Abstract
The human papilloma virus (HPV) is a DNA virus associated with benign and malignant lesions of skin and mucous membranes and is the most common sexually transmitted viral infection worldwide. We investigated the prevalence of HPV infection and associated risk factors in Italian and Turkish women population attending the gynecology outpatients clinic in Naples (Italy) and Pamukkale (Turkey). Women were enrolled from the Department of Obstetrics and Gynecology of the University of Campania “Luigi Vanvitelli” in Naples (Italy) and of “Pamukkale University” in Denizli (Turkey) between January 2014 and June 2015. A questionnaire that included sociodemographic and sexual behavior characteristics, questions about HPV awareness, vaccine status, and reasons for not wanting to get vaccinated, and HPV-related knowledge was completed for each participant, and cervical cytology samples were collected. The prevalence of HPV infection was higher in the Italian group (52.6% vs 32.6%, p < 0.001), while the distribution of genotypes is similar (p=0.325). Moreover, the differences in cytological alterations in these patients are significant (p < 0.001). The analysis showed a higher prevalence of sexual behavioral characteristics (p < 0.001) and better attention to the execution of the screening test in the Italian population (p < 0.001). Italian women showed more knowledge and propensity to vaccination compared to Turkish women (p < 0.001). Our data highlighted three relevant aspects: the different prevalence of cytological abnormalities, the different distribution of risk factors and, above all, the different attitude of women towards the primary prevention of cervical cancer between an Italian and a Turkish population group.
1. Introduction
Human papilloma virus (HPV) is a double-stranded DNA virus belonging to the Papillomaviridae family and is the most common sexually transmitted viral infection worldwide, and it is associated with the occurrence of condylomas and a variety of cancers in both women and men [1, 2]. The HPV-DNA contains sequences that encode E6 and E7, two proteins with oncogenic capacity, that can disrupt the function of two tumor suppressor genes, p53 and pRb, respectively, causing different cellular pathways alterations and uncontrolled cell growth [3]. There are more than 100 HPV types, and the outcome of HPV infection depends on the specific HPV type/s present and can be from asymptomatic infection until high squamous cell malignancies [4]. Low-risk HPV types, such as types 6 and 11, are associated with anogenital warts and mild dysplasia, while high-risk types, such as 16 and 18, are associated with high-grade dysplasia and cancers of the cervix, vulva, vagina, urethra, penis, anus, and oropharynx. Moreover, cervical cancer is the fourth most common type of cancer for women worldwide and the second most common female cancer in women between 15 and 44 years in Europe [5]. Literature evidences that the protection offered by HPV vaccines is enduring and extended vaccination could deliver substantial health economic benefits [6, 7]. Otherwise, monitoring the impact of HPV vaccine effectiveness, in Europe, is challenging because of the variety of factors that need consideration, like the different policies (targeting both sexes and time of vaccination), health system outcomes, and biologic outcomes as infection, risk factors, and sexual behavioral characteristics [8–10]. Vaccination in Italy is recommended and offered to adolescents of both sexes, preferably around 12 years of age: from 9 to 14 years, a bivalent or quadrivalent vaccine was administered in two doses at 0 and 6 months. In later ages, vaccines were administered in three doses at 0, 1, and 6 (bivalent) or 0, 2, and 6 months (quadrivalent vaccine) [11, 12]. The bivalent Cervarix® HPV vaccine (GSK Aspen) targets HPV types 16 and 18, while quadrivalent Gardasil® HPV vaccine (MSD) targets HPV types 6, 11, 16, and 18. A new vaccine will expand coverage against five more oncogenic types (HPV 31, 33, 45, 52, and 58) in addition to the four original types included in quadrivalent vaccine. In Campania, in the south of Italy, the pap test still largely appears as the only screening resource for cervical cancer while the vaccination coverage showed the difficulty of reaching high levels [13, 14]. In Turkey, instead, the National Standards of Cervical Cancer Screening recommends having a smear at least once between the ages of 35 and 40 years with repeat screening every five years, until the age of 65, with two consecutive negative tests. Patients with a cytological or positive anomaly to HPV types 16/18 undergo colposcopy. However, within the same Turkish territory, this screening programme has a highly inhomogeneous distribution because of different and conflicting socioeconomic realities between the territories of western and eastern Turkey. Otherwise, vaccination is only available through private demand [15, 16]. In order to optimize the vaccination programs, it is important to support the beneficial effects of the vaccine and spread awareness about high-risk sexual behavior through the media [17, 18]. Our study aims to compare the Turkish and the Italian population in order to assess the prevalence of HPV infection and the distribution of cytological lesions and the prevalence of sexual behavioral characteristics and environmental risks associated with HPV infection.
