Chronic myelomonocytic leukemia (CMML), a myeloid neoplasm with overlapping features of myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPN), is characterized by peripheral blood (PB) monocytosis [absolute monocyte count (AMC) ≥1x109/L and ≥10% of white blood cell count/WBC) differential], bone marrow (BM) dysplasia, and an inherent risk for leukemic transformation (LT) (15-30% over 3-5 years).1 Gene mutations commonly encountered in CMML include, TET2 (approx. 60%), SRSF2 (approx. 50%), ASXL1 (approx. 40%) and oncogenic RAS pathway (NRAS, KRAS, CBL and PTPN11) mutations.2–4 Primary myelofibrosis (PMF), a prototypic MPN, is characterized by the presence of megakaryocytic proliferation, atypia and clustering, reticulin fibrosis, and the presence of characteristic driver mutations; JAK2V617F (approx. 60%), CALR (approx. 20%) and MPL (approx. 10%).5,6 Absolute monocytosis has been documented in approximately 15% of patients with PMF, and has been associated with adverse outcomes.7 While patients with CMML can have MPN-features, such as leukocytosis, circulating immature myeloid cells (IMC), BM fibrosis, splenomegaly and associated constitutional symptoms, classical MPN associated-driver mutations are infrequent; JAK2V617F (approx. 10%), MPL (<1%) and CALR (<1%).1 In fact, the 2016 World Health Organization (WHO) guidelines state that the presence of these driver mutations tends to support a diagnosis of MPN with monocytosis, rather than CMML.2,5,8 In addition, while megakaryocytic atypia can be seen in CMML (hypolobated or dwarf megakaryocytes), in PMF, the megakaryocytes are often enlarged with hypersegmented nuclear lobes and associated clusters.9 Given the spectrum of overlap of CMML with MPN/PMF, we carried out this study to assess: i) clinical correlates; ii) prognostic impact; and iii) survival outcomes in CMML patients with MPN associated-driver mutations.
Three hundred and twenty-four Mayo Clinic patients with CMML defined according to WHO 2016 criteria, diagnosed from 1994 to 2017 were identified from the institutional database.5 All patients had BM aspirates, core biopsies and cytogenetic studies performed at diagnosis. Reticulin staining to assess BM fibrosis was available for assessment in 133 (41%) patients. The distinction between CMML and MPN with monocytosis was made on morphological grounds, based on the WHO 2016 diagnostic criteria.5 Although we have developed a multi-parametric flow cytometry-based monocyte repartitioning assay to help distinguish patients with CMML from MPN with monocytosis, this assay has not been validated on viably frozen PB and BM samples and was not used in this study.10 A 29-gene panel next generation sequencing (NGS) assay was carried out on BM DNA specimens on all patients obtained at diagnosis, or at the time of first referral after diagnosis to our institution, for the following genes; TET2, DNMT3A, IDH1, IDH2, ASXL1, EZH2, SUZ12, SRSF2, SF3B1, ZRSR2, U2AF1, PTPN11, Tp53, SH2B3, RUNX1, CBL, NRAS, KRAS, JAK2, CSF3R, FLT3, KIT, CALR, MPL, NPM1, CEBPA, IKZF,ETNK1 and SETBP1, by previously described methods.4
The performance characteristics of this NGS assay are as follows: single base substitutions and insertion/deletion events, accuracy >99%; reproducibility 100% (intra- and inter-assay); sensitivity 2-5% variant allele fraction (VAF), with minimum depth coverage of 250X. JAK2V617F mutational analysis was also carried out by allele specific polymerase chain reaction (PCR) (sensitivity approx. 0.01%), in all patients, by previously described methods.11 For the NGS studies, base-calling was performed using Illumina’s Real Time Analyser (RTA) v.1.17.21.3. Genesifter® software was utilized (PerkinElmer, Danvers, MA, USA) to analyze sequencing data. Nucleotide variants were called using the Genome Analysis Toolkit (GATK, Broad Institute, Cambridge, MA, USA).
All statistical analyses considered parameters obtained at time of CMML diagnosis. Differences in the distribution of continuous variables between categories were analyzed either by Mann-Whitney or Kruskal-Wallis tests. Patient groups with nominal variables were compared by χ2 test. Overall survival (OS) was calculated from the date of diagnosis to date of death or last contact. Leukemia-free survival (LFS) was calculated from the date of diagnosis to date of LT or death/last contact. Thrombosis-free survival (TFS) was calculated from the date of diagnosis to the date of first thrombotic event after diagnosis, or death/last contact. Overall, LFS and TFS curves were prepared by the Kaplan-Meier method and compared by the log-rank test. Cox proportional hazard regression model was used for multivariable analysis. The JMP® Pro 13.0.0 software from SAS Institute, Cary, NC, USA, was used for all calculations.
The median age of the study cohort was 71 years (range, 18-95 years) and 67% were male. Thirty-one (9.5%) patients had CMML with MPN associated-driver mutations; 30 (97%) with JAK2V617F and 1(3%) with a MPLW515L mutation. Given the rarity, the patient with the MPL mutation was not included in further analyses of the study cohort. The JAK2V617F mutation was detected by NGS testing in 26 (87%) patients (median VAF 37%, range, 3-90%), while it was detected by allele specific PCR in 4 (13%) patients with negative NGS testing (VAF 0.01%, 0.07%, 0.5% and not known). There were no patients with CALR or JAK2 exon 12 mutations. Additional signal-pathway mutations included NRAS (15%), KRAS (4%), CBL (15%), PTPN11 (3%), CSF3R (1%), and SH2B3 (1%).
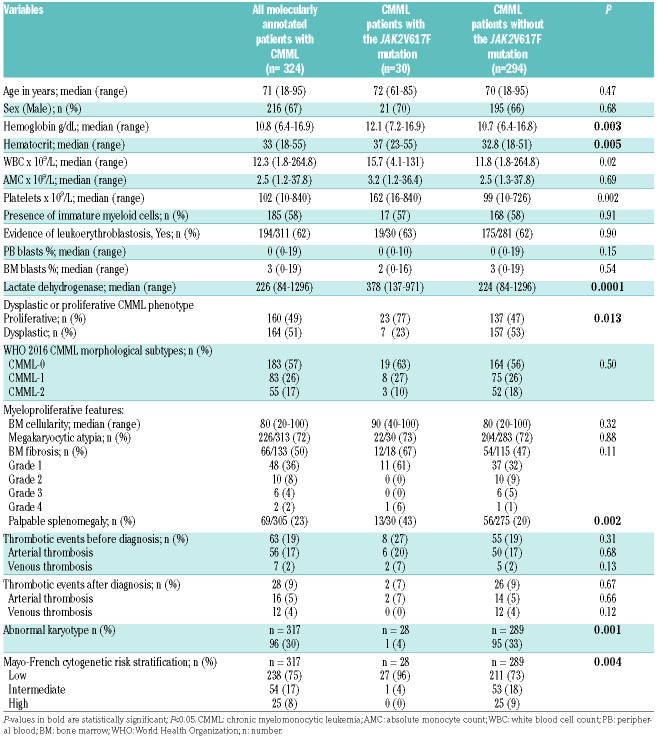
Concerning clinical correlates, the median age of JAK2V617F mutated CMML patients was 72 years (range, 61-85) and 70% were male (Table 1). The WHO morphological subtypes included: CMML-0 (63%), CMML-1 (27%), and CMML-2 (10%), while 96% of patients had a normal karyotype. The distribution of additional mutations in this group included: TET2 (74%), SRSF2 (48%), ASXL1 (48%), RUNX1 18%, SETBP1 (11%) and EZH2 (11%), NRAS (7%), SF3B1 7% (7%), and U2AF1, CBL, PTPN11, FLT3-TKD, and Tp53, all 4% each, respectively. Risk stratification by the Mayo Molecular Model included high (17%), intermediate-2 (38%), intermediate-1 (31%), and low risk (14%), respectively. In comparison to CMML patients without the JAK2V617F mutation, those with, had a higher hemoglobin (HB, P=0.003) and hematocrit (HCT, P=0.005), were more likely to have leukocytosis (P=0.02) and elevated lactate dehydrogenase (LDH) levels (P=0.0001), less likely to have thrombocytopenia (P=0.002), more likely to have a “proliferative” CMML phenotype (MP-CMML, P=0.001) with palpable splenomegaly (P=0.002), more likely to have a normal karyotype (P=0.001), and more likely to have mutations involving TET2 (P=0.01). There were no differences between the two groups with regards to BM fibrosis (P=0.11), BM cellularity (P=0.32), degree of PB monocytosis (P=0.69), circulating IMC (P=0.91), PB (P=0.15) and BM (P=0.54) blasts, and the presence of BM megakaryocytic atypia (P=0.88). These statistics, including BM fibrosis, did not change after excluding the four patients with CMML who had a detectable JAK2V617F mutation by PCR analysis only.
Table 1.
Clinical and laboratory characteristics of 324 patients with chronic myelomonocytic leukemia (CMML), obtained at CMML diagnosis, stratified by as to whether or not they had the JAK2V617F mutation.
Seven (23%) JAK2V617F mutant patients were classified as having “dysplastic” CMML at diagnosis (MD-CMML; none were on cytoreductive agents such as hydroxyurea); with a median JAK2V617F VAF of 11% (range, 0.01-30%). One JAK2V617F mutant MD-CMML patient did eventually evolve into MP-CMML six years after diagnosis. With the exception of one patient with a NRAS mutation, there were no additional signal pathway mutations seen in the JAK2V617F mutant MD-CMML group. We compared the JAK2V617F mutant MP-CMML (n=23) patients with MP-CMML patients with other signal pathway mutations (n=69) (Online Supplementary Table S1) and found that, in comparison to the other signal pathway mutation group, JAK2V617F mutant patients were more likely to have higher HB (P=0.04) levels, higher platelet counts (P=0.02), higher LDH levels (P=0.02), higher frequency of TET2 mutations (P=0.01), a normal karyotype (P=0.001), with fewer PB immature cells (P=0.03), and PB blasts (P=0.01). There was no difference in BM morphological features including BM fibrosis, or difference in survival between the two groups (P=0.61). We then compared JAK2V617F mutant CMML patients (n=30) with PMF patients with monocytosis (n=73; JAK2V617F 66%, type 1 CALR 15%, type 2 CALR 4% and MPL 10%) (Online Supplementary Table S2) and found that the JAK2V617F mutant CMML patients were older in age (P=0.03), more likely to have higher HB (P=0.03) levels and higher AMC (P=0.03), had lower LDH (P=0.004) levels, and lower PB blasts (P=0.0001), were less likely to have ≥grade 2 BM fibrosis (P=0.0001), palpable splenomegaly (P=0.0005) and an abnormal karyotype (P=0.0002), and were more likely to have TET2 mutations (P=0.001).
Twenty-eight (9%) thrombotic events (n=16, arterial; n=12, venous) were documented in the CMML cohort; 2 (6%) in JAK2V617F mutant CMML patients and 26 (9%) in those without (P=0.67). Both thrombotic events in the JAK2V617F mutant group were acute coronary syndromes. There was no difference in thrombotic events between the JAK2V617F mutated CMML patients and the PMF patients with monocytosis.
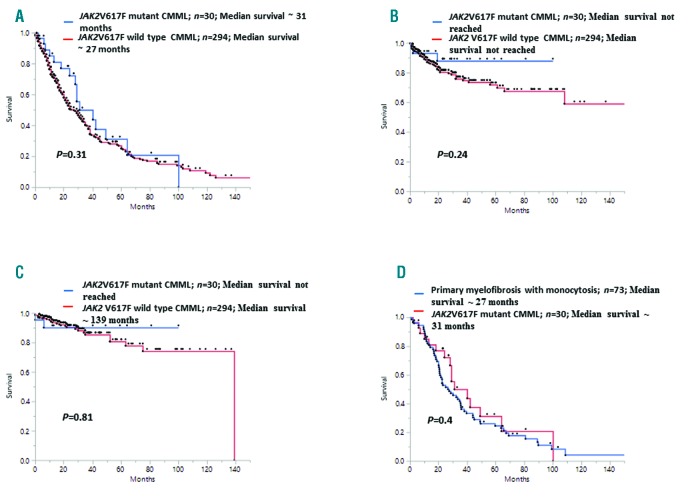
Concerning prognostic impact and survival outcomes, at last follow up, 221 (68%) deaths and 57 (18%) LT were documented, of which 16 (53%) deaths (P=0.07) and 3 (10%) LT (P=0.22) occurred in the JAK2V617F mutant group. The median OS for the entire cohort was 29 months (range, 22-32), 31 months for JAK2V617F mutant CMML patients and 27 months for those without (P=0.31) (Figure 1). On a univariate analysis, survival was adversely impacted by age >65 years (P<0.0001), low HB (P<0.0001), high WBC count (P=0.001) and AMC (P=0.0002), circulating IMC (P=0.01), PB (P=0.0007) and BM blasts (P=0.02), palpable splenomegaly (P=0.01), abnormal karyotype (P=0.0001), presence of DNMT3A (P=0.006), ASXL1 (P=0.009), and Tp53 (P=0.03) mutations and the absence of TET2 (P=0.0008) mutations. BM fibrosis did not impact survival (P=0.2). On a multivariable analysis that included the aforementioned significant variables, only age >65 years (HR 1.4, 95%CI: 1.07-2.06; P=0.02), HB <10 gm/dL (HR 1.4, 95%CI: 1.1-2.1; P=0.01), AMC>5 x 109/L (HR 1.4, 95%CI: 1.03-1.95; P=0.02), abnormal karyotype (HR 1.69, 95%CI: 1.2-2.3; P=0.0009), absence of TET2 mutations (HR 1.4, 95%CI: 1.07-1.9; P=0.014), and the presence of DNMT3A (HR 2.6, 95%CI: 1.3-4.6; P=0.006) mutations retained significance. The presence of JAK2V617F mutations in CMML did not impact OS (P=0.31), LFS (P=0.24), or TFS (P=0.81). There was also no difference in survival between JAK2V617F mutant CMML patients and PMF patients with monocytosis (P=0.04) (Figure 1D). In patients with ET, the presence of a JAK2V617F mutation is associated with higher HB/HCT levels, higher neutrophil counts, increased incidence of thrombosis, and evolution to PV.13 In MDS-ring sideroblasts (RS), the acquisition of a JAK2V617F mutation results in thrombocytosis with concomitant dysplasia (MDS/MPN-RS-T).14 Similarly in CMML, we demonstrate that JAK2V617F can be seen in approximately 9% of patients, is associated with higher HB/HCT levels, higher WBC, and platelet counts, a MP-CMML phenotype, normal karyotype, and TET2 mutations. While 23% of JAK2V617F mutant CMML patients had a MD-CMML phenotype, all of these patients had low JAK2V617F VAF, indicating the possible sub-clonal nature of these mutations, with one case eventually progressing to MP-CMML. Within the MP-CMML group, in comparison to other signal pathway mutations, JAK2V617F conferred higher HB/HCT levels, higher platelet counts, with no difference in the degree of BM fibrosis or survival outcomes.
Figure 1.
Kaplan-Meier survival analysis curves demonstrating the impact of the JAK2V617F mutation on overall survival (OS), leukemia-free survival and thrombosis-free survival, in 324 patients with chronic myelomonocytic leukemia (CMML) and in comparison with 73 patients with primary myelofibrosis (PMF) and monocytosis. (A-C) All patients stratified by whether or not they had the JAK2V617F mutation. (A) OS in 324 patients with CMML. (B) Leukemia-free survival in 324 patients with CMML. (C) Thrombosis-free survival in 324 patients with CMML. (D) OS analysis between 30 JAK2V617F mutant CMML patients and 73 patients with PMF with monocytosis.
The presence of a JAK2V617F mutation in CMML makes it challenging to assess as to whether the patient has CMML or PMF with monocytosis. In this study, we show that apart from diagnostic BM morphological features, especially ≥grade 2 BM fibrosis, the JAK2V617F mutant CMML cases had higher HB levels and higher AMC, with a lower frequency of palpable splenomegaly and karyotypic abnormalities, with no difference in OS between the two groups. The significant co-occurrence of TET2 mutations with the JAK2V617F mutation in CMML is a novel finding, needing further elucidation. The successful use of a multi-parametric flow cytometry-based monocyte repartitioning assay to help distinguish CMML from MPN with monocytosis has been documented, but needs prospective validation in a larger cohort of patients.10 With the JAK2V617F mutation being associated with clonal hematopoiesis of indeterminate potential (CHIP), the contribution of this mutation, especially at low VAF, to disease biology remains controversial.15 In addition, the JAK2V617F mutation has been associated with an increased thrombotic risk in patients with CHIP and MPN; an association that was not seen in our CMML study.6,15 In summary, we demonstrate that JAK2V617F is the predominant MPN associated-driver mutation in CMML and is associated with “proliferative” disease features, higher hemoglobin/hematocrit levels and platelet counts, and frequently co-occurs with TET2 mutations, with no impact on thrombosis-free, leukemia-free, or overall survival.
Supplementary Material
Footnotes
Funding: this current publication is supported in part by grants from the The Henry J. Predolin Foundation for Research in Leukemia, Mayo Clinic, Rochester, MN, USA. This publication was supported by CTSA Grant Number KL2 TR000136 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Patnaik MM, Tefferi A. Chronic myelomonocytic leukemia: 2018 update on diagnosis, risk stratification and management. Am J Hematol. 2018;93(6):824–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patnaik MM, Barraco D, Lasho TL, et al. DNMT3A mutations are associated with inferior overall and leukemia-free survival in chronic myelomonocytic leukemia. Am J Hematol. 2017;92(1):56–61. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik MM, Itzykson R, Lasho TL, et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia. 2014;28(11):2206–2212. [DOI] [PubMed] [Google Scholar]
- 4.Patnaik MM, Lasho TL, Vijayvargiya P, et al. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood Cancer J. 2016;6:e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A, Nicolosi M, Mudireddy M, et al. Driver mutations and prognosis in primary myelofibrosis: Mayo-Careggi MPN alliance study of 1,095 patients. Am J Hematol. 2018;93(3):348–355. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Shah S, Mudireddy M, et al. Monocytosis is a powerful and independent predictor of inferior survival in primary myelofibrosis. Br J Haematol. 2018;183(5):835–838. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik MM, Vallapureddy R, Yalniz FF, et al. Therapy related-chronic myelomonocytic leukemia (CMML): Molecular, cytogenetic, and clinical distinctions from de novo CMML. Am J Hematol. 2018; 93(1):65–73. [DOI] [PubMed] [Google Scholar]
- 9.Chapman J, Geyer JT, Khanlari M, et al. Myeloid neoplasms with features intermediate between primary myelofibrosis and chronic myelomonocytic leukemia. Mod Pathol. 2018;31(3):429–441. [DOI] [PubMed] [Google Scholar]
- 10.Patnaik MM, Timm MM, Vallapureddy R, et al. Flow cytometry based monocyte subset analysis accurately distinguishes chronic myelomonocytic leukemia from myeloproliferative neoplasms with associated monocytosis. Blood Cancer J. 2017;7(7):e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tefferi A, Lasho T, Patnaik M, et al. JAK2 germline genetic variation affects disease susceptibility in primary myelofibrosis regardless of V617F mutational status: nullizygosity for the JAK2 46/1 haplotype is associated with inferior survival. Leukemia. 2009;24(1):105–109. [DOI] [PubMed] [Google Scholar]
- 12.Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2015 update on diagnosis, risk-stratification and management. Am J Hematol. 2015;90(2):162–173. [DOI] [PubMed] [Google Scholar]
- 13.Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366(9501):1945–1953. [DOI] [PubMed] [Google Scholar]
- 14.Patnaik MM, Lasho TL, Finke CM, et al. Predictors of survival in refractory anemia with ring sideroblasts and thrombocytosis (RARS-T) and the role of next-generation sequencing. Am J Hematol. 2016; 91(5):492–498. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.