Abstract
Introduction
Fecal microbiota transfer (FMT) is highly effective in the treatment and prevention of recurrent Clostridioides difficile infection (rCDI) with cure rates of about 80% after a single treatment. Nevertheless, the reasons for failure in the remaining 20% remain largely elusive. The aim of the present study was to investigate different potential clinical predictors of response to FMT in Germany.
Methods
Information was extracted from the MicroTrans Registry (NCT02681068), a retrospective observational multicenter study, collecting data from patients undergoing FMT for recurrent or refractory CDI in Germany. We performed binary logistic regression with the following covariates: age, gender, ribotype 027, Eastern Co-operative Oncology Group score, immunosuppression, preparation for FMT by use of proton pump inhibitor, antimotility agents and bowel lavage, previous recurrences, severity of CDI, antibiotic induction treatment, fresh or frozen FMT preparation, and route of application.
Results
Treatment response was achieved in 191/240 evaluable cases (79.6%) at day 30 (D30) post FMT and 78.1% at day 90 (D90) post FMT. Assessment of clinical predictors for FMT failure by forward and confirmatory backward-stepwise regression analysis yielded higher age as an independent predictor of FMT failure (p = 0.001; OR 1.060; 95%CI 1.025–1.097).
Conclusion
FMT in Germany is associated with high cure rates at D30 and D90. No specific pre-treatment, preparation or application strategy had an impact on FMT success. Only higher age was identified as an independent risk factor for treatment failure. Based on these and external findings, future studies should focus on the assessment of microbiota and microbiota-associated metabolites as factors determining FMT success.
Keywords: Fecal microbiota transfer, recurrent Clostridioides difficile infection, microbiota, risk factors
Introduction
The clinical application of fecal microbiota transfer (FMT) for the treatment of recurrent Clostridioides difficile infections (rCDI) has increased rapidly since the first publication of a randomized controlled trial in 2013.1 In the following years, its efficacy has been confirmed, not only in further randomized clinical trials, but also in clinical practice.2,3 In 2013, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) included FMT in the guidelines for the treatment of rCDI with an AI recommendation.4
In Germany, FMT is subject to the German Medicines Act (Arzneimittelgesetz; AMG), meaning that FMT can only be performed as an individualized clinical trial (treatment of last resort, based on an unregistered drug in a setting where all registered options failed to achieve a clinical response), unless preparations are manufactured in a certified Good Manufacturing Practice facility. To date, such a facility has not been approved in Germany. As a consequence, FMT is poorly standardized at a national level. In 2015, the German Clinical Microbiome Study Group (GCMSG) created the MicroTrans Registry in an attempt to capture all active sites and FMT strategies. The first data analysis was published in the following year, confirming the high efficacy and acceptable safety of FMT in the treatment of rCDI, while at the same time, revealing significant variability of treatment standards between centers.2 Recently, a first effort has been made to standardize the performance of FMT in Europe, but further work in this area is required.5,6
From a statistical point of view, heterogeneity is usually perceived as a drawback; however, in the context of a multicenter registry, it may help to identify factors contributing to treatment success. Previous analyses of this kind suggest that specific risk factors, including early use of antibiotics after FMT, female sex, inadequate bowel preparation, surgery prior to FMT, inpatient status, severity of CDI, as well as the number of previous CDI recurrences are associated with FMT failure;7–11 however, none of these analyses was performed within the extremely diverse treatment context of Germany. When searching for potential reasons for limited treatment success, the ideal route of FMT application may be of high relevance. To date, there is no randomized study designed as a head to head comparison of all available applications, namely via a duodenal tube or upper gastrointestinal tract endoscopy, a colonoscopy, capsules or an enema. To answer this question in the context of a real-life treatment setting, we performed an analysis of data captured in the MicroTrans registry with a focus on clinical predictors of FMT failure.