2. Materials and Methods
We performed a retrospective cohort study of outpatient women who came for gynecological examination, between January 2014 and June 2015, to two referral centers: the Department of Obstetrics and Gynecology of the University of Campania “Luigi Vanvitelli” in Naples (Italy) and of “Pamukkale University” in Denizli (Turkey). The Local Institutional Review Board approved the study. All participants before enrollment signed a full written consent form. The study was conducted in accordance with the principles of Helsinki Declaration. Inclusion criteria were being 25 years of age or older and active sexual history. Patients with autoimmune diseases or any diseases involving the immune system, with HIV infection, previously vaccinated against HPV, with a presumed or confirmed pregnancy, with a diagnosis of any malignancies or with a history of chemotherapy or radiotherapy, and with a history of total uterine or cervical resection, were excluded from the study. The study was performed according to the STROBE (strengthening the reporting of the observational studies in epidemiology) guidelines [19]. Cervical cytology samples were collected, strictly during the nonmenstrual period, by three gynecologists using the Abbott cervi-collect specimen collection kit (Abbott) and transported in ThinPrep® PreservCyt® Solution (HOLOGIC™). These specimens were stored at 15 to 20°C and transported to the laboratory within 24 hours of collection. 15 ml of cervical samples was centrifuged at 2000 rpm for 15 minutes at 4°C for cervical cells concentration. Cell pellet was suspended in 2.5 ml of PBS, and 5 aliquots of 500 μl for each sample were obtained. One aliquot was used for DNA extraction. DNA extraction from a 500 μl cell pellet aliquot of the cervical sample was carried out using the linear array test (Roche Molecular Diagnostics, Milan, Italy), a qualitative in vitro test for the detection of HPV in clinical specimens. The test utilizes amplification of target DNA by polymerase chain reaction and nucleic acid hybridization and detects 37 anogenital HPV-DNA genotypes (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73 (MM9), 81, 82 (MM4), 83 (MM7), 84 (MM8), IS39, and CP6108) in cervical cells collected in PreservCyt solution. Three experienced cytology experts performed cervical liquid-based cytology tests and formulated cytological reports according to the Bethesda System [20]. PreservCyt specimens were stored at 15 to 20°C for as long as five weeks, in case the sample had to be retested. Women positive for high-risk HPV were recalled and underwent a colposcopic examination and, if there were an evident lesion, also a targeted biopsy. A targeted biopsy was performed to all those HPV-positive patients with cytological lesions. The cytohistological and viral information obtained was inserted in a specific database. All charts recorded in the database were reviewed carefully by two authors. Data were anonymized before analysis. All patients completed a questionnaire that included sociodemographic and lifestyle information, questions about HPV awareness, vaccine status, and reasons for not wanting to get vaccinated and HPV-related knowledge in order to determine personal risks factors that could increase the susceptibility to HPV. Data were shown as means ± standard deviation (SD) for continuous variables or as number (percentage). Comparisons between the two groups were assessed with Student's t-test. Comparison groups were assessed with Pearson's chi-square test and Fisher's exact test for categorical variables and Student's t-test for continuous variables. A p-value < 0.05 was considered statistically significant. Statistical analysis was made using SPSS for Windows (version 15.0, SPSS, Chicago, IL).
3. Results
Seven hundred three women were recruited for the study and 570 participants (aged between 25 and 65 years, median 35.6 ± 4.5 years) were enrolled. One hundred thirty-three patients were excluded according to the exclusion criteria as shown in Figure 1.
Figure 1.
