Abstract
Global implementation of a birth‐dose hepatitis B (HB) vaccine has significantly reduced the prevalence of hepatitis B virus (HBV) carriers. Durable and sufficient titers of antibodies to hepatitis B surface antigen (anti‐HBs) are desirable for vaccinees to gain resistance to HBV exposure. However, the existence of primary nonresponders and vaccinees who lost anti‐HBs over time remains a challenge for the strategy of HBV elimination. We thus aim to clarify the mechanisms of acquisition and maintenance of vaccine‐induced anti‐HBs in healthy adults. We retrospectively analyzed the vaccination records of 3,755 first‐time HB‐vaccinated students and also traced the acquired antibody transition of 392 first‐time vaccinees for 10 consecutive years. To understand the cellular and humoral immune response, we prospectively examined peripheral blood from 47 healthy first‐time HB‐vaccinated students, 62 booster‐vaccinated health care workers, and 20 individuals who maintained their anti‐HBs. In responders, a significant increase of follicular helper T (Tfh) cells, activated plasmablasts, and plasma cells was observed in first‐time‐vaccinated but not booster‐vaccinated persons. We also discovered memory B cells and antibody‐secreting cells were more abundant in individuals who maintained anti‐HBs. According to vaccination records, higher anti‐HBs antibody titer acquisition was related to the longer term maintenance of anti‐HBs, the level of which was positively correlated with prevaccination levels of serum interferon‐γ and related chemokines. The second series of vaccination as a booster provided significantly higher anti‐HBs antibody titers compared to the initial series. Conclusion: Coordinated activation of Tfh and B‐cell lineages after HB vaccination is involved in the acquisition and maintenance of anti‐HBs. Our findings support the rationale of preconditioning the immune status of recipients to ensure durable vaccine responses.
Abbreviations
- anti‐HBs
antibody to hepatitis B surface antigen
- ASC
antibody‐secreting cell
- Bmem cell
memory B cell
- CCR7
chemokine (C‐C motif) receptor 7
- CD
clusters of differentiation
- cTfh cell
circulating follicular helper T cell
- CXCL
chemokine (C‐X‐C motif) ligand
- CXCR
chemokine (C‐X‐C motif) receptor
- HB
hepatitis B
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HLA
human leukocyte antigen
- IFN‐γ
interferon‐γ
- Ig
immunoglobulin
- PB
plasmablast
- PC
plasma cell
- Tfh cell
follicular helper T cell
- Th cell
T helper cell
Hepatitis B virus (HBV) infection is one of the largest public health issues worldwide. To prevent the mother‐to‐child transmission (MTCT) of HBV, the World Health Organization (WHO) recommends that hepatitis B (HB) vaccination be given within 24 hours of birth.1 In general, three‐dose HB vaccination provides detectable HB surface antigen (HBsAg)‐specific antibodies (anti‐HBs) in more than 95% of children without major complications.2 Significant progress has been made in providing HB vaccination, and global coverage with the three doses of HB vaccine in infancy reached 84% according to the Global Hepatitis Report 2017.3 Consequently, in the Western Pacific region, it has been estimated that 10 million cases of chronic HB have been prevented, with a reduction of approximately 2.5 million HB‐related deaths since 2003.4 However, despite WHO recommendations, the global coverage of birth‐dose vaccination is still low, and significant regional differences exist. In 2014, only 96 of 194 (49%) countries offered birth‐dose HB vaccination as a national immunization schedule, and fewer than 38% of babies born worldwide received timely birth‐dose vaccination within 24 hours after birth.5
Adults and children who are immunocompetent, having acquired anti‐HBs ≥10 mIU/mL approximately 1 month after a complete three‐dose HB vaccination, are considered seroprotected and deemed vaccine responders.6 Although vaccine‐induced anti‐HBs usually declines over time and sometimes becomes undetectable, booster vaccination for adults is not currently recommended by the Centers for Disease Control and Prevention.7 One rationale for this policy is a report that acquired specific immunity, at least to a vaccine, is maintained for more than 30 years after the birth dose.8 However, Stramer et al.9 reported that HBV‐DNA was detected in 6 HB‐vaccinated study donors in the analysis of 520 randomly selected samples from blood donors in the United States. The anti‐HBs titers of the 6 persons were all less than 100 mIU/mL. Accordingly, a low titer (<100 mIU/mL) of vaccine‐induced anti‐HBs is potentially insufficient to prevent HBV infection, especially with viral genotypes other than the vaccine genotype.
