS and NCGS.24 Increased IP in response to gluten in HLA-DQ2/DQ8 positive IBS-D patients has been shown.34 Unfortunately, we did not have data on gluten sensitivity or HLA genotyping in our IBS patients. Alteration in gut microbiome and mucosal inflammation are likely to contribute to TJ dysfunction in IBS.6
Serum I-FABP levels in IBS patients were not increased, suggesting that altered permeability observed in our study is likely not due to an increased rate of enterocyte death. Although studies have shown that I-FABP is significantly higher in patients with untreated CeD, levels tend to normalize within 6 weeks of gluten elimination.23 As our samples were collected within 6 weeks of initial diagnosis of CeD, the majority of our participants had initiated a GFD. This might explain why the levels of I-FABP in patients with CeD were higher (but not significantly different) compared to controls. It is also interesting to note that I-FABP levels in patients with IBS-C were significantly lower compared to HC, a finding that was also seen in a previous study.25 This decreased enterocyte turnover noted in IBS patients might be another potential pathogenic factor in IBS, which needs to be explored further in future studies.
Our study has a few limitations. Firstly, we did not have data on extra-intestinal symptoms such as depression or anxiety. Secondly, we did not correlate the biomarkers with other measures of IP such as LMA or immunohistochemistry for TJ proteins. This was an exploratory study, so we did not have data on gluten sensitivity, HLA genotyping or gut microbiome composition in these patients, factors known to modulate zonulin expression and release. We also did not have data on clinical symptoms such as IBS severity, severity of bowel dysfunction etc. in all patients with IBS due to the retrospective nature of the study design, which used a pre-existing sera repository. A recent study suggests the commercially available ELISA kit for zonulin used in our study could identify a variety of proteins structurally and possibly functionally related to zonulin, suggesting the existence of a family of zonulin proteins, rather than a single member of permeability-regulating proteins.35 Thus, our findings need to be interpreted with caution until they are confirmed in future studies.
In this study, we show that zonulin might have utility as a simple serological biomarker for altered IP in patients with IBS. Despite normal small mucosal histology, patients with IBS-D have TJ dysfunction (measured using zonulin levels) comparable to active CeD. Serum zonulin levels also correlate with diarrhea severity in patients with IBS-D. Further studies are needed to confirm our findings and investigate the role of zonulin in IBS. Identifying IBS patients with zonulin-mediated intestinal TJ dysfunction could enable mechanistically targeted treatment in IBS (e.g. larazotide acetate).
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Disclosures
AL has worked as a consultant for Salix, Takeda, Ironwood, and Allergen.
Ethics approval
Ethics approval was obtained from Institutional Review Board (IRB) (Protocol # 2018P000009, 16 January 2018).
Funding
This work was supported in part by RO1 AT008573-03 (AL) and T32DK007760 (PS).
Informed consent
The need for informed consent was waived by the IRB for HC and IBS patients in gastrointestinal sera repository as the repository used discarded sera. Written informed consent was obtained from all patients with CeD.
References
- 1.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterol. Epub ahead of print 18 February 2016. DOI: 10.1053/j.gastro.2016.02.031.