Methods
The MicroTrans Registry (NCT02681068) is a retrospective observational multicenter study, collecting data from patients undergoing FMT for recurrent or refractory CDI in Germany. Currently, 35 sites are contributing data to this network. Documentation was performed by use of an online eCRF at ClinicalSurveys.Net from January 2014 to February 2018. Data capture included age, gender, height, weight, underlying disease, ECOG (Eastern Cooperative Oncology Group) status, indication for FMT, previous treatments for CDI, antibiotic induction treatment, chemotherapy, immunosuppression, ribotype 027, bowel-movement pre/post-transplant, proton pump inhibitor use, antimotility agent use and bowel lavage in preparation for FMT, fresh or frozen FMT preparation, duration of storage, type of administration, response to treatment, primary cure, secondary cure, follow-up, treatment-related adverse event (AE) assessment, and associated day 30 (D30) mortality. AEs were documented only if considered at least potentially related to FMT. Data was entered into the database based on a chart review. Data from all participating sites were centrally exported, tabulated, and analyzed.
Patients were included into the analysis, if they were treated for rCDI and post-treatment observational data covered at least 10 days. Figure 1 illustrates identification of patients for the analysis.
Figure 1.
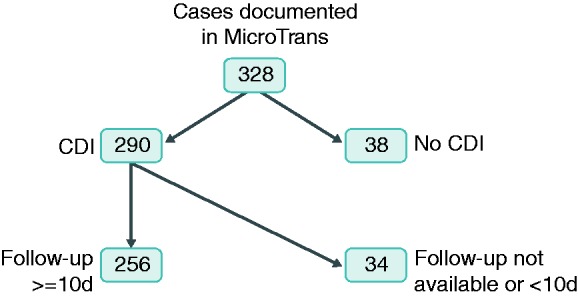
Selection of patients for analysis.
rCDI was defined as per current ESCMID guidelines.4,12 CDI was defined as severe when leukocytes were ≥15 × 103/μL and/or when creatinine was >1.5 mg/dL on the day of CDI diagnosis. Response to FMT treatment was defined as absence of diarrhea after FMT for at least 48 h. Primary cure D30 and day 90 (D90) were defined as resolution of CDI after FMT until the respective time points. For determination of secondary cure rates, response after multiple FMTs was assessed at D30 and D90. Recurrence was defined as the occurrence of CDI after an initial response to treatment. Treatment failure was defined as a lack of any response to treatment or recurrence before D30 or D90, respectively.
For statistical analysis, IBM SPSS Statistics software (version 21, IBM Corporation, Armonk, NY, USA) was used. We performed descriptive statistics as appropriate to evaluate the dataset. All risk factors for FMT failure identified during a literature search were first analyzed using univariate statistics (Student's t-test for continuous and Fisher's exact test for dichotomous variables). To assess independence of covariates, we performed binary logistic regression with FMT failure as the dependent variable and potential risk factors as covariates. Based on a review of the literature, the following variables were initially included into the model: age, gender, ribotype 027, ECOG status, immunosuppression, proton pump inhibitor use, antimotility agent use and bowel lavage in preparation for FMT, number of recurrences, severity of CDI, antibiotic induction treatment, fresh or frozen FMT preparation, and type of administration. We then eliminated covariates from the analysis by a stepwise-forward approach, using Wald's statistic with an exclusion margin of 0.1 to improve the model further. As a second step, a stepwise-backward model with an inclusion margin of 0.2 was used to validate the findings. Patients receiving combination treatment were not included into the analysis.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Since this is a retrospective registry, collecting an anonymized dataset, applicable German law does not require informed consent. Initial approval of the study was obtained from the ethics committee of the University Hospital Cologne (ID: 14–295, 29.12.2014). Subsequently, ethical approval was obtained from participating sites.
Results
Out of 327 cases in the database, 38 were excluded due to not meeting inclusion criteria and 34 based on incomplete data, yielding a total of 256 evaluable patients (Table 1). Median age was 75 years (interquartile range (IQR): 63.0–81.0), with the majority of patients being female (n = 157; 61.3%). Median ECOG performance was 3 (IQR: 1–4). The most frequent comorbidities reported were cardiovascular (n = 154; 60.2%), gastrointestinal (except CDI) (n = 90; 35.2%), endocrine (n = 81; 31.6%), and kidney diseases (n=75; 29.3%). A total of 18 patients received chemotherapy (7.0%) and 48 (18.8%) received other immunosuppressive medication. Some patients underwent allogenic stem cell transplantation (n = 4; 1.6%) or solid organ transplantation (n = 8; 3.1%).
Table 1.
Patient characteristics at first fecal microbiota transfer (FMT).
| Number of patients – no. | 256 |
| Age – years | |
| Median (IQR) | 75 (63–81) |
| Female – no. (%) | 157 (61.3) |
| BMI | |
| Median (range) | 22.6 (14.87–54.82) |
| ECOG performance score – value | |
| Median (IQR) | 3 (1–4) |
| Comorbidities – no. (%) | |
| Cardiovascular | 154 (60.2) |
| Gastrointestinal (except CDI) | 90 (35.2) |
| Endocrinological | 81 (31.6) |
| Nephrological | 75 (29.3) |
| Hematological/oncological | 68 (26.6) |
| Pulmonary | 52 (20.3) |
| Neurological | 37 (14.5) |
| Psychiatric | 34 (13.3) |
| Orthopedic | 23 (9.0) |
| Rheumatological | 15 (5.9) |
| Urological | 10 (3.9) |
| Othera | 28 (10.9) |
| None | 12 (4.7) |
| Transplantation – no. (%) | |
| Solid organ | 8 (3.1) |
| Allogenic stem cell | 4 (1.6) |
| Immunosuppression – no. (%) | |
| Chemotherapy | 18 (7.0) |
| Other immunosuppressive medication | 48 (18.8) |
| CDI recurrences before FMT – no. | |
| Median (IQR) | 3 (2–4) |
| Antibiotic pre-treatmentb – no. (%) | 237 (92.6) |
| Sequential Metro+Vanco | 91 (35.5) |
| Sequential Metro+Vanco+Fidaxo | 72 (28.1) |
| Vancomycin only | 31 (12.1) |
| Sequential Fidaxo+Vanco | 16 (6.3) |
| Sequential Metro+Vanco+Rifax | 11 (4.3) |
| Otherc | 16 (6.3) |
| Unknown | 19 (7.4) |
BMI = body mass index; CDI = Clostridioides difficile infection; ECOG = Eastern Cooperative Oncology Group (range 0–5); IQR = interquartile range; SD = standard deviation.
Other: dermatological n = 5 (2.0%), ophthalmological n = 5 (2.0%), immunosuppressive n = 6 (2.3%), infective n = 4 (1.6%), coagulative n = 3 (1.2%), gynecological n = 4 (1.6%), otorhinolaryngological n = 1 (0.4%).
Antibiotic pre-treatment: any kind of antibiotic ever given to the patient during previous CDI episodes
Other: metronidazole only n = 7, sequential metronidazole + vancomycin + fidaxomicin + rifaximin n = 3, fidaxomicin only n = 2, sequential rifaximin + vancomycin n = 1, sequential metronidazole + vancomycin + fidaxomicin + rifaximin + tigecycline n = 1, drug sequence unknown n = 2.
The median number of recurrences prior to FMT was 3 (IQR: 1–4). The two most common sequential antibiotic treatments used to treat CDI before FMT were metronidazole followed by vancomycin (n = 91; 35.5%), as well as the sequence of metronidazole, vancomycin, and fidaxomicin (n = 72; 28.1%).
Procedural characteristics are listed in Table 2. A total of 77.7% of the patients received an antibiotic induction as a preparation for FMT (n = 199); for this purpose, vancomycin was the most frequently used drug (n = 154; 60.2%), whereas in 46 patients (18.0%) FMT was performed without antibiotic induction. Approximately every fourth patient was treated with frozen preparations (n = 68; 26.6%) and 66.8% received a bowel lavage prior to FMT (n = 171). Of note, 3.9% of patients received a bowel lavage despite choosing an oral way of application.
Table 2.
Procedural characteristics of first FMT.
| Number of patients – no. | 256 |
| Antibiotic inductiona – no. (%) | 199 (77.7) |
| Metronidazole only | 3 (1.2) |
| Vancomycin only | 154 (60.2) |
| Fidaxomicin only | 15 (5.9) |
| Rifaximin only | 6 (2.3) |
| Combination | 21 (8.2) |
| None | 46 (18.0) |
| Unknown | 12 (4.7) |
| Antimotility agent – no. (%) | 28 (10.9) |
| Proton pump inhibitor – no. (%) | 10 (3.9) |
| Frozen preparations – no. (%) | 68 (26.6) |
| Bowel lavage – no. (%) | 171 (66.8) |
| Type of application – no. (%) | |
| Gastric | 7 (2.7) |
| direct endoscopic | 7 (2.7) |
| Duodenal/jejunal | 102 (39.8) |
| direct endoscopic | 78 (30.5) |
| tube | 24 (9.4) |
| Rectum/colon/terminal ileum | 107 (41.8) |
| direct endoscopic | 106 (41.4) |
| enema | 1 (0.4) |
| Combinationb | 4 (1.6) |
| Capsule | 45 (17.6) |
| Patients with adverse event (%) | 19 (7.4) |
| Type of adverse event – no. (%) | |
| Nausea | 6 (2.3) |
| Fever | 3 (1.2) |
| Belching | 3 (1.2) |
| Abdominal pain | 2 (0.8) |
| Emesis | 2 (0.8) |
| Food intolerancec | 2 (0.8) |
| Aspiration pneumonia | 2 (0.8) |
| Others (retrosternal pressure, hemorrhage, pharyngeal pain, irritable bowel syndrome, loss of a tooth, polyneuropathy, weight gain,d bloody diarrhea, hypertension, increased peristaltic activity) | Each 1 (0.4) |
| No adverse events | 216 (84.4) |
Antibiotic induction: antibiotic given before the FMT treatment as a preparation.
Direct endoscopic gastric/duodenal/jejunal + direct colonoscopic.
1 × spinach; 1 × food intolerance not specified.
10 kg over 12 months after FMT (body mass index change from 25.7 to 29.4).
The most frequently used route of administration was colonoscopic administration, excluding enemas (n = 106; 41.4), followed by duodenal/jejunal application (n = 102; 39.8%) and orally by using capsules (n = 45; 17.6%).
Overall, 19 patients suffered treatment-related AEs (7.4%), most of them being of transient gastrointestinal nature. Nausea (n = 6; 2.3%), fever (n = 3; 1.2%), and belching (n = 3; 1.2%) were the most frequent AEs following FMT. Two cases of aspiration pneumonia (0.8%) and one case of hemorrhage (0.4%) in association with endoscopy were reported.
Primary treatment success was achieved in 191 out of 240 cases (79.6%) at D30 post FMT. At D90, 153 of 196 evaluable patients remained without recurrence (78.1%). Response by route of administration is detailed in Table 3.
Table 3.
Primary response on D30 and D90 by route of application.
| Route of application (%) | D30 | D90 |
|---|---|---|
| All | 191/240 (79.6) | 153/196 (78.1) |
| Upper GIT | 79/104 (76.0) | 68/93 (73.1) |
| Lower GIT | 84/97 (86.6) | 63/73 (86.3) |
| Oral capsule | 33/44 (75.0) | 25/33 (75.8) |
| Combinationa | 4/4 (100.0) | 2/2 (100.0) |
Upper + lower GIT endoscopy, GIT = gastrointestinal tract.
A second FMT was administered in 40 patients (15.6%). In two cases, a third FMT was necessary. The secondary response at D30 was 89.6% and remained steady at D90 (89.8%).
Assessment of clinical predictors for FMT failure by forward-stepwise regression analysis yielded only higher age as an independent, statistically significant predictor of FMT failure (Table 4). This result did not change, if a backward-stepwise approach was used.
Table 4.