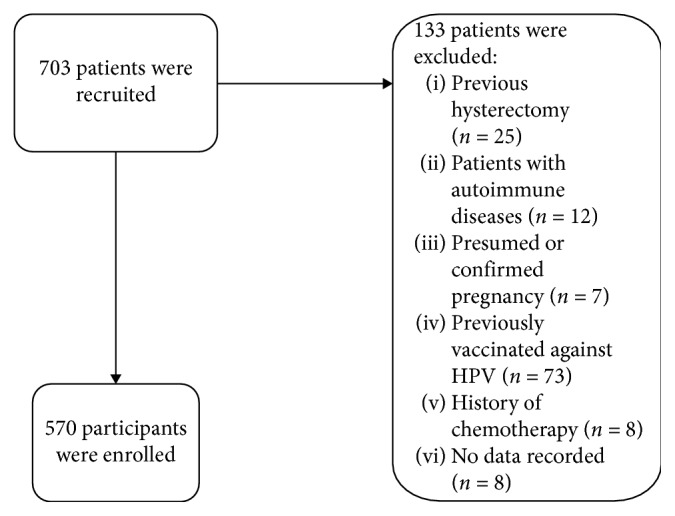
Strengthening the reporting of observational studies in epidemiology (STROBE) statement.
Patients were divided into two groups according to the country of the enrollment: 300 patients in the Italian group (group A) and 270 patients in the Turkish group (group B). The comparison between the two groups showed that the prevalence of HPV infection was higher in the Italian group than in the Turkey one: 52.6% (n=158) vs 32.6% (n=88), p < 0.05. The distribution of genotypes is similar in the groups of positive HPV patients (p=0.325) as shown in Table 1.
Table 1.
Distribution of genotyping in HPV-positive patients.
| Group A (Italian) (n=158) | Group B (Turkish) (n=88) | p value | |
|---|---|---|---|
| Low | 41 (26.0%) | 23 (26.2%) | 1.000 |
| Intermediate | 12 (7.5%) | 5 (5.6%) | 0.793 |
| High | 105 (66.5%) | 60 (68.2%) | 0.887 |
Table 2 showed a higher incidence of cytological alterations in group A: the percentage of high-grade lesions (H-SIL) and low-grade lesions (L-SIL), in fact, is higher in group A (20.3% vs 8.0%, p < 0.05, and 23.4% vs 6.8%, p < 0.05, respectively). Otherwise, group B showed a greater frequency of atypical squamous cells of undeterminated significance (ASCUS) (43.2% vs 25.3%, p < 0.05).
Table 2.
Incidence of cytological alterations in HPV-positive patients.
| Group A (Italian) (n=158) | Group B (Turkish) (n=88) | p value | |
|---|---|---|---|
| Negative | 49 (31%) | 37 (42%) | 0.094 |
| ASCUS | 40 (25.3%) | 38 (43.2%) | 0.004 |
| L-SIL | 37 (23.4%) | 6 (6.8%) | 0.0008 |
| H-SIL | 32 (20.3%) | 7 (8.0%) | 0.0110 |
ASCUS: atypical squamous cells of undeterminated significance; L-SIL: low-grade squamous intraepithelial lesions; H-SIL: high-grade squamous intraepithelial lesions.
Women positive for high-risk HPV were recalled and underwent a colposcopic examination and, if there were an evident lesion, also a targeted biopsy. The results of the biopsies showed a higher incidence of CIN I and CIN II-III in the Italian group and also if not significant (p=1.000 and p=0.688, respectively) as shown in Table 3.
Table 3.
Outcome of biopsies.
| Group A (Italian) (n=83) | Group B (Turkish) (n=13) | p value | |
|---|---|---|---|
| Negative | 56 (67.5%) | 11 (84.6%) | 0.332 |
| CIN-1 | 12 (14.5%) | 1 (7.7%) | 1.000 |
| CIN-2/3 | 15 (18%) | 1 (7.7%) | 0.688 |
CIN: cervical intraepithelial neoplasia.
The questionnaire revealed a greater prevalence of risk factors associated with HPV infection in the Italian group, in particular for low-age first sexual intercourse and the high number of partners in the last three months (p < 0.05). Other risk factors like smoking, use of oral contraceptives, and sexually transmitted infections were more prevalent in the Italian group but not statistically significant. Moreover, the Italian group showed greater attention to the execution of the pap test as a screening test (p < 0.05), probably related to a higher degree of education (p < 0.05). The distribution of risk factors is shown in Table 4.
Table 4.