- 2.Makharia GK, Verma AK, Amarchand R, et al. Prevalence of irritable bowel syndrome: A community based study from northern India. J Neurogastroenterol Motil 2011; 17: 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hungin APS, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: Prevalence, symptom patterns and impact. Aliment Pharmacol Ther 2005; 21: 1365–1375. [DOI] [PubMed] [Google Scholar]
- 4.Canavan C, West J and Card T. Review article: The economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther 2014; 40: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 5.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712–721.e4. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012; 303: G775–785. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil 2007; 19: 545–552. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, Madsen K, Spiller R, et al. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 2012; 24: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M, Oduyebo I, Halawi H. Chemical and molecular factors in irritable bowel syndrome: Current knowledge, challenges, and unanswered questions. Am J Physiol Gastrointest Liver Physiol 2016; 311: G777–G784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gecse K, Róka R, Séra T, et al. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion 2012; 85: 40–46. [DOI] [PubMed] [Google Scholar]
- 11.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 2006; 101: 1288–1294. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009; 146: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: Validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol 2011; 301: G919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: Involvement of soluble mediators. Gut 2009; 58: 196–201. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Park DI, Kim HJ, et al. The relationship between small-intestinal bacterial overgrowth and intestinal permeability in patients with irritable bowel syndrome. Gut Liver 2009; 3: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerckhoffs APM, Akkermans LMA, de Smet MBM, et al. Intestinal permeability in irritable bowel syndrome patients: Effects of NSAIDs. Dig Dis Sci 2010; 55: 716–723. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability: A new target for disease prevention and therapy. BMC Gastroenterol 2014; 14: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci 2012; 1258: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fasano A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 2012; 10: 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visser J, Rozing J, Sapone A, et al. Tight junctions, intestinal permeability, and autoimmunity: Celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci 2009; 1165: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khaleghi S, Ju JM, Lamba A, et al. The potential utility of tight junction regulation in celiac disease: Focus on larazotide acetate. Ther Adv Gastroenterol 2016; 9: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adriaanse MPM, Tack GJ, Passos VL, et al. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharmacol Ther 2013; 37: 482–490. [DOI] [PubMed] [Google Scholar]
- 23.Adriaanse MPM, Mubarak A, Riedl RG, et al. Progress towards non-invasive diagnosis and follow-up of celiac disease in children: A prospective multicentre study to the usefulness of plasma I-FABP. Sci Rep 2017; 7: 8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbaro MR, Cremon C, Caio G, et al. 247 Zonulin serum levels are increased in non-celiac gluten sensitivity and irritable bowel syndrome with diarrhea. Gastroenterol 2015; 148: S–56. [Google Scholar]
- 25.Undseth R, Berstad A, Valeur J. Systemic symptoms in irritable bowel syndrome: An investigative study on the role of enterocyte disintegrity, endotoxemia and inflammation. Mol Med Rep 2016; 14: 5072–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997; 11: 395–402. [DOI] [PubMed] [Google Scholar]
- 27.Anbardan SJ, Daryani NE, Fereshtehnejad S-M, et al. Gender role in irritable bowel syndrome: A comparison of irritable bowel syndrome module (ROME III) between male and female patients. J Neurogastroenterol Motil 2012; 18: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Gao M, Zhang W, et al. Zonulin regulates intestinal permeability and facilitates enteric bacteria permeation in coronary artery disease. Sci Rep. 6, Epub ahead of print 29 June 2016. DOI: 10.1038/srep29142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bueno L. Protease activated receptor 2: A new target for IBS treatment. Eur Rev Med Pharmacol Sci 2008; 12(Suppl 1): 95–102. [PubMed] [Google Scholar]
- 30.Róka R, Rosztóczy A, Leveque M, et al. A pilot study of fecal serine-protease activity: A pathophysiologic factor in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2007; 5: 550–555. [DOI] [PubMed] [Google Scholar]
- 31.Smecuol E, Bai JC, Vazquez H, et al. Gastrointestinal permeability in celiac disease. Gastroenterology 1997; 112: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 32.Duerksen DR, Wilhelm-Boyles C, Veitch R, et al. A comparison of antibody testing, permeability testing, and zonulin levels with small-bowel biopsy in celiac disease patients on a gluten-free diet. Dig Dis Sci 2010; 55: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 33.Peters SA, Edogawa S, Sundt WJ, et al. Constipation-predominant irritable bowel syndrome females have normal colonic barrier and secretory function. Am J Gastroenterol 2017; 112: 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterol 2013; 144: 903–911.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheffler L, Crane A, Heyne H, et al. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol. 9, Epub ahead of print 5 February 2018. DOI: 10.3389/fendo.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]