Introduction
Sickle cell disease (SCD) is a clinical syndrome caused by the presence of hemoglobin S (HbS), in which glutamic acid in position 6 of the β chain of hemoglobin is substituted by valine (βGlu6Val). It is generally recognized as an autosomal recessive disorder, in that individuals who have inherited one copy of the HbS allele and one normal HbA allele (i.e. have HbAS or sickle cell trait, SCT), are typically asymptomatic and spared the serious complications associated with possessing two copies of the mutant allele (i.e. HbSS). It is estimated that 300 million people (~5% of the world’s population) carry the HbS allele, and nearly 5.5 million births are affected annually.1
Individuals with SCD die prematurely, but the life expectancy of individuals with SCT is similar to that of people without the trait.2 However, early literature attributed a number of possible disease associations to this heterozygous state, partly as a result of unreliable diagnostic laboratory testing for hemoglobinopathies and partly due to questionable conclusions drawn from uncontrolled observational studies, individual case reports, and small case series.3,4 Nevertheless, while some of the associations historically attributed to SCT are unfounded, recent meta-analyses found high-quality evidence that SCT is indeed a risk factor for a handful of complications common to SCD.2
Exercise in individuals with sickle cell trait
Sudden death is the most feared complication of SCT. Despite a lack of clear evidence, early concerns about SCT-related sudden death led to the initial adoption of universal SCT screening in the 1970s for all United States (US) Armed Forces recruits and mandatory occupational restrictions for those recruits found to have SCT. The complication of sudden death was reinforced in a study in 1987 which evaluated deaths among two million US military recruits during training; African-American recruits with SCT had a 28-fold increase in relative risk [95% confidence interval (CI): 9-100] of sudden unexplained death compared to those without SCT.5 Although the relative risk was seemingly very high, the study was limited by the small absolute number of deaths in each group and its inability to examine separately subsets of sudden unexplained death (cardiac, exertional heat stroke, heat stress, and rhabdomyolysis), which may not share the same disease mechanism. Various other studies have examined physiological responses to exercise – e.g. aerobic metabolism, energy expenditure, maximal oxygen consumption, and maximal exercise performance – in subjects with HbAS and have found no difference compared to those in subjects with normal HbAA,6–9 even when intravascular sickling in subjects with SCT is observed. Additionally, a recent longitudinal analysis of a large cohort found no association of SCT with fitness, or with the development of hypertension, diabetes, and metabolic syndrome.7
On the civilian side, the sudden death of a college football player during training in 2006 led to a lawsuit whose settlement prompted the National Collegiate Athlete Association (NCAA) to adopt universal SCT screening for incoming Division I athletes in 2010, a policy that was later extended to Division II and III athletes.10 A retrospective analysis of athletes in the NCAA also found a 37-fold increase of exertional death in Division I football players with SCT compared to those without, but again the absolute risk was low.11
A more recent retrospective study of 47,944 black soldiers showed that with adoption of universal preventive measures, the risk of sudden death attributed to SCT appears to be completely mitigated.12 An outcome of these events and reports is the recommendation that preventive measures should be universally adopted to protect all soldiers and athletes, rather than singling out individuals with SCT with mandatory screening practices.4 Indeed, there are a handful of studies showing that hydration and progressive exercise training may reverse various hematologic abnormalities observed during exertion in SCT, such as increased blood viscosity, red blood cell rigidity, oxidative stress, endothelial activation, and red blood cell sickling.12–17
Nonetheless, soldiers with SCT appear to be at higher risk of developing exertional rhabdomyolysis;12 the risk is modest (hazard ratio, 1.54; 95% CI: 1.12-2.12), under conditions of extreme exertion, as also supported by a recent systematic review.2
Renal manifestations of sickle cell trait
The renal medulla with its unique hyperosmolar and acidotic environment, together with the low oxygen tension and low-flow state in medullary vasa recta, create optimal conditions for HbS polymerization,3 leading to a reduced number of vasa recta and loss of its normal vascular architecture.18 Hyposthenuria (a defect in concentrating urine) results, and in turn may predispose individuals to dehydration, postulated to be a contributory factor to the exertional rhabdomyolysis associated with SCT.
Among the renal manifestations associated with SCT, the most common are hematuria and proteinuria. In one large-scale study, hematuria was found to be twice as common in hospitalized African-American patients with HbAS than in those with normal hemoglobin.19 Urographic evaluation in a separate case series revealed that ~50% of hematuria cases in individuals with SCT were related to renal papillary necrosis.1,20 A much rarer cause of hematuria is renal medullary carcinoma, an aggressive malignancy found almost exclusively in young black patients with SCT.21 The tumor is hypothesized to arise from the distal collecting duct epithelium as a result of abnormal proliferation stimulated by chronic ischemia.22 The prognosis of patients with renal medullary carcinoma is poor, with a typical median survival of <1 year, and response to traditional chemotherapy is limited.23 It has been proposed that individuals with SCT presenting with new-onset hematuria should undergo urological evaluation.24
The chronic microvascular damage in the renal medulla also predisposes individuals with SCT to proteinuria and chronic kidney disease.2 A large study combining data from several African-American population-based prospective cohorts showed that the presence of SCT imparts a 1.86-fold higher odds of albuminuria (95% CI: 1.49-2.31).25 SCT is also a recognized risk factor for chronic kidney disease, for which a 1.5- to 2-fold increased risk is attributed to SCT.25,26 It is important to note that several co-morbid conditions such as type 2 diabetes and hypertension, as well as co-inherited genetic risk factors could influence the risk of chronic kidney disease, which is a potential explanation for the lack of association of SCT and chronic kidney disease in smaller cohort studies involving different ethnic groups.27,28 Additionally, SCT may increase the risk of proteinuria and retinopathy in individuals with diabetes.2,29,30
A common genetic risk modifier of renal disease in the African-American population is APOL1; inheritance of the G1 and G2 risk alleles is believed to account for much of the excess risk of chronic kidney disease and end-stage renal disease in individuals of African ancestry overall.31 APOL1 risk alleles have also been associated with proteinuria in patients with SCD,32,33 but so far, no genetic interactions between SCT and APOL1 risk alleles have been observed.25,26,34 The evidence for SCT itself being a risk factor for the development of end-stage renal disease remains inconclusive.
Another well-known genetic modifier of disease severity in SCD is α-thalassemia trait, co-inherited in ~35% of individuals of African descent. Co-inherited α-thalassemia reduces intracellular HbS concentration, a key determinant of polymerization kinetics. The protective effect of α-thalassemia on anemia and chronic kidney disease in individuals with SCT has been demonstrated in a cohort from the Jackson Heart Study.35
Other complications related to sickle cell trait
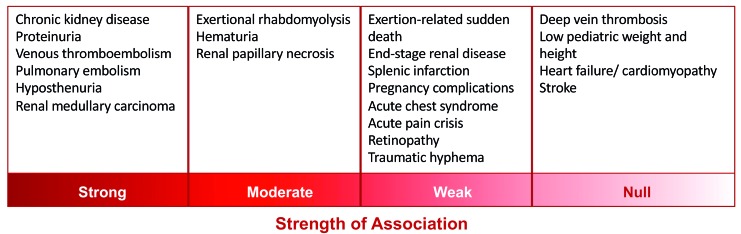
Despite numerous reported associations with SCT, few complications are supported by strong evidence (Figure 1). One final strong association is that of venous thromboembolic disease and pulmonary embolism in particular.2 In a study of 65,154 hospitalized African-Americans, HbS carriers had a slightly higher relative risk of pulmonary embolism, but the study was limited by the lack of radiological confirmation.19 Subsequent large cohort studies have identified SCT as a risk factor for venous thromboembolism36 as well as pulmonary embolism, but not deep vein thrombosis.36–38 One study suggested that the risk of venous thromboembolism attributable to SCT among blacks is higher than the risk attributable to the prothrombin G20210A mutation among whites.36 However, the reason that HbS might predispose a subject to pulmonary embolism over deep vein thrombosis is unknown and merits further investigation.
Figure 1.
The strength of association of sickle cell trait with various complications reported in the literature.
A number of other reported associations – e.g. splenic infarction, pregnancy complications, acute chest syndrome, retinopathy and traumatic hyphema – are backed by at times significant anecdotal evidence and have been reviewed in recent publications.1,2,4 This perspective article is limited in its ability to explore all reported associations in depth, but it is important to note that interpretation of these associations may not be straightforward. For example, the occurrence of splenic infarction in individuals with SCT has been documented in a number of case reports, often but not always in men exposed to high altitudes.39–41 However, as discussed in the next section, detailed genetic testing was not performed in these cases, so it is not possible to draw the conclusion that the splenic infarction was a complication of SCT alone.
SCT has also been reported to be associated with a variety of maternal and fetal complications during pregnancy or the puerperium, but the existing evidence is conflicting.1,4 Some of these associations are concerning, such as potentially higher rates of preeclampsia, maternal infections, and fetal loss.42,43 However, in a prospective Nigerian cohort, pregnant women with SCT did not experience more morbidity than women with HbAA, and in fact, had fewer attacks of malaria during pregnancy.44 In yet another study the observed risk of venous thromboembolism was higher in pregnant subjects with SCT (relative risk, 1.6; 95% CI: 0.5-5.5),45 but the magnitude of the difference did not suggest that the risk of venous thromboembolism was increased above that associated with SCT alone.
A recent analysis found moderate-quality evidence for a null association between SCT and low pediatric height and weight, as well as between SCT and heart failure/cardiomyopathy and stroke.2 In a field in which many questions remain unanswered, it is crucial to recognize and promote such null findings in order to prevent unnecessary concern, unfounded stigmatization, and psychological harm to carriers of HbS.
Molecular factors worsening the HbAS phenotype
The key event in the pathophysiology of SCD is polymerization of the deoxygenated HbS, which under certain conditions can be irreversible, leading to distortion of the erythrocyte and loss of deformability, ultimately causing vaso-occlusion in the microvasculature and hemolysis. The tendency for HbS to polymerize is highly dependent on the hemoglobin composition in the erythrocyte – mainly the concentration of intracellular HbS, as well as the concentration and type of non S hemoglobin. HbA and HbF present in the cell reduce the concentration of HbS, but HbF additionally has an inhibitory effect on HbS polymerization. Individuals with SCT, who have HbA in excess of HbS (30-40% of the total hemoglobin), do not typically suffer from the effects of sickling and are asymptomatic. Apart from the intracellular hemoglobin composition, other factors that influence the likelihood of HbS polymerization include oxygen saturation, intracellular pH and 2,3-diphosphoglycerate levels. Environmental and co-inherited genetic factors can change these parameters, modulating the kinetics of HbS polymerization in individuals with SCT.
Clinically symptomatic HbAS individuals could be considered to fall under four genetic categories: (i) co-inheritance of HbS with a genetic modifier; (ii) dominant forms of HbS alleles; (iii) apparent heterozygosity for HbS; and (iv) non-Mendelian inheritance of HbS.
Co-inheritance of HbS with a genetic modifier
Compound heterozygosity for HbS and genetic variants causing non-hemoglobin red cell (i.e. membrane and enzyme) disorders, while uncommon, can have important modifying effects on the clinical outcome of SCT. Two cases of HbS carriers experiencing typical SCD complications of chronic hemolytic anemia, recurrent acute pain, recalcitrant leg ulceration and end-stage renal disease, have been reported.46,47 Each of the probands had co-inherited mutations in the PKLR gene, causing a deficiency of pyruvate kinase protein. Pyruvate kinase is a key enzyme in the final step of glycolysis; it converts phosphoenolpyruvate to pyruvate, generating 50% of the total red cell ATP that is essential for metabolism of the red blood cell. Of particular relevance to SCD, a reduction in pyruvate kinase activity also leads to accumulation of the upstream enzyme substrates, including 2,3-diphospho-glycerate which decreases oxygen affinity, favoring polymerization of deoxy-HbS. In one case, fresh blood was available for in-vitro functional assays that supported the role of 2,3-diphosphoglycerate as a key factor in HbS polymerization.46
Another example is the co-inheritance of hereditary spherocytosis, implicated as the cause of splenic infarction in a handful of SCT cases.48 The presence of hereditary spherocytosis is believed to increase mean corpuscular hemoglobin concentration and intracellular HbS concentration, thereby increasing the propensity to HbS polymerization. Along the same lines, glucose-6-phosphate dehydrogenase deficiency, a common red cell enzyme disorder in individuals of African ancestry, might be expected to increase hemolysis and modify the clinical phenotype of SCD and SCT. However, there is a lack of evidence addressing co-inheritance of SCT and glucose-6-phosphate dehydrogenase deficiency, and even the evidence for SCD is mixed, with no consistent differences in red cell indices, degree of anemia, hemolysis, frequency of vaso-occlusive complications, or stroke risk in subjects who have co-inherited glucose-6-phosphate dehydrogenase deficiency.49–51 These conflicting reports could be related to limitations of the methodologies used for the enzyme assays, or the panel of glucose-6-phosphate dehydrogenase variants genotyped. These reports serve as an important caveat when examining past anecdotal evidence of complications in SCT, as in-depth genetic investigation is often lacking in reported cases of symptomatic SCT.
Dominant forms of HbS alleles
Rare HbS alleles that behave dominantly have been reported. One example is that found in a 19-month old girl, identified as having SCT through newborn screening; she developed a vaso-occlusive crisis with splenic sequestration during a flight and required splenectomy.52 Subsequent DNA sequencing analysis confirmed that the baby had inherited a maternal normal HbA allele but had acquired a new mutation, βLeu68Phe, cis to the paternal HbS allele. βLeu68Phe has previously been identified as Hb Rockford, a known Hb variant with reduced affinity for oxygen. The double mutant, named Hb Jamaica Plain (JP, βGlu6Val,Leu68Phe), has similar electrophoretic motility as HbS, hence it was missed at the newborn screening. The βLeu68Phe mutation in Hb JP causes it to desaturate easily at lower oxygen tension, thus polymerizing more readily than typical HbS, converting it to a dominant mutation.52
Two other Hb variants with double mutations, HbS Antilles (βGlu6Val, Val23Ile) and HbS-Sao Paolo (βGlu6Val, Lys65Glu), also promote polymerization through reduced oxygen affinity in heterozygotes.53,54 In addition, HbS Antilles has lower solubility and HbS-Sao Paolo forms more stable polymers than HbS, potentially further enhancing irreversible polymerization and red blood cell sickling. In the case of HbS Antilles, family studies led to the identification of 24 HbA/S Antilles individuals in the proband’s family, many of whom had recurrent sickle pain crises, chronic hemolytic anemia, and splenomegaly,54 a phenotype similar to that of HbSC disease.
Apparent heterozygosity for HbS
Compound heterozygotes of βS and very mild β-thalassemia mutations (βS/β++-thalassemia) can appear as having SCT, but the giveaway here is an excess of HbS over HbA. A 38-year old, previously healthy man, presented with a 6-month history of worsening pruritis, jaundice and ascites. Extensive work-up for causes of liver disease was negative, but hemoglobin electrophoresis showed 49.6% HbS and 41.3% HbA. The patient had not received any blood transfusion. While this result could easily be misinterpreted as HbS trait, given the slight increase of HbS over HbA, DNA and family studies were pursued. He was revealed as a compound heterozygote for βS and a novel, very mild β-thalassemia mutation (β IVS2-844 C→A) that was transmitted to both of his sons, and the liver pathology was ascribed as sickle-related.55
Another diagnostic conundrum is the discrepant findings of HbSS on hemoglobin electrophoresis but HbAS on genetic testing. Two such cases have been reported, where the individuals presented with typical hematologic and clinical phenotypes of SCD.56 In both instances, DNA testing showed that the individual possessed one HbA and one HbS allele, but expression of the HbA allele was abolished by a deletion of the upstream β locus control region, resulting in sole expression of the HbS allele and, thereby, a functionally homozygous HbS phenotype.56
Non-Mendelian inheritance of HbS
Homozygosity due to uniparental disomy of chromosome 11 is another rare genetic defect. Two individuals were reported to have inherited HbS from one parent and normal HbA from the other parent but presented later in life with phenotypic SCD. In-depth DNA analysis revealed that post-zygotic mitotic recombination had occurred, leading to mosaic segmental isodisomy.57,58 These individuals had dual populations of HbSS and HbAS erythroid progenitors and peripheral red blood cells, with HbSS erythrocytes accounting for the chronic hemolytic phenotype of SCD.
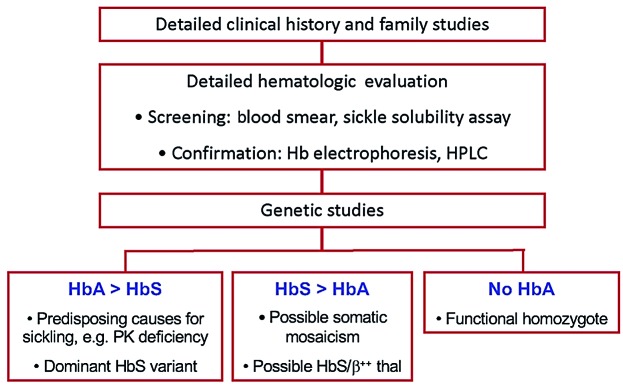
These cases demonstrate that the diagnosis of sickle trait can be nuanced and deserves further workup if the individual has a phenotype of SCD. In such diagnostically challenging scenarios, family studies are extremely useful, and detailed hemoglobin evaluation is essential (Figure 2). A relative excess of HbA over HbS with no other Hb variant present indicates that only one copy of abnormal allele is present. If phenotypic and genetic workup both show HbAS, a search for modifiers that promote HbS polymerization should be undertaken.
Figure 2.
Aids to unraveling the molecular basis of sickle complications in individuals with purported sickle cell “trait”. Individuals with purported sickle cell trait suffering complications of sickle cell disease may have an unrecognized rare genetic alteration or co-inherited red cell disorder and present a diagnostic challenge. The first step is to obtain a detailed clinical and family history, and to perform family studies if family members are available. Detailed hematologic evaluation, including hemoglobin electrophoresis, high-performance liquid chromatography (HPLC), examination of the peripheral blood smear, and qualitative sickle solubility assay, are essential. The hematologic data should always be interpreted in conjunction with genetic data. HbA in excess of HbS validates heterozygosity for the βS allele and, if found, should prompt investigation into whether the βS allele could be dominantly inherited with a double mutation. The patient could also have co-inherited other genetic variants [e.g. pyruvate kinase (PK) deficiency] that increase the likelihood of HbS polymerization. If HbS is in excess of HbA, and the inheritance pattern from parents is consistent with HbAS, somatic mosaicism should be considered. If hemoglobin electrophoresis or HPLC shows only HbS, genetic testing shows heterozygosity for HbS, and only one parent has HbAS, one should consider the possibility of a “functional homozygote” with the trans β gene structurally intact but functionally inactivated, such as can be seen in deletion of the trans upstream β locus control region.
Conclusion
While HbAS represents an asymptomatic carrier state, clinical and epidemiological studies have shown that SCT is certainly not an entirely harmless condition. The presence of HbS in SCT may contribute to specific disease processes, particularly under extreme conditions that promote HbS polymerization. Additionally, individuals with HbAS can present with complications typical of the SCD phenotype. Such cases of unusually severe HbAS can pose a diagnostic challenge, but elucidating their molecular basis provides further insight into the pathophysiology of SCD and help to identify genetic risk modifiers in SCT.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/6/1106
References
- 1.Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med. 2009;122(6):507–512. [DOI] [PubMed] [Google Scholar]
- 2.Naik RP, Smith-Whitley K, Hassell KL, et al. Clinical outcomes associated with sickle cell trait: a systematic review. Ann Intern Med. 2018;169(9):619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Key NS, Derebail VK. Sickle-cell trait: novel clinical significance. Hematology Am Soc Hematol Educ Program. 2010;2010:418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naik RP, Haywood C., Jr Sickle cell trait diagnosis: clinical and social implications. Hematology Am Soc Hematol Educ Program. 2015;2015:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med. 1987;317(13):781–787. [DOI] [PubMed] [Google Scholar]
- 6.Connes P, Reid H, Hardy-Dessources MD, Morrison E, Hue O. Physiological responses of sickle cell trait carriers during exercise. Sports Med. 2008;38(11):931–946. [DOI] [PubMed] [Google Scholar]
- 7.Liem RI, Chan C, Vu TT, et al. Association among sickle cell trait, fitness, and cardiovascular risk factors in CARDIA. Blood. 2017;129(6):723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin TW, Weisman IM, Zeballos RJ, Stephenson SR. Exercise and hypoxia increase sickling in venous blood from an exercising limb in individuals with sickle cell trait. Am J Med. 1989;87(1):48–56. [DOI] [PubMed] [Google Scholar]
- 9.Monchanin G, Connes P, Wouassi D, et al. Hemorheology, sickle cell trait, and alpha-thalassemia in athletes: effects of exercise. Med Sci Sports Exerc. 2005;37(7):1086–1092. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari R, Parker LS, Grubs RE, Krishnamurti L. Sickle cell trait screening of collegiate athletes: ethical reasons for program reform. J Genet Couns. 2015;24(6):873–877. [DOI] [PubMed] [Google Scholar]
- 11.Harmon KG, Drezner JA, Klossner D, Asif IM. Sickle cell trait associated with a RR of death of 37 times in National Collegiate Athletic Association football athletes: a database with 2 million athlete-years as the denominator. Br J Sports Med. 2012;46(5): 325–330. [DOI] [PubMed] [Google Scholar]
- 12.Nelson DA, Deuster PA, Carter R, 3rd, Hill OT, Wolcott VL, Kurina LM. Sickle cell trait, rhabdomyolysis, and mortality among U.S. srmy soldiers. N Engl J Med. 2016;375(5):435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aufradet E, Monchanin G, Oyonno-Engelle S, et al. Habitual physical activity and endothelial activation in sickle cell trait carriers. Med Sci Sports Exerc. 2010;42(11): 1987–1994. [DOI] [PubMed] [Google Scholar]
- 14.Bergeron MF, Cannon JG, Hall EL, Kutlar A. Erythrocyte sickling during exercise and thermal stress. Clin J Sport Med. 2004;14(6):354–356. [DOI] [PubMed] [Google Scholar]
- 15.Chirico EN, Martin C, Faes C, et al. Exercise training blunts oxidative stress in sickle cell trait carriers. J Appl Physiol. 2012;112(9):1445–1453. [DOI] [PubMed] [Google Scholar]
- 16.Diaw M, Samb A, Diop S, et al. Effects of hydration and water deprivation on blood viscosity during a soccer game in sickle cell trait carriers. Br J Sports Med. 2014;48(4):326–331. [DOI] [PubMed] [Google Scholar]
- 17.Tripette J, Loko G, Samb A, et al. Effects of hydration and dehydration on blood rheology in sickle cell trait carriers during exercise. Am J Physiol Heart Circ Physiol. 2010;299(3):H908–914. [DOI] [PubMed] [Google Scholar]