Distribution of risk factors.
| Group A (Italian) (n=158) | Group B (Turkish) (n=88) | p value | |
|---|---|---|---|
| Age (mean ± SD) | 32.5 ± 11.4 | 30.6 ± 9.6 | 0.187 |
|
| |||
| Education | |||
| Low levels of education | 23 (14.6%) | 28 (31.8%) | <0.001 |
| Upper secondary education | 108 (68.4%) | 47 (53.4%) | |
| Graduate education | 27 (17.0%) | 13 (14.8%) | |
|
| |||
| Marital status | |||
| Married or married-like situation | 118 (74.7%) | 48 (54.5%) | <0.001 |
| Never married | 35 (22.1%) | 38 (43.2%) | |
| Divorced | 5 (3.2%) | 2 (2.3%) | |
| Widow | 0 | 0 | |
|
| |||
| Smoke | |||
| Ever smoked | 82 (52.0%) | 35 (40.0%) | 0.067 |
| Current or ex-smokers | 76 (48.0%) | 53 (60.0%) | |
|
| |||
| Number of pregnancies | |||
| None | 85 (53.8%) | 40 (45.4%) | 0.315 |
| From 1 to 3 | 66 (41.8%) | 41 (46.6%) | |
| More than 3 | 7 (4.4%) | 7 (8.0%) | |
|
| |||
| Combined oral contraceptive | |||
| User | 80 (50.6%) | 35 (39.8%) | 0.101 |
| Not user | 78 (49.4%) | 53 (60.2%) | |
|
| |||
| Age first sexual intercourse (mean ± SD) | 19.8 ± 3.4 | 26.4 ± 5.3 | <0.001 |
|
| |||
| Number of partners (in the last three months) | |||
| Only 1 | 68 (43.3%) | 68 (77.0%) | <0.001 |
| From 2 to 3 | 60 (37.8%) | 13 (15.2%) | |
| From 4 to 9 | 30 (18.9%) | 7 (7.8%) | |
|
| |||
| Condom | |||
| Never used during their sex life | 45 (28.5%) | 28 (31.9%) | 0.582 |
| Usually used during their sex life | 113 (71.5%) | 60 (68.1%) | |
|
| |||
| History of sexually transmitted infection | |||
| Yes | 27 (17.1%) | 10 (11.4%) | 0.228 |
| No | 131 (82.9%) | 78 (88.6%) | |
|
| |||
| Pap test done in the last 3 years | |||
| Yes | 92 (58.2%) | 29 (33.0%) | <0.001 |
| No | 66 (41.8%) | 59 (67.0%) | |
The two groups showed an overlapping level of knowledge about the screening for cervix cancer, but, surprisingly, group A showed more knowledge about the vaccine and a higher propensity to use it compared to the Turkish group (p < 0.05). Moreover, the Turkish group showed a lower willingness to have their children vaccinated against HPV (p < 0.05). Data are shown in Table 5.
Table 5.
Knowledge and approach of patients in comparison of HPV and vaccine.
| Group A (Italian) (n=158) | Group B (Turkish) (n=88) | p value | |
|---|---|---|---|
| Knowledge of the motivation of the screening with the pap test | |||
| Yes | 111 (70.2%) | 53 (60.2%) | 0.109 |
| No | 47 (29.8%) | 35 (39.8%) | |
|
| |||
| HPV knowledge | |||
| Yes | 90 (57.0%) | 28 (31.9%) | <0.001 |
| No | 68 (43.0%) | 60 (68.1%) | |
|
| |||
| Knowledge of the anti-HPV vaccine | |||
| Yes | 71 (44.9%) | 18 (20.4%) | <0.001 |
| No | 87 (55.1%) | 70 (79.6%) | |
|
| |||
| Knowledge about how the HPV vaccine works | |||
| Yes | 41 (26.0%) | 18 (20.4%) | 0.333 |
| No | 117 (74.0%) | 70 (79.6%) | |
|
| |||
| Positive propensity for personal use of the vaccine | |||
| Yes | 111 (70.2%) | 37 (42.0%) | <0.001 |
| No | 47 (29.8%) | 51 (58.0%) | |
|
| |||
| Positive propensity for the vaccine for children | |||
| Yes | 111 (70.2%) | 37 (42.0%) | <0.001 |
| No | 47 (29.8%) | 51 (58.0%) | |
4. Discussion
HPV is the most common viral infection of the reproductive tract, and most people are infected with HPV shortly after the onset of sexual activity [21, 22]. Although most precancerous lesions resolve spontaneously, there is a risk for all women that HPV infection may become chronic and precancerous lesions progressing to invasive cervical cancer in 15 to 20 years [3, 23]. In the era of the vaccine approach as a preventive strategy against cancer associated with HPV infection, epidemiological studies about the distribution of high-risk HPV in different geographical regions are a key tool for several objectives. We need to identify specific risk factors that can be removed with targeted prevention campaigns, to adopt vaccination strategies tailored to every single national reality, to develop vaccines that include prevalent high-risk HPV in different populations, and to identify high-risk genotypes poorly represented in the general population, such as immigrants [24]. Otherwise, our study has some limitations since its results cannot be generalized as it was conducted in two hospitals from two different countries. Moreover, the results of the study are limited to the period when data were collected. However, data showed a similar distribution between the Italian and Turkish groups about the different HPV genotypes but a higher incidence of high-grade cytological lesions in the Italian group. This result could be related to some sexual risk factors that were more frequent in the Italian population, and this point needs to be further studied. As regards the data emerging from the questionnaires, one of the greatest risk factors is represented by smoking due to its potential immunosuppressive effect which can increase the persistence of HPV infection. Literature indicates that the increased risk of cervical cancer is about 2-fold higher among woman smokers compared to nonsmokers, even after appropriate adjustments for sexual habits [25, 26]. However, use of oral contraceptives could promote cervical ectropion that facilitates the exposure of the squamocolumnar junction to potential carcinogens and increased risk of vulvovaginitis and predisposition to dysplasia [27–29]. Alternatively, the use of estrogen and progesterone could maintain infection by increasing cell proliferation and papilloma virus transcription [30, 31]. An innovative aspect of our work is related to recent literature evidence suggesting a role for eating habits and the nutritional status of patients in the progression of cervical carcinoma. In particular, it has been suggested a protective role of folate and vitamins C and E against cervical cancer: they may increase the immune response of the cervix mucosa to HPV infection or act as blockers of free radicals and oxidants that can cause damage to DNA, proteins, and lipids and inhibit the formation of DNA adducts, produced by tobacco smoke [32–34]. In this regard, it was interesting to observe the different eating habits in the two groups. In the typical diet of the Turkish population, there is a high prevalence of daily consumption of raw vegetables and fruit, rich in vitamins and antioxidants: these factors have been called into question with a protective role against the risk of cervical cancer [35, 36]. Moreover, our data showed a better knowledge about HPV of Italian patients compared to Turkish women and higher willingness to have their children vaccinated against HPV. These data are in agreement with two previous studies that revealed that Turkish women have limited awareness and knowledge about the HPV vaccine and a greater need for knowledge [37, 38]. Furthermore, they are likely to reflect the different territorial realities. In Italy, instead, information campaigns for HPV are widespread and the vaccine has been institutionalized since 2007/2008 for all girls from 12 years of age; in 2014, a national coverage of 71% was achieved, with profound differences between the different regional realities ranging between 27% and 86%. In particular, in Campania, the vaccination program reached coverage of 56.1% for a completing vaccination (3 doses) for the 1997 birth cohort on 31/12/2017 [14, 39]. The Italian National Plan of Vaccination Prevention 2017–2019 established the achievement, in girls in the twelfth year of life, of vaccination coverage for a complete cycle of anti-HPV ≥95%; in 2017, the introduction of anti-HPV vaccination for 11-year-old males with the initiation of an active call for the 2006 cohort; and in 2018, the completion of the anti-HPV vaccination in favor of 11-year-old males for the cohort of those born in 2007, with the completion of the recovery of the cohort of those born in 2006 if not achieved in 2017. It should be noted that, in Italy also, the adhesion to screening programs for cervical cancer is very low, with a national average of about 40% [40–42]. Otherwise, in Turkey, there is no similar national vaccine prevention plan for HPV and there is also a lack of a campaign to spread awareness about HPV. Moreover, vaccination is only available through private demand. On the other hand, the level of risk for HPV infection and the prevalence of the infection are much lower than that of Italian women [43, 44]. In order to assess an effective cervical cancer prevention program, it is necessary to implement both strategies: to achieve greater adhesion of the pediatric and adolescent population to the vaccination program, and therefore work to improve communication aspects to families through complete, clear, and transparent information. From this point of view, it would be advisable to make communication more effective by involving other professionals in support of public health facilities such as pediatricians, consultants, general practitioners, gynecologists, and referents of screening programs. Moreover, it should be highlighted the importance to increase adherence to cervical screening programs through the pap smear, which remains an indispensable tool for the cohort of women who got vaccinated.
5. Conclusions
Our data underline three relevant epidemiological aspects that could, if confirmed by larger case studies, address the prevention strategies of the cervical cancer and personalize them on the needs of specific realities: the different prevalence of cytological abnormalities, the different distribution of risk factors among the populations under examination and, above all, the different attitude of women towards the primary prevention of cervical cancer. While in Turkey, it is a priority to create a national database of screening and prevention of HPV that is currently lacking and to launch an information campaign and a vaccination plan. In Italy, instead, the level of knowledge and perception regarding the cervical disease, HPV vaccination, and screening for cervical cancer is still not satisfied, and other studies are necessary to give insight regarding knowledge, perception, and acceptance about the HPV vaccine.
Acknowledgments
We would like to say thanks to the University of Campania “Luigi Vanvitelli” and “Pamukkale University” for giving the opportunity for doing this thesis and for their financial support. Last but not the least, we would like to thank our study participants.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
Ethical clearance was obtained from the Department of Obstetrics and Gynecology of the University of Campania “Luigi Vanvitelli” in Naples (Italy) and of “Pamukkale University” in Denizli (Turkey).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
All authors contributed to the design of this study. All authors reviewed and revised the draft further and approved the final version for submission.
References
- 1.Wang X., Zeng Y., Huang X., Zhang Y. Prevalence and genotype distribution of human papillomavirus in invasive cervical cancer, cervical intraepithelial neoplasia, and asymptomatic women in Southeast China. BioMed Research International. 2018;2018:10. doi: 10.1155/2018/2897937.2897937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Handisurya A., Schellenbacher C., Kirnbauer R. Erkrankungen durch humane Papillomviren (HPV) Journal der Deutschen Dermatologischen Gesellschaft. 2009;7(5):453–467. doi: 10.1111/j.1610-0387.2008.06988_supp.x. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nature Reviews Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 4.Duensing S., Münger K. Mechanisms of genomic instability in human cancer: insights from studies with human papillomavirus oncoproteins. International Journal of Cancer. 2004;109(2):157–162. doi: 10.1002/ijc.11691. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Human Papillomavirus (HPV) and Cervical Cancer. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 6.Kent A. HPV vaccination and testing. Reviews in Obstetrics & Gynecology. 2010;3(1):33–34. [PMC free article] [PubMed] [Google Scholar]
- 7.Prue G., Baker P., Graham D., Nutting C., Greenhouse P., Lawler M. It is time for universal HPV vaccination. The Lancet. 2018;392(10151):913–914. doi: 10.1016/s0140-6736(18)31821-x. [DOI] [PubMed] [Google Scholar]
- 8.Sheikh S., Biundo E., Courcier S., et al. A report on the status of vaccination in Europe. Vaccine. 2018;36(33):4979–4992. doi: 10.1016/j.vaccine.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Brotherton J. M. L., Giuliano A. R., Markowitz L. E., Dunne E. F., Ogilvie G. S. Monitoring the impact of HPV vaccine in males-Considerations and challenges. Papillomavirus Research. 2016;2:106–111. doi: 10.1016/j.pvr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu P., Bhattacharya C., Biswas J., Singh P., Banerjee D. Efficacy and safety of human papillomavirus vaccine for primary prevention of cervical cancer: a review of evidence from phase III trials and national programs. South Asian Journal of Cancer. 2013;2(4):187–192. doi: 10.4103/2278-330x.119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Intesa tra il Governo, le Regioni e le Province autonome di Trento e Bolzano sul documento recante “Piano Nazionale Prevenzione Vaccinale (PNPV) 2012–2014”. Intesa ai sensi dell’articolo 8, comma 6, della legge 5 giugno 2003, n. 131.
- 12.Petrelli A., Giorgi Rossi P., Francovich L., et al. Geographical and socioeconomic differences in uptake of Pap test and mammography in Italy: results from the National Health Interview Survey. BMJ Open. 2018;8(9) doi: 10.1136/bmjopen-2018-021653.e021653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giambi C. Stato di Avanzamento della Campagna Vaccinale per l’HPV: Dati di Copertura Vaccinale al 31/12/2011. Rome, Italy: Istituto Superiore di Sanitá; 2011. https://www.epicentro.iss.it/hpv/pdf/Aggiornamento_datiHPV_31_12_2011_validato.pdf. [Google Scholar]
- 14.Adamo B., Simonetti A. Andamento della campagna vaccinale contro l’Hpv nella Asl di Napoli. Naples, Italy: Azienda Sanitaria Locale: Napoli 1 Centro Servizio Epidemiologia e Prevenzione; 2011. https://www.epicentro.iss.it/territorio/campania/pdf/HPV_Napoli1_301012.pdf. [Google Scholar]
- 15.Sahin H. G., Kolusari A., Guducuoglu H. Prevalence of high risk human papillomavirus (HPV) infection and abnormal cervical cytology and knowledge about HPV vaccine in Eastern Turkey. European Journal of Gynaecological Oncology. 2017;38(2):241–244. [PubMed] [Google Scholar]
- 16.Saglik T. C., Bakanligi Türkiye Halk Sagligi Kurumu Kanserle Savas Dairesi Baskanligi . Serviks Kanseri Taramasi Ulusal Standartlari (National Standards of Cervical Cancer Screening) Ankara, Turkey: Bakanligi Türkiye Halk Sagligi Kurumu Kanserle Savas Dairesi Baskanligi; 2007. in Turkish, http://kanser.gov.tr/Dosya/tarama/serviks.pdf. [Google Scholar]
- 17.Gupta S., Palmer C., Bik E. M., et al. Self-sampling for human papillomavirus testing: increased cervical cancer screening participation and incorporation in international screening programs. Front Public Health. 2018;6:p. 77. doi: 10.3389/fpubh.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vorsters A., Arbyn M., Baay M., et al. Overcoming barriers in HPV vaccination and screening programs. Papillomavirus Research. 2017;4:45–53. doi: 10.1016/j.pvr.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Elm E., Altman D. G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet. 2007;370(9596):1453–1457. doi: 10.1016/s0140-6736(07)61602-x. [DOI] [PubMed] [Google Scholar]
- 20.Smith J. H. Bethesda 2001. Cytopathology. 2002;13(1):4–10. doi: 10.1046/j.1365-2303.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- 21.Brianti P., De Flammineis E., Mercuri S. R. Review of HPV-related diseases and cancers. New Microbiologica. 2017;40(2):80–85. [PubMed] [Google Scholar]
- 22.Harper D. M., DeMars L. R. HPV vaccines—a review of the first decade. Gynecologic Oncology. 2017;146(1):196–204. doi: 10.1016/j.ygyno.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Campitiello M. R., De Franciscis P., Mele D., et al. Endometrial LGR7 expression during menstrual cycle. Fertility and Sterility. 2011;95(8):2511–2514. doi: 10.1016/j.fertnstert.2011.01.124. [DOI] [PubMed] [Google Scholar]
- 24.Laganà A. S., Gavagni V., Musubao J. V., Pizzo A. The prevalence of sexually transmitted infections among migrant female patients in Italy. International Journal of Gynecology & Obstetrics. 2015;128(2):165–168. doi: 10.1016/j.ijgo.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Winkelstein W., Jr. Smoking and cancer of the uterine cervix: hypothesis. American Journal of Epidemiology. 1977;106(4):257–259. doi: 10.1093/oxfordjournals.aje.a112460. [DOI] [PubMed] [Google Scholar]
- 26.Siciliano R. A., Mazzeo M. F., Spada V., et al. Rapid peptidomic profiling of peritoneal fluid by MALDI-TOF mass spectrometry for the identification of biomarkers of endometriosis. Gynecological Endocrinology. 2014;30(12):872–876. doi: 10.3109/09513590.2014.943718. [DOI] [PubMed] [Google Scholar]
- 27.Smith J. S., Bosetti C., Muñoz N., et al. Chlamydia trachomatisand invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. International Journal of Cancer. 2004;111(3):431–439. doi: 10.1002/ijc.20257. [DOI] [PubMed] [Google Scholar]
- 28.Torella M., De Franciscis P., Russo C., et al. Stress urinary incontinence: usefulness of perineal ultrasound. La Radiologia Medica. 2014;119(3):189–194. doi: 10.1007/s11547-013-0317-4. [DOI] [PubMed] [Google Scholar]
- 29.Watts D. H., Fazarri M., Minkoff H., et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-Infected and high-risk HIV-1-Uninfected women. The Journal of Infectious Diseases. 2005;191(7):1129–1139. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- 30.De Franciscis P., Cobellis L., Fornaro F., Sepe E., Torella M., Colacurci N. Low-dose hormone therapy in the perimenopause. International Journal of Gynecology & Obstetrics. 2007;98(2):138–142. doi: 10.1016/j.ijgo.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Schettino M. T., Ammaturo F. P., Labriola D., De Franciscis P., Colacurci N., Torella M. Betulinic acid and possible influence on the clearance of Human Papilloma Virus: cytological and virological followup. Minerva Ginecologica. 2013;65(6):661–668. [PubMed] [Google Scholar]
- 32.Zhao W., Hao M., Wang Y., et al. Association between folate status and cervical intraepithelial neoplasia. European Journal of Clinical Nutrition. 2016;70(7):837–842. doi: 10.1038/ejcn.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schettino M. T., Ammaturo F. P., Grimaldi E., et al. Persistent papillomavirus type-31 and type-45 infections predict the progression to squamous intraepithelial lesion. Taiwanese Journal of Obstetrics and Gynecology. 2014;53(4):494–497. doi: 10.1016/j.tjog.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Simonelli A., Guadagni R., De Franciscis P., et al. Environmental and occupational exposure to bisphenol A and endometriosis: urinary and peritoneal fluid concentration levels. International Archives of Occupational and Environmental Health. 2017;90(1):49–61. doi: 10.1007/s00420-016-1171-1. [DOI] [PubMed] [Google Scholar]
- 35.Guo L., Zhu H., Lin C., et al. Associations between antioxidant vitamins and the risk of invasive cervical cancer in Chinese women: a case-control study. Scientific Reports. 2015;5(1):p. 13607. doi: 10.1038/srep13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schettino M. T., Mainini G., Ammaturo F. P., et al. The role of human papillomavirus in cervical pre-neoplastic lesions: the relationship between virus genotype and persistence or clearance of the infection. European Journal of Gynaecological Oncology. 2018;39(4):564–568. [Google Scholar]
- 37.Ozyer S., Uzunlar O., Ozler S., et al. Awareness of Turkish female adolescents and young women about HPV and their attitudes towards HPV vaccination. Asian Pacific Journal of Cancer Prevention. 2013;14(8):4877–4881. doi: 10.7314/apjcp.2013.14.8.4877. [DOI] [PubMed] [Google Scholar]
- 38.Baykal C., Al A., Uğur M. G., Cetinkaya N., Attar R., Arioglu P. Knowledge and interest of Turkish women about cervical cancer and HPV vaccine. European Journal of Gynaecological Oncology. 2008;29(1):76–79. [PubMed] [Google Scholar]
- 39.Ministero della Salute della Repubblica Italiana (CNESPS, ISS) EpiCentro Dati, Coperture Vaccinali al 31/12/2017 per HPV, epicentro.iss.it. Rome, Italy: Ministero della Salute della Repubblica Italiana; http://www.salute.gov.it/imgs/C_17_tavole_27_allegati_iitemAllegati_0_fileAllegati_itemFile_1_file.pdf. [Google Scholar]
- 40.Napolitano F., Navaro M., Vezzosi L., Santagati G., Angelillo I. F. Primary care pediatricians’ attitudes and practice towards HPV vaccination: a nationwide survey in Italy. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194920.e0194920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baussano I., Lazzarato F., Ronco G., Franceschi S. Impacts of human papillomavirus vaccination for different populations: a modeling study. International Journal of Cancer. 2018;143(5):1086–1092. doi: 10.1002/ijc.31409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Igidbashian S., Schettino M. T., Boveri S., et al. Tissue genotyping of 37 in situ and invasive cervical cancer with a concomitant negative HC2 HPV DNA test. Journal of Lower Genital Tract Disease. 2014;18(1):87–91. doi: 10.1097/lgt.0b013e3182909f86. [DOI] [PubMed] [Google Scholar]
- 43.Dursun P., Senger S. S., Arslan H., Kuşçu E., Ayhan A. Human papillomavirus (HPV) prevalence and types among Turkish women at a gynecology outpatient unit. BMC Infectious Diseases. 2009;9(1):p. 191. doi: 10.1186/1471-2334-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dursun P., Ayhan A., Mutlu L., et al. HPV types in Turkey: multicenter hospital based evaluation of 6388 patients in Turkish gynecologic oncology group centers. Turkish Journal of Pathology. 2013;29(3):210–216. doi: 10.5146/tjpath.2013.01188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


