Abstract
Introduction
Hepatic ischemic reperfusion injury occurs in multiple clinical settings. Novel potential protective agents are still needed to attenuate this injury. Apelin preconditioning protects against ischemic reperfusion injury in different organs. However, the protective mechanism of apelin on hepatic ischemic reperfusion injury is not yet clear.
Aim
Evaluate the effect of apelin-13 preconditioning on hepatic ischemic reperfusion injury and clarify possible interactions between apelinergic, renin-angiotensin systems and endothelial nitric oxide synthase.
Methods
In total, 60 rats were assigned to four groups: control sham-operated, ischemic reperfusion, apelin-treated ischemic reperfusion and apelin + N-nitro-L-arginine methyl ester-treated ischemic reperfusion. Apelin 2 µg/kg/day and N-nitro-L-arginine methyl ester 10 mg/kg/day were injected intraperitoneally daily for 3 days and 2 weeks respectively before hepatic ischemic reperfusion. Serum aminotransferase, aspartate aminotransferase, hepatic malondialdehyde, apelin, gene expression of caspase-3, endothelial nitric oxide synthase and angiotensin type 1 receptor and liver histopathology were compared between groups.
Results
Apelin significantly reduced serum aminotransferase, aspartate aminotransferase, hepatic malondialdehyde, caspase-3 and angiotensin type 1 receptor expression, whereas hepatic apelin and endothelial nitric oxide synthase expression were significantly increased with improved hepatic histopathology. N-nitro-L-arginine methyl ester co-administration partially reversed this hepatoprotective effect.
Conclusion
Apelin-13 reduced hepatic ischemic reperfusion injury. This protection could be related to the suppression of hepatic angiotensin type 1 receptor expression and elevation of hepatic apelin level and endothelial nitric oxide synthase expression, which counteracts the pathologic effects of Ang II/angiotensin type 1 receptor. An interaction exists between apelinergic, renin-angiotensin systems and endothelial nitric oxide synthase in hepatic ischemic reperfusion pathophysiology.
Keywords: Angiotensin type 1 receptor (AT1R), apelin, endothelial nitric oxide synthase (eNOS), hepatic ischemia reperfusion injury (I/R), N-nitro-L-arginine methyl ester (L-NAME)
Key summary
Apelin exerts a protective role against several models of ischemia reperfusion injury in the kidney, heart and brain acting through several signaling pathways.
The only study regarding apelin’s protective effect against hepatic ischemia reperfusion injury was published by Sagiroglu et al. (2014).
The mechanism through which apelin exerts its hepatoprotective effect remains to be elucidated.
This study, to the best of our knowledge, is the second to prove that exogenous apelin-13 preconditioning provided marked hepatic protection against hepatic ischemia reperfusion injury in the experimental rat model (evidenced by significantly reduced serum aminotransferase and aspartate aminotransferase and improved the hepatic histopathological damage).
This study is the first to delineate the mechanism through which apelin exerts its hepatoprotective effect against hepatic ischemia reperfusion injury. The apelin hepatoprotective effect is probably through modulating the oxidative stress with its antiapoptotic effect (apelin significantly decreased hepatic malondialdehyde and caspase-3 gene expression).
This study is also the first to clarify the interaction between apelinergic, renin-angiotensin systems and endothelial nitric oxide synthase in hepatic ischemia reperfusion injury. Apelin’s hepatoprotective effect involves suppression of hepatic angiotensin type 1 receptor expression and elevation of hepatic apelin level, whereas the hepatic expression of endothelial nitric oxide synthase was significantly increased.
Co-administration of N-nitro-L-arginine methyl ester with apelin caused the partial reversal of the hepatoprotective effect of apelin.
Introduction
Hepatic ischemic reperfusion (I/R) injury, a major cause of liver damage, occurs in multiple clinical settings including liver resection, liver transplantation, thermal injury, severe trauma and shock.1 In severe cases, it can result in liver failure in association with remote organ failure, both of which lead to high morbidity and mortality.2 Hepatic I/R injury is also responsible for nearly a third of delayed graft function cases in liver transplantation.3
Hepatic I/R injury is a complex phenomenon,4 characterized by derangement of sinusoidal blood flow, significant inflammatory processes and apoptotic cell death after reperfusion.5 Recently, a number of peptides have been developed to attenuate hepatic I/R injury in several animal models.6 However, novel potential protective agents are still needed, which need to show promising results for alleviating hepatic I/R injury with the potential to increase the number of livers suitable for transplantation.
Apelin, a small regulatory peptide (an adipocytokine), is the endogenous ligand of the G protein coupled receptor APJ.7 It has various isoforms,8 among which apelin-13 is the most active isoform binding to the APJ receptor.9 The apelin-APJ axis is widely expressed in hepatic parenchymal, Kupffer, stellate and endothelial cells.10
The apelin/APJ system is involved in regulating a number of physiological functions and pathophysiological statuses. Although a line of evidence indicates the primary role of apelin signaling is in development of cardiovascular diseases,11 the investigations increasingly focus on the effect of the apelin/APJ system on I/R injury.12 Recently, exogenously administered apelin was shown to protect the heart against I/R injury mainly via inhibiting cardiac cell apoptosis and resisting oxidation effects, and PI3K/Akt, ERK, endothelial nitric oxide synthase (eNOS) signalling pathways are involved in this.12 In addition, apelin protects against brain I/R injury primarily through activation of the PI3K/Akt and ERK1/2 signalling pathway, as well as suppression of the apoptosis of neurons.6 However, the protective mechanism of apelin on hepatic I/R injury is not yet clear.
The aim of this study is to assess the effect of apelin-13 preconditioning on hepatic I/R injury in rats and evaluate its effect on hepatic expression of angiotensin type 1 receptor (AT1R), eNOS and hepatic tissue level of apelin in an attempt to clarify the possible interaction between the apelinergic, renin-angiotensin system (RAS) and eNOS.
Materials and Methods
Experimental animals and study design
This study was conducted on 60 male albino rats (local strain) weighing 180–200 g at the animal house of the Faculty of Medicine, Cairo University in accordance with the guidelines approved by the Ethical Animal Research Committee of Cairo University. Rats were randomly assigned to one of the following four groups (15 rats each): control sham-operated, I/R, apelin-treated I/R and apelin + N-nitro-L-arginine methyl ester (L-NAME)-treated I/R. Animals were housed in wire mesh cages at room temperature, with 12-hour light-dark cycle and were fed a commercial rat chow diet with free access to water.
Chemicals and reagents
Apelin-13 powder (Apelin®, Phoenix Pharmaceutical, Belmont, CA, USA) and L-NAME (non-selective eNOS inhibitor) (St Louis, MO, USA) were purchased, dissolved in 0.5 ml saline, and administrated intraperitoneally at dose of 2 µg/kg/day starting 3 days prior to surgical hepatic I/R procedure,13 and 10 mg/kg/day starting 2 weeks prior to the surgical procedure.14 The same amount of saline was administrated intraperitoneally in the control and I/R groups throughout the experimental period.
Surgical procedure
After an overnight fasting period, each rat was anaesthetized by thiopental sodium (40 mg/kg) administered intraperitoneally prior to surgery.15 Using a sterile technique, all animals underwent laparotomy. The hepatoduodenal ligament (liver) was exposed.16 The control sham group underwent liver exposure without any clamping and rats were left open for about 2.5 hours after which serum and liver specimens were taken.
In groups I/R, I/R+ apelin and I/R + apelin + L-NAME, an atraumatic bulldog clamp was placed across the hepatoduodenal ligament for 30 minutes. Ischemia was confirmed by the pale blanching of the ischemic lobes.17 After 2 hours of reperfusion, serum samples were obtained from retro-orbital plexus and stored at –70℃ for measurement of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Then the animals were sacrificed and hepatic tissue was harvested from each rat. Tissue specimens for biochemical analysis (malondialdehyde (MDA), apelin, gene expression of caspase-3 (CASP-3), eNOS and AT1R) were stored at –80℃, whereas those for histopathological analysis were fixed immediately in 10% formalin then impregnated in paraffin.
Liver function assay
Serum ALT and AST (ALT and AST kits respectively, Lab Biotechnology, USA) were measured.
Liver tissue examination
MDA assay
MDA concentration in the liver tissue, an indicator of lipid peroxidation, was assayed by a thiobarbituric acid test.
Semi-quantitation of hepatic CASP-3, eNOS and AT1R gene expression by reverse transcriptase polymerase chain reaction18
Hepatic tissue homogenate was centrifuged, then the clear lysate was subjected to the following:
(a) RNA extraction
Total RNA was extracted from hepatic tissue using an SV Total RNA isolation system (Promega, Maidson, WI).
(b) Reverse transcription into cDNA
(c) DNA amplification
Sequence of the primers used for real-time polymerase chain reaction (PCR)
CASP-3 Forward primer: 5-ACTCTTGTGGGCA AACCCAA-3
Reverse primer: 5-CTCTCCATGAGCAGTAGCC G-3
eNOS Forward primer: 5’-GACCAGAAACTGTC TCACCTG-3
Reverse primer: 5’-CGAACATCGAACGTCTCA CA-3
AT1R Forward primer: 5’-CAGCTTGGTGGTGA TTGTC-3
Reverse primer: 5′-GCCATCGGTATTCCATAGC-3
(d) Data analysis
At the end of the quantitative PCR running with SYBR Green chemistry, the relative quantification was used according to applied biosystem software.
Apelin assay
The apelin concentration was measured using an enzyme-linked immunosorbent assay kit (RayBiotech Inc, Parkway Lane, Norcross, GA).
Histopathological examination
Slides were stained with haematoxylin and eosin and examined under a Leica DM4000B microscope. An experienced pathologist evaluated the sections and the histological severity of the liver damage was graded using a Suzuki score. It comprised three components (sinusoidal congestion, hepatocyte vacuolation and necrosis), which were graded from 0 to 4 (Table 1).
Table 1.
Suzuki’s score for grading histological severity of liver damage.
| Score | Congestion | Vacuolation | Necrosis |
|---|---|---|---|
| 0 | None | None | None |
| 1 | Minimal | Minimal | Single-cell necrosis |
| 2 | Mild | Mild | <30% |
| 3 | Moderate | Moderate | 30–60% |
| 4 | Severe | Severe | 60% |
Statistical analysis
The results were analysed using the SPSS computer software package, version 10.0 (Chicago, IL, USA). Data were presented as mean ± SD. Differences among the four groups were compared by one-way analysis of variance. To study the relationship between the variables, Pearson’s correlation coefficient was calculated. The results were considered statistically significant at p < 0.05.
Results
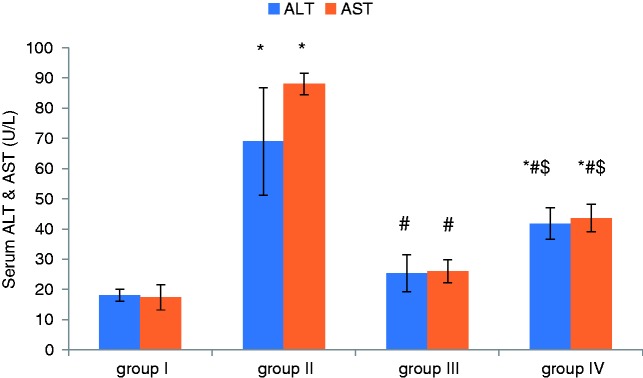
Effect of apelin preconditioning on serum ALT and AST (Figure 1)
Figure 1.
Comparison of the mean values of ALT and AST among all studied groups.
Values are presented as mean ± SD.
*Statistically significant compared to corresponding value in group I (p < 0.001).
#Statistically significant compared to corresponding value in group II (p < 0.001).
$Statistically significant compared to corresponding value in group III (p < 0.001).
ALT: alanine aminotransferase; AST: aspartate aminotransferase.
Following the induction of hepatic I/R, our results revealed marked hepatic damage as shown by a significant increase in serum ALT and AST in group II (I/R) compared to the control (group I), whereas apelin-pretreatment provided marked hepatic protection with significant reduction in the serum levels of ALT and AST in group III (Apelin + I/R) compared with the I/R.
Effect of apelin preconditioning on hepatic MDA, apelin and CASP-3 gene expression (Table 2)
Table 2.
Comparison of the mean values of hepatic MDA, apelin and CASP-3 gene expression among the studied groups.
| Group I (control) | Group II (I/R) | Group III (apelin + I/R) | Group IV (apelin + L-NAME + I/R) | |
|---|---|---|---|---|
| MDA (nmol/mg) | 1.19 ± 0.22 | 17.17 ± 3.11a | 3.16 ± 1.11b | 8.25 ± 1.42a,b,c |
| CASP-3 Gene expression | 1.03 ± 0.03 | 11.27 ± 1.59a | 3.64 ± 0.88a,b | 6.61 ± 1.27a,b,c |
| Apelin (ng/mg) | 2.93 ± 0.15 | 0.61 ± 0.38a | 1.60 ± 0.40a,b | 0.78 ± 0.20a,c |
CASP-3: caspase-3; I/R: ischemic reperfusion; MDA: malondialdehyde; L-NAME: N-nitro-L-arginine methyl ester.
Compared to the control group (group I), hepatic I/R (group II) was associated with marked oxidative stress with significant increase in hepatic MDA. Also, the hepatic apoptosis marker CASP-3 gene expression was significantly increased whereas the hepatic apelin level was significantly decreased. However, the apelin-pretreatment (group III) induced a significant decrease in both hepatic MDA and CASP-3 gene expression whereas the hepatic apelin level was significantly increased in comparison with the I/R group (group II).
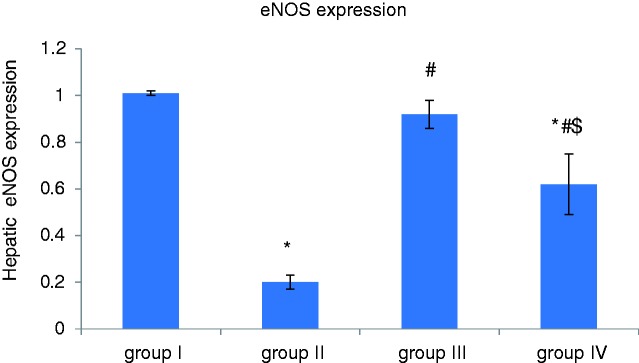
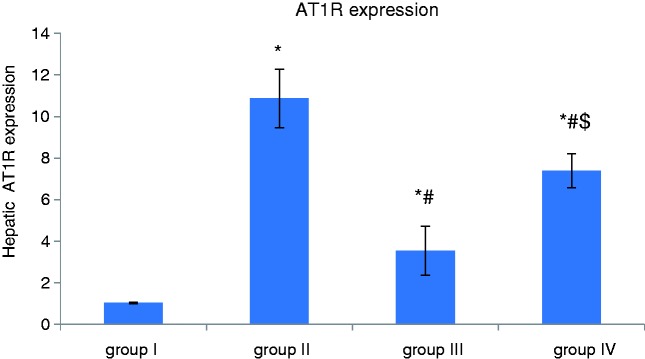
Effect of apelin preconditioning on the hepatic expression of eNOS and AT1R (Figures 2 and 3)
Figure 2.
Comparison of the mean values of hepatic expression of eNOS among all studied groups.
Values are presented as mean ± SD.
*Statistically significant compared to corresponding value in group I (p < 0.001).
#Statistically significant compared to corresponding value in group II (p < 0.001).
$Statistically significant compared to corresponding value in group III (p < 0.001).
eNOS: endothelial nitric oxide synthase.
Figure 3.
Comparison of the mean values of hepatic expression of AT1R among all studied groups.
Values are presented as mean ± SD.
*Statistically significant compared to corresponding value in group I (p < 0.001).
#Statistically significant compared to corresponding value in group II (p < 0.001).
$Statistically significant compared to corresponding value in group III (p < 0.001).
AT1R: Angiotensin-II type 1 receptor.
It is obvious from our results that induction of hepatic I/R (group II) was associated with a significant decrease in the hepatic expression of eNOS whereas hepatic AT1R expression was significantly increased compared with the control group (group I). However, apelin-pretreatment (group III) induced a significant increase in the hepatic expression of eNOS, whereas hepatic AT1R expression was significantly decreased in comparison with the I/R group (group II).
Effect of co-administration of L-NAME with apelin preconditioning on all measured parameters
Regarding co-adminstration of L-NAME with apelin (group IV), L-NAME caused a partial reversal of the hepatoprotective effect of apelin as evidenced by significantly:
-
–
Elevated serum ALT and AST compared to group III (Apelin + I/R). However, values were still significantly lower than group II (I/R).
-
–
Elevated hepatic MDA and CASP-3 gene expression compared to group III (Apelin + I/R). However, values were still significantly lower than group II (I/R).
-
–
Decreased hepatic eNOS expression compared to group III (Apelin + I/R). However, values were still significantly higher than group II (I/R).
-
–
Decreased hepatic apelin levels compared to group III (Apelin + I/R).
-
–
Increased hepatic AT1R expression compared to group III (Apelin + I/R). However, values were still significantly lower than group II (I/R).
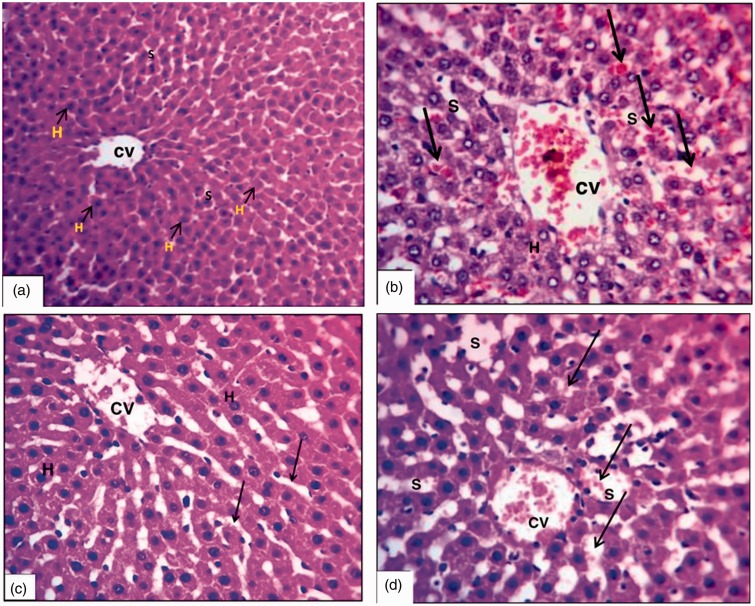
Histopathological results (Figure 4 and Table 3)
Figure 4.