Until 2016 when the birth‐dose vaccination was introduced, babies born from mothers who were HBV‐positive have been selectively given HB vaccine and HB immunoglobulin (Ig) to prevent MTCT in Japan. Although this strategy has successfully reduced the number of chronic HBV carriers, horizontal HBV infection during infancy had not been completely prevented. Moreover, the low proportion of individuals positive for anti‐HBs among adolescents and young adults is attributable to a gradual increase of sporadically observed patients with acute HB as a sexually transmitted disease in Japan.
HB vaccination is recommended for health care workers or persons at high risk of exposure to HBV‐containing body fluids if they did not receive the birth‐dose vaccination. In our university, medical students and staff have received HB vaccinations. Every year, approximately 10% of vaccinees have been unable to acquire a detectable level of anti‐HBs; therefore, elucidating the immunologic mechanism of the failure is crucial. To generate and maintain anti‐HBs by the HB vaccine, several steps of immune responses are expected to be required. We classified the vaccine‐induced immune reactions into three steps: (1) the acquisition of anti‐HBs after the first HB vaccination; (2) the maintenance of detectable levels of anti‐HBs; and (3) the response to a booster vaccination in recipients who have lost their anti‐HBs.
In this study, we sought to clarify the pivotal immunologic factors involved in these three steps. We also analyzed the cumulative vaccination records of thousands of HB vaccine recipients to determine the long‐term persistence of anti‐HBs after HB vaccination in healthy adults who were stratified by vaccine‐induced anti‐HBs titers. Based on these analyses, we aim to understand immune reactions to the HB vaccine to improve the efficacy of the vaccine for prevention or as a therapeutic agent.
Participants and Methods
HB Vaccination Program and Study Populations
At Showa University, medical students who have never received the HB vaccine or been infected, confirmed by the absence of anti‐HBs or HBsAg, are vaccinated before clinical training. The HB vaccination series consists of three injections: the initial injection and injections given 1 and 6 months after the initial shot. One month after the third shot, vaccine‐induced anti‐HB titers were measured with the Architect‐i4000 system (Abbott Japan, Tokyo, Japan). Subjects with anti‐HBs ≥10 mIU/mL are deemed to be vaccine responders. These anti‐HBs data have been archived as vaccination records in the Showa University Health Service Center, and we analyzed the records of first‐time HB vaccinations between 2004 and 2015 (n = 3,755; Table 1). Three different kinds of HB vaccine were used in each fiscal year based on their availability: genotype C HBV vaccine containing Pre‐S2 protein (Meinyu; Meiji Dairies Co., Tokyo, Japan) (n = 1,166); genotype C HBV vaccine (Bimmugen; Kaketsuken, Kumamoto, Japan) (n = 2,216); and genotype A HBV vaccine (Heptavax‐II; MSD K.K., Tokyo, Japan) (n = 373) (Supporting Table S1).
Table 1.
Retrospective Analysis of Anti‐HBs Antibody Acquisition Rates and Titers Induced by Three Kinds of HB Vaccine in First‐Time‐Vaccinated Students
| Meinyu | Bimmugen | Heptavax‐II | Total | |
|---|---|---|---|---|
| Antigen | adr + PreS2 (10 μg) | adr (10 μg) | adw (10 μg) | |
| Year | 2004‐2008 | 2009, 2010, 2012‐2015 | 2011 | 2004‐2015 |
| Participants | ||||
| Total | 1,166 | 2,216 | 373 | 3,755 |
| Male | 698 | 983 | 174 | 1,855 |
| Female | 468 | 1,233 | 199 | 1,900 |
| Age (mean ± SD, years) | ||||
| Total | 23.2 ± 1.76 | 21.7 ± 1.23 | 21.8 ± 1.73 | 22.2 ± 1.69 |
| Male | 23.2 ± 1.56 | 22.1 ± 1.19 | 21.3 ± 1.44 | 22.4 ± 1.76 |
| Female | 23.0 ± 2.07 | 21.4 ± 1.07 | 22.0 ± 2.19 | 21.9 ± 1.76 |
| Responders: numbers (response rate) | ||||
| Total | 1,085 (93.1%) | 2,036 (91.9%) | 318 (85.3%) | 3,439 (91.6%) |
| Male | 636 (91.1%) | 865 (88.0%) | 137 (78.7%) | 1,638 (88.3%) |
| Female | 449 (95.9%) | 1,171 (95%) | 181 (91%) | 1,801 (94.8%) |
| Acquired anti‐HBs antibody titer (mIU/mL): number (% of total) | ||||
| HBsAb < