Abstract
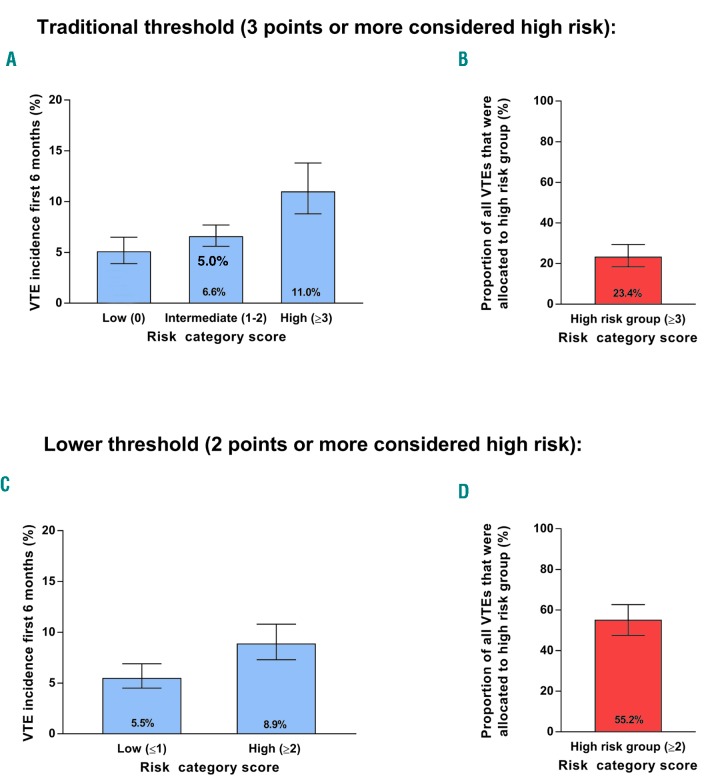
We aimed to evaluate the performance of the Khorana score in predicting venous thromboembolic events in ambulatory cancer patients. Embase and MEDLINE were searched from January 2008 to June 2018 for studies which evaluated the Khorana score. Two authors independently screened studies for eligibility, extracted data, and assessed risk of bias. Additional data on the 6-month incidence of venous thromboembolism were sought by contacting corresponding authors. The incidence in each Khorana score risk group was estimated with random effects meta-analysis. A total of 45 articles and eight abstracts were included, comprising 55 cohorts enrolling 34,555 ambulatory cancer patients. For 27,849 patients (81%), 6-month follow-up data were obtained. Overall, 19% of patients had a Khorana score of 0 points, 64% a score of 1 or 2 points, and 17% a score of 3 or more points. The incidence of venous thromboembolism in the first six months was 5.0% (95%CI: 3.9-6.5) in patients with a low-risk Khorana score (0 points), 6.6% (95%CI: 5.6-7.7) in those with an intermediate-risk Khorana score (1 or 2 points), and 11.0% (95%CI: 8.8-13.8) in those with a high-risk Khorana score (3 points or higher). Of the patients with venous thromboembolism in the first six months, 23.4% (95%CI: 18.4-29.4) had been classified as high risk according to the Khorana score. In conclusion, the Khorana score can be used to select ambulatory cancer patients at high risk of venous thromboembolism for thromboprophylaxis; however, most events occur outside this high-risk group.
Introduction
Venous thromboembolism (VTE) is a burdensome and frequent complication in patients with active cancer. The estimated overall 12-month incidence is approximately 6-8% but varies widely across tumor types.1,2 VTE is associated with substantial morbidity and mortality,3 decreases quality of life,4 and can lead to interruption or discontinuation of cancer treatment. Although thromboprophylaxis effectively reduces the risk of VTE,5 current guidelines recommend against its routine use in ambulatory cancer patients, probably due to the high number that require treatment, the fear of bleeding, and the considerable burden associated with daily injections of low-molecular-weight heparins.6
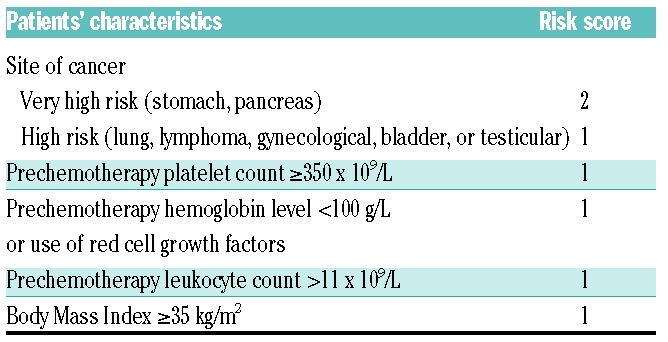
Risk stratification tools may help to reduce the number requiring treatment by guiding selection of cancer patients at high risk of VTE. An ideal risk score would help clinicians identify both patients with a negligible risk as well as those at very high risk needing intervention. The best-known risk stratification tool is the Khorana score, which was introduced in 2008. This score assigns points to five clinical and pre-chemotherapy laboratory parameters: primary tumor site (+1 or 2 points), platelet count of 350x109/L or more (+1 point), hemoglobin concentration of 100 g/L or lower or use of erythropoiesis-stimulating agents (+1 point), leukocyte count of 11x109/L or higher (+1 point), and a Body Mass Index of 35 kg/m2 or higher (+1 point) (Table 1).7 A sum score of 0 points classifies patients as being at low risk of VTE, 1 or 2 points at intermediate risk, and those with 3 or more points at high risk. The Khorana score is endorsed by the latest guideline updates of the American Society of Clinical Oncology and the National Comprehensive Cancer Network to select ambulatory cancer patients for thromboprophylaxis.6,8
Table 1.
Khorana risk score.
Over 50 studies have evaluated the score since its publication, but reported results were often conflicting. A clear interpretation of these findings is further hampered by the substantial variation in study design, cancer types included, and duration of follow up, ranging from a median of 2 to 79 months.9,10
To obtain valid and interpretable summary estimates of the performance of the Khorana score, based on the evidence available, we performed a systematic review and meta-analysis, specifically focusing on 6-month follow-up outcomes of all published relevant studies by obtaining additional data, thereby minimizing between-study heterogeneity. Our findings provide physicians with clinically useful data on the absolute risks of VTE associated with a low-, intermediate-, and high-risk Khorana score in ambulatory patients with cancer.
Methods
This report adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance (See checklist in Online Supplementary Table S1).11
Search strategy and data collection
A comprehensive search was performed in Embase and MED-LINE from January 2008 to June 2018 to identify studies that had evaluated the Khorana score in ambulatory cancer patients. In addition, studies presented as abstracts at conferences of the American Society of Hematology (ASH) or the International Society on Thrombosis and Haemostasis (ISTH) were identified by a manual search. Two reviewers (FIM and MC) independently screened studies and assessed bias with the Quality in Prognosis Studies (QUIPS) tool.12 The search strategy is shown in Online Supplementary Table S2, and a full explanation of study selection, data extraction, and bias assessment is provided in Online Supplementary list 1.
Additional data
Because the number of events are expected to increase with the duration of follow up, we evaluated the incidence of VTE during a pre-specified follow-up duration to minimize between-study heterogeneity in observation time. Since the majority of venous thromboembolic events occur in the first six months after start of chemotherapy,1 this 6-month follow-up period was considered most relevant. Corresponding authors of included studies not reporting the 6-month period were contacted and invited to provide additional data for this period.
Statistical analysis
The primary outcome measure was the proportion of cancer patients who developed VTE during the first six months of study follow up in those with a low (0 points), intermediate (1-2 points), or high (3 or more points) Khorana score. VTE was defined as the composite of radiologically confirmed symptomatic or incidental distal or proximal lower-extremity deep-vein thrombosis, upper-extremity deep-vein thrombosis, or pulmonary embolism. Studies with a fixed follow-up time less than six months in their study design were not included in the analysis of the 6-month outcomes. The derivation cohort of the Khorana score was excluded from analysis.7 As currently ongoing clinical trials (clinicaltrials.gov identifier: 02048865 and 02555878) select patients with a score of 2 or more for thromboprophylaxis; the primary outcome was also assessed for this alternative positivity threshold. Secondary outcome measures included the proportion of patients with VTE during overall follow up, the proportion of VTE occurring in the high-risk group, and the relative risk of VTE for patients with a high-risk score (≥3 points) versus those with a low-to-intermediate risk score (0-2 points) in the first six months and during complete follow up. A sensitivity analysis was performed restricted to studies not judged to be at high risk of bias in any of the domains.
A random effects model with logit transformation and inverse variance weighting was used to calculate summary estimates. Forest plots are presented with back-transformed study-specific estimates and corresponding 95% confidence and prediction intervals. Between-study heterogeneity was assessed by calculating tau-squared (τ2) using restricted maximum likelihood estimation. Differences between subgroups were tested for significance with a χ2 test. P<0.05 was considered statistically significant. Publication bias was explored with a funnel plot using the relative risk between high- and low-to-intermediate risk patients on the x-axis.13 Analyses were performed with R computing software, version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org), in particular using the meta package version 4.9-0.
Results
Search results
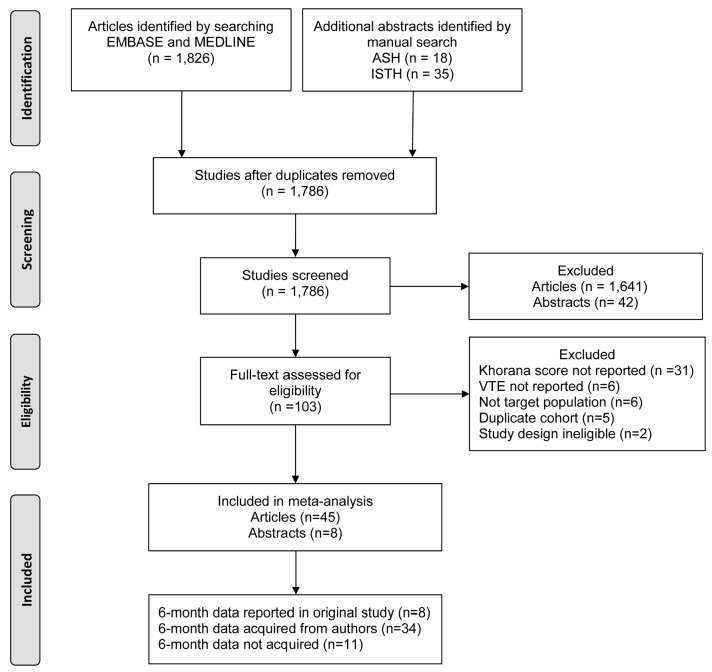
The database and manual search yielded 1,826 unique articles and 53 abstracts, of which 1,641 were excluded on the basis of title and abstract (Figure 1). Another 50 studies were excluded after full-text assessment because the Khorana score was not reported (n=31), VTE incidence was not reported (n=6), the study population only comprised patients with VTE (n=6), the cohort was a duplicate report (n=5), or the study had a case-control design (n=2).
Figure 1.
PRISMA flow chart. ASH: American Society of Hematology; ISTH: International Society on Thrombosis and Haemostasis.
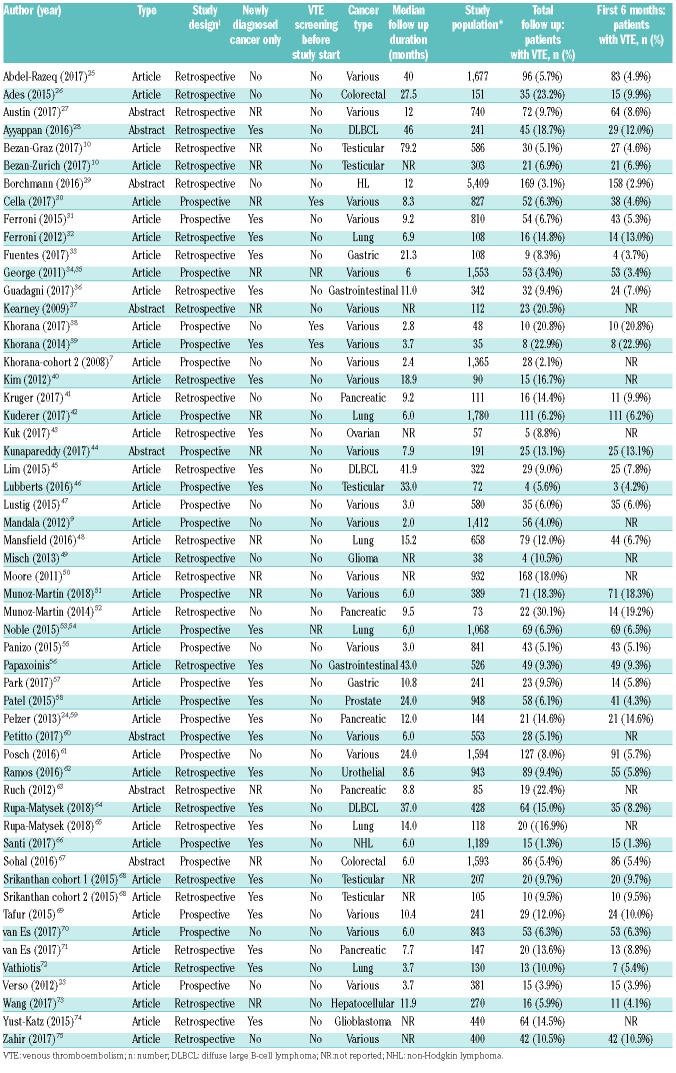
A total of 45 articles and eight abstracts were included in the analysis, comprising 55 cohorts and 34,555 ambulatory cancer patients, of whom 2,386 (6.9%) were diagnosed with VTE during follow up. Most studies included patients with various tumors (n=22; 42%), while others had confined recruitment to patients with gastrointestinal (n=12; 23%), lung (n=6; 11%), urogenital (n=6; 11%), hematologic (n=5; 9%), or central nervous system cancer (n=2; 4%). Almost half of the studies had a prospective design (n=25; 47%); the majority also included incidentally detected VTE as outcome event (n=32; 60%). Study group size ranged from 35 to 5,409 patients. Median follow-up duration ranged from 2 to 79 months. Key study characteristics of included studies are shown in Table 2.
Table 2.
Studies with relevant characteristics.
The 6-month follow-up data were reported in eight of the included studies. For 11 studies, no additional data were obtained after contacting the corresponding author because the authors did not reply despite reminders (n=8), were not able to retrieve the data (n=1), or where not willing to share the data (n=2). For 34 studies, additional data were obtained, yielding available 6-month data for 27,849 of the available 34,555 patients (81%).
Risk of bias
Using the pre-specified Quality in Prognosis Studies (QUIPS) criteria, 25 studies were judged to be at high risk of bias for one or more of the bias domains. All eight included abstracts and four articles were judged to be at high risk of bias because of insufficient reporting on methods. Other reasons were a high risk of bias in the applicability of the Khorana score (n=1), patient selection (n=4), outcome (n=3), study attrition (n=2), participation (n=4), prognostic factor measurement (n=3), outcome measurement (n=5), and confounding factors (n=4). Online Supplementary Table S4 summarizes the risk of bias assessment for all studies. A funnel plot did not indicate evidence of publication bias (Online Supplementary Figure S1).
Risk classification by the Khorana score
Overall, 6,319 patients (19%) had a Khorana score of 0 points (low risk), 21,172 patients (64%) a score of 1 or 2 points (intermediate risk), and 5,614 patients (17%) a score of 3 or more points (high risk). The group with a Khorana score of 0 or 1 point comprised 15,107 patients (53%), and the group with a score of 2 points or higher 13,148 (47%).
Incidence of venous thromboembolism in the Khorana score risk groups
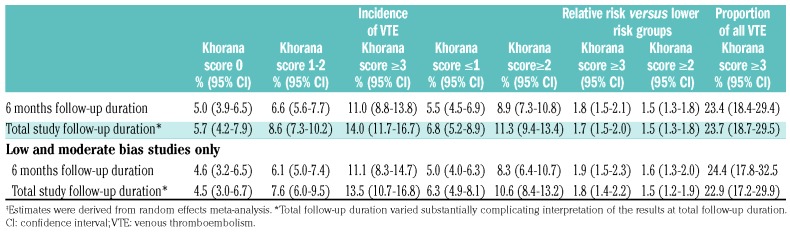
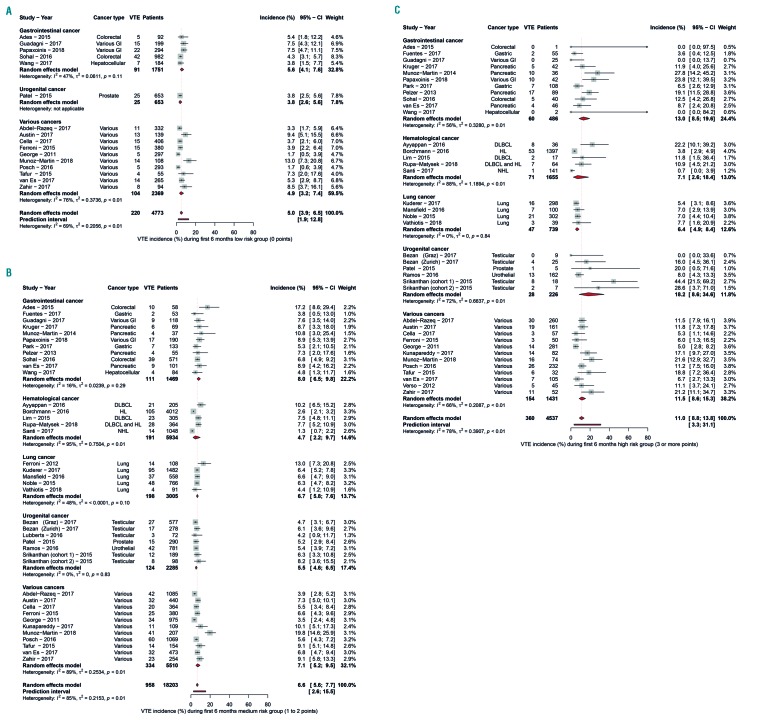
The incidence of VTE in the first 6-month period was 5.0% (95%CI: 3.9-6.5) in patients with a low-risk Khorana score (0 points), 6.6% (95%CI: 5.6-7.7) in those with an intermediate-risk Khorana score (1 or 2 points), and 11.0% (95%CI: 8.8-13.8) in those with a high-risk Khorana score (3 points or higher) (Table 3 and Figure 2A-C). The relative risk of VTE in the first six months was 1.8 (95%CI: 1.5-2.1) for patients with a score of 3 or higher compared to those with a score of 2 or lower (Online Supplementary Figure S2).
Table 3.
Summary estimates for 6-month and total follow-up duration.‡
Figure 2.
Venous thromboembolism incidence in the low-, intermediate-, and high-risk group over six months. Venous thromboembolism incidence in the low-risk (A), intermediate-risk (B), and high-risk (C) groups according to the Khorana score, over six months follow up.
In the high-risk Khorana score group, the reported 6-month risk of VTE was lower in studies including patients with lung cancer (6.4%; 95%CI: 4.9-8.4) or hematologic malignancies (7.1%; 95%CI: 2.6-18.4) compared to studies with gastrointestinal (13.0%; 95%CI: 8.5-19.6), urogenital cancer (18.2%; 95%CI: 8.6-34.6), or various cancers (11.5%; 95%CI: 8.6-15.3, lung vs. various, P=0.0008; hematologic vs. various, P=0.000). The 6-month incidence in the group with a Khorana score of 1 point or lower was 5.5% (95%CI: 4.5-6.9) compared to 8.9% (95%CI: 7.3-10.8) in the group with a score of 2 or more points, corresponding to a relative risk of 1.5 (95%CI: 1.3-1.8).
During the overall study follow-up period, that ranged from a median of two to 79 months, the summary incidence of VTE was 5.7% (95%CI: 4.2-7.9) in patients with a low-risk Khorana score (0 points), 8.6% (95%CI: 7.3-10.2) in those with an intermediate-risk Khorana score (1 or 2 points), and 14.0% (95%CI:11.7-16.7) in those with a high-risk Khorana score (3 points or higher) (Table 3 and Online Supplementary Figure S3A-C).
Distribution of venous thromboembolic events over the Khorana score risk groups
Of all patients who developed VTE in the first six months, 23.4% (95%CI: 18.4-29.4) had been classified as high risk with the Khorana score (3 points or higher). All other thromboembolic events occurred in the intermediate- or low-risk groups (76.6%; 95%CI:70.6 -81.6). For the total follow-up duration, the proportion of events occurring in the high-risk group was 23.7% (95%CI: 18.7-29.5).
Sensitivity analyses
Results were consistent in the sensitivity analysis in which studies judged to be at high risk of bias in one or more of the bias domains were excluded (Table 3). When excluding these studies, the 6-month risks of VTE in patients with a Khorana score of 0, 1 to 2, and 3 points or higher were 4.6% (95%CI: 3.2-6.5), 6.1% ((95%CI: 5.0-7.4), and 11.1% (95%CI: 8.3-14.7), respectively. The inci dence in the group with a score of 2 points or higher was 8.3% (95%CI: 6.4-10.7). The relative risk of patients with a score of 3 or higher compared to those with a lower score was 1.9 (95%CI: 1.5-2.3).
Discussion
This systematic review and meta-analysis examined the performance of the Khorana score in predicting VTE in over 34,000 patient ambulatory patients with various types of cancer. To minimize between-study heterogeneity and obtain clinically relevant estimates, the main analysis was restricted to the first six months of follow up. During this period, the summary estimate of the risk of VTE in patients with a high-risk Khorana score was 11.0%, which was significantly higher than in those with a low-risk (5.0%) or intermediate-risk (6.6%) score. These findings indicate that the Khorana score may help clinicians in selecting patients at high risk of VTE for thromboprophylaxis, which is in support of the suggestions presented in current guidelines.
The analyses also highlight several limitations of the score. Within the high-risk group, the estimated risk of VTE was considerably lower for patients with lung cancer and hematologic malignancies than for those with other cancer types (Figure 2C). Hence, the Khorana score appears to be less informative for these two large groups of patients. Furthermore, the VTE incidence in patients with a low-to-intermediate risk score was 5-7%, which indicates that the residual risk in this group is still substantial. Therefore, the Khorana score is of limited use in ruling out a future venous thromboembolic event. Lastly, the Khorana score is designed to select patients in the high-risk group for thromboprophylaxis. However, about one in four (23.4%, 95%CI: 18.4-29.4) of the venous thromboembolic events occur in patients with a high-risk Khorana score. This means that a substantial amount of cancer patients with subsequent venous thromboembolic events will not be identified with this form of risk stratification, and will, therefore, not benefit from thromboprophylaxis.
A major strength of this study is the additional data obtained from 34 studies on the 6-month incidence of VTE after starting chemotherapy, representing 81% of cancer patients in the available relevant literature. This approach minimized between-study heterogeneity related to the broad range of reported median follow-up durations. We considered this 6-month period to be clinically most relevant. Prediction of VTE only for the first few months of chemotherapy may be too short, since the risk remains elevated throughout the first six months. On the other hand, the Khorana score calculated with pre-chemotherapy laboratory data likely predicts less well for longer term (>6 months) than for shorter term intervals. The inclusion of more than 50 studies enabled the meta-analysis for various subgroups of cancer patients, showing that the performance of the Khorana score varies across tumor types. A potential limitation is the substantial proportion of studies judged to be at high risk of bias (Online Supplementary Table S4). However, the sensitivity analyses restricted to studies at low risk of bias did not materially alter the results (Table 3). When the analysis was restricted to studies with a prospective design or to studies without systematic VTE screening preceding study, results were comparable (data not shown). Additional data for the first six months could not be obtained for eleven studies, possibly introducing sampling bias. We believe, however, that the magnitude of this risk of bias is at best modest since 6-month data were available in the final analyses for 81% of all patients. Some studies included more types of venous thromboembolic events than specified in our primary outcome. However, these types of venous thromboembolic events occur infrequently. A large proportion of the studies (n=32, 60%) included incidentally detected VTE, unlike the outcome in the derivation study of the Khorana score.7 However, we believe these events should also be considered since clinical outcomes in patients with incidental VTE are similar to those with symptomatic events.14–16 Consequently, international guidelines regard incidental VTE events as clinically relevant and recommend anticoagulant treatment, as for patients with symptomatic VTE.6,17 Despite minimizing bias due to differ ences in follow up by using 6-month outcome data, considerable residual heterogeneity was observed in the analyses. This is expected in meta-analyses of predictive model performance, especially when evaluating risk assessment tools across various cancers.18 Nonetheless, we believe the presented estimates overall and for subgroups by cancer type are the most reliable ones based on the current literature, and can help clinicians to decide whether to use the score in their practice.
Two currently ongoing randomized trials use the Khorana score to select cancer patients at high risk of VTE for thromboprophylaxis (clinicaltrials.gov identifier: 02048865 and 02555878). Interestingly, these studies apply a positivity threshold of 2 points rather than the conventional 3 points. Our analyses demonstrate that this approach increases the proportion of patients classified as high risk (17-47%) while in parallel decreasing the absolute risk of VTE in this group (11-9%). As a consequence, the proportion of thromboembolic events that occur in the high-risk group increases from 23% to 55% (Figure 3). It is a matter of debate whether the 9% risk of VTE during the first six months is considered high enough to justify thromboprophylaxis.
Figure 3.
Estimated incidence of venous thrombosis and proportion in the high-risk group over six months. Estimated incidence of venous thrombosis (A and C) and proportion of venous thromboembolic events allocated to the high-risk group (B and D). When considering two points or more as high-risk (C and D) instead of three points or more (traditional threshold, A and B), the proportion of venous thromboembolic events allocated to the high risk groups increases, but also results in a lower incidence. VTE: venous thromboembolism.
The primary aim of risk stratification with the Khorana score is to select cancer patients with a high risk of VTE suitable for long-term thromboprophylaxis. A meta-analysis of randomized trials that compared low-molecular-weight heparins in prophylactic doses in cancer patients with placebo showed an absolute risk reduction of approximately 50% during a median follow-up of ten months (RR 0.54; 95%CI: 0.38-0.75), with an increase in major bleeding events (RR 1.44; 95%CI: 0.98-2.11).19 As the estimated 6-month incidence of VTE in cancer patients with a high Khorana score is 11.0%, thromboprophylaxis with low-molecular-weight heparins for cancer patients in this group could result in a number requiring treatment of approximately 19 when extrapolating the relative risk reduction of 0.54. When considering patients with 2 points or more as high-risk, thromboprophylaxis with low-molecular-weight heparins could result in a number requiring treatment of 24. Recent trials showed an acceptable safety profile of therapeutic doses of direct oral anticoagulants in cancer patients compared to low-molecular-weight heparins.20,21 Since their oral administration makes these drugs more convenient, long-term thromboprophylaxis would be less burdensome and, therefore, more likely to be accepted by clinicians and patients. Whether the safety and efficacy of prophylactic doses of direct oral anticoagulants are comparable to that of low-molecular-weight heparin in cancer patients needs to be established.
The present meta-analysis shows that the Khorana score can select high-risk patients for thromboprophylaxis overall. These findings indicate that the Khorana score may help clinicians in selecting patients at high risk of VTE for thromboprophylaxis, which is in support of the suggestions presented in some guidelines and could accelerate their implementation in clinical practice. However, several limitations of the Khorana need to be taken into account, including the different in predicted performance across cancer types and the modest proportion of patients with VTE assigned to the high-risk group. Several other VTE prediction tools for cancer patients have been introduced, which may have a better performance than the Khorana score;22–24 these scores, however, require prospective validation. Development of risk prediction models for bleeding events in patients with prophylactic anticoagulants could help to carefully weigh the benefit risk trade-off for thromboprophylaxis in cancer patients. In addition, future prediction tools should aim to address the limitations of the Khorana score, as outlined by this analysis. Novel biomarkers or genetic information from tumor biopsies could improve prediction of VTE and, therefore, merit investigation.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/6/1277
CAT-prediction collaborators
Abdel-Razeq H, King Hussein Cancer Center, Jordan; Ades S, University of Vermont, Burlington, VT, USA; Ayappan SR, The Ohio State University Comprehensive Cancer Center (OSUCCC-James), Columbus, OH, USA; Borchmann S, University Hospital Cologne, Germany; Cella CA, Federico II University, Naples, Italy; Fankhauser CD, University Hospital Zürich, Switzerland; Ferroni P, San Raffaele Roma Open University, Italy; Fuentes HE, John Stronger Jr. Hospital, Chicago, IL, USA; Kruger S, Ludwig-Maximilians-University of Munich, Germany; Lim SH, Samsung Medical Center, Seoul, Republic of Korea; Lubberts S, University Medical Center Groningen, the Netherlands; Lustig DB, University of Ottawa, ON, Canada; Mansfield AS, Mayo Clinic, Rochester, MN, USA; Munõz Martín AJ, Medical Oncology Service, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Noble S, Cardiff University, UK; Panizo E, University Clinic of Navarra, Pamplona, Spain; Papaxoinis G, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester, UK; Park K, Pusan National University Yangsan Hospital, Republic of Korea; Patel JN, Levine Cancer Institute, NC, USA; Posch F, Medical University of Vienna, Austria; Ramos JD, University of Washington, Seattle, WA, USA; Roselli M, University of Rome Tor Vergata, USA; Santi R, A.O.SS. Antonio e Biagio e Cesare Arrigo of Alessandria, Italy; Sohal D, Cleveland Clinic, OH, USA; Srikanthan A, Princess Margaret Cancer Centre, University of Toronto, ON, USA; Tafur AJ, University of Oklahoma Health Sciences Center, USA; Terbuch A, Medical University, Graz, Austria; Thomas M, University College London Hospitals NHS Foundation Trust, UK; Vathiotis O, Oncology Unit, Sotiria General Hospital, University of Athens, Greece; Wang R, Graduate School of Tianjin Medical University, PRC; Zahir MN, Aga Khan University Hospital, Pakistan.
Funding
This study was supported by an unrestricted grant from LeoPharma. The sponsor had no influence on study design, data collection, analysis, writing of the manuscript, or in the decision to submit the manuscript for publication.
References
- 1.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of venous thrombosis. Blood. 2013;122(10):1712–1723. [DOI] [PubMed] [Google Scholar]
- 2.Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. Thromb Haemost. 2016;117(1):57–65. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpa tient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd AJ, Dewilde S, Noble S, Reimer E, Lee AYY. What Impact Does Venous Thromboembolism and Bleeding Have on Cancer Patients’ Quality of Life? Value Heal. 2018;21(4):449–455. [DOI] [PubMed] [Google Scholar]
- 5.Di Nisio M, Porreca E, Ferrante N, Otten HM, Cuccurullo F, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2012;2(12):CD008500. [DOI] [PubMed] [Google Scholar]
- 6.Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33(6):654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streiff MB, Holmstrom B, Ashrani A, et al. Cancer-associated venous thromboembolic disease, version 1.2015: Featured updates to the NCCN Guidelines. JNCCN J Natl Compr Cancer Netw. 2015;13(9):1079–1095. [DOI] [PubMed] [Google Scholar]
- 9.Mandalá M, Clerici M, Corradino I, et al. Incidence, risk factors and clinical implications of venous thromboembolism in cancer patients treated within the context of phase I studies: The “sendo experience”. Ann Oncol. 2012;23(6):1416–1421. [DOI] [PubMed] [Google Scholar]
- 10.Bezan A, Posch F, Ploner F, et al. Risk stratification for venous thromboembolism in patients with testicular germ cell tumors. PLoS One. 2017;12(4):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden JA, Van Der Windt DA, Cartwright JL, Co P. Research and reporting methods annals of internal medicine assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. [DOI] [PubMed] [Google Scholar]
- 13.Sterne JAC, Harbord RM. Funnel plots in meta-analysis. Stata J. 2004;4(2):127–141. [Google Scholar]
- 14.den Exter PL, Hooijer J, Dekkers OM, Huisman MV. Risk of recurrent venous thromboembolism and mortality in patients with cancer incidentally diagnosed with pulmonary embolism: a comparison with symptomatic patients. J Clin Oncol. 2011;29(17):2405–2409. [DOI] [PubMed] [Google Scholar]
- 15.Font C, Carmona-Bayonas A, Beato C, et al. Clinical features and short-term outcomes of cancer patients with suspected and unsuspected pulmonary embolism: The EPIPHANY study. Eur Respir J. 2017;49(1):1600282. [DOI] [PubMed] [Google Scholar]
- 16.Sahut D’Izarn M, Caumont Prim A, Planquette B, et al. Risk factors and clinical outcome of unsuspected pulmonary embolism in cancer patients: a case-control study. J Thromb Haemost. 2012; 10(10):2032–2038. [DOI] [PubMed] [Google Scholar]
- 17.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 SUPPL):419–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debray TPA, Damen JAAG, Snell KIE, et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ. 2017;356:i6460. [DOI] [PubMed] [Google Scholar]
- 19.Di Nisio M, Porreca E, Ferrante N, Otten HM, Cuccurullo F, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2012;(2):CD008500. [DOI] [PubMed] [Google Scholar]
- 20.Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018; 36(20):2017–2023. [DOI] [PubMed] [Google Scholar]
- 21.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615–624. [DOI] [PubMed] [Google Scholar]
- 22.Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018;5(7):e289–e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7(3):291–292. [DOI] [PubMed] [Google Scholar]
- 24.Pelzer U, Sinn M, Stieler J, Riess H. Primäre medikamentöse thromboembolieprophy- laxe bei ambulanten patienten mit fortgeschrittenem pankreaskarzinom unter chemotherapie? Dtsch Med Wochenschr. 2013;138(41):2084–2088. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Razeq H, Mansour A, Abdulelah H, et al. Thromboembolic events in cancer patients on active treatment with cisplatin-based chemotherapy: another look! Thromb J. 2018;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ades S, Kumar S, Alam M, et al. Tumor oncogene (KRAS) status and risk of venous thrombosis in patients with metastatic colorectal cancer. J Thromb Haemost. 2015;13(6):998–1003. [DOI] [PubMed] [Google Scholar]
- 27.Austin K, Borrowman J, Blake L, Scully M, Thomas M. Retrospective cohort study of venous thromboembolism (VTE) rates in ambulatory cancer patients, and association with Khorana Score. Res Pract Thromb Haemost. 2017;11–1451. [Google Scholar]
- 28.Ayyappan SR, Gupta V, Diamond A, et al. Venous thromboembolic events in diffuse large B cell lymphoma patients: risk factors and outcomes. Blood. 2016; 128(22):3611. [Google Scholar]
- 29.Borchmann S, Hude I, Müller H, et al. thrombosis in Hodgkin lymphoma patients: incidence, time points, risk factors and impact of stage and treatment. Blood. 2016;128(22):4151. [Google Scholar]
- 30.Cella CA, Di Minno G, Carlomagno C, et al. Preventing Venous Thromboembolism in Ambulatory Cancer Patients: The ONKOTEV Study. Oncologist. 2017; 22(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferroni P, Riondino S, Formica V, et al. Venous thromboembolism risk prediction in ambulatory cancer patients: clinical significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio. Int J Cancer. 2015;136(5):1234–1240. [DOI] [PubMed] [Google Scholar]
- 32.Ferroni P, Martini F, Portarena I, et al. Novel high-sensitive D-Dimer determination predicts chemotherapy-associated venous thromboembolism in intermediate risk lung cancer patients. Clin Lung Cancer. 2012;13(6):482–487. [DOI] [PubMed] [Google Scholar]
- 33.Fuentes HE, Oramas DM, Paz LH, Wang Y, Andrade XA, Tafur AJ. Venous thromboembolism is an independent predictor of mortality among patients with gastric cancer. J Gastrointest Cancer. 2017;1–7. [DOI] [PubMed] [Google Scholar]
- 34.George D, Agnelli G, Fisher W, et al. Venous thromboembolism (VTE) prevention with semuloparin in cancer patients initiating chemotherapy: benefit-risk assessment by VTE risk in SAVE-ONCO. Blood. 2011;118(21):206 LP-206. [Google Scholar]
- 35.Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366(7):601–609. [DOI] [PubMed] [Google Scholar]
- 36.Guadagni F, Riondino S, Formica V, et al. Clinical significance of glycemic parameters on venous thromboembolism risk prediction in gastrointestinal cancer. World J Gastroenterol. 2017;23(28):5187–5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearney JC, Rossi S, Glinert K, Henry DH. Venous thromboembolism (VTE) and survival in a cancer chemotherapy outpatient clinic: a retrospective chart review validation of a VTE predictive Model. Blood. 2009;114(22):2503. [Google Scholar]
- 38.Khorana AA, Francis CW, Kuderer NM, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thromb Res. 2017;151:89–95. [DOI] [PubMed] [Google Scholar]
- 39.Khorana AA, Rubens D, Francis CW. Screening high-risk cancer patients for VTE: A prospective observational study. Thromb Res. 2014;134(6):1205–1207. [DOI] [PubMed] [Google Scholar]
- 40.Kim SY, Burns ZT, Henry DH. The assessment of thrombotic risk using a predictive model in metastatic cancer patients undergoing first-line therapy. Thromb Res. 2012; 130(6):967–970. [DOI] [PubMed] [Google Scholar]
- 41.Kruger S, Haas M, Burkl C, et al. Incidence, outcome and risk stratification tools for venous thromboembolism in advanced pancreatic cancer – A retrospective cohort study. Thromb Res. 2017;157:9–15. [DOI] [PubMed] [Google Scholar]
- 42.Kuderer NM, Poniewierski MS, Culakova E, et al. Predictors of venous thromboembolism and early mortality in lung cancer: results from a global prospective study (CAN-TARISK). Oncologist. 2018;23(2):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuk A, Magnowska M, Suchy W, et al. Retrospective evaluation of Thromboembolism risk in ovarian cancer patients treated with Bevacizumab. Target Oncol. 2017;12(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunapareddy G, Switzer B, Conces M, et al. Implementation of an Electronic medical record tool for early detection of deep vein thrombosis in the Ambulatory oncology Setting: the Cleveland Clinic Experience. Blood. 2017;130(Suppl 1):3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim SH, Woo SY, Kim S, Ko YH, Kim WS, Kim SJ. Cross-sectional study of patients with diffuse large B-cell lymphoma: assessing the effect of host status, tumor burden and inflammatory activity on venous thromboembolism. Cancer Res Treat. 2015;48(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lubberts S, Boer H, Altena R, et al. Vascular fingerprint and vascular damage markers associated with vascular events in testicular cancer patients during and after chemotherapy. Eur J Cancer. 2016;63:180–188. [DOI] [PubMed] [Google Scholar]
- 47.Lustig D Ben, Rodriguez R, Wells PS. Implementation and validation of a risk stratification method at the Ottawa Hospital to guide thromboprophylaxis in ambulatory cancer patients at intermediate-high risk for venous thrombosis. Thromb Res. 2015;136(6):109–1102. [DOI] [PubMed] [Google Scholar]
- 48.Mansfield AS, Tafur AJ, Wang CE, Kourelis TV, Wysokinska EM, Yang P. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost. 2016;14(9):1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misch M, Czabanka M, Dengler J, et al. D-dimer elevation and paresis predict thromboembolic events during bevacizumab therapy for recurrent malignant glioma. Anticancer Res. 2013;33(5):2093–2098. [PubMed] [Google Scholar]
- 50.Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J Clin Oncol. 2011;29(25):3466–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muñoz Martín AJ, Ortega I, Font C, et al. Multivariable clinical-genetic risk model for predicting venous thromboembolic events in patients with cancer. Br J Cancer. 2018; 118(8):1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muñoz Martín AJ, García Alfonso P, Rupérez Blanco AB, et al. Incidence of venous thromboembolism (VTE) in ambulatory pancreatic cancer patients receiving chemotherapy and analysis of Khorana’s predictive model. Clin Transl Oncol. 2014; 16(10):927–930. [DOI] [PubMed] [Google Scholar]
- 53.Noble S, Robbins A, Alikhan R, Hood K, Macbeth F. Prediction of venous thromboembolism in lung cancer patients receiving chemotherapy. Int Soc Thromb Haemost. 2015;131–997. [Google Scholar]
- 54.Macbeth F, Noble S, Evans J, et al. Randomized phase III trial of standard therapy plus low molecular weight heparin in patients with lung cancer: FRAGMATIC trial. J Clin Oncol. 2016;34(5):488–494. [DOI] [PubMed] [Google Scholar]
- 55.Panizo E, Alfonso A, García-Mouriz A, et al. Factors influencing the use of thromboprophylaxis in cancer outpatients in clinical practice: A prospective study. Thromb Res. 2015;136(6):1145–1148. [DOI] [PubMed] [Google Scholar]
- 56.Papaxoinis G, Kamposioras K, Germetaki T, et al. Predictive factors of thromboembolic complications in patients with esophagogatric adenocarcinoma undergoing preoperative chemotherapy. Acta Oncol. 2018; 57(6):1–9. [DOI] [PubMed] [Google Scholar]
- 57.Park K, Ryoo B-Y, Ryu M-H, et al. Incidence of venous thromboembolism and the role of D-dimer as predictive marker in patients with advanced gastric cancer receiving chemotherapy: A prospective study. World J Gastrointest Oncol. 2017;9(4):176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel JN, Jiang C, Hertz DL, et al. Bevacizumab and the risk of arterial and venous thromboembolism in patients with metastatic, castration-resistant prostate cancer treated on Cancer and Leukemia Group B (CALGB) 90401 (Alliance). Cancer. 2015;121(7):1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pelzer U, Opitz B, Deutschinoff G, et al. Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: Outcomes from the CONKO-004 trial. J Clin Oncol. 2015;33(18):2028–2034. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez Petitto G, Escalante CP, Richardson MN, Rojas Hernandez C. modified Khorana models for prediction of cancer-associated venous thromboembolism: an exploratory study. Blood. 2017; 130(Suppl 1):4635. [Google Scholar]
- 61.Posch F, Riedl J, Reitter E-M, et al. Hypercoagulabilty, venous thromboembolism, and death in patients with cancer. Thromb Haemost. 2016;115(04):817–826. [DOI] [PubMed] [Google Scholar]
- 62.Ramos JD, Casey MF, Bamias A, et al. The Khorana Score in predicting venous thromboembolism for patients with metastatic urothelial carcinoma and variant histology treated with chemotherapy. Clin Appl Thromb. 2017; 23(7):755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruch JM, Bellile E, Hawley AE, Anderson MA, Wakefield TW, Sood SL. Prediction of venous thromboembolism (VTE) in patients with Pancreatic cancer using clinical data, biomarkers, and VTE risk models. Blood. 2012;120(21):3398. [Google Scholar]
- 64.Rupa-Matysek J, Gil L, Kaźmierczak M, Barańska M, Komarnicki M. Prediction of venous thromboembolism in newly diagnosed patients treated for lymphoid malignancies: validation of the Khorana Risk Score. Med Oncol. 2018;35(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rupa-Matysek J, Lembicz M, Rogowska EK, Gil L, Komarnicki M, Batura-Gabryel H. Evaluation of risk factors and assessment models for predicting venous thromboembolism in lung cancer patients. Med Oncol. 2018;35(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santi RM, Ceccarelli M, Bernoccol E, et al. Khorana score and histotype predicts incidence of early venous thromboembolism in non-Hodgkin lymphomas. Thromb Haemost. 2017;117(8):1615–1621. [DOI] [PubMed] [Google Scholar]
- 67.Sohal DPS, Kuderer NM, Shepherd FA, et al. Predictors of venous thmromboembolism in colorectal cancer: results from a global prospective study. Blood. 2016; 128(22):1422. [Google Scholar]
- 68.Srikanthan A, Tran B, Beausoleil M, et al. Large retroperitoneal lymphadenopathy as a predictor of venous thromboembolism in patients with disseminated germ cell tumors treated with chemotherapy. J Clin Oncol. 2015;33(6):582–587. [DOI] [PubMed] [Google Scholar]
- 69.Tafur AJ, Dale G, Cherry M, et al. Prospective evaluation of protein C and factor VIII in prediction of cancer-associated thrombosis. Thromb Res. 2015; 136(6):1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Es N, Di Nisio M, Cesarman G, et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: A prospective cohort study. Haematologica. 2017;102(9):1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Es N, Franke VF, Middeldorp S, Wilmink JW, Büller HR. The Khorana score for the prediction of venous thromboembolism in patients with pancreatic cancer. Thromb Res. 2017;150:30–32. [DOI] [PubMed] [Google Scholar]
- 72.Vathiotis I, Dimakakos EP, Boura P, et al. Khorana Score: new predictor of early mortality in patients With lung adenocarcinoma. Clin Appl Thromb. 2018;24(8):1347–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Attar BM, Fuentes HE, Yu J, Zhang H, Tafur AJ. Performance of Khorana Risk Score for prediction of Venous thromboembolism in patients with hepatocellular carcinoma. Clin Appl Thromb Hemost. 2018; 24(3):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yust-Katz S, Mandel JJ, Wu J, et al. Venous thromboembolism (VTE) and glioblastoma. J Neurooncol. 2015;124(1):87–94. [DOI] [PubMed] [Google Scholar]
- 75.Zahir MN, Shaikh Q, Shabbir-Moosajee M, Jabbar AA. Incidence of venous thromboembolism in cancer patients treated with cisplatin based chemotherapy - a cohort study. BMC Cancer. 2017;17(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.