Photomicrographs of liver sections showing (a) control group (X200), (b) I/R group (X200), (c) apelin + I/R group (X200) and (d) I/ R + apelin + L-NAME group (X200).
CV: central vein; H: hepatocytes; I/R: ischemic reperfusion; L-NAME: N-nitro-L-arginine methyl ester; S: sinusoidal spaces.
Table 3.
Results of Suzuki’s score in the studied groups.
| Group | Congestion | Vacuolation | Necrosis | Scoring |
|---|---|---|---|---|
| Group I | None | None | None | 0 |
| Group II (I/R) | Severe | Severe | Severe | 4 |
| Group III (apelin + I/R) | Minimal | Minimal | Minimal | 1 |
| Group IV (apelin + L-NAME + I/R) | Mild | Mild | Mild | 2 |
I/R: ischemic reperfusion; L-NAME: N-nitro-L-arginine methyl ester.
Classic hepatic architecture was shown in the sham group where hepatocytes (H) were arranged in parallel cords separated by narrow sinusoidal spaces (S) (Figure 4(a)). Induction of I/R injury was associated with marked dilatation and congestion of the central vein (CV), dilatation of S with severe congestion (arrows), and degeneration of H with fragmentation of their nuclei (Figure 4(b)). However, apelin pretreatment reduced the hepatic histological damage in the form of mild dilatation of the CV with minimal congestion, and the H were normally arranged, separated by narrow S (arrows) (Figure 4(c)). Co-adminstration of L-NAME with apelin caused reversal of the hepatoprotective effect of apelin as shown in Figure 4(d).
Correlation between the hepatic apelin level and other measured parameters
It was revealed that the hepatic apelin level was positively correlated with hepatic expression of eNOS, although it was negatively correlated with serum ALT and AST, hepatic MDA, hepatic expression of CASP-3 and AT1R as shown in Table 4.
Table 4.
Correlation between ALT, AST, hepatic MDA, hepatic CASP-3, eNOS and AT1R expression with the hepatic apelin level in the studied groups.
| Apelin (ng/mg) | ||
|---|---|---|
| ALT (U/L) | R | −0.590 |
| p value | <0.001 | |
| AST (U/L) | R | −0.614 |
| p value | <0.001 | |
| MDA (nmol/mg) | R | −0.700 |
| p value | <0.001 | |
| CASP-3 Gene expression | R | −0.683 |
| p value | <0.001 | |
| eNOS Gene expression | R | 0.655 |
| p value | <0.001 | |
| AT1R Gene expression | R | −0.628 |
| p value | <0.001 | |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; AT1R: angiotensin type 1 receptor; CASP-3: caspase-3; eNOS: endothelial nitric oxide synthase; MDA: malondialdehyde.
Discussion
Apelin exerts a protective role against several models of I/R injury in the kidney,19 heart,20 and brain21 acting through several signaling pathways. However, the only study regarding its protective effect against hepatic I/R injury was published by Sagiroglu et al. (2014).13
The underlying mechanisms of hepatic I/R are complex. The mechanism through which apelin exerts its hepatoprotective effect remains to be elucidated. To the best of our knowledge, the present study is the first to delineate the protective mechanism of exogenous apelin-13 on hepatic I/R and the interaction between apelinergic, RAS systems and eNOS.
In the present study, hepatic tissue damage was evidenced in the I/R group by the significant elevation in serum ALT and AST levels and histopathological tissue damage. Hepatic levels of MDA, which is used as a biomarker of oxidative stress,22 and hepatic CASP-3 expression, which is thought to be a specific indicator of apoptosis, were significantly increased.
NO derived from eNOS is an important endogenous vasodilator and can inhibit platelet aggregation and neutrophil adhesion to the vascular endothelium. Moreover, it is an important antioxidant, abolishing mitochondrial oxidant damage.22 Also, NO is an antiapoptotic agent as it is involved in activating the molecular signalling pathways in cell survival. Therefore, eNOS is thought to have a protective role in ischemic injury.
Indeed, in the current study, the hepatic expression of eNOS was significantly decreased in the I/R group by 80.20%. This finding is in line with previous studies as eNOS activity is greatly affected by a lack of oxygen and increasing acidosis, reported during I/R.20,23
Ang II is considered a key regulator of ischemic injury but the potential mechanisms by which the RAS system may contribute to damage in hepatic I/R are poorly understood.24
Interestingly, high homology was identified between AT1R and apelin/APJ receptor,25 yet the apelin/APJ system has been found to exert opposing actions to Ang II/AT1R in a number of physiologic and pathophysiologic settings.26 Moreover, apelin peptides have been shown to be specific substrates of ACE2, a carboxypeptidase enzyme that also cleaves the C-terminal phenylalanine residue of either Ang-I or Ang-II to form Ang-(1–7), a functional antagonist of Ang II.27
According to our data, the I/R group showed a significant increase in hepatic AT1R gene expression accompanied by a significant decrease in the hepatic apelin level (by 79.18%). In agreement with this, decreased plasma and tissue apelin levels were observed after stroke,28 myocardial I/R injury20 and renal I/R injury.14 This might indicate a protective role for apelin against hepatic I/R injury.
One of the mechanisms that has been proposed to underline the drop in the tissue level of apelin during reperfusion injury is the activation of the RAS system.29 Indeed, the negative correlation observed in this study between hepatic AT1R expression and hepatic apelin provides evidence for the interaction between both systems in hepatic tissue during I/R.
Interestingly, most published studies on apelin function in the liver showed increased apelin level in rats and humans with chronic liver disease such as cirrhosis and delivery of APJ antagonist improved liver pathology30 but so far little is known about the role of exogenous apelin in acute conditions such as hepatic I/R injury.
In the present study, the protective effect of apelin-13 as a pharmacological preconditioning agent in hepatic I/R was confirmed by the significantly reduced serum ALT and AST levels and by the improved liver histopathology. This recorded protective effect is consistent with the published data by Sagiroglu et al. (2014).13
Moreover, apelin significantly reduced the hepatic MDA level by 81.60% and the hepatic expression of CASP-3 by 67.70%; both were negatively correlated with the hepatic apelin level. These results suggested the hepatoprotective effect of apelin on hepatic I/R injury is related to its antioxidative and antiapoptotic effects.
In agreement with these results, Yang et al.21 reported that apelin protects against I/R injury via the suppression of oxidative stress and upregulation of antioxidant defense systems. Also, Boal et al.31 demonstrated that apelin reduced hypoxia-induced ROS production through regulating the FoxO1 phosphorylation pathway, which prevents hypoxia-induced mitochondrial O2 and H2O2 generation, causing irreversible oxidative damage to mitochondrial DNA, proteins and lipids.
Moreover, Yang et al.12 provided evidence that apelin-13 upregulated the AMPK phosphorylation level, which participated in the mechanism of apelin-mediated cytoprotection through reduced apoptosis of cells and downregulated CASP-3, leading to reduced cell death.
Regarding the effect of apelin preconditioning on the hepatic expression of eNOS and AT1R in hepatic I/R, it is evident from results of the current study that apelin-13 significantly increased the hepatic expression of eNOS with a positive correlation between the hepatic apelin level and hepatic eNOS expression. Previous studies have also shown that apelin acutely protects against I/R injury via the activation of PI3K/Akt/eNOS and promotion of NO release.32
However, apelin administration significantly decreased hepatic AT1R expression by 67.43%. Importantly, the negative correlation noticed between the hepatic expression of AT1R and hepatic apelin level provides evidence that the hepatoprotective effect of apelin is related to suppression of RAS system and antagonism of the Ang II/AT1R.
The antagonism between apelin and Ang II could be mediated through the convergence of two independent intracellular signaling pathways or via the direct physical interaction of apelin receptor/APJ with AT1R to form a GPCR heterodimer. AT1R heterodimerizes with GPCRs including apelin receptor, leading to significant changes in AT1R function.33
In support of these findings, Zhang et al.34 reported that activated apelin/PI3K/Akt signalling pathways lead to proliferation of the endothelial progenitor cells (EPCs). EPCs can form new vessels through differentiation into endothelial cells, thus are important in the expression of eNOS and prevention of I/R injury as endothelial dysfunction leads to impairment of the NO-dependent vasodilatation effect.35
Co-administration of L-NAME to apelin, resulting in significantly reduced hepatic expression of eNOS, caused a partial reversal of the beneficial effect of apelin and deterioration of liver functions as evidenced by significantly higher serum ALT, AST, hepatic MDA and hepatic expression of CASP-3. However, values were still significantly lower than the untreated I/R group and this was reflected in the histopathological assessment. It is to be noted that the partial reversal of the beneficial effect of apelin by L-NAME may be due to the dose used or may suggest the involvement of other NO-independent mechanisms.
The present study sought to determine whether a decrease in eNOS might be linked to changes in hepatic apelin level and expression of AT1R. In this line, the results of this study showed significant suppression of apelin levels accompanied by significant overexpression of hepatic AT1R after co-administration of L-NAME with apelin.