- 18.Nath KA, Hebbel RP. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol. 2015;11(3):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller P, Best WR, Nelson RB, Becktel J. Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenase deficiency in hospitalized black male patients. N Engl J Med. 1979;300(18):1001–1005. [DOI] [PubMed] [Google Scholar]
- 20.Eckert DE, Jonutis AJ, Davidson AJ. The incidence and manifestations of urographic papillary abnormalities in patients with S hemoglobinopathies. Radiology. 1974;113(1):59–63. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez O, Rodriguez MM, Jordan L, Sarnaik S. Renal medullary carcinoma and sickle cell trait: a systematic review. Pediatr Blood Cancer. 2015;62(10):1694–1699. [DOI] [PubMed] [Google Scholar]
- 22.Kiryluk K, Jadoon A, Gupta M, Radhakrishnan J. Sickle cell trait and gross hematuria. Kidney Int. 2007;71(7):706–710. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe IC, Billis A, Guimaraes MS, et al. Renal medullary carcinoma: report of seven cases from Brazil. Mod Pathol. 2007;20(9):914–920. [DOI] [PubMed] [Google Scholar]
- 24.Pecker LH, Naik RP. The current state of sickle-cell trait: implications for reproductive and genetic counseling. Blood. 2018. November 28 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2014;312(20):2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naik RP, Irvin MR, Judd S, et al. Sickle cell trait and the risk of ESRD in blacks. J Am Soc Nephrol. 2017;28(7):2180–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dueker ND, Della-Morte D, Rundek T, Sacco RL, Blanton SH. Sickle cell trait and renal function in Hispanics in the United States: the Northern Manhattan Study. Ethn Dis. 2017;27(1):11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukendi K, Lepira FB, Makulo JR, Sumaili KE, Kayembe PK, Nseka MN. Sickle cell trait is not associated with chronic kidney disease in adult Congolese patients: a clinic-based, cross-sectional study. Cardiovasc J Afr. 2015;26(3):125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajayi AA, Kolawole BA. Sickle cell trait and gender influence type 2 diabetic complications in African patients. Eur J Intern Med. 2004;15(5):312–315. [DOI] [PubMed] [Google Scholar]
- 30.Lonsdorfer A, Comoe L, Yapo AE, Lonsdorfer J. Proteinuria in sickle cell trait and disease: an electrophoretic analysis. Clin Chim Acta. 1989;181(3):239–247. [DOI] [PubMed] [Google Scholar]
- 31.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashley-Koch AE, Okocha EC, Garrett ME, et al. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol. 2011;155(3):386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saraf SL, Shah BN, Zhang X, et al. APOL1, alpha-thalassemia, and BCL11A variants as a genetic risk profile for progression of chronic kidney disease in sickle cell anemia. Haematologica. 2017;102(1):e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hicks PJ, Langefeld CD, Lu L, et al. Sickle cell trait is not independently associated with susceptibility to end-stage renal disease in African Americans. Kidney Int. 2011;80(12): 1339–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raffield LM, Ulirsch JC, Naik RP, et al. Common alpha-globin variants modify hematologic and other clinical phenotypes in sickle cell trait and disease. PLoS Genet. 2018;14(3):e1007293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin H, Key NS, Benson JM, et al. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007;110(3): 908–912. [DOI] [PubMed] [Google Scholar]
- 37.Bucknor MD, Goo JS, Coppolino ML. The risk of potential thromboembolic, renal and cardiac complications of sickle cell trait. Hemoglobin. 2014;38(1):28–32. [DOI] [PubMed] [Google Scholar]
- 38.Folsom AR, Tang W, Roetker NS, et al. Prospective study of sickle cell trait and venous thromboembolism incidence. J Thromb Haemost. 2015;13(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheikha A. Splenic syndrome in patients at high altitude with unrecognized sickle cell trait: splenectomy is often unnecessary. Can J Surg. 2005;48(5):377–381. [PMC free article] [PubMed] [Google Scholar]
- 40.Seegars MB, Brett AS. Splenic infarction associated with sickle cell trait at low altitude. Hematology. 2015;20(10):607–609. [DOI] [PubMed] [Google Scholar]
- 41.Goodman J, Hassell K, Irwin D, Witkowski EH, Nuss R. The splenic syndrome in individuals with sickle cell trait. High Alt Med Biol. 2014;15(4):468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larrabee KD, Monga M. Women with sickle cell trait are at increased risk for preeclampsia. Am J Obstet Gynecol. 1997;177(2):425–428. [DOI] [PubMed] [Google Scholar]
- 43.Hamdi IM, Karri KS, Ghani EA. Pregnancy outcome in women with sickle cell trait. Saudi Med J. 2002;23(12):1455–1457. [PubMed] [Google Scholar]
- 44.Adeyemi AB, Adediran IA, Kuti O, Owolabi AT, Durosimi MA. Outcome of pregnancy in a population of Nigerian women with sickle cell trait. J Obstet Gynaecol. 2006;26(2):133–137. [DOI] [PubMed] [Google Scholar]
- 45.Porter B, Key NS, Jauk VC, Adam S, Biggio J, Tita A. Impact of sickle hemoglobinopathies on pregnancy-related venous thromboembolism. Am J Perinatol. 2014;31(9):805–809. [DOI] [PubMed] [Google Scholar]
- 46.Cohen-Solal M, Prehu C, Wajcman H, et al. A new sickle cell disease phenotype associating Hb S trait, severe pyruvate kinase deficiency (PK Conakry), and an alpha2 globin gene variant (Hb Conakry). Br J Haematol. 1998;103(4):950–956. [DOI] [PubMed] [Google Scholar]
- 47.Alli N, Coetzee M, Louw V, et al. Sickle cell disease in a carrier with pyruvate kinase deficiency. Hematology. 2008;13(6):369–372. [DOI] [PubMed] [Google Scholar]
- 48.Ustun C, Kutlar F, Holley L, Seigler M, Burgess R, Kutlar A. Interaction of sickle cell trait with hereditary spherocytosis: splenic infarcts and sequestration. Acta Haematol. 2003;109(1):46–49. [DOI] [PubMed] [Google Scholar]
- 49.Bernaudin F, Arnaud C, Kamdem A, et al. Biological impact of alpha genes, beta haplotypes, and G6PD activity in sickle cell anemia at baseline and with hydroxyurea. Blood Adv. 2018;2(6):626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benkerrou M, Alberti C, Couque N, et al. Impact of glucose-6-phosphate dehydrogenase deficiency on sickle cell anaemia expression in infancy and early childhood: a prospective study. Br J Haematol. 2013;163(5):646–654. [DOI] [PubMed] [Google Scholar]
- 51.Bouanga JC, Mouele R, Prehu C, Wajcman H, Feingold J, Galacteros F. Glucose-6-phosphate dehydrogenase deficiency and homozygous sickle cell disease in Congo. Hum Hered. 1998;48(4):192–197. [DOI] [PubMed] [Google Scholar]
- 52.Geva A, Clark JJ, Zhang Y, Popowicz A, Manning JM, Neufeld EJ. Hemoglobin Jamaica Plain–a sickling hemoglobin with reduced oxygen affinity. N Engl J Med. 2004;351(15):1532–1538. [DOI] [PubMed] [Google Scholar]
- 53.Jorge SE, Petruk AA, Kimura EM, et al. Hb S-Sao Paulo: a new sickling hemoglobin with stable polymers and decreased oxygen affinity. Arch Biochem Biophys. 2012;519(1):23–31. [DOI] [PubMed] [Google Scholar]
- 54.Monplaisir N, Merault G, Poyart C, et al. Hemoglobin S antilles: A variant with lower solubility than hemoglobin S and producing sickle cell disease in heterozygotes. Proc Natl Acad Sci U S A. 1986;83(24):9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cross TJ, Berry PA, Akbar N, Wendon J, Thein SL, Harrison PM. Sickle liver disease-an unusual presentation in a compound heterozygote for HbS and a novel beta-thalassemia mutation. Am J Hematol. 2007;82(9):852–854. [DOI] [PubMed] [Google Scholar]
- 56.Koenig SC, Becirevic E, Hellberg MS, et al. Sickle cell disease caused by heterozygosity for Hb S and novel LCR deletion: report of two patients. Am J Hematol. 2009;84(9):603–606. [DOI] [PubMed] [Google Scholar]
- 57.Swensen JJ, Agarwal AM, Esquilin JM, et al. Sickle cell disease due to uniparental disomy in a child who inherited sickle cell trait. Blood. 2010;116(15):2822–2825. [DOI] [PubMed] [Google Scholar]
- 58.Vinatier I, Martin X, Costa JM, Bazin A, Giraudier S, Joly P. A late onset sickle cell disease reveals a mosaic segmental uniparental isodisomy of chromosome 11p15. Blood Cells Mol Dis. 2015;54(1):53–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.