Stepwise-forward multivariate analysis on risk factors for treatment failure at D30.
| Dependent variable: treatment failure at day 30 | ||||
|---|---|---|---|---|
| Univariate |
Multivariate (only remaining variables) |
95% confidence interval | ||
| Variable | p-value | p-value | odds ratio | |
| Age | 0.001 | 0.001 | 1.060 | 1.025–1.097 |
| Gender | 0.546 | |||
| ECOG status | 0.144 | |||
| Immunosuppression | 0.093 | |||
| Ribotype 027 | 0.766 | |||
| Severe CDI | 0.374 | 0.083 | 0.311 | 0.083–1.166 |
| Number of recurrences | 0.054 | 0.080 | 1.477 | 0.955–2.284 |
| Antibiotic induction | 0.688 | |||
| Proton pump inhibitor | 0.114 | |||
| Antimotility agent | 0.925 | |||
| Bowel lavage | 0.191 | |||
| Frozen FMT preparation | 0.283 | |||
| Route of application | ||||
| Capsules | 0.093 | |||
| Upper GIT | 0.214 | |||
| Lower GIT | 0.254 | |||
CDI = Clostridioides difficile infection, ECOG = Eastern Cooperative Oncology Group, FMT = fecal microbiota transfer, GIT = gastrointestinal tract.
Discussion
The MicroTrans Registry was introduced to capture the current status within the German FMT landscape. The data presented here confirms that FMT, independent of its application strategy or baseline status of the patient, is a viable and safe option in the treatment of rCDI, with proof of efficacy beyond clinical trials in a larger cohort than previously reported by us.2 Longitudinal comparisons with our previous analysis,2 as well as real-life data from other groups, reveal trends indicating the transition of novel findings into clinical practice. Firstly, the number of immunocompromised patients treated with FMT is rising. Interestingly, the likelihood of experiencing a serious AE attributable to FMT does not seem to be significantly elevated in this group of vulnerable patients, underlining the safety of FMT.2,10,13,14 Secondly, our study is the first to compare encapsulated FMT preparations to the standard upper and lower gastrointestinal tract application routes in a European real-life setting. Based on monovariate analysis, clinical effectiveness of encapsulated preparations appears to be slightly inferior (Table 3), logistic regression analysis, however, reveals this finding to be confounded by the higher age of patients opting for a capsule-based approach, as age was the only independent predictor of FMT failure. This is fully in line with our clinical experience, as specifically older patients and their relatives are concerned that an endoscopic approach and the necessary preparations might represent an additional and avoidable risk.
On the other hand, age has not been previously identified as a risk factor for FMT failure.7–10,14 Our dataset, however, introduced many variables, e.g. route of application, specific co-medications (proton pump inhibitors, antibiotic induction treatment, bowel lavage and antimotility agents), frozen FMT preparations, and presence of ribotype 027, that in their entirety were not part of previous regression analyses. It is possible that the introduction of these variables may have further refined the regression and therefore have produced results that differ from previous analyses. Unfortunately, we were not able to assess the impact of early non-CDI directed antibiotics, as this variable was not available for all patients.
If we can assume that the route of application has no significant impact on treatment outcome, it seems noteworthy to point out that in rare cases, application of an FMT preparation via the upper gastrointestinal tract may indeed harbor serious risks. Two documented cases of aspiration pneumonia occurred during upper gastrointestinal tract endoscopy. Similar cases have been identified by other authors.13 Even though these events are rare and the current basis of evidence may not support any conclusions based on statistical significance, these cases might have been avoided by use of another application strategy.
The fact that specific preparation of the patient using an antibiotic induction regimen, proton pump inhibitors, or antimotility agents did not impact treatment success is also noteworthy, as this insight facilitates the practical planning and conduct of a FMT. These factors have not been analyzed in depth.
Our analysis is limited by a lack of randomization, its retrospective documentation scheme, and the low sample size. Even though we asked for the origin of feces in our registry (relative, partner, friend, anonymous, other, or unknown), this information has not been provided in a high number of cases.
Finally, the fact that our analysis demonstrates the lack of impact of a large number of clinical factors corroborates a major gap in knowledge on the mechanisms of FMT in rCDI as of today. There is rapidly growing evidence for the crucial role of specific microbiota-associated metabolites,15–17 as well as personalized selection of microbiota to be transferred.18
Recognizing the importance of additional information on microbiota and their associated metabolomics, we are aiming to integrate, expand and harmonize fecal sampling strategies of biobanks existing at different sites of the MicroTrans Registry network to facilitate improved analyses in the future. In conclusion, we were able to show that various FMT strategies are transitioning into clinical practice to help the continuously growing target population. We found that no specific preparation or application strategy had an impact on FMT outcome. Only higher age was identified as an independent risk factor for treatment failure, however, the associated odds ratio is low. Based on our findings and previously published data, factors determining FMT success are likely to be related to microbiota signatures and their associated metabolites.15–17
Declaration of Conflicting Interests
CL has served as a speaker and consultant to Astellas and MSD Sharp & Dohme. FT has served as a speaker and consultant to Astellas. MJGTV has served at the speakers’ bureau of Akademie für Infektionsmedizin, Ärztekammer Nordrhein, Astellas Pharma, Basilea, Gilead Sciences, Merck/MSD, Organobalance, and Pfizer, received research funding from 3M, Astellas Pharma, DaVolterra, Gilead Sciences, MaaT Pharma, Merck/MSD, Morphochem, Organobalance, Seres Therapeutics, Uniklinik Freiburg/Kongress und Kommunikation, and is a consultant to Alb-Fils Kliniken GmbH, Ardeypharm, Astellas Pharma, Berlin Chemie, DaVolterra, Ferring, MaaT Pharma, and Merck/MSD. MS has served at the speakers’ bureau of Astellas and MSD. All other authors have no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed Consent
Since this is a retrospective registry collecting an anonymized dataset, applicable German law does not require informed consent.
Ethics Approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Initial approval of the study was obtained from the ethics committee of the University Hospital Cologne (ID: 14–295, 29.12.2014). Subsequently, ethical approval was obtained from participating sites.
References
- 1.Van Nood E, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407–415. [DOI] [PubMed] [Google Scholar]
- 2.Hagel S, et al. Fecal microbiota transplant in patients with recurrent Clostridium difficile infection. Dtsch Arztebl Int 2016; 113: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quraishi MN, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017; 46: 479–493. [DOI] [PubMed] [Google Scholar]
- 4.Debast SB, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014; 20(Suppl 2): 1–26. [DOI] [PubMed] [Google Scholar]
- 5.Cammarota G, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017; 66: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullish BH, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J Hosp Infect 2018; 100(Suppl 1): S1–S31. [DOI] [PubMed] [Google Scholar]
- 7.Meighani A, et al. Predictors of fecal transplant failure. Eur J Gastroenterol Hepatol 2016; 28: 826–830. [DOI] [PubMed] [Google Scholar]
- 8.Fischer M, et al. Predictors of early failure after fecal microbiota transplantation for the therapy of Clostridium difficile infection: a multicenter study. Am J Gastroenterol 2016; 111: 1024–1031. [DOI] [PubMed] [Google Scholar]
- 9.Allegretti JR, et al. Early antibiotic use after fecal microbiota transplantation increases risk of treatment failure. Clin Infect Dis 2018; 66: 134–135. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YW, et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: a multicenter experience. Am J Transplant 2019; 19: 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ianiro G, et al. Predictors of failure after single faecal microbiota transplantation in patients with recurrent Clostridium difficile infection: results from a 3-year cohort study: authors' reply. Clin Microbiol Infect 2017; 23: 891. [DOI] [PubMed] [Google Scholar]
- 12.Crobach MJ, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 2016; 22(Suppl 4): S63–S81. [DOI] [PubMed] [Google Scholar]
- 13.Kelly CR, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 2014; 109: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahtinen P, et al. Faecal microbiota transplantation in patients with Clostridium difficile and significant comorbidities as well as in patients with new indications: a case series. World J Gastroenterol 2017; 23: 7174–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald JAK, et al. Inhibiting growth of Clostridioides difficile by restoring valerate, produced by the intestinal microbiota. Gastroenterology 2018; 155: 1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seekatz AM, et al. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe 2018; 53: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott SJ, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 2017; 152: 799–811. [DOI] [PubMed] [Google Scholar]
- 18.Smillie CS, et al. Strain tracking reveals the determinants of bacterial engraftment in the human gut following fecal microbiota transplantation. Cell Host Microbe 2018; 23: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]


