Abstract
This guideline presents recommendations for the management of coeliac disease (CD) and other gluten-related disorders both in adults and children. There has been a substantial increase in the prevalence of CD over the last 50 years and many patients remain undiagnosed. Diagnostic testing, including serology and biopsy, should be performed on a gluten-containing diet. The diagnosis of CD is based on a combination of clinical, serological and histopathological data. In a group of children the diagnosis may be made without biopsy if strict criteria are available. The treatment for CD is primarily a gluten-free diet (GFD), which requires significant patient education, motivation and follow-up. Slow-responsiveness occurs frequently, particularly in those diagnosed in adulthood. Persistent or recurring symptoms necessitate a review of the original diagnosis, exclude alternative diagnoses, confirm dietary adherence (dietary review and serology) and follow-up biopsy. In addition, evaluation to exclude complications of CD, such as refractory CD or lymphoma, should be performed. The guideline also deals with other gluten-related disorders, such as dermatitis herpetiformis, which is a cutaneous manifestation of CD characterized by granular IgA deposits in the dermal papillae. The skin lesions clear with gluten withdrawal. Also, less well-defined conditions such as non-coeliac gluten sensitivity (NCGS) and gluten-sensitive neurological manifestations, such as ataxia, have been addressed. Newer therapeutic modalities for CD are being studied in clinical trials but are not yet approved for use in practice.
Keywords: Coeliac disease, seronegative coeliac disease, dermatitis herpetiformis, non-coeliac gluten sensitivity, gluten ataxia, neurocoeliac, coeliac neuropathy, slow-responder coeliac, refractory coeliac disease, enteropathy associated T-cell lymphoma
Introduction and methodology
Aim of the guidelines
This clinical guideline addresses the management of gluten-related disorders including coeliac disease (CD), non-coeliac gluten sensitivity (NCGS) and extra-intestinal manifestations related to gluten.
The need for updated guideline
New guidelines dealing with CD and other gluten-related disorders are necessary taking into consideration that the currently available international guidelines, both for children and adults, are outdated. In recent years there was a plethora of new data that need to be critically evaluated and incorporated in a structured manner in an updated guideline.
The board members of the European Society for the Study of Coeliac Disease (EScCD), a trans-national and multidisciplinary group including both paediatricians and adult gastroenterologists, have undertaken the task of providing up-to-date guidelines dealing with gluten-related disorders. Furthermore, two of the board representatives were nominated by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).
Writing the manuscript
At first there was a detailed revision of the currently available guidelines. The second step included an extensive search of the literature using the PubMed database. We searched for articles published from 1 January 1 1990 to present using the terms “celiac”, “coeliac”, “non-tropical sprue” and “gluten”. This is in addition to the terms “dermatitis herpetiformis”, “enteropathy” and “ataxia'', with no language restrictions. In our selection of articles, we emphasised those published since 2000, but included older landmark publications of scientific and historical relevance. We mostly selected cohort and case–control studies and the few randomised trials performed in this subject area and also smaller, non-controlled clinical studies of particular relevance. There was significant input from distinguished reviewers who have extensive experience in the field of diagnosis and management of CD. The literature search and preparing the first draft of the manuscript was conducted between January and August 2018.
The board members of the EScCD reviewed critically each section of the manuscript. The group initially made contact via email, which was followed by a meeting in Vienna, Austria, parallel to the United European Gastroenterology (UEG) week, 20–24 October 2018. Then a final draft of the guidelines was written.
Recommendations and grades of evidence
Each section provides specific recommendations. The GRADE system was used to evaluate the quality of supporting evidence.1 A “strong” recommendation is made when the benefits clearly outweigh the negatives and the result of no action. “Conditional” is used when some uncertainty remains about the balance of benefit /potential harm. The quality of the evidence is graded from high to low. “High”-quality evidence indicates that further research is unlikely to change the authors' confidence in the estimate of effect. “Moderate”-quality evidence indicates that further research would be likely to have an impact on the confidence of the estimate, whereas “low”-quality evidence indicates that further study would likely have an important impact on the confidence in the estimate of the effect and would likely change the estimate.
Supplement File 1 gives an overview of the GRADE system.
Overview
1. Review of the evidence and recommendations
1.1. Definitions
1.2. Epidemiological factors
1.2.1. Genetics
1.2.2. Environmental factors
2. Serology in CD diagnosis
2.1. Who should be tested for CD?
2.2. The role of serology in CD diagnosis
2.2.1. IgA-Anti-gliadin antibodies (AGA)
2.2.2. Tissue transglutaminase (TG2) and endomysium (EMA) testing
2.2.3. Deamidated gliadin peptides (IgA and IgG-DGP)
2.3. IgA deficiency
2.4. Other serology assay methods
2.5. Interpretation of serological results
2.6. Point-of-care tests
2.7. Serological test in saliva and faeces
3. Endoscopy and histopathology
3.1. Endoscopic findings and biopsy
3.2. Histopathological findings
3.3. The histopathology report
3.4. Differential diagnosis based on histopathology
3.5. Correlation of mucosal damage with serological findings
4. Other issues in CD diagnosis
4.1. Novel diagnostic methods
4.2. HLA-DQ2/8 typing
4.3. Other tests in CD diagnosis
4.3.1. Video capsule endoscopy (VCE)
4.3.2. Intestinal permeability tests
4.3.3. Intestinal fatty acid binding protein (I-FABP)
4.3.4. Radiology
5. Establishing diagnosis of CD
5.1. Requirements for CD diagnosis
5.2. Decision-making in special scenarios
5.2.1. Positive serology with normal biopsy
5.2.2. Normal villous architecture with duodenal lymphocytosis (Marsh-1)
5.2.3. Negative serology with duodenal biopsy consistent with CD
5.2.4. CD diagnosis in patients already following a GFD
6. Dietary management
6.1. Gluten-free diet
6.1.1. Safe gluten intake
6.1.2. Role of the dietician
6.1.3. Benefits of a GFD
6.2. Nutritional deficiencies/excess in CD and a GFD
6.2.1. Micronutrient deficiencies
6.2.2. Other nutritional deficiencies in the GFD
6.2.3. The metabolic syndrome in CD after a GFD
7. Management of severe presentations of CD
8. Follow-up of CD in adult patients
8.1. Systemized follow-up
8.2. Assessment of adherence to a GFD
8.3. Who may perform the follow-up?
8.4. At-risk family members
8.5. Who needs to be vaccinated?
8.6. Bone disease
9. Slow-responders and refractory CD
9.1. Slow-responders
9.2. Refractory CD
9.2.1. Diagnostic approach to RCD
9.2.2. Pathogenesis of RCD
9.2.3. Treatment options
9.2.4. Prognosis of RCD
9.3. Risk of malignancies in CD
9.3.1. Enteropathy-associated T cell lymphoma (EATL)
9.3.2. Other malignancies
10. Special issues concerning CD in childhood and adolescence
10.1. Diagnostic aspects
10.1.1. Who should be tested for CD in childhood and adolescence?
10.1.2. Approach for a child with symptoms/signs suggestive of CD
10.1.3. Diagnosis of CD without duodenal biopsies
10.1.4. Approach for an asymptomatic child with an increased risk for CD
10.1.5. Gluten challenge
10.2. Follow-up
10.3. Transition from childhood to adulthood in CD
10.3.1. The process of transfer of care
10.3.2. Issues that need to be discussed during transfer
11. Non-coeliac gluten sensitivity
11.1. Clinical aspects
11.2. Pathogenesis
11.3. Diagnosis
11.4. Management
12. CD-related skin and oro-dental disorders
12.1. Dermatitis herpetiformis
12.1.1. Histopathology
12.1.2. DH versus CD
12.1.3. Diagnostic approach
12.1.4. Treatment
12.1.5. Refractory DH
12.1.6. Follow-up
12.2. Other skin disorders
12.2.1. Psoriasis
12.2.2. Non-specific skin conditions
12.3. Oro-dental abnormalities in CD
13. Neuro-psychiatric manifestation related to gluten
13.1. The link to gluten
13.2. Pathophysiology
13.2.1. Genetics
13.2.2. Immunological basis
13.2.3. Serotonergic effects
13.3. Overview of neuro-psychiatric manifestations related to gluten
13.3.1. Gluten ataxia (GA)
13.3.2. Peripheral neuropathy
13.3.3. Gluten encephalopathy
13.3.4. Other neurological disorders
13.3.5. Psychiatric disorders
14. Quality of life
14.1. Studies in adults
14.2. Studies in children
15. Novel therapies for CD
15.1. The need for therapeutic measures other than diet
15.2. Overview of potential therapeutic options
15.3. Summary of results of novel therapies
16. Areas of uncertainty and future research
1. Review of the evidence and recommendations
1.1. Definitions
Gluten ingestion has been linked with a range of clinical disorders, collectively called gluten-related disorders, which have gradually emerged as an epidemiologically relevant phenomenon. Besides CD, the spectrum of these disorders includes dermatitis herpetiformis and disorders such as gluten-sensitive ataxia and NCGS. Gluten is the water-insoluble protein mass that remains when wheat dough is washed to remove starch, albumins and other water-soluble proteins.2 Gluten and gluten-related proteins are present in wheat, rye and barley and are used widely in food processing to give dough the desired baking properties, add flavours and improve texture. Coeliac disease is a chronic, multi-organ autoimmune disease that affects the small-bowel in genetically predisposed persons precipitated by the ingestion of gluten.2,3 Historically, it used to be known as coeliac sprue, gluten-sensitive enteropathy or non-tropical sprue. A subgroup of CD is regarded as a “potential” CD because they have a normal small-bowel mucosa but positive CD-serology along with HLA-DQ2 and/or -DQ8 positivity.
Depending on certain clinical, immunological and histopathological characteristics, CD may be subdivided into different categories, such as seronegative, slow-responders and refractory CD. These will be further defined in the dedicated sections.
Dermatitis herpetiformis (DH) is a cutaneous manifestation of CD characterized by herpetiform clusters of pruritic urticated papules and vesicles on the skin and granular IgA deposits in the dermal papillae. The skin lesions usually clear with gluten withdrawal but not in all adults.4
Gluten ataxia is defined as an otherwise idiopathic sporadic ataxia in association with positive coeliac serology with or without enteropathy.5 Other alternative explanations of ataxia such as genetic disorders, ischaemia and paraneoplastic phenomena need to be excluded. Non-coeliac gluten sensitivity is a condition characterized by irritable bowel syndrome (IBS)-like symptoms and extra-intestinal manifestations, occurring in a few hours or days after ingestion of gluten-containing food, improving rapidly with gluten withdrawal and relapsing soon after gluten challenge. Pre-requisite for suspecting NCGS is the exclusion of both CD and wheat allergy (WA) when the patient is still on a gluten-containing diet. Besides gluten, other potential culprits of this syndrome are amylase-trypsin inhibitors (ATIs) and fructans (rich in fermentable oligo di-mono-saccharides and polyols or FODMAPs), which are all components of wheat and other gluten-containing and non-gluten foodstuffs.6,7
1.2. Epidemiological factors
The prevalence of CD has significantly increased over the past 50 years. There has been a substantial increase in the numbers of new cases, partly due to better diagnostic tools and thorough screening of individuals considered to be at high risk for the disorder.8,9 CD still represents a statistical iceberg, with still more cases that need to be diagnosed.8,9 The majority of patients with CD remain undetected world-wide.
In western countries, the prevalence is around 0.6% histologically confirmed and 1% in serological screening of the general population. The female-to-male ratio ranges from 1:3 to 1.5:1. CD is known to affect all age groups, including the elderly; more than 70% of new patients are diagnosed above the age of 20 years.10 Some of these adults probably have had undetected disease since childhood; in other cases they have contracted the disease in adulthood.11
The risk of having CD is much greater in first-degree relatives (5–10%) but lesser in second-degree relatives, as well as in individuals with type 1 diabetes mellitus (T1DM) and other autoimmune diseases, Down syndrome, and a number of other associated diseases.12,13 Studies on twins showed a significantly higher concordance in monozygotic twins than in dizygotic twins.14 Monozygotic and dizygotic twins had 70% and 9% cumulative probability of having symptomatic or silent forms of CD, respectively, within 5 years.
Clinically severe manifestations may occur postpartum, especially during the puerperium in 15–20% of coeliac women.13
1.2.1. Genetics
The specific role of the HLA-DQA1 and HLA-DQB1 genes in the presentation of gluten peptides as antigens makes the MHC-HLA locus the most important genetic factor in the development of CD.15–17 The majority (in some populations 90–95%) of CD patients carry HLA-DQ2.5 heterodimers, encoded by DQA1*05 and DQB1*02 alleles, which may be inherited together on the same chromosome (cis configuration) or separately on the two homologous chromosomes (trans configuration).18,19 The remaining patients (5–10%) carry either HLA-DQ8 heterodimers encoded by DQA1*03 with DQB1*03:02 or they carry HLA-DQ2.2. Some rare patients (<1%) not carrying these heterodimers express the other half of the DQ2.5 heterodimer (DQ7.5).17,19
Homozygous DQ2.5 carries the highest CD risk up to 30%, versus 3% risk in heterozygous genotype. HLA-DQ2.5 homozygosity is associated with a more classical presentation and complicated CD.20
The presence of human leukocyte antigen (HLA) risk alleles is a necessary, but not a sufficient, factor for the development of CD. 21 Although key to the pathogenesis of CD, HLA haplotypes alone confer approximately 35–40% of the genetic risk.19,21 Additional non-HLA genomic regions identified as being associated with CD appear to explain some of the genetic heritability.21
1.2.2. Environmental factors
Gluten exposure is essential for the development of CD. However, the duration of breast feeding and/or time of gluten introduction have no impact on the risk of developing CD.
There is currently no evidence to recommend avoiding either an early (at 4 months of age) or a late (at or after 6 or even 12 months) gluten introduction in children at risk of CD.22–24
Loss of gluten tolerance can occur at any time in life as a consequence of other triggers besides gluten. Gastrointestinal infections, medications, α-interferon and surgery have also been implicated as trigger factors.25–27
2. Serology in CD diagnosis
2.1. Who should be tested for CD?
CD may present in many different ways. Traditionally patients with CD presented with malabsorption dominated by diarrhoea, steatorrhea, weight loss or failure to thrive. However, CD can present with a wide range of symptoms and signs, including anaemia, vague abdominal symptoms (often similar to IBS), reflux oesophagitis, eosinophilic oesophagitis, neuropathy, ataxia, depression, short stature, osteomalacia and osteoporosis, unexplained liver transaminitis, adverse pregnancy outcomes and even lymphoma.28
Malabsorption in CD, if present, results from damage to the small-bowel mucosa with loss of absorptive surface area, reduction of digestive enzymes (both luminal and also pancreatic enzymes) with consequent impaired absorption of micronutrients such as fat-soluble vitamins, iron, B12 and folic acid.29,30 In addition, the inflammation causes net secretion of fluid that can result in diarrhoea. Weight loss might be due to failure of absorption of adequate calories. Furthermore, malabsorption results in abdominal pain and bloating.31 Abdominal pain could be also attributed to small-bowel distension, inflammation with thickening of the proximal jejunal wall and because of this intermittent intussusception. Also, pain may be due to an associated IBS.32
In children it is often characterized by failure to thrive, diarrhoea, muscle wasting, poor appetite and abdominal distension.33 Many of these children also show signs of emotional distress, “change of mood” and lethargy. Others may have constipation and abdominal pain.
Currently, active case-finding (serological testing for CD among individuals with only subtle or atypical symptoms, and in risk groups) is a favoured strategy to increase detection of CD. Data from Finland suggested that this strategy and the increased alertness to the condition have made efficient diagnosis of CD possible.34
The frequency of CD is substantially increased in persons who have a first-degree family member affected with CD.12,13 One multicentre study reported a rate of 5% in both first- and second-degree relatives.9 Other studies show a rate of up to 20% in siblings and 10% in other first-degree relatives.12 The risk is highest in monozygous twins, next in HLA-matched siblings, siblings, and finally parents and children of patients with CD.12 A lower rate probably applies to second-degree relatives.13 HLA typing, if available, can be considered as the first line test for first-degree relatives; no further workup is needed on those who are negative for HLA-DQ2/8. Members of families who have more than one individual identified with CD are at higher risk of CD, and recommendations for screening should extend to all other family members, including second-degree relatives.10,12,32
Patients with unexplained elevation of liver enzymes should be assessed for CD. There are considerable data showing that gluten-dependent hypertransaminasaemia will normalize in most patients (>95%) on a GFD.35,36 Rarely, CD can be associated with severe liver disease and even liver failure.37
In patients with T1DM there is evidence that CD is substantially more common than in the general population. The estimates vary between 3 and 10%.38,39 In comparison to those with isolated T1DM, patients with undiagnosed CD and T1DM have a higher prevalence of retinopathy (58% vs. 25%) and nephropathy (42% vs. 4%).40,41
Several reports suggested that various operations, particularly upper GI operations, may unmask undiagnosed CD, such as fundoplication, gastrectomy, pancreaticoduodenectomy and bariatric gastric bypass. This phenomenon may be related to altered nutrient absorption, motility, perioperative stress and hormone derangements.42,43
Table 1 summarizes the indications for CD testing.
Table 1.
Who should be tested for CD?
| Endoscopy and duodenal biopsy even if CD serology is negative (1) Chronic ( non-bloody) diarrhoea (2) Diarrhoea with features of malabsorption, especially weight loss (3) Iron deficiency anaemia in absence of other causes (4) GI symptoms with a family history of CD (5) Gl symptoms in patient with autoimmune disease or IgA deficiency (6) Failure to thrive in children (7) Skin biopsy-proven DH (8) Patient with video capsule findings suggestive for villous atrophy (9) Unexplained high output ileo-(colo-)stomy | CD serology is indicated: biopsy is needed only when serology is positive (1) IBS (2) Elevated otherwise unexplained liver transaminases (3) Chronic GI symptoms without a family history of CD or a personal history of autoimmune disease (4) Microscopic colitis (5) Hashimoto's thyroiditis and Graves' disease (6) Osteopenia/osteoporosis (7) Unexplained ataxia or peripheral neuropathy (8) Recurrent aphthous ulcerations/dental enamel defects (9) Infertility, recurrent miscarriage, late menarche, early menopause (10) Chronic fatigue syndrome (11) Acute or chronic pancreatitis after excluding other known causes (12) Epilepsy; headaches including migraines; mood disorders; or attention-deficit disorder/cognitive impairment (13) Hyposplenism or functional asplenia (14) Psoriasis or other skin lesions than DH (15) Down's or Turner's syndrome (16) Pulmonary haemosiderosis (17) IgA nephropathy |
2.2. The role of serology in CD diagnosis
2.2.1. IgA-anti-gliadin antibodies (AGA)
Several antibody tests have been developed to detect CD. IgA-AGA has been used for decades and is reasonably accurate (sensitivity 85% and specificity 90%) when there is a high pre-test prevalence of CD but performs rather poorly in the general population setting.44,45
Nowadays AGA-testing has been replaced by more accurate serological assays largely because of poor specificity.46
2.2.2. Tissue transglutaminase (TG2) and endomysium (EMA) testing
It was with the advent of autoantibodies, first directed against reticulin, then EMA and finally TG2 antibodies, that the truly coeliac-specific testing was developed. The identification of TG2 as the target antigen for IgA-EMA antibodies was a major breakthrough.47 The sensitivity and also specificity of TG2 for untreated CD is about 95%.46 The higher the titre of anti-TG2, the greater is the likelihood of a true positive result.
The test is based on an enzyme-linked immunosorbent assay (ELISA) and less commonly on radioimmunoassay (RIA).45,46 ELISA-TG2 assays demonstrated high sensitivity and specificity with lower cost and greater reproducibility than RIA. Although performance characteristics of assays vary, overall TG2 testing is reliable and inexpensive. For these reasons it has become the most common test for coeliac diagnosis and monitoring.45
The anti-TG2 test is the most sensitive test for CD, whereas IgA-EMA is the most specific test.45 Therefore, serological testing for CD relies on anti-TG2 as the first step. IgA-EMA may be used as a confirmatory test, particularly when TG2 has a low titre (<2 times the upper normal limit (ULN)), although in these patients biopsy is usually indicated.29
2.2.3. Deamidated gliadin peptides (IgA and IgG-DGP)
DGPs bind with high affinity to HLA-DQ2 or DQ8 on coeliac patients' antigen-presenting cells to potently stimulate the inflammatory T cell response observed in the small-bowel mucosa of patients with CD.48 Testing for anti-DGPs displays a higher specificity for CD than antibodies to native gluten.49
Depending on the populations studied, IgA anti-DGP can be nearly as sensitive and specific as IgA-TG2. However, IgA-TG2 performs significantly better, and it is significantly less costly than IgA anti-DGP testing.49,50 Notably, an isolated positivity for IgA -and/ or IgG-DGP in patients at low risk for CD is predictive of CD only in 15% of cases, being a false-positive result in the remaining cases.50
IgG-DGP together with IgG-TG2 are regarded as the best tool for identifying CD in patients with selective IgA-deficiency.45,49
2.3. IgA deficiency
This affects 2–3% of patients with CD.51 Total IgA levels needs to be measured concurrently with serology testing to determine whether IgA levels are sufficient. Incorporating IgG-based testing into the serology panel would be the next step in case of documented IgA-deficiency. IgG-DGPs and/or IgG-TG2 would then be the preferred test.49 Furthermore, finding of IgA-deficiency should prompt evaluation for other diseases that may cause villus atrophy (VA), such as giardiasis, small-bowel bacterial overgrowth (SIBO) or common variable immunodeficiency (CVID).52
2.4. Other serology assay methods
There is multiplex kit to simultaneously measure TG2 and DGP antibody levels.53 The IgA-based kit includes a novel “IgA Verification Bead” to check for IgA-deficiency to ensure that these patients are identified and tested using the IgG-based kit. These kits have been used in a limited number of biopsy-proven CD patients. The results show a high sensitivity and specificity for detecting IgA deficiency without pre-screening with a separate IgA assay.
The Quanta Flash® IgA anti-TG2 antibodies were measured in patients without a diagnosis of CD.54 A very high concordance (99%) between anti-TG IgA and EMA was found, with sensitivity and specificity of 99% and 100%, respectively. Quanta Flash® IgA assay alone may be regarded as a reliable approach for screening of CD, with no need to perform EMA detection. More data are needed to confirm these findings.
2.5. Interpretation of serological results
No one test for CD has a perfect sensitivity or specificity. Thus, individual tests may be combined in commercially available panels. This strategy may increase the sensitivity if any positive test is regarded as an overall positive result.55 There is a high strength of evidence that both the IgA-TG2 test and IgA-EMA are associated with high (>95%) sensitivity and specificity.45,56 Table 2 shows the sensitivity and specificity of different tests.
Table 2.
Sensitivity and specificity of different serological tests.
| Antigen | Antibody type | Sensitivity, % (range) | Specificity, % (range) |
|---|---|---|---|
| Gliadin | IgA | 85 (57–100) | 90 (47–94) |
| IgG | 80 (42–100) | 80 (50–94) | |
| Endomysium | IgA | 95 (86–100) | 99 (97–100) |
| IgG | 80 (70–90) | 97 (95–100) | |
| Tissue transglutaminase | IgA | 98 (78–100) | 98 (90–100) |
| IgG | 70 (45–95) | 95 (94–100) | |
| Deamidated gliadin peptide | IgA | 88 (74–100) | 90 (80–95) |
| IgG | 80 (70–95) | 98 (95–100) |
The antibodies directed against gliadin or its deamidated products as well as the self-antigen TG2 are dependent on the ingestion of gluten. The reduction or total elimination of dietary gluten leads to a decrease in the levels of antibodies directed against gliadin or TG2. A weakly positive antibody titre may become negative within weeks of strict adherence to a GFD. After 6–12 months of adhering to a GFD, 80% of subjects will test negative by serology. By 5 years, more than 90% of those adhering to the GFD will have negative serology.57
2.6. Point-of-care tests (POCT)
Several POCTs for CD have been developed. The results on the usefulness of these POCTs in adults thus far are conflicting, and therefore these tests have not yet gained widespread acceptance. The subjective nature of the POCT interpretation may have contributed to these results.58 Further evidence from diagnostic performance studies on larger numbers and in low-prevalence cohorts, not only from western countries but also from the rest of the world, would support a wider utility of POCT.
2.7. Serological test in saliva and faeces
Salivary tests for detection of TG2 antibodies are under active investigation. There are a few reports showing that it could be possible to make a simple, reproducible, non-invasive, inexpensive and highly sensitive screening test for CD using the saliva of paediatric patients with suspected CD.59,60 Although these results are encouraging, there is still not enough evidence to make a recommendation for their use.
The sensitivity of faecal IgA antibodies against TG2 was as low as 10%, which is not suitable for accurate screening for CD.61
Recommendations
Who should be tested for CD?
(1) Adult patients with symptoms, signs or laboratory evidence suggestive of malabsorption should be tested with serology for CD. (Strong recommendation, high level of evidence)
(2) Screening of asymptomatic first-degree family member of CD patient is recommended. If available, HLA-typing may be offered as the first-line test; if negative, no further work-up is needed. (Conditional recommendation, high level of evidence)
(3) CD should be excluded in patients with unexplained elevation of serum aminotransferase levels. (Strong recommendation, high level of evidence)
(4) T1DM should be screened regularly for CD. (Strong recommendation, high level of evidence)
Role of serology in CD diagnosis
(1) IgA-TG2 antibody is the preferred single test for detection of CD at any age. (Strong recommendation, high level of evidence)
(2) Total IgA level needs to be measured concurrently with serology testing to determine whether IgA levels are sufficient. (Strong recommendation, moderate level of evidence)
(3) In patients with selective total IgA-deficiency, IgG-based testing (IgG-DGPs or IgG-TG2) should be performed at diagnosis and follow-up. (Strong recommendation, moderate level of evidence)
(4) All diagnostic serologic testing should be done while patients on a gluten-containing diet. (Strong recommendation, high level of evidence)
(6) Antibodies directed against native gliadin (AGA) are not recommended for the primary detection of CD. (Strong recommendation, high level of evidence)
3. Endoscopy and histopathology
3.1. Endoscopic findings and biopsy
Endoscopic features of CD are well described in the literature, including mucosal fissuring, nodular mucosa (mosaicism), bulb atrophy with visible submucosal vessels and loss, and reduction or scalloping of Kerckring folds. These features have high sensitivity and specificity for CD.62 Approximately one-third of newly diagnosed cases of CD have an endoscopic appearance that is entirely normal.63 Therefore, when CD is suspected, biopsies should be taken even when the endoscopic appearance of the duodenum is normal.
The pathological findings in CD can be patchy and can affect areas of the duodenum with varying degrees of severity.64 Therefore, multiple biopsies of duodenum (at least four) should be performed if the diagnosis of CD is considered.65
Adding biopsies of the duodenal bulb might increase the diagnostic yield.66 Also, there are some reports on what is called ultrashort CD, where the enteropathy may be limited to the duodenal bulb, with a mild clinical phenotype and infrequent nutritional deficiencies.67,68
There are enough data to recommend that only a single biopsy specimen should be obtained with each pass of the biopsy forceps.69 This improves the orientation of biopsy specimens and captures more severe villous atrophy compared with double bite. Moreover, specimens obtained with the double bite technique were more often architecturally damaged.
3.2. Histopathological findings
The diagnosis of CD relies on a combination of clinical, serological and histopathological findings. Because of the changing presentation of disease and the recognition of many potential histopathological mimics, communication between pathologists and gastroenterologists is essential for appropriate interpretation of small-bowel biopsy specimens.
Based on the dynamic development pattern of coeliac lesions and on the frequent finding of cases of CD with mild lesions, Marsh proposed a staging system for the histological changes in CD.70 Subsequently Rostami and later Oberhuber proposed a standardized report, based on the Marsh classification, in which stage 3 was split into 3A, 3B and 3C, characterized by mild villous flattening, marked villous flattening and completely flat mucosa, respectively.71,72 At present, this modified Marsh classification is used by most pathologists both for diagnosis and to assess the regression of the lesions after a GFD, although Marsh himself has argued against the subclassification of Marsh 3 type lesions.73 Later, Corazza and Villanacci proposed a simpler grading system hoping to minimize disagreement between pathologists and to facilitate the comparison between serial follow-up biopsies.74
There are other methods regarded as quantitative histology aiming to provide objective measures of histological changes.75 There are algorithms suggested to provide a standardized, objective and quantitative histology scoring system for use as a clinical or research application. These methods need to be further refined, and at present they are time consuming compared to the available semi-quantitative or subjective histology. A firm recommendation on using these methods at the present time cannot be yet made.
3.3. The histopathology report
Histopathological evaluation of small-bowel biopsies should be performed on biopsy pieces that contain three to four consecutive villous-crypt units visualized in their entirety and arranged parallel to each other.
The normal ratio of villous height to crypt depth ranges from 3:1 to 5:1 and a ratio of 2:1 has been suggested to be normal for the duodenal bulb.76 Scattered Intraepithelial lymphocytes (IELs) are present normally, which are more prominent along the lateral edge of villi, decreasing in number from the villous base towards the tip, the so-called decrescendo pattern.77 Biopsies from patients with CD displaying normal villous and crypt architecture lack this pattern as a result of increased density of lymphocytes at the proximal portions of villi, especially the villous tips, causing an even distribution of IELs along the villous length or an inversion of the normal pattern. The presence of diffuse and uniform infiltration of IELs is the most sensitive but still non-specific feature of CD.78 A count of at least 25 IEL/100 epithelial cells represents a definite increase in IELs.79 Immunohistochemistry for CD3 is helpful to highlight the distribution pattern of IELs. Counting IELs with or without the aid of an immunohistochemical stain for CD3 is helpful in cases with patchy or mild increases in IELs. Immunophenotypic studies have shown that the increased IELs represent an expansion of both cytotoxic αβ-T cells and γδ-T cells; the former predominate and 60% to 70% express CD8, whereas the latter are mostly CD8 −ve. The γδ-T cells comprise 1–10% of IELs in normal small-bowel mucosa, but increase in patients with CD, in whom they can represent up to 15–30% of all IELs.80
Microscopic examination of the small-bowel biopsies should be performed in a sequential manner, ensuring inspection and evaluation, not only of the mucosa and submucosa (when present) but also the luminal aspect, to identify adherent or free-floating infectious micro-organisms, e.g., Giardia, foreign objects and so forth.77
Notably, it is found that a population of plasma cells from duodenal biopsies of patients with CD express MHC-II; this is the most abundant cell type presenting the immunodominant gluten peptide DQ2.5-glia-α1a in the tissues from these patients. These results indicate that plasma cells in the gut can function as antigen-presenting cells and might promote and maintain inflammation in patients with CD.81
The following should be clearly stated in the histopathology report:
Number of biopsies (including duodenal bulb) and orientation.
Architectural features (normal, partial, sub-total or total VA). Presence of crypt hyperplasia, villous height: crypt depth ratio and subepithelial collagen.
Comment on the content of the lamina propria: in CD there is infiltration with lymphocytes, plasma cells and eosinophils, and occasionally neutrophils. Cryptitis and crypt abscesses should suggest other pathology. The absence of plasma cells suggests CVID.82
Presence of Brunner's glands.
Percentage of IELs (use of immunohistochemistry for CD3 in equivocal cases).
The report should provide a conclusion stated according to the modified Marsh classification.
3.4. Differential diagnosis based on histopathology
Lymphocytic duodenosis (Marsh-1) is present in 3.8% of a population negative for coeliac serology.83 Only around 16% of cases of lymphocytic duodenosis were found to have CD.84 Similarly there are causes of villous atrophy in duodenal biopsies other than CD. Table 3 shows other causes of Marsh-1 and VA. Helicobacter pylori infection is frequently associated with Marsh-1 histology, and its eradication may lead to normalization of duodenal IEL count.85 Concomitant gastric biopsies or performing serology is needed when H. pylori is suspected.
Table 3.
Causes of histological mimics of CD in seronegative patients.
| Differential diagnosis of CD with or without
villous atrophy | |
|---|---|
| Normal villous architecture and increased IELs | VA ± increased IELs |
| Food hypersensitivity (cow's milk, soy, fish, eggs, etc.) Peptic ulcer disease Helicobacter pylori-associated gastroduodenitis Drugs (NSAIDs, proton pump inhibitors) Infections (e.g., viral enteritis, Giardia, Cryptosporidium) Immune dysregulation (rheumatoid arthritis, Hashimoto's thyroiditis, SLE, multiple sclerosis, autoimmune enteropathy) CVID Graft-versus-host disease (GVHD) Inflammatory bowel disease (IBD) Bacterial overgrowth Blind loop syndrome Microscopic colitis (lymphocytic and collagenous) IBS NCGS | Infections (tropical sprue, Giardia, Whipple disease, Mycobacterium avium complex, AIDS enteropathy) Collagenous sprue Autoimmune enteropathy CVID GVHD IBD (Crohn disease) Drugs (mycophenolate mofetil, colchicine, olmesartan, losartan) Chemoradiation therapy Immunomodulatory therapy (anti-CTLA4 antibody) Eosinophilic gastroenteritis Bacterial overgrowth Enteropathy-associated T cell lymphoma (EATL) Nutritional deficiency Amyloidosis |
3.5. Correlation of mucosal damage with serological findings
The degree of mucosal damage has been shown to correlate with the presence and titres of both anti-TG2 and EMA. Studies have shown that EMA-seropositivity correlates with more severe VA, but not with the presence of gastrointestinal symptoms or the clinical mode of disease presentation.86 Other studies have shown that anti-TG2 levels of 100 units or greater occur almost exclusively in adults and children manifesting severe degrees of VA.87
Normalization of architectural changes of the duodenal mucosa can be variable and may take from 6 to 24 months after starting a GFD; recovery may remain incomplete in some adults for longer periods.88 Studies have shown that adhering to strict a GFD for more than 1 year, up to 75% had remission of symptoms and biopsies showed normal villous architecture, but 50–70% still had increased IELs.83,84 A normal anti-TG2 level at follow-up does not predict recovery of VA.89,90
Recommendations
(1) When CD is suspected biopsies, should be taken even when the endoscopic appearance of the duodenum is normal. (Strong recommendation, high level of evidence)
(2) Duodenal biopsy is an essential component of the diagnostic evaluation for adults with suspected CD and is recommended to confirm the diagnosis. (Strong recommendation, high level of evidence)
(3) Multiple biopsies of the duodenum (at least four of the second part of duodenum) are recommended to confirm the diagnosis of CD. (Strong recommendation, high level of evidence)
(4) The addition of two biopsies of the duodenal bulb might increase the diagnostic yield. (Conditional recommendation, low level of evidence)
(5) An increase in IEL infiltration in the absence of VA in duodenal biopsies (Marsh 1) is not specific for CD and other causes should be excluded. (Strong recommendation, high level of evidence)
(6) H. pylori infection is frequently associated with Marsh 1 histology and its eradication may lead to normalization of duodenal IEL count. Concomitant gastric biopsies or performing serology is needed when H. pylori is suspected. (Strong recommendation, high level of evidence)
(7) If CD is highly suspected, duodenal biopsy should be done even if serology is negative. (Strong recommendation, moderate level of evidence)
4. Other issues in CD diagnosis
4.1. Novel diagnostic methods
In a large cohort of CD patients and controls, it has been shown that determination of small-bowel mucosal TG2-specific IgA autoantibody deposits is a valuable tool in CD diagnostics.91 Autoantibody deposits were found in all untreated CD patients even when these autoantibodies were not present in the serum. This technique might be a way of defining early or potential CD. However, this is still experimental.91 Another diagnostic method that requires further evaluation is EMA- and TG2-assay in culture medium of small-bowel biopsies.92
Promising results were found using flow cytometry of IELs, which shows increased numbers of γδ- IELs in active CD (≥15% have a 97% specificity for CD diagnosis)80,93 and a test for HLA-DQ–gluten tetramer in blood for detection of gluten-specific CD4 + T cells.94 The latter is a non-invasive test for CD and has high sensitivity and specificity, even if the subject is on a GFD, but is not available outside the research setting so far.
Interestingly, it has been reported that interferon (IFN)-γ-secreting T cells reactive to gluten can be detected in the peripheral blood of CD patients after short-term consumption of gluten-containing food. IFN-γ can be transiently detected by using the enzyme-linked immunospot (ELISPOT) assays or by flow cytometry tetramer technology. The main limitations of the wide use of this technique for clinical practice are limited sensitivity and specificity compared to available serology tests, and the high cost of ELISPOT and tetramers immune assays.95,96 Others reported that in CD patients, a single gluten challenge is followed by an increased level of serum IL-2, and to lesser extent IL-8 and IL-10, at 4 hours thereafter.97
There are new techniques associated with endoscopy to enhance the diagnosis of CD, but these are still limited by availability, expertise, tolerability and cost.98
4.2. HLA-DQ2/8 typing
It is important to recognize the capability of the performing laboratory to identify HLA-DQ2 heterodimers, as individual carriage of one-half of the DQ2 molecule still confers a small risk of CD. Thus, HLA-DQ2.5 (very high predisposition) and HLA-DQ2.2 (low predisposition) must be separated.
Testing negative for HLA-DQ2/8 makes CD diagnosis very unlikely (positive predictive value >99%).17,99,100 HLA testing is recommended in the following situations:
A negative HLA test is helpful to exclude the possibility of CD. This is especially helpful in those already on a GFD before testing.
When diagnosis of CD is uncertain, e.g., negative serology, but histology suggestive of CD.
To distinguish siblings who can be reassured that it is unlikely that they will develop CD from those who need to be monitored. Furthermore, the data on the quality of life on a GFD in those patients detected by screening are conflicting, but there is a trend towards improvement.101,102 Also, the lack of understanding of the natural history of undiagnosed CD may justify screening asymptomatic persons.
In subjects with other autoimmune diseases and some genetic disorders who should be investigated for CD.
4.3. Other tests in CD diagnosis
4.3.1. Video capsule endoscopy (VCE)
A meta-analysis showed that VCE had a sensitivity of 89% and specificity of 95% for diagnosis of CD.103 VCE had better overall sensitivity for detection of macroscopic features of atrophy compared with regular upper endoscopy (92% vs. 55%). The sensitivity of VCE is less when there is partial villous atrophy, and all non-atrophic lesions (Marsh I-II) may escape detection.103
VCE can detect complications associated with CD.104 Extensive mucosal damage detected by VCE was associated with low albumin and type II refractory CD. Capsule findings among patients with slow-responsive CD include stenosis, erosions, ulcers and lymphoma. In these patients, VCE may be used to assess the need for further evaluation with devise-assisted enteroscopy, especially among patients with clinical suspicion of lymphoma, adenocarcinoma or ulcerative jejunitis.105
It is important not to misdiagnose ulcerative jejunitis as Crohn disease.
4.3.2. Intestinal permeability tests
Although permeability tests (e.g., d-xylose breath test, sucrose, lactulose-mannitol ratio) can detect the gross changes of intestinal permeability associated with CD, their sensitivity and specificity are quite variable, and these tests are not recommended for diagnosis of CD.106
4.3.3. Intestinal fatty acid binding protein (I-FABP)
I-FABP is a cytosolic protein, expressed by epithelial cells of the small bowel. Upon cellular damage, it is released into the systemic circulation. Serum I-FABP might be useful in identifying dietary non-adherence and unintentional gluten intake.107,108
4.3.4. Radiology
It is important that clinicians and radiologists are aware of certain radiological findings that may suggest CD, e.g., a decreased number of jejunal folds, an increased number of ileal folds, small-bowel dilatation, wall thickening, intussusception, (cavitating-) mesenteric lymphadenopathy, vascular changes and splenic atrophy.109,110
Recommendations
HLA-DQ2/8 Typing in CD diagnosis:
(1) HLA-DQ2/DQ8 testing should not be used routinely in the initial diagnosis of CD. It is recommended that the results of such testing should be included along with a caution that patients at risk should be serologically tested for CD without changing their diet. (Strong recommendation, moderate level of evidence)
(2) HLA-DQ2/DQ8 testing should be used to rule out CD in selected clinical situations, including:
(a) Marsh 1–2 histology in seronegative patients; (b) Evaluation of patients in whom no testing for CD was done before being started on GFD; (c) When the results of coeliac-specific serology and histology are discrepant. (Strong recommendation, moderate level of evidence)
Other tests in CD diagnosis:
(1) VCE is not used for initial diagnosis of CD except for patients with positive coeliac-specific serology who are unwilling or unable to undergo endoscopy with biopsy. (Strong recommendation, moderate level of evidence)
(2) VCE is important in detecting complications associated with CD. (Strong recommendation, moderate level of evidence)
(3) Intestinal-permeability tests are neither sensitive nor specific and are not recommended for CD diagnosis. (Strong recommendation, moderate level of evidence)
(4) Serum I-FABP might be useful in identifying dietary non-adherence and unintentional gluten intake. (Strong recommendation, moderate level of evidence)
5. Establishing a diagnosis of CD
5.1. Requirements for CD diagnosis
There is a great overlap in (non-)gastrointestinal symptoms in CD and other GI disorders. Improvement of symptoms or exacerbation after re-introduction of gluten has a very low predictive value for CD and should not be used for diagnosis in the absence of other supportive evidence. A positive CD-specific serology (TG2, DGP and EMA) in patients with VA confirms the diagnosis of CD.111
IgA-TG2 may be negative in 5–15% of patients with biopsy-confirmed CD tested while on a gluten-containing diet.112 Histological response to a GFD in patients with VA strongly supports a diagnosis of CD but requires a follow-up biopsy. HLA typing and histological response may help to rule out or confirm the diagnosis of CD in patients with seronegative CD.112
Small-bowel biopsy has been central to the confirmation of the diagnosis of CD. In adult patients there are some data suggesting that the diagnosis of CD may be made on the basis of serology alone without confirmatory biopsy; however, this issue is currently under scrutiny and need more data before a firm recommendation can be made.113–117 Furthermore, in adults endoscopy may disclose other disorders associated with CD such as eosinophilic oesophagitis, autoimmune gastritis and lymphocytic gastritis.118,119 Lymphocytic gastritis is variably reported in CD patients, is not associated with H. pylori infection and improves after a GFD. Moreover, CD at onset in adults can be already associated with complications such small-bowel adenocarcinoma (SBA) and enteropathy-associated T cell lymphoma (EATL). Finally, adult CD may not quickly respond to a GFD (slow-responders) or more rarely is refractory. In these cases it is very useful to have index histology to be compared with the histological findings after a GFD.120–122
5.2. Decision-making in special scenarios
5.2.1. Positive serology with normal biopsy
False-positive TG2 results do occur and usually show low titre. Hypergammaglobulinemia, autoimmune diseases, chronic liver disease, congestive heart failure and enteric infections have shown false-positive results.45
The initial step in evaluation of such patients should be a review of the biopsies for subtle abnormalities. The next step will be to confirm that the patient was on a full gluten-containing diet at the time of endoscopy. If the patient was on a low gluten diet, then it is recommended to repeat the biopsy after gluten challenge for 2–6 weeks. HLA-DQ2/8 typing should be requested. In the presence of HLA-DQ2/8, those who have a positive serology but a normal small-bowel mucosa are regarded as having a “potential CD”. In addition to anti-TG2, testing for other antibodies, e.g., EMA antibodies, is mandatory. If more than one serological test is positive, that strengthens the argument that the patient has a true CD.55 In symptomless patients, the decision to perform a duodenal biopsy may be delayed by repeating serology at 3–6 months.
5.2.2. Normal villous architecture with duodenal lymphocytosis (Marsh-1)
Increased IEL levels in duodenal biopsies lacks specificity. It can be found in CD, but more commonly with other disorders and medications.123 Reported aetiologies are shown in Table 2.
Determining the aetiology can be challenging and relies on assessment of clinical, serological and histopathological data.124 Serology correlates with degree of mucosal injury; therefore, negative serology alone does not exclude CD in patients with Marsh-1.86
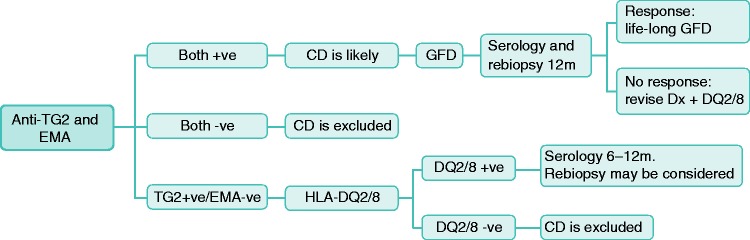
There is as yet no firm evidence-based recommendation that can be made regarding the best diagnostic approach for these patients. In symptomatic patients and/or abnormal laboratory tests, if there is no other apparent cause, then we suggest the following expert opinion-based approach: If both anti-TG2 and EMA are positive, then CD is likely and a GFD needs to be started. In an adult patient repeat of duodenal biopsy and serology after a period of about 12 months is advisable. A response, both histological and serological, confirms the diagnosis of CD. If EMA is negative, then the absence of HLA-DQ2/8 excludes CD, while in the presence of HLA-DQ2/8, it is advisable to repeat serology after 6–12 months. Re-biopsy may also be considered. This approach is summarized in Figure 1.
Figure 1.
Suggested approach for patients with Marsh I histology with positive serology.
5.2.3. Negative serology with duodenal biopsy consistent with CD
At diagnosis, 2–15% of patients with CD are seronegative.112 The term seronegative CD (SNCD) should be strictly used to denote those patients with VA who show response to a GFD but negative coeliac serology (IgA/IgG-EMA, IgA/IgG-TG2 and IgG-DGP), with the presence of HLA-DQ2/or-DQ8 and excluding other causes of seronegative VA.112,125,126
Differentiation of SNCD from alternate causes of enteropathy is a clinical challenge and requires integration of clinical, genetic and histopathological criteria. Other than SNCD, possible aetiologies in patients with VA but negative coeliac serology include CD patients on a GFD at the time of testing and non-coeliac enteropathy (NCE).127 Causes of the latter are shown in Table 3.
SNCD may be seen in the early stages of CD development and in those patients who have adopted a reduced-gluten diet before testing. It can also result from impaired immunoregulation, concomitant CVID and use of immunosuppressants. Compared to seropositive-CD, patients with SNCD were older at diagnosis, more likely to have typical symptoms, and were associated with more severe VA and coexisting autoimmune diseases.127 Interestingly, TG2 deposits in the small-bowel mucosa in patients with SNCD, despite their seronegativity.128 Anti-TG2 antibodies are bound to bowel TG2 with high avidity, rendering the antibodies unable to enter the circulation to cause seropositivity. In patients where the diagnosis is uncertain or findings are atypical, biopsies should be reviewed by a GI pathologist with an interest in CD. If the initial biopsies are unavailable or prove to be non-diagnostic after re-evaluation, repeat endoscopy with biopsy should be performed. Further, obtain HLA-DQ2/DQ8 and DGP testing and consider causes of NCE. As a part of serological assessment, IgG-based DGP testing should be considered in patients with IgA-deficiency because they have a 10- to 20-fold greater risk of developing CD.129 If these are found to be positive, then the patient is labelled as seropositive.
5.2.4. CD diagnosis in patients already following a GFD
The specific serological and histological features of CD do not normalize immediately upon the initiation of a GFD. If the duration of GFD has been brief (1–3 months), serology and histology are often still abnormal. Some patients will quickly revert to normal on a GFD. Hence, normal serological and histological findings on a GFD cannot be used to exclude CD definitively.130 A negative HLA-DQ2/8 genotyping result obviates the need for further workup.
Gluten challenge is needed to enable diagnostic testing in a patient already treated with a GFD.131 Gluten challenge with a diet containing at least 10 g/day for 6–8 weeks has long been the norm; however, there are few data to support that.
One study130 found that diagnostic histological changes are seen in most CD patients after only 2 weeks of gluten ingestion, while another could not show the same.132
In the future, alternatives to long-term challenge might be provided by flow cytometry of IELs80,93 or testing for HLA-DQ–gluten tetramer in blood if these tests are further validated and made available for clinical use.94 Furthermore, as mentioned earlier, detection of transient cytokine release such as IFN-γ, serum IL-2, IL-8 and IL-10 after only 3 days consumption of gluten-containing food may provide an alternative to long-term challenge.95–97 These investigations are ongoing.
Recommendations
(1) The confirmation of a CD diagnosis should be based on clinical data, positive serology and duodenal histology. (Strong recommendation, high level of evidence)
(2) Improvement of symptoms or exacerbation after re-introduction of gluten has a very low predictive value for CD and should not be used for diagnosis in the absence of other supportive evidence. (Strong recommendation, high level of evidence)
(3) A positive CD-specific serology in patients with VA confirms the diagnosis of CD. (Strong recommendation, high level of evidence)
(4) In asymptomatic patients with positive (but low titre) coeliac serology, the decision to perform biopsy may be preceded by repeating serology test at 3–6 months. (Conditional recommendation, low level of evidence)
(5) In case of elevated TG2-titre and normal histology: biopsies should be reviewed by a pathologist familiar with CD. It is recommended to repeat biopsy after gluten challenge if the patient was not on gluten-containing diet before testing. HLA-DQ2/8 typing is mandatory. Testing for other antibodies, e.g. DGP and/or EMA, may be of added value. (Strong recommendation, moderate level of evidence)
(6) In symptomatic patients and/or abnormal laboratory tests with Marsh 1: If both anti-TG2 and EMA are positive then CD is likely; If EMA is negative, then the absence of HLA-DQ2/8 excludes CD; while in the presence of HLA-DQ2/8, it is advisable to repeat serology after 6–12 months. (Conditional recommendation, low level of evidence)
(7) Seronegative CD requires careful assessment with HLA-DQ2/8 testing and a response to a GFD after excluding other causes of seronegative VA. Coeliac serology, both IgA- and IgG-based, should be negative. (Strong recommendation, moderate level of evidence)
(8) In patients who are already following GFD prior to testing, serology and HLA typing are needed. If serology is positive, then biopsy is the next step. Gluten challenge should be undertaken when serology is negative but HLA DQ2/DQ8 positive. (Strong recommendation, high level of evidence)
(9) In adults there are some data suggesting that the diagnosis of CD may be made without confirmatory biopsy; however, these data are currently under scrutiny and need more confirmation before a firm recommendation can be made.
6. Dietary management
6.1. Gluten-free diet
The mainstay of treatment for CD is a GFD. Patients with CD should be educated to avoid cereals and food products derived from wheat, barley or rye and food made from gluten-contaminated cereals that are normally gluten-free like maize, oats, etc.133 Oats uncontaminated by gluten are safe for almost all patients with CD.134,135 A small percentage of patients with CD may be sensitive to oats and develop symptoms or even mucosal damage.134 Patients should be instructed for using separate cooking utensils, cooking surfaces and toasters. However, this might not be necessary if these shared items are thoroughly cleaned with soap and water between use in the case of the first two items, and toaster bags can avoid the need for two toasters. Food labelling is important; available lists should be checked for allowable foodstuffs.136 Patients should be advised to eat a high-fibre diet.137
There is evidence that compliance with a GFD is improved in those who are more knowledgeable about CD and the diet. Also support by health providers and families has a positive impact. In most countries, high-quality gluten-free products are available in supermarkets, specialized health food stores and on the internet.
GFD foodstuffs are generally more expensive than the equivalent wheat-based foods, and some countries reimburse patients on this diet. Coeliac support groups might be of help especially for underprivileged and migrant populations.
6.1.1. Safe gluten intake
The susceptibility to gluten contamination of food varies among patients with CD. A review article on ‘safe’ gluten levels argues that daily intakes of <10 mg have no effect on mucosal histology, whereas definite alterations are caused by a daily intake of 500 mg and observable alterations by 100 mg.138 A calculated daily intake of 30 mg seems not to harm the mucosa. Therefore, at present, a safe limit could be set at between 10 and 100 mg.138
A systematic review (35 studies) suggests that while the amount of tolerable gluten varies among people with CD, a daily gluten intake of <10 mg is unlikely to cause significant histological abnormalities.139
In 2008 the Codex Alimentarius Commission of the WHO issued guidelines for gluten content of processed food, and a law from the European Commission (EC41/2009), effective since January 2012, specified that foods labelled as ‘gluten-free’ should contain ≤ 20 ppm of gluten, which is regarded to be safe for people with coeliac disease.
In addition to foodstuffs, drug products also need to be clearly labelled as gluten-free or gluten-containing.140
6.1.2. Role of the dietician
Newly diagnosed patients should be referred to a dietitian to discuss dietary management.141 An availability of dietitians with a subspecialism for CD is highly desirable for evaluating patients for potential current and future dietary nutrient deficiencies and educate them on how to maintain a strict GFD with provision of healthy alternatives to gluten.
6.1.3. Benefits of GFD
A poor dietary adherence is negative for specific health problems, such as the risk of lymphoma and pregnancy outcome. Poor foetal outcome in pregnant women with undiagnosed CD compared to those with CD on a GFD has been reported.142
Adherence to GFD typically leads to vast improvement in symptomatology and mucosal healing associated with decreased risk for cardiovascular disease and malignancy.143–145 Data indicate that a strict GFD might be of help in reaching ideal body weight, whether an individual is underweight or obese at diagnosis.146
Untreated CD is associated with an increased prevalence of low bone mineral density (BMD), which improves on GFD in both adults and children.147 GFD reduces the risk of infertility, spontaneous abortions, preterm deliveries and delivery of low birth weight infants.142,148
6.2. Nutritional deficiencies/excess in CD and GFD
6.2.1. Micronutrient deficiencies
Adherence to a GFD usually leads to improvement in nutrient absorption. However, a GFD itself has limitations in nutrient value and vigilant dietary monitoring is necessary.
Iron deficiency is present in 7–80% of coeliac patients at diagnosis.149 CD is present in 2–5% of patients with iron deficiency anaemia (IDA).150 With a strict GFD, iron stores typically improve. Eating foods rich in iron is necessary. Intravenous iron therapy may be needed especially in severe cases of deficiency and in those who are intolerant or unresponsive for oral therapy. Folate deficiency improves as the underlying enteropathy improves. A GFD is typically low in folate. Supplementation of folate and vitamin B12 helped improve anxiety and depression and might be needed for years, especially in slow-responders.151 Vitamin B12 deficiency is present in 5–41% of untreated cases of CD.151 B12 deficiency is typically corrected with a GFD but should be treated with B12 supplementation in the short term.152 Vitamin D absorption is decreased due to fat malabsorption. Further, elimination of milk products in CD with concomitant lactose intolerance will lead also to vitamin D deficiency. Several studies report vitamin D and calcium levels can normalize within 1–2 years of a strict GFD and, in some patients, reverse bone loss.153 Calcium and vitamin D should be supplemented in coeliac patients with documented low serum levels, those with loss of BMD or those who cannot achieve adequate intake via diet.154 Zinc deficiency can lead to growth arrest and diminished protein synthesis. With a strict GFD, zinc deficiencies resolve, and long-term supplementation is not needed.155 Malabsorption may lead to deficiency of copper in CD.156 With copper repletion, the haematological manifestations typically resolve, but the neurological deficits can be irreversible. Screening for copper deficiency needs to be considered at diagnosis of CD, especially when any associated deficiency symptoms are identified. Screening for pyridoxine (vitamin B6) is indicated.157 In general, these deficiencies are more common in adults than in children.
6.2.2. Other nutritional deficiencies in the GFD
Macronutrients and energy intake are usually imbalanced both at the diagnosis of CD and also with adherence to a GFD. Overweight in CD patients is becoming more prevalent with one study showing 40% of patients with CD being overweight at diagnosis and 13% in the obese range.158
The GFD is usually low in fibre.137,159 This can lead to constipation, as well as removal of other health benefits of soluble and insoluble fibre. Children on a GFD were found to have increased intake of simple sugars, fats and proteins, with higher energy intakes than controls.160 Many processed gluten-free products have an increased glycaemic index with increased fat and lower proteins compared with gluten-containing meals.
6.2.3. The metabolic syndrome in CD after a GFD
There are a few reports raising concern of the development of the metabolic syndrome and also hepatic steatosis in CD patients on a GFD.161,162 In contrast, other studies showed the converse.163,146
Patients should be informed about this possible risk and advised about having a balanced diet and an active lifestyle. The link of the metabolic syndrome to a GFD needs to be confirmed by further studies involving a large number of patients.
Recommendations
(1) Patients with CD should adhere to a lifelong GFD. (Strong recommendation, high level of evidence)
(2) Oats are safely tolerated by the majority of CD patients; its introduction into the diet should be cautious and patients should be monitored for possible adverse reaction. (Strong recommendation, moderate level of evidence)
(3) Patients with CD should be referred to a dietitian who is well-trained concerning CD in order to get a detailed nutritional assessment, education on the GFD and subsequent monitoring. (Strong recommendation, moderate level of evidence)
(4) A newly diagnosed adult CD patient should undergo testing to uncover deficiencies of essential micronutrient, e.g. iron, folic acid, vitamin D and vitamin B12. (Strong recommendation, moderate level of evidence)
(5) Patients should be advised to eat a high-fibre diet supplemented with whole-grain rice, maize, potatoes and ample vegetables. (Strong recommendation, moderate level of evidence)
7. Management of severe presentations of CD
Rarely CD may present with an acute onset or rapid progression of GI symptoms requiring hospitalization and/or parenteral nutrition – a scenario called coeliac crisis. These patients may have signs of severe dehydration – hemodynamic instability or orthostatic hypotension, neurological and renal dysfunction, metabolic acidosis, hypoalbuminaemia, electrolyte disturbances and significant weight loss.164
Although it is still unclear what triggers this more aggressive disease course, current scientific evidence suggests a combination of severe mucosal inflammation and immune activation. Approximately 50% of patients have an inciting event such as surgery, infection or pregnancy within months of their crisis.165
The treatment includes admission to the hospital for intravenous hydration, electrolyte repletion and the institution of a GFD. About half of patients may require the initiation of total parenteral nutrition and/or steroids.
8. Follow-up CD in adult
8.1. Systemized follow-up
Dietary adherence improves by having regular follow-up within the setting of a specialist coeliac clinic.166 One of the important elements concerning adherence is dietetic input. Optimally, the clinic should have a gastroenterologist and dietitian, both with a special interest in CD. Patients should be encouraged to join national coeliac societies or other disease-specific patient support groups.
In the first year after establishing the diagnosis, follow-up needs to be frequent to optimize the chance of dietary adherence, provide psychological support and to optimally motivate the patient to adapt to new situation.
Once the disease is stable and the patients manage their diet without problems, annual or biennial follow-ups should be initiated. The physician should check the integrity of small-bowel absorption, associated autoimmune conditions (in particular thyroid disorders and T1DM), liver disease and dietary adherence by measuring coeliac-specific antibodies (anti-TG2 or EMA/DGP).111 Liver enzyme abnormalities, if present at presentation, need to be followed-up. If these abnormalities are persistent then further assessment (immunological, radiological and/or histopathological) is needed.
Key endpoints at follow-up of CD patients are absence of symptoms and achieving mucosal healing.167 We suggest a systemized follow-up scheme as seen in Table 4.
Table 4.
Suggested follow-up scheme for adult CD patients.
| At diagnosis (physician and dietitian) | Physical examination including BMI Education on CD Dietary counselling by a skilled dietician Recommend family screening (DQ2/D8 and coeliac serology) Recommend membership of coeliac national society or support group Coeliac serology (if not previously obtained) Routine tests (complete blood count, iron status, folate, vitamin B12, thyroid function tests, liver enzymes, calcium, phosphate, vitamin D)/bone densitometry at diagnosis but not later than 30-35 years of age |
| At 2nd visit 3–4 months (physician and dietitian) | Assess symptoms and coping skills Dietary review Coeliac serology (IgA-TG2) |
| At 6 months (physician) (by telephone) | Assess symptoms Dietary review Coeliac serology Repeat routine tests (if previously abnormal) |
| At 12 months (physician and dietitian) | Assess symptoms Physical examination (on indication) Dietary review Coeliac serology Repeat routine tests Small-bowel biopsy (not routinely recommended, see text) |
| At 24 months (physician) | Assess symptoms Consider dietary review Coeliac serology Thyroid function tests Other tests as clinically indicated |
| At 36 months (physician); thereafter every 1–2 years | Bone densitometry (if previously abnormal) Assess symptoms Consider dietary review Coeliac serology Thyroid function tests Other tests as clinically indicated |
8.2. Assessment of adherence to GFD
There are four complimentary steps to assess dietary adherence:
Clinical assessment: A strict adherence to the GFD is a pre-requisite to control symptoms, improve quality of life and decrease the risk of complications. Nutritional status, height and weight need to be assessed.
Dietetic review: There is extensive evidence to support the central role of a dietitian in slow-responder patients or if gluten contamination is suspected.168 A dietetic review supported by questionnaires evaluating self-reported GFD adherence and food frequency is a useful tool to rule out inadvertent gluten intake and to provide education for a balanced and adequate but not excessive nutrient intake.167
Serology and other markers: All coeliac-associated antibodies are gluten-dependent. A decrease from baseline values is expected within months of strict adherence to the GFD.169 Lack of declining values and/or persistently positive serology 1 year after starting a GFD strongly suggests gluten contamination. Persistently positive serology was seen in only 1% of patients who underwent annual follow-up during a 5-year period.89 It is reasonable to assume that positive antibody titres indicate some gluten intake. IgG-TG2 titres (in those with IgA deficiency) also show decline with time but may not reach normalization despite strict diet. A significant decrease (or normalization) of markers of malabsorption, such as steatorrhea, should be expected after a GFD. A POCT may help streamline the follow-up process by providing TG2 or DGP results during the consultation and facilitate the decision-making regarding the onward management plan such as the necessity of follow-up duodenal biopsy.170 Recent studies have reported that gluten immunogenic peptides (GIPs) are considered to be the most immunodominant peptide within gluten in CD.171–174 GIPs were also found in stool and urine of coeliac patients on a presumably GFD, showing the capacity to resist and be absorbed and excreted from the body, providing the first simple and objective means to assess adherence to the GFD. Detection of these GIPs in stool or urine may help detect dietary gluten exposure. This may provide a useful tool when clinically available.
Follow-up biopsy: In adults, neither symptoms nor serology is reliable to predict small-bowel damage.175,176 Serum antibodies have poor sensitivity for persistent VA. Early biopsy (at 6 months) is not considered to be optimal. A degree of villous atrophy is present in about 40% of patients who are rebiopsied at 1 year despite good dietary compliance.144, 177
Currently, there are no studies indicating an absolute necessity for performing routine follow-up biopsy for all patients. However, there is a need for distinguishing asymptomatic patients with negative serology from symptomatic patients who need repeated biopsies to rule out refractory CD (RCD) or malignancies.178 Data from Finland suggested a more personalized follow-up, wherein the repeat biopsy is conducted after a few years and only for a selected group based on age, initial disease severity and response to the GFD.179
It may be reasonable to do a follow-up biopsy in adults after 1-2 years of starting a GFD to assess for mucosal healing, especially in patients older than 40 years or in those having initially severe presentations.
It seems logical to perform a follow-up biopsy in patients with serology-negative coeliac patients because this is the only way possible to confirm response to GFD.
8.3. Who may perform the follow-up?
It is not clear who should perform follow-up of patients with CD and at what frequency. In a survey of patients, the preferred method of follow-up was to see a dietitian with a doctor being available.166,180 In a population-based cohort 56% of visits were conducted with primary care providers and 39% with gastroenterologists.180 A nationwide study from Finland, with probably the highest prevalence of recognised CD in the world, showed that medical follow-up by primary care providers was effective.181
Primary care physicians may take the responsibility of the follow-up if they have enough experience in dealing with CD.
8.4. At-risk family members
It is advisable to follow-up these individuals with serology. The time interval is not defined but 3–5 years would be reasonable. Those who get positive serology or develop symptoms should have duodenal biopsy examination.
8.5. Who needs to be vaccinated?
Hyposplenism or functional asplenia in association with CD may result in impaired immunity to encapsulated bacteria, and an increase in such infections has been demonstrated in CD.182,183 Hyposplenism is considered to be present if the size of the spleen is small at imaging, or in the presence of circulating Howell–Jolly bodies, mild degrees of thrombocytosis and leucocytosis.184 Those patients who are known to be hyposplenic should receive the pneumococcal vaccine.180 However, it is unclear whether vaccination with the conjugated vaccine is preferable in this setting and whether additional vaccination against Haemophilus, meningococcus and influenza should be considered if not previously given.183
8.6. Bone disease
This manifests as osteopenia and early onset of osteoporosis and osteomalacia.185 Rickets is also frequently reported, especially in children from developing countries.186 The risk of osteoporosis and bone fracture is increased in CD patients.187,188 The excess risk is reduced with good dietary adherence and reduction in VA.188 Patients with severe CD had lower serum calcium and higher parathyroid hormone levels compared with mild cases.189,190 Bone density increases during the first year of GFD adherence.189
It is strongly recommended to measure calcium, alkaline phosphatase and vitamin D levels at diagnosis and replace as necessary. A baseline bone density measurement (DEXA) is needed in adults. The first DEXA scan may be performed at diagnosis, especially in those with malabsorption or those at high risk if there is a long delay in diagnosis or there are clinical presentations suggestive of bone disease. Certainly, in the presence of other risk factors for low BMD, including perimenopause or menopause in women, age >50 years in men and a history of fragility fracture.185 In other patients, it would be appropriate not to delay the DEXA scan after the age of 30–35 years and then to repeat measurements at 5-year intervals in those with normal baseline measurement. But the interval needs to be shorter (generally after an interval of 2–3 years) in patients who have low bone density on index measurement or who have evidence of ongoing VA or poor dietary adherence.
Loss of bone density at a greater than expected rate should prompt dietary review of adherence, consideration of repeat duodenal biopsy and excluding other risk factors such as hypogonadism.191
The ESsCD board advises the use of intravenous bisphosphonates in documented cases of osteoporosis in newly diagnosed CD to overcome the uncertainty about absorption of medications, although that necessitates further confirmation. Starting calcium and vitamin D supplementation before instituting bisphosphonates is strongly advised to overcome the risk of tetany in patients who also have osteomalacia.185
Recommendations
(1) CD patients should be monitored regularly for persistent or new symptoms, adherence to GFD and assessment for complications. (Strong recommendation, moderate level of evidence)
(2) Periodic medical follow-up should be performed by a gastroenterologist or physician with special expertise in CD. (Moderate recommendation, low level of evidence)
(3) Dietary revision should be performed by a dietitian with special expertise in CD especially in slow-responders to exclude gluten contamination. (Strong recommendation, moderate level of evidence)
(4) Monitoring of adherence to GFD should be based on a combination of history and serology. (Strong recommendation, moderate level of evidence)
(5) A normal anti-TG2 level at follow-up does not predict recovery of VA.
(6) A follow-up duodenal biopsy is recommended for monitoring in cases of lack of clinical response or relapse of symptoms despite a GFD. (Strong recommendation, moderate level of evidence)
(7) Monitoring of coeliacs should include verification of normalization of laboratory abnormalities detected during initial investigation. (Strong recommendation, moderate level of evidence)
(8) It is advisable to follow-up at-risk family members with serology. Those who have positive serology or develop symptoms should have duodenal biopsy examination. (Conditional recommendation, low level of evidence)
(9) CD patients who are known to be hyposplenic should receive the pneumococcal vaccine. (Strong recommendation, moderate level of evidence)
(10) DEXA should be measured in those at high risk of osteoporosis. It may be performed at diagnosis especially in those with malabsorption or those at high risk if there is a long delay in diagnosis or there are clinical presentations suggestive of bone disease. In others, not later than age of 30–35 years and then to be repeated at 5-year intervals. A shorter interval (2–3 years) is needed in case of low bone density on index measurement, evidence of ongoing VA or poor dietary adherence. (Strong recommendation, moderate level of evidence)
9 Slow-responders and refractory CD
9.1. Slow-responders
A considerable percentage of adult CD patients (between 7 and 30%) continue to have persistent symptoms, signs or laboratory abnormalities of CD despite at least 6–12 months of GFD.121,192 These are regarded as slow-responders. The use of the term non-responsive CD to denote these patients is strongly discouraged because most of them will improve over time on a strict GFD or they have another treatable cause for their complaints.
Careful evaluation with emphasis on the differential diagnosis is needed to identify and treat the specific cause. The first step in the evaluation is to review the available small-bowel histology and serology obtained at the time of diagnosis. If the diagnosis of CD is incorrect, then alternative diagnoses and treatments must be considered.120,121 In patients with confirmed CD the ingestion of gluten, either purposeful or inadvertent, is the most common cause of slow-response, being identified in 35–50% of cases.122,193 Therefore, a careful dietary evaluation is the next important step in the assessment. This evaluation should also look for other food intolerances, e.g., to lactose or fructose, medications, etc. Coeliac serology is helpful, so might testing for gluten peptides be in urine or stool.171–174 If it is positive, then the most likely cause of slow-response is gluten exposure.121,194 However, a normal serology does not exclude intermittent or low-level gluten ingestion sufficient to cause persistent CD activity.
Once dietary causes have been excluded, duodenal biopsy should be repeated. The presence of an active inflammatory enteropathy with VA is consistent with gluten exposure, RCD or other causes of VA.120,122 Normal or near normal small-bowel histology (Marsh 0–1) suggests other aetiologies such as IBS, microscopic colitis, food intolerances, SIBO or exocrine pancreatic insufficiency.194,195
CD and microscopic colitis do overlap and an association between these two entities is variably reported.195 CD-like enteropathy has been reported in association with certain medications, such as olmesartan, mycophenolate and losartan.196
Meanwhile, immunohistochemistry and flow cytometric analysis on duodenal biopsies should be carried out to rule out RCD. The possibility of EATL development needs to be explored when indicated. Figure 2 summarizes the diagnostic approach to symptomatic CD or laboratory abnormalities despite GFD.
Figure 2.
Diagnostic approach to symptomatic CD or laboratory abnormalities despite GFD.
9.2. Refractory CD
RCD may be defined as persistent or recurrent symptoms and signs of malabsorption with VA despite a strict GFD for more than 12 months and in the absence of other disorders including overt lymphoma. 122,193,194 Currently, two types of RCD are being recognized depending on the presence or absence of aberrant IELs (cells lacking surface-CD3 and generally CD8 but expressing intracellular-CD3 (iCD3)).122,194 When the percentage of aberrant T cells is below 20%, then this is regarded as RCD-I, while more than this defines RCD-II. The latter can be considered as pre-lymphoma (Pr-EATL) or low-grade lymphoma because of the high risk of transformation into EATL.197–199
RCD is mostly diagnosed after the age of 50 years, but younger cases have been recognized. Incidence rates range from 0.04 to 1.5% for both types of RCD.200 RCD patients may experience persisting symptoms after diagnosis of CD and despite a GFD (primary refractoriness) or may develop recurring of symptoms despite initial response to a GFD (secondary refractoriness).197
The distinction between RCD-I and RCD-II is mandatory because of different treatment strategies and prognosis. RCD-II becomes more likely when severe malnutrition with wasting, protein-losing enteropathy and ulcerative jejunitis are present.197,201 Symptoms are notably less severe in RCD-I, and endoscopic and histological features are similar to those found in uncomplicated active CD. The diagnosis of RCD-I may therefore be difficult, and the distinction between slow-responders, especially in the elderly, inadvertent gluten ingestion and RCD-I may be sometimes impossible. Ulcerative jejunitis is by definition RCD-II.197
9.2.1. Diagnostic approach to RCD
Assessment of dietary adherence to GFD: Monitoring of anti-TG2 titre is generally suitable for this purpose. In addition, all patients should be referred to a dietitian to systematically review their diet.197
Revision of initial CD diagnosis: Absence of CD-related genotypes and/or negative serology at time of initial CD diagnosis are highly suggestive of misdiagnosis.
Endoscopy and histological evaluation: Endoscopic features of RCD may be similar to those found in uncomplicated CD. The finding of mucosal ulcerations in the duodenum and jejunum supports the diagnosis RCD-II.197 When histology reveals persisting VA, the evaluation should focus on identifying other causes of VA (Table 3).
Identification of aberrant IELs: IELs are markedly increased both in uncomplicated CD as well as in RCD-I. They have a normal T cell phenotype. The majority of these cells carry the αβ T cell receptor. While up to 14% of IELs carry the γδ T cell receptor, ≥15% have a high specificity for CD diagnosis.80,202 Furthermore, a small proportion of IELs consist of cells lacking surface-CD3 and generally CD8 but expressing intracellular-CD3 (iCD3). Normally, such cells typically constitute <10% of IELs.203 In RCD-II, aberrant T cells may also be isolated from extra-intestinal sites such as the paranasal sinuses. Flow cytometric analysis is able to differentiate cytoplasmic from membranous CD3 expression. It is superior to T cell receptor (TCR) clonality analysis in identifying patients at risk of developing an EATL.203 However, new generations of TCR analysis may provide a very good sensitivity and specificity for detection of aberrant cells.204 It has been postulated that RCD-II constitutes a low-grade lymphoma (Pre-EATL) and that the expansion of aberrant cells occurs as the consequence of clonal expansion of such cells. The identification of a monoclonal pattern upon TCR rearrangements analysis may contribute to the diagnosis of RCD-II.201 It was found that 97% of patients characterized as RCD-II based on the presence of increased numbers of aberrant cells above 50% by immunohistochemistry displayed clonality of the TCR γ chain versus 0% of RCD-I patients.201 However, a subsequent report from Amsterdam showed that clonality analysis lacks sensitivity and specificity and is of limited value in separating RCD-I from RCD-II.202
Other endoscopic investigations: VCE is useful in determining the extent of lesions and is less invasive than other endoscopic techniques.104,205 Balloon enteroscopy can efficiently detect suspected lesions in jejunum, especially when suggested by imaging modalities.105
Radiological imaging: Mesenteric lymphadenopathy, bowel-wall thickening and spleen atrophy (spleen volume <100 cm3) are more commonly detected in RCD-II and EATL. 184,206 PET-scan can help to exclude the presence of lymphoma.207,208 MR-enteroclysis is complementary to VCE in the analysis of small bowel.109
9.2.2. Pathogenesis of RCD
The only known genetic risk factors that have been associated with RCD-II are the same HLA alleles that are associated with CD susceptibility, although RCD-II patients are more often homozygous for HLA-DQ2 alleles (44–60%) than patients with CD.209 This observation suggests that the strength of the gluten-specific T cell response influences RCD-II and EATL development.210
Recently, a novel single nucleotide polymorphism associated with progression of CD to RCD-II has been identified.211
No specific immune mechanisms have been shown in RCD-I. It was postulated that interleukin-15 (IL-15) impairs control of autoreactive cells, which accumulate and sustain immune responses that becomes independent of gluten intake.211 The hallmark of RCD-II is the expansion of IEL with an aberrant phenotype. Only patients harbouring the most mature aberrant IELs may develop an EATL.212 These cells express an IL-15 receptor. An overproduction of IL-15 by enterocytes leads to persistent activation of IELs. The increased IL-15 response results in the expression of cytotoxic proteins and stimulates production of IFN-γ and NKG2D-dependent cytotoxicity resulting in severe enteropathy. The strong anti-apoptotic effect of IL-15 might explain the accumulation and eventually expansion of these aberrant cells. Next to IL-15, it is shown that IL-2, IL-21 and tumour necrosis factor (TNF) from gluten-specific CD4+T cells induce proliferation of aberrant IEL cell lines from RCD-II patients.213
Recently, a new variant of EATL has been reported that originates from γδ-IELs (γδ-pre-EATL), rather than from aberrant IELs.214
9.2.3. Treatment options
To date, there is no curative treatment for RCD, and management relies on a combination of nutritional support and immunosuppressive or ablative treatments.
Treatment of RCD-I
A combination of nutritional support and immunosuppressive treatment is needed. Immunosuppressive drugs suggested for RCD-I include steroids, thiopurines and infliximab. SR-mesalamine might be effective; however, histological response is disappointing.215
Oral budesonide resulted in clinical improvement but no histological change in up to 90% of patients.216 Budesonide administered as an open capsule to maximize its effect on the proximal small bowel has been reported.217 A favourable clinical and histological response has been seen both in RCD-I and RCD-II. If this result is going to be confirmed by controlled trials, then it may provide a good treatment option, particularly in mildly affected patients.
Combining azathioprine and prednisone might exert a better histological restoration, although complete normalization of villi is only seen in 50% of patients, and both lymphoma development and steroid-related side effects are of concern.218
Treatment with infliximab may induce responses, but only a few cases have been reported.219
Thioguanine is well tolerated and it has good bioavailability despite the presence of intestinal villous atrophy. A clinical and histological response was observed in 83% of RCD-I.220 This drug might be associated with a small risk of nodular regenerative hyperplasia of the liver.221
We recommend the following strategy in treating RCD-I:
Nutritional support.
A trial of budesonide (open capsule or if available non-slow release) (3 mg, 3 times a day) for at least 3 months may be given.216,217 Following a response on steroids, azathioprine (2–2.5 mg/kg/day) can be initiated. Duodenal re-biopsy should be performed after 3 months on azathioprine to assess response. 6-Thioguanine seems to be a safe alternative.220
Failure to respond should prompt a re-evaluation of the diagnosis of RCD-I and warrants optimizing dosing of thiopurines.
Patients who respond should undergo annual endoscopy and biopsy with quantification of aberrant IELs. Withdrawing azathioprine after 2–3 years of complete response may be considered.
Treatment of RCD-II
Immunosuppressive drugs have a limited role.218 Open capsule or non-SR budesonide seems encouraging.217
Given the high percentage of RCD-II patients that develop an EATL, the ultimate treatment goal in RCD-II is to eradicate the aberrant cell population. Clinical and histological remission accompanied by a reduction of aberrant cells has been documented using cladribine (2-CDA).222,223 Responders have a 5-year survival of 83% versus 22% in non-responders. Exclusion of EATL is necessary before this treatment.
One possible alternative strategy includes high-dose chemotherapy followed by autologous haematopoietic stem cell transplantation (auto-SCT).224,225 Auto-SCT is well tolerated with a significant improvement in the clinical and biochemical markers and quality of life (QoL). Quite surprisingly, the reduction of aberrant T cells is not sustained in the treated patients, and therefore long-term outcome, notably the onset of EATL, is still awaited.225 Auto-SCT is indicated only in symptomatic patients and not purely to try to eradicate the aberrant IEL population in asymptomatic patients. The high risk of malignant transformation requires a treatment strategy encompassing an inhibitor of common downstream signalling molecules of the different receptors expressed by aberrant IELs. Tofacitinib is a Jak3 inhibitor that blocks signalling through γ-chain receptors for several cytokines.226 Inhibition of this may result in downregulating of the immune activation and the anti-apoptotic effects in RCD-II.
Combination therapy integrating Jak3-inhibitor with conventional therapy (2-CDA + open capsule/non-SR budesonide) holds some promise but has not been clinically tested. Further, as mentioned above, the identification of the anti-apoptotic pathway mediated by IL-15 may provide treatment avenues for this devastating disorder. Anti-IL-15 monoclonal antibody (AMG 714) is currently undergoing clinical trial in a subset of RCD-II patients.227 Preliminary results demonstrated proof-of-mechanism and proof-of-concept for halting the progression of RCD-II/Pre-EATL, with an acceptable safety profile.
We recommend the following strategy in treating RCD-II. These patients should be managed in conjunction with referral centres having good experience in complicated CD. These centres should have gastroenterological, immunological and haematological units experienced with CD.
In relatively stable patients a trial of open capsule/non-SR budesonide (3 mg, 3 times a day) or oral prednisone (0.5–1 mg/kg/day) may be given.
In seriously ill patients, intravenous prednisone is needed, to be changed later to enteral therapy.
Purine analogue inhibitors such as cladribine or fludarabine could be used at this stage at 0.15 mg/kg/day for 5 days. Those who show a promising response may receive a second course if they relapse after 6–12 months.
Patients who continue to experience a symptomatic decline following cladribine therapy should undergo a re-evaluation of their diagnosis. 2-CDA-auto-SCT step-up therapy is the next step.228
Patients may be included in trials to test other options such as anti-IL15 or Jak3-inhibitor if available.
9.2.4. Prognosis of RCD
RCD-I generally runs a benign course with 80–96% 5-year survival rates.197,198 Main causes of death in this group were either unrelated to CD or nutrition related. It should be noted that lymphoma development in this category of patients was not observed.
RCD-II, on the other hand, is associated with a poor prognosis with a 44% and 58% 5-year survival. The higher mortality associated with RCD-II can be largely attributed to the much higher risk of developing EATL, which occurs in between 33 and 52% within 5 years after diagnosis.198, 228
9.3. Risk of malignancies in CD
9.3.1. Enteropathy-associated T cell lymphoma (EATL)
This is a rare GI lymphoma that accounts for <1% of all non-Hodgkin's lymphomas (NHL).228 Transformation of RCD-II to EATL is a prominent risk and may run an aggressive course with poor overall prognosis.
Currently, two groups of EATL are recognized: EATL type-I accounts for 80–90% of all cases and is exclusively associated with CD; EATL type-II (de novo) usually has not been associated with pre-existent CD or precedes the diagnosis of CD.229,230 In RCD-II, abnormal IELs may be found in mesenteric lymph nodes, blood, bone marrow and in different epitheliums, such as lung and skin.228 Extra-intestinal dissemination of RCD-II IELs explains why EATL does not develop exclusively in the intestine. EATL may notably arise from RCD-II cutaneous lesions.
Histologically, there is infiltration by medium to large lymphoid cells expressing CD30 in more than 80% of cases.231
PET-scan can further guide realization of radiologically guided biopsy or explorative laparoscopy.
The most widely used treatment in clinical practice is anthracycline-based combination chemotherapy, followed by auto-SCT in eligible individuals. The overall response rate ranges from 30% to 60%.229,231 The most frequent causes of death are bowel perforation and bleeding. Further, the poor prognosis of EATL is determined by extent of disease at diagnosis, multifocal small-bowel involvement, poor general health and presence of complications including perforation that preclude chemotherapy.232 Debulking surgery for large ulcerated small-bowel tumours is recommended for limiting the risk of perforation or bleeding during chemotherapy. Pre-existent RCD-II is associated with a poor prognosis. A small proportion of patients may benefit from second-line therapy.233
Brentuximab is an anti-CD30 chimeric antibody conjugated to the potent antimitotic agent monomethyl auristatin E (MMAE). After binding to CD30 receptors, brentuximab is internalized and transported to lysosomes, where MMAE is cleaved and, once released, will bind to tubulin and cause cell cycle arrest and apoptosis.234 Except one case report, no data seem to be available describing its use or benefit in EATL.234 The excellent clinical and radiographic response reported in this case highlights the need to further evaluate its role in patients with EATL. A phase-2a trial is currently in process. In conclusion, the current Clinical Practice Guidelines in Oncology recommend using multi-agent chemotherapy followed by stem cell transplantation for EATL.235 This regimen should still be the preferred therapy in eligible patients who can tolerate it, because current evidence supports its use. Until further data are available, brentuximab may be considered in patients who have a poor tolerance of chemotherapy or in the absence of other standard options.
9.3.2. Other malignancies
The incidence of malignancies in connection with CD differs depending on the type and site of cancer, as well as the period relative to diagnosis. Although some studies report no increase in the overall incidence of cancer, a higher risk for developing lymphoproliferative disease (EATL and less frequently B cell NHL) is unanimously reported.236,237
After the first years of CD or DH diagnosis, there is a decrease in the overall incidence of solid cancers, NHL and all GI cancers.238 On the contrary, one report suggested a high lymphoma risk independent of adherence to GFD.144 There is an association between CD and SBA and unexpectedly for oesophageal squamous-cell carcinoma.239
Recommendations
(1) Patients showing slow response should be evaluated carefully to exclude dietary inconsistencies and also identify other specific aetiologies. (Strong recommendation, high level of evidence)
(2) Evaluation of slow-responders should include review initial diagnosis, coeliac serology, a dietary review and follow-up duodenal biopsy. (Strong recommendation, high level of evidence)
(3) Distinction should be made between RCD-I and RCD-II to select appropriate management and determine the prognosis. (Strong recommendation, moderate level of evidence)
(4) T-cell flow cytometry is the most reliable method to make a distinction between RCD-I and RCD-II. (Strong recommendation, moderate level of evidence)
(5) The nutritional status of RCD patients should be monitored closely. Nutritional support including parenteral nutrition forms an essential part of the management. (Strong recommendation, high level of evidence)
(6) EATL should be confidently excluded before starting treatment with medications in RCD-II. (Strong recommendation, moderate level of evidence)
(7) RCD-II patients need to be treated in referral centres by an experienced gastroenterologist in CD in collaboration with haematologist. (Strong recommendation, moderate level of evidence)
(8) EATL needs to be excluded in any CD patient with abdominal pain, fever, obstruction, anaemia, gastrointestinal bleeding or unexplained weight loss. (Strong recommendation, moderate level of evidence)
(9) In EATL: debulking surgery for large ulcerated small bowel tumours is recommended for limiting the risk of perforation or bleeding during chemotherapy. (Strong recommendation, moderate level of evidence)
10: Special issues concerning CD in childhood and adolescence
10.1. Diagnostic aspects
This has been dealt with by the ESPGHAN guidelines in great detail.240 Important issues are summarized here.
10.1.1. Who should be tested for CD in childhood and adolescence?
It is important to diagnose CD not only in children with obvious GI symptoms but also in those with a less clear clinical picture, with only extra-intestinal manifestations or even in those who are asymptomatic because the disease may have negative health consequences in the future. Testing for CD should be offered to the following groups:
Group 1: Those with otherwise unexplained symptoms and signs of chronic or intermittent diarrhoea, failure to thrive, weight loss, stunted growth, delayed puberty, amenorrhoea, IDA, nausea or vomiting, chronic abdominal pain, cramping or distension, chronic constipation, chronic fatigue, recurrent aphthous stomatitis, DH-like rash, fracture with inadequate traumas/osteopenia/osteoporosis and abnormal liver biochemistry.
Group 2: Asymptomatic with an increased risk for CD such as T1DM, Down syndrome, autoimmune thyroid disease, Turner syndrome, Williams syndrome, selective IgA deficiency, autoimmune liver disease and first-degree relatives with CD.
10.1.2. Approach for a child with symptoms/signs suggestive of CD
IgA-TG2 is recommended as the first test to identify those who need further investigation to diagnose CD. If total serum IgA is not known, then this should be measured. In the presence of total IgA-deficiency, at least one test measuring IgG class antibodies should be done (IgG-TG2, IgG-DGP or IgG-EMA). If the first test was POCT, then the result should be confirmed by a quantitative test. In unclear cases, small-bowel biopsies and HLA testing are strongly required.
10.1.3. Diagnosis of CD without duodenal biopsies
In children and adolescents with signs or symptoms suggestive of CD the ESPGHAN guidelines recommend that the diagnosis of CD may be made without confirmatory biopsy in those with high TG2-antibody levels (>10 times ULN) confirmed by EMA-positivity and the presence of HLA-DQ2/8 haplotypes. The diagnosis is confirmed by an antibody decline and a clinical response to a GFD.
10.1.4. Approach for an asymptomatic child with an increased risk for CD
HLA testing should be offered as the first line test. In the absence of DQ2/DQ8 haplotypes no further serological tests are needed. If the patient is DQ2 and/or DQ8 positive, then an IgA-TG2 test should be performed plus total IgA. If antibodies are negative, then repeated testing for CD-specific antibodies is recommended for at-risk children periodically. Genetic technological progress now enables HLA typing from buccal cell samples, reducing stress associated with venepuncture in high-risk populations.241
If initial antibody testing was a POCT, then a positive test result always should be confirmed by a quantitative test.
To avoid unnecessary biopsies in those with low CD-specific antibody levels (i.e., < 3 times ULN), it is recommended to test for EMA. If the EMA test is positive, then the child should be referred for duodenal biopsies. If the EMA test is negative, then repeated serological testing on a gluten-containing diet at 3- to 6-monthly intervals is recommended.
10.1.5. Gluten challenge
When there is doubt about the initial diagnosis, then gluten challenge is necessary. It should be preceded by HLA typing and assessment of mucosal histology. It should be discouraged before the age of 5 years and during the pubertal growth spurt. The gluten intake during the challenge period should contain no less than 5 g/day. The diagnosis of CD is confirmed if CD-antibodies become positive and a clinical and/or histological change is observed. If antibodies remain negative and no histological change is observed, then CD is excluded, taking into consideration that a delayed disease development may occur later in life.
10.2. Follow-up
The family should receive dietary counselling for a GFD preferably from an expert dietist/nutritionist if available. The patients should be followed-up regularly for symptomatic improvement and normalization of CD-specific antibody tests. It is unnecessary to perform follow-up small-bowel biopsies; however, if there is no clinical response to the GFD, then a careful assessment to exclude lack of dietary adherence and further investigations including new biopsies are required.
Paediatric patients should be seen at 3- to 6-month intervals for the first year after diagnosis. Once symptoms have resolved and serology has normalized, an annual follow-up visit is recommended.
DEXA should only be considered for those at high risk (e.g., history of low-energy bone fractures, dietary non-adherence, established persistent VA or low body mass index).242
10.3. Transition from childhood to adulthood in CD
Children and adolescents with CD need to be sufficiently prepared for the transfer to adult care, and the process of transition needs to be well organized. Generally, the transition from paediatric to adult care should be a collaborative process involving patients, their parents or caregivers, the physician and the dietician.243–246
The transition process should gradually parallel the evolution of becoming an adult and include an incremental shift in knowledge and decisions to the adolescent patient with CD.244 The physical, mental and psychosocial development is central to transition, which varies between individuals. Some adolescents and young adults with CD will experience a delay in pubertal and sexual development and may continue so beyond the expected age of pubertal completion.247,248
10.3.1. The process of transfer of care
One path to facilitate transition of care is to create a ‘transition letter’ by the paediatrician.249 Such a transition letter should contain details of the basis for the diagnosis and information during follow-up such as serology, anthropometric data, comorbidities and dietary adherence.
10.3.2. Issues that need to be discussed during transfer
Some young adults may question their diagnosis and feel that re-evaluation is needed especially if the diagnosis is only based on serology. If the existing diagnostic guidelines have not been met and the diagnosis needs re-evaluation, a new diagnostic approach should be instituted. Serology, HLA-DQ2/8 genotyping and histology may be part of this approach.
Adherence to a GFD can be difficult, particularly with new challenges: peer pressure and the stigma of ‘being different’ and increasing independence from parents.
Financial issues: gluten-free products are expensive; this becomes more relevant for adolescents/young adults who now live on a limited budget after moving away from home.
Recommendations
(1) The confirmation of a CD diagnosis in some children should be based on a combination of clinical data, positive serology and duodenum biopsies. (Strong recommendation, moderate level of evidence)
(2) Duodenal histology in some children is recommended to confirm the diagnosis of CD. (Strong recommendation, high level of evidence)
(3) CD diagnosis may be made without duodenal biopsy in symptomatic children with high TG2 levels (>10 times ULN) and EMA in the presence of HLA-DQ2/8. The diagnosis is confirmed by an antibody decline and preferably a clinical response to a GFD. (Conditional recommendation, moderate level of evidence)
(4) Gluten challenge is indicated when the CD diagnosis in doubt with negative serology before starting a GFD. It should not be performed before the child is 5 years old or during the pubertal growth spurt. (Conditional recommendation, moderate level of evidence)
(5) A GFD should be introduced only when CD diagnosis has been made conclusively. (Strong recommendation, high level of evidence)
(6) The duration of breast feeding and/or gluten introduction while the infant is still breast fed had no impact on the risk of developing CD. (Moderate recommendation, moderate level of evidence)
(7) There is currently no evidence to recommend avoiding either an early (at 4 months of age) or a late (at or after 6 or even 12 months) gluten introduction in children at risk of CD. (Moderate recommendation, moderate level of evidence)
(8) A gradual transfer of medical care of an adolescent with CD to adult care is recommended. The transfer should be structured and include as the minimum written information on the base of diagnosis, follow-up, anthropometric data, comorbidities and dietary adherence.
11: Non-coeliac gluten sensitivity
11.1. Clinical aspects
NCGS is characterized by IBS-like symptoms and extra-intestinal manifestations, occurring in a few hours or days after gluten ingestion, improving rapidly with gluten withdrawal and relapsing soon after gluten challenge.
Pre-requisite for suspecting this condition is the exclusion of both CD and WA when the patients are still on a gluten-containing diet. Besides gluten, other potential culprits of this syndrome are ATIs and fructans (rich in FODMAPs), which are all components of wheat and other gluten-containing cereals”.6,7,250–252
There is substantial overlap in symptoms between CD and NCGS. It appears that NCGS does not have a strong hereditary basis and is not associated with severe malabsorption or malignancy. It is less frequently associated with autoimmunity than CD, although in this syndrome there is a high prevalence of autoimmune thyroiditis and ANA-positivity.253
11.2. Pathogenesis
This is poorly understood. NCGS potentially involves many triggers as are seen in CD and IBS. The inciting event mainly involves exposure of bowel epithelium to dietary gluten leading to immune-mediated and/or non-immune-mediated responses. Due to the lack of evidence for T cell involvement and the possible contribution from toll-like receptors (e.g., TLR-1, TLR-2), NCGS may be more of an innate rather than adaptive immune response.6,254 Changes in the gut microbiome produced by gluten consumption may also influence NCGS.254
Recent data have shown that in NCGS patients TLR4 might play a role in transducing the effect of gliadin through a myeloid differentiation factor 88 (MYD88) adaptor protein. Circulating bacterial components, such as Lipopolysacharides (LPS) and flagellin, bind to TLR4 on macrophages and dendritic cells with the result of signals through the MYD88-dependent pathway. MYD88 signalling increases the expression of pro-inflammatory cytokines causing systemic effects. This systemic immune activation may play a role in the pathogenesis of NCGS.254
11.3. Diagnosis
There are no specific biochemical, immunological or histopathological markers associated with NCGS. The diagnosis should be considered in patients with persistent intestinal and/or extra-intestinal complaints showing a normal result of the CD and WA serological markers on a gluten-containing diet, usually reporting worsening of symptoms after eating gluten-rich food. Unfortunately, many of these patients are already on the GFD when first seen at the specialty clinic.
The following multi-step approach is suggested to make the diagnosis of NCGS:255
Step 1: A full clinical and laboratory evaluation to exclude CD and WA while still on a gluten-containing diet. If highly suspicious of CD, the clinician can proceed for obtaining a duodenal biopsy. If the biopsy indicates low CD probability (Marsh 0-1) then the clinician can proceed to the following steps: 1. establish baseline symptoms while the patient is on a gluten-containing diet; 2. follow GFD for at least 6 weeks; and 3. re-evaluate symptoms. NCGS is excluded in subjects failing to show symptomatic improvement.
Step 2: Gluten challenge is required in patients responding to treatment with the GFD and in those who are already on a GFD before testing and are willing to establish the diagnosis. Whether this should be done with regular bread or any other vehicle where FODMAP is excluded is a matter of debate. Ideally the clinical evaluation should include serially repeated specific laboratory tests.
NCGS might be difficult to differentiate from a category of IBS patients (both IBS-D and IBS-C) showing clinical response after adhering to GFD.256,257
11.4. Management
A GFD helps resolve the intestinal and extra-intestinal symptoms. Obviously, a less stringent GFD might be sufficient compared to those with CD.
Recommendations
(1) NCGS may be considered in patients with gluten-related intestinal and/or extra-intestinal complaints showing a normal result of the CD and WA serological markers on a gluten-containing diet. (Strong recommendation, moderate level of evidence)
(2) Serology and small-bowel histology (while the patient is on a gluten-containing diet) and HLA-DQ typing (to rule out CD if negative) are needed to differentiate between CD and NCGS. (Strong recommendation, moderate level of evidence)
(3) The diagnosis of NCGS is excluded in subjects failing to show symptomatic improvement after 6 weeks of GFD. (Strong recommendation, moderate level of evidence)
(4) A less-stringent GFD might be enough compared with those with CD. (Conditional recommendation, low level of evidence)
(5) Patients showing a negative gluten challenge should be investigated for other possible causes of IBS-like symptoms. (Conditional recommendation, low level of evidence)
12 CD-related skin and oro-dental disorders
12.1. Dermatitis herpetiformis
This is considered the specific cutaneous manifestation of CD. Both diseases occur in gluten-sensitive individuals, share the same HLA haplotypes and improve following a GFD.4
12.1.1. Histopathology
The IgA deposits against TG2 can be seen in the small-bowel mucosa of DH patients.258 Pathognomonic granular IgA deposits can be detected by direct immunofluorescence (IF) in the papillary dermis.258 The autoantigen for deposited cutaneous IgA was found to be epidermal TG3.259 Currently, the most valid immunopathogenesis hypothesis is that DH starts from hidden CD in the gut with TG2 antibody response, eventually evolving to immune-complex deposition of high avidity IgA antibodies with TG3 enzyme in the papillary dermis.260
The typical findings in the lesional skin of DH consist of subepidermal vesicles and blisters with accumulation of neutrophils at the papillary tips.253
12.1.2. DH versus CD
Small-bowel enteropathy: Histological changes similar to that occurring in CD have been reported in 75% of patients with DH, and the remaining have minor changes consistent with latent CD. Patients who present with CD and concurrent DH are more likely to have more severe intestinal damage than those with a DH-predominant presentation.261
Serology: Patients with DH usually show CD-specific antibodies. IgA-TG3 antibodies are considered specific and sensitive serological markers for DH but are not available for clinical use.259
HLA haplotypes: As in CD, virtually all patients with DH carry either HLA-DQ2 or HLA-DQ8.
Epidemiology: Overall, the ratio between CD and DH in different populations has been 10–20:1.262 DH in childhood seems to be rare.263 No significant differences exist in adults. The mean age at diagnosis of DH is 39 (range 11–80) years and that of CD is 44 (range 1–85) years.
GI symptoms and nutritional status: Minor GI complaints and occasional loose stools are the most common findings in DH. Signs of malabsorption are rare.264 Nutritional deficiencies are similar between the two groups.
Body mass index: In untreated adults with CD, the BMI is lower than in general population, but still 28% are overweight and 11% obese.265 An Italian study suggested that BMI was even higher in patients with DH than in those with CD.266
Bone mineral density: there is no increased risk for fractures in treated DH compared with controls.267
Associated autoimmune diseases: this is similar in CD and DH.268
Risk of lymphoma: As in CD, the risk of NHL is significantly increased in DH. A strict GFD for more than 5 years seems to protect against lymphoma in DH.269
Mortality: Adherence to GFD reduces the all-cause mortality in DH.270
12.1.3. Diagnostic approach
The first step is testing serum anti-TG2 and a biopsy of perilesional skin for Direct Immunoflourescence (DIF). The diagnosis of DH should always be confirmed by DIF examination of perilesional skin showing granular IgA deposits in the papillary dermis. DIF has sensitivity and specificity close to 100% for the diagnosis of DH. If the patient is on a GFD, a gluten-containing diet should be administered and the biopsy taken after at least 1 month.271
In case of typical results from DIF and positive anti-TG2, the diagnosis of DH and CD can be confirmed. If TG2 is negative, HLA DQ2/DQ8 testing is suggested. If negative, DH can be excluded, but if positive, then EMA and anti-DGP should be tested. If EMA or anti-DGP is positive, DH can be confirmed. If negative, then duodenal biopsy is needed prior to starting a GFD. The diagnosis of DH is excluded in case of negative DIF and DQ2/DQ8 or negative DIF and serology.272
12.1.4. Treatment
GFD: The mainstay for treatment of DH is a strict GFD. It resolves both the gastrointestinal and the cutaneous manifestations.273,274 While it takes an average of 1–2 years of a GFD for the complete resolution of the cutaneous lesions, the gastrointestinal symptoms usually resolve in an average of 3-6 months. IgA antibodies may disappear from the dermal–epidermal junction after many years of a strict GFD. Few studies have suggested that DH can go into remission in up to 20% of the cases, and, therefore, clinicians should continually re-evaluate the need for a GFD for patients with well-controlled DH.274 However, other studies are required to confirm whether the GFD can be safely discontinued.
Dapsone: Although no reports from randomized controlled trials are available about its use, dapsone is considered an essential therapeutic option for patients with DH during the 6- to 24-month period until the GFD is effective.260,261 In some patients it may be needed for up to 10 years. The starting dose should be 50 mg/day in order to minimize the potential side effects. Then the dosage can be increased up to 200 mg/day until the disease is under control; in the maintenance phase, 0.5–1 mg/kg/day generally can control itching and the development of new skin lesions. Several side effects are associated with dapsone use. They are usually dose-dependent and more frequent in patients with comorbidities, such as anaemia, cardiopulmonary disease and glucose-6-phosphate dehydrogenase deficiency. Because of side effects, patients using dapsone should be carefully monitored. Before starting the therapy, complete blood count, glucose-6-phosphate dehydrogenase, methaemoglobinaemia, liver and renal functions, as well as urinalysis should be investigated.
Other drugs: If dapsone fails to control the symptoms or in case of adverse effects, sulfasalazine, sulfapyridine and sulfamethoxypyridazine can be valid alternatives for the treatment of patients with DH.275,276 Topical steroids can be used to control the skin symptoms in patients with DH especially in cases with localized disease to reduce pruritus and the appearance of new lesions. Accordingly, systemic steroids or antihistamines can control, at least in part, itching and burning sensation, although their effectiveness is considered quite low. Topical dapsone, immunosuppressors such as cyclosporine A or azathioprine, colchicine, tetracycline, heparin nicotinamide, mycophenolate and rituximab have been shown to be effective in some reports.276 Finally, several new experimental approaches for the treatment of CD are currently under investigation.
12.1.5. Refractory DH
Despite long adherence to strict GFD, a small proportion of patients (about 2%) with DH need to continue treatment with dapsone to control the active rash.277 The term refractory DH has been suggested to describe this condition. No lymphoma or abnormal IELs have been found yet in these patients indicating that refractory DH is a benign condition. Refractory DH differs from those with refractory CD by showing a clear response to a GFD in the small-bowel mucosa. However, the rash remains active with persistent cutaneous IgA deposits. A case report showed that one elderly patient with resistant DH achieved complete clinical and serological remission following therapy with rituximab, a monoclonal antibody targeting CD20-positive B cells.278
12.1.6. Follow-up
Patients with DH should be evaluated at regular intervals (3 months, 6 months after diagnosis and then yearly) by a multidisciplinary team involving at least a physician and a dietitian. The purposes of these visits are to assess the compliance with the GFD and the presence of dyslipidaemia, and to evaluate the possible development of intestinal malabsorption, autoimmune diseases and complications such as refractory CD, or lymphoma. Together with the visits, laboratory investigations, including TG2 antibodies, and evaluation of intestinal malabsorption should be performed.
12.2. Other skin disorders
12.2.1. Psoriasis
CD-specific antibodies were reported in subjects with psoriasis with levels correlating with the severity of psoriasis.279 Concomitant CD is present in 1–4% of people with psoriasis.280,281 This association, if present, may be explained by several mechanisms including vitamin D deficiency, abnormal Th1/Th17 response, common genetic background and increased intestinal permeability.282 There is good evidence to suggest that psoriatic patients, either with concomitant CD or asymptomatic gluten intolerance, may benefit from GFD with resolution of skin lesions.283
Thus, it is justifiable to monitor patients with either condition for clinical evidence of the other. Vitamin D should be regularly controlled in patients with CD. GFD should be considered in psoriatic patients with serological evidence of gluten intolerance even in the absence of clinical signs of CD.