Conclusions
In conclusion, the results of this study provide evidence that exogenous apelin-13 preconditioning exerts a protective effect against hepatic I/R injury in the experimental rat model, probably by modulating the oxidant stress together with its antiapoptotic effect. Several signaling pathways may be involved including suppression of hepatic AT1R expression and elevation of hepatic apelin levels. The hepatic apelinergic system may be implicated in vasodilatory effects during hepatic I/R injury through increasing the hepatic expression of eNOS, which counteracts the pathologic effects of the Ang II/AT1R system. These results clearly demonstrate a strong interaction between the apelin/APJ system, RAS and eNOS signaling pathways in hepatic I/R injury pathophysiology.
Further studies are needed to fully characterize the proper timeframe, dose range and duration of apelin treatment to exhibit more potent therapeutic effects against hepatic I/R injury. Moreover, conjugation with traditional therapeutic drugs that significantly decrease I/R injury could be another important modification strategy. Such treatment modalities may have important clinical implications for the future, particularly in view of the increasing use of marginal donor livers in hepatic transplantation programs with their greater susceptibility to I/R injury.
Conflicts of interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, educational grants, participation in speakers’ bureaus, membership, employment, consultancies, stock ownership, or other equity interest, and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethics approval
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Not applicable.
References
- 1.Yang J, Wu R, Qiang X, et al. Human adrenomedullin and its binding protein attenuate organ injury and reduce mortality after hepatic ischemia-reperfusion. Ann Surg 2009; 249(2): 310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanaoka J, Shimada M, Utsunomiya T, et al. Beneficial effects of enteral nutrition containing with hydrolyzed whey peptide on warm ischemia/reperfusion injury in the rat liver. Hepatol Res 2014; 44(1): 114–21. [DOI] [PubMed] [Google Scholar]
- 3.Wertheim JA, Petrowsky H, Saab S, et al. Major challenges limiting liver transplantation in the United States. Am J Transplant 2011; 11(9): 1773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000; 190(3): 255–66. [DOI] [PubMed] [Google Scholar]
- 5.Yamada T, Kotake Y, Nagata H, et al. Atrial natriuretic peptide reduces hepatic ischemia-reperfusion injury in rabbits. J Anesth 2013; 27(6): 901–8. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Wang X, Zhou X, et al. Temporal expression of apelin/apelin receptor in ischemic stroke and its therapeutic potential. Front Mol Neurosci [Internet] 2017; 10: 1–8. Available from: http://journal.frontiersin.org/article/10.3389/fnmol.2017.00001/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Su T, Li F, Li L, et al. PI3K / Akt signaling transduction pathway is involved in rat vascular smooth muscle cell proliferation induced by apelin-13. Acta Biochim Biophys Sin 2010; 42: 396–402. [DOI] [PubMed] [Google Scholar]
- 8.Folino A, Montarolo PG, Samaja M, et al. Effects of apelin on the cardiovascular system. Heart Fail Rev 2015; 20(4): 505–18. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Zhang X, Li F, Chen L, et al. 14-3-3 mediates apelin-13-induced enhancement of adhesion of monocytes to human umbilical vein endothelial cells. Acta Biochim Biophys Sin (Shanghai) 2010; 42(6): 403–9. [DOI] [PubMed] [Google Scholar]
- 10.Kwon MH, Tuvshintur B, Kim WJ, et al. Expression of the apelin-APJ pathway and effects on erectile function in a mouse model of vasculogenic erectile dysfunction. J Sex Med 2013; 10(12): 2928–41. [DOI] [PubMed] [Google Scholar]
- 11.Koguchi W, Kobayashi N, Takeshima H, et al. Cardioprotective effect of apelin-13 on cardiac performance and remodeling in end-stage heart failure. Circ J [Internet] 2012; 76(1): 137–44. Available from: http://joi.jlc.jst.go.jp/JST.JSTAGE/circj/CJ-11-0689?from=CrossRef. [DOI] [PubMed] [Google Scholar]
- 12.Yang S, Li H, Tang L, et al. Apelin-13 protects the heart against ischemiareperfusion injury through the RISK-GSK-3β-mPTP pathway. Arch Med Sci 2015; 11(5): 1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagiroglu T, Aksoy MB, Sagiroglu G, et al. Effect of leptin and apelin preconditioning on hepatic ischemia reperfusion injury in rats. Indian J Surg 2014; 76(2): 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samy D, Ismail C, Deif A, et al. Induction of apelin by losartan in renal ischemia/reperfusion injury in rats-implication of endothelial nitric oxide synthase (eNOS) phosphorylation. J Physiol Pharmacol Adv 2014; 1(4): 465–77. [Google Scholar]
- 15.Anderson CD, Pierce J, Nicoud I, et al. Modulation of mitochondrial calcium management attenuates hepatic warm ischemia-reperfusion injury. Liver Transplant 2005; 11(6): 663–8. [DOI] [PubMed] [Google Scholar]
- 16.Tsung A, Stang MT, Ikeda A, et al. The transcription factor interferon regulatory factor-1 mediates liver damage during ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 2006; 290(6): G1261–8. [DOI] [PubMed] [Google Scholar]
- 17.Heijnen BHM, Elkhaloufi Y, Straatsburg IH, et al. Influence of acidosis and hypoxia on liver ischemia and reperfusion injury in an in vivo rat model. J Appl Physiol [Internet] 2002; 93(1): 319–23. Available from: http://jap.physiology.org/lookup/doi/10.1152/japplphysiol.01112.2001. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res [Internet] 2001; 29(9): 45e–45. Available from: https://academic.oup.com/nar/article-lookup/doi/10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagiroglu T, Torun N, Yagci M, et al. Effects of apelin and leptin on renal functions following renal ischemia/reperfusion: An experimental study. Exp Ther Med 2012; 3(5): 908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, McKinnie SMK, Patel VB, et al. Loss of apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: Therapeutic potential of synthetic apelin analogues. J Am Heart Assoc 2013; 2(4): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Zhang X, Cui H, et al. Apelin-13 protects the brain against ischemia/reperfusion injury through activating PI3K/Akt and ERK1/2 signaling pathways. Neurosci Lett [Internet] 2014; 568: 44–9. Available from: 10.1016/j.neulet.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Xu Z, Park SS, Mueller RA, et al. Adenosine produces nitric oxide and prevents mitochondrial oxidant damage in rat cardiomyocytes. Cardiovasc Res 2005; 65(4): 803–12. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Liu X, Wei X, et al. Losartan, an angiotensin II type 1 receptor blocker, ameliorates cerebral ischemia-reperfusion injury via PI3K/Akt-mediated eNOS phosphorylation. Brain Res Bull [Internet] 2012; 89(1–2): 65–70. Available from: 10.1016/j.brainresbull.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Guo L, Richardson KS, Tucker LM, et al. Role of the renin-angiotensin system in hepatic ischemia reperfusion injury in rats. Hepatology [Internet] 2004; 40(3): 583–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15349896. [DOI] [PubMed] [Google Scholar]
- 25.Chapman NA, Dupré DJ, Rainey JK. The apelin receptor: Physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR1. Biochem Cell Biol [Internet] 2014; 92(6): 431–40. Available from: http://www.nrcresearchpress.com/doi/abs/10.1139/bcb-2014-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oudit GY, Penninger JM. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Curr Heart Fail Rep 2011; 8(3): 176–83. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, McKinnie SMK, Farhan M, et al. Angiotensin-converting enzyme 2 metabolizes and partially inactivates Pyr-apelin-13 and apelin-17: Physiological effects in the cardiovascular system. Hypertension 2016; 68(2): 365–77. [DOI] [PubMed] [Google Scholar]
- 28.Jeong K, Oh Y, Kim SJ, et al. Apelin is transcriptionally regulated by ER stress-induced ATF4 expression via a p38 MAPK-dependent pathway. Apoptosis 2014; 19(9): 1399–410. [DOI] [PubMed] [Google Scholar]
- 29.Iwanaga Y, Kihara Y, Takenaka H, et al. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: Possible role of angiotensin II-angiotensin type 1 receptor system. J Mol Cell Cardiol 2006; 41(5): 798–806. [DOI] [PubMed] [Google Scholar]
- 30.Yokomori H, Oda M, Yoshimura K, et al. Overexpression of apelin receptor (APJ/AGTRL1) on hepatic stellate cells and sinusoidal angiogenesis in human cirrhotic liver. J Gastroenterol 2011; 46(2): 222–31. [DOI] [PubMed] [Google Scholar]
- 31.Boal F, Timotin A, Roumegoux J, et al. Apelin-13 administration protects against ischaemia/reperfusion-mediated apoptosis through the FoxO1 pathway in high-fat diet-induced obesity. Br J Pharmacol 2016; 173(11): 1850–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azizi Y, Faghihi M, Imani A, et al. Post-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damagethrough reduction of oxidative injury and nitric oxide enhancement in the ratmodel of myocardial infarction. Peptides [Internet] 2013; 46: 76–82. Available from: 10.1016/j.peptides.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Siddiquee K, Hampton J, McAnally D, et al. The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition. Br J Pharmacol 2013; 168(5): 1104–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Gong Y, Wang Z, et al. Apelin inhibits the proliferation and migration of rat PASMCs via the activation of PI3K/Akt/mTOR signal and the inhibition of autophagy under hypoxia. J Cell Mol Med 2014; 18(3): 542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C, Dai C, Gong K, et al. Apelin-13 protects neurovascular unit against ischemic injuries through the effects of vascular endothelial growth factor. Neuropeptides [Internet] 2016; 60: 67–74. Available from: 10.1016/j.npep.2016.08.006. [DOI] [PubMed] [Google Scholar]