12.2.2. Non-specific skin conditions
Patients with CD frequently report non-specific dermatological issues, including dry skin, easy bruising, brittle nails and thinning hair.284 Zinc deficiency is particularly associated with these skin lesions. Iron, zinc and fat-soluble vitamins are most often deficient in patients with newly diagnosed CD, and repletion can accelerate clinical improvement.
Alopecia areata can be a coexisting autoimmune disorder in adults and children with CD, although it is less common.285 This condition does not typically respond to a GFD and might be progressive. Consequently, patients with CD and severe hair loss should be referred to a dermatologist for evaluation.
12.3. Oro-dental abnormalities in CD
CD can develop at any age when solid foods are introduced into the diet; however, if CD occurs in children while the permanent teeth are developing, i.e., before 7 years of age, abnormalities in the structure of the dental enamel can occur.286 It has been seen in children 3–4 years old, and we think that this forms a window of opportunity to recognize CD. These defects are seen most commonly in the permanent dentition and tend to appear symmetrically and chronologically in all four quadrants, with more defects in the maxillary and mandibular incisors and molars. Both hypoplasia and hypomineralization of the enamel can occur. A band of hypoplastic enamel, often with intact cusps, is common. A hiatus in enamel and dentin formation can occur at a developmental stage corresponding to the onset of GI symptoms.287 The exact mechanism leading to these defects is not clear, but immune-mediated damage is suspected.288 Recurrent aphthous ulcers can also occur in CD.289
Recommendations
(1) The diagnosis of DH should always be confirmed by DIF examination of perilesional skin showing granular IgA deposits in the papillary dermis. (Strong recommendation, high level of evidence)
(2) In case of typical results from DIF and positive anti-TG2, the diagnosis of DH and CD can be confirmed. (Strong recommendation, high level of evidence)
(3) If the patient is on a GFD, a gluten-containing diet should be administered and the biopsy taken after at least 1 month. (Strong recommendation, moderate level of evidence)
(4) GFD is essential in the management of DH. (Strong recommendation, high level of evidence)
(5) Dapsone is considered an essential therapeutic option for patients with DH during the 6–24-month period until the GFD is effective. (Strong recommendation, moderate level of evidence)
(6) GFD should be considered in patient with psoriasis and serological evidence of gluten intolerance even in the absence of clinical signs of CD. (Conditional recommendation, low level of evidence)
The following tips may be of help to dentists encountering oral symptoms and signs in a patient:
(1) Consider CD as a possible diagnosis in patient with dental enamel defects, recurrent oral aphthous ulcers or both.
(2) Question about other clinical symptoms of CD, including abdominal pain, diarrhoea, weight loss, poor growth, anaemia and fatigue.
(3) Inquire about the presence of other autoimmune diseases, especially T1DM and thyroiditis. The presence of these will further increase the probability of CD.
(4) Consider adding CD to the list of disorders that you inquire about during family history screening.
(5) If CD is suspected, the dentist or dental hygienist should consult the patient’s primary care physician or specialist.
(6) Do not recommend a GFD to a patient suspected of having CD without confirmation of the diagnosis.
13 Neuro-psychiatric manifestation related to gluten
13.1. The link to gluten
Gluten-induced neurological manifestations, including gluten ataxia, are seen in adult CD and occur also in children. They may either precede or be present at onset of CD.5,290 In established CD, a 10-22% prevalence of neurological dysfunction is reported.5,290Also, these complications may be the prime presentation of CD and NCGS.291 Because of the lack of gut involvement in NCGS, neurocoeliac disease may easily go unrecognized.
13.2. Pathophysiology
To date, both the causative factors and pathophysiological mechanisms of neurological involvement in CD remain elusive. The nervous system may be one of the selective sites of gluten-mediated reactions, including cross-reacting antibodies, immune-complex deposition, direct T cell cytotoxicity, immune-cytotoxicity and deficiency of vitamins and other nutrients secondary to chronic malabsorption.292,293
13.2.1. Genetics
In the Sheffield neurology cohort HLA-DQ8 was more common in patients who had no enteropathy compared with CD patients, i.e., having proven enteropathy. This may represent a separate entity from ataxia complicating CD.292
13.2.2. Immunological basis
Current research suggests that neurocoeliac manifestations are immune-mediated. Post-mortem examination from patients with gluten ataxia showed patchy loss of Purkinje cells and infiltration of T-lymphocytes within the cerebellum. Lymphocytic infiltrates are found in dorsal root ganglia in coeliac patients with sensory neuronopathy or with myopathy.292,294 Anti-TG6 has been found in up to 85% of coeliac patients with neurological involvement.295
13.2.3. Serotonergic effects
Gluten intolerance may interfere with serotonergic functioning. GFD might improve depressive and behavioural problems and increase free L-tryptophan levels.296
13.3. Overview of neuro-psychiatric manifestations related to gluten:
13.3.1. Gluten ataxia (GA)
This is the most frequently reported neurological disturbance in CD. The data from Sheffield suggest a 20% prevalence of GA among all patients with ataxias and 40% among patients with sporadic ataxias.297 The use of gliadin antibodies to prove the link between ataxia and gluten created some scepticism towards the association with gluten.
GA is infrequently related to intestinal coeliac manifestations or vitamin deficiencies, and improvement with a GFD is possible. Less than 10% of patients with GA have GI symptoms but a third have enteropathy on biopsy.297 Few studies, mainly case reports, suggest overall favourable effect of GFD.298
13.3.2. Peripheral neuropathy
Gluten neuropathy is defined as idiopathic neuropathy in the absence of an alternative aetiology with serological evidence of gluten sensitivity. Presentations include symmetrical sensorimotor axonal peripheral neuropathy, asymmetrical neuropathy, sensory ganglionopathy and small-fibre neuropathy.299 Only a third has enteropathy.
Effect of a GFD on peripheral neuropathy is disappointing.300,301 A strict GFD ameliorates the overall pain and health change scores, indicating better QoL.302 Furthermore, nutritional deficiencies such as vitamin B12 or copper could lead to neurological sequelae in CD.
Copper deficiency is a rare cause of myelopathy indistinguishable from subacute combined degeneration due to B12 deficiency. If proven, these deficiencies need to be promptly addressed.152,156
13.3.3. Gluten encephalopathy
This has a wide range of symptoms, such as headaches resembling migraine responsive to a GFD to severe debilitating headaches associated with focal neurological deficits and white matter abnormalities on MRI at the other end. The white matter abnormalities can be diffuse or focal and do not resolve after a GFD.290
13.3.4. Other neurological disorders
A link between epilepsy and CD has been reported with prevalence of 4–8%. Gluten sensitivity and temporal lobe epilepsy with hippocampal sclerosis might be associated.303 GFD might benefit these patients.304 In relapsing-remitting or secondary-progressive multiple sclerosis, there is no evidence of an increase in prevalence of gluten sensitivity.305 Cases of gradually progressive neurological disease and gluten sensitivity associated with white matter lesions, mimicking multiple sclerosis, have been described.306 Adult CD patients often complain of mild cognitive symptoms called “foggy brain”, which improves when gluten-restriction is started, but re-appears with dietary contamination.307,308 Concentration and attention difficulties, episodic memory deficits, word-retrieval problems, reduced mental acuity and episodes of confusion or disorientation are common recognized features in CD.309
13.3.5. Psychiatric disorders
There are reported associations of psychiatric disorders with CD, including depression, bipolar disorder, apathy, excessive anxiety, schizophrenia, eating disorders, attention-deficit/hyperactivity disorder, autism and sleep complaints.310–312 Anxiety disorders are usually reactive in CD patients and improve with a GFD. Depressive disturbances may significantly impair QoL and are a good predictor of lack of dietary compliance.310 A prolonged GFD might improve some patients. The association between autism and CD is debated.313
Summary
(1) Awareness for CD in neurology outpatient clinics should be improved.
(2) Patients with CD might be more susceptible to the development of neurological dysfunction if they continue to consume gluten.
(3) Neurological manifestations in patients with CD may either precede or follow the disease.
(4) The prevalence of gluten ataxia might be up to 20% among all patients with idiopathic ataxias.
(5) Up to 25% of coeliacs on a GFD have neurophysiological evidence of peripheral neuropathy.
(6) New diagnostic tools are becoming available (e.g. anti-TG6), which will enable identification of patients with neuro-CD.
(7) Neurological manifestations in relation to gluten are most likely immune-mediated.
(8) Removal of the immunological trigger (gluten) forms the basis of treatment and should be recommended once the diagnosis is properly made. (Strong recommendation, low level of evidence)
(9) Depression may significantly impair QoL and is a good predictor of lack of dietary compliance. Prolonged GFD might improve some patients.
14 Quality of life
In recent years the health-related QoL of coeliac patients has attracted interest both in medical research and in clinical practice.314 Measurement of this allows the impact of medical interventions to be evaluated more comprehensively. The great majority of QoL studies in CD have been cross-sectional and conducted among adults with the classical gastrointestinal disorder. These studies showed that a wide variety of issues can affect the QoL of patients.315 Besides age and gender, clinical manifestations and compliance with diet are strongly associated with the health-related QoL in CD.
14.1. Studies in adults
The disease may be quite burdensome, especially in patients with abdominal complaints with signs of malnutrition, which explains the reduced health-related QoL observed in untreated coeliac patients.314–316 The initiation of a GFD has usually resulted in significant improvement. The persistence of poor QoL despite appropriate GFD might be explained by the significant burden and social restrictions caused by the treatment.316 Women with CD may have an increased risk for anxiety despite appropriate dietary treatment.317
In DH, no difference in health-related QoL between patients and healthy controls was found, either at diagnosis or while on treatment.318
In patients with gluten neuropathy, physical dysfunctioning is the major determinant of QoL. A strict GFD ameliorates the overall pain and health change scores, indicating better QoL.302
CD patients who are found by screening may have only subtle symptoms or may even be completely asymptomatic. It is essential to evaluate whether early diagnosis is truly beneficial for the QoL in these subjects.101,102
14.2. Studies in children
The perception of good health-related QoL may change with age. Special questionnaires for young children and for adolescents are usually required. Children seem to have better health-related QoL than adults with CD.319,320 For those asymptomatic children diagnosed after screening, QoL did not improve.
15 Novel therapies for CD
15.1. The need for therapeutic measures other than diet
It is still quite challenging to eliminate the “hidden gluten” that often contributes to the ongoing signs and symptoms and incomplete mucosal healing in a significant proportion of CD patients. Difficulty also exists during travel to places where food choices may be very limited. Additionally, patient dissatisfaction with the restrictive nature of the diet and the high cost of gluten-free foods all add to the burden of treatment and can lead to suboptimal adherence.321 These concerns and observations have given way to increased interest in therapeutics which may either replace or act in conjunction with a GFD.
With better understanding of the pathogenesis of CD many potential therapeutic targets have emerged, and there is increasing interest in identifying and testing novel therapies for CD.
15.2. Overview of potential therapeutic options
Clinical trials are being conducted to test the efficacy of inhibiting one or more steps in the pathophysiological pathway of CD. Different agents targeted the following steps:
Dietary modification (gluten replacement or removal).322,323
Oral enzyme supplements (gluten digestion by endopeptidases).324–328 Latiglutinase (IMGX-003 or ALV-003), AN-PEP (prolyl endopeptidases derived from Aspergillus niger) and STAN1 (a cocktail of microbial enzymes) have been tested.
Enhancing tight junction integrity using larazotide acetate.329–331
TG2-inhibition and modulation of the immune system.332 A European phase-II gluten re-challenge study with ZED1227 (an irreversible TG2 inhibitor) has been started.
Blocking HLA-DQ2.333 Based on experience from other parts of medicine, it is unlikely that this strategy will succeed.
Induction of tolerance to gluten using Nexvax2, which is a novel, peptide-based, epitope-specific immunotherapy based on the principle of desensitization therapy for allergic conditions using whole proteins, or induction of infection with parasites to regulate host immune system by upregulation of Th2 response.334,335
15.3. Summary of results of novel therapies
A number of current clinical studies have shown promising results, especially compounds for enzymatic gluten detoxification or to modulate tight junction integrity, but these have been of a short duration and low power; therefore, studies with longer duration and greater power will be needed to assess both efficacy and long-term safety.
As with any novel therapy the projected costs of the new therapies will need to be weighed against the value and benefit provided compared to that of a GFD alone. Ultimately health insurance plans would need to compensate these costs to permit patients to afford such medications, as they already face considerable costs to adhere to a GFD.
16. Areas of uncertainty and future research
Future research should focus on exploring the following areas and provide appropriate answers:
In screening studies: There is a lack of understanding of the natural history of undiagnosed CD; this needs further clarification also to justify screening of asymptomatic persons. Sensitivity/specificity of anti-TG2 testing and the role of EMA in patients with low or intermediate anti-TG2 level needs to be defined. Further, we need to determine the time intervals mandatory to repeat testing in subjects in high-risk populations.
The role of POCT in high-incidence areas with limited access to medical care needs to be determined.
Studies are needed to validate the following tests and make them widely available for clinical use: HLA-DQ-gluten tetramer in blood, serum assays for I-FABP and T cell flow cytometry.
Studies are needed to validate the results of GWAS suggesting that genetic susceptibility to CD might be distinct from the progression to RCD-II.
Role of biopsy in diagnosis and follow-up: The debate on the diagnosis of CD without biopsy needs more clarification. Also, the indication for and timing of biopsy at follow-up should be further explored.
Marsh-1 pathology: An evidence-based approach is needed.
The management of bone disease should be optimized, e.g., by determining the age of screening for osteoporosis and the place for intravenous bisphosphonates particularly in the early years after diagnosis.
Treatment of RCD: The results of ongoing trials using anti-IL15 and tofacitinib are being awaited. Strategies such as combination or step-up therapies need more elaboration.
NCGS: The future research should explore the genetic background, histological characteristics, susceptibility and risk factors for NCGS in addition to developing reliable biomarkers.
In neuro-coeliac disease: More data are needed to clarify the gluten–brain link and to develop preventive and therapeutic strategies.
The development of non-dietary therapies might alleviate symptoms especially after an inadvertent gluten exposure, and an effective replacement of the GFD could greatly enhance the QoL of patients struggling with the diet.
Supplemental Material
Supplemental Material for European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders by Abdulbaqi Al-Toma, Umberto Volta, Renata Auricchio, Gemma Castillejo, David S Sanders, Christophe Cellier, Chris J Mulder and Knut E A Lundin in United European Gastroenterology Journal
References
- 1.See supplementary material.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders by Abdulbaqi Al-Toma, Umberto Volta, Renata Auricchio, Gemma Castillejo, David S Sanders, Christophe Cellier, Chris J Mulder and Knut E A Lundin in United European Gastroenterology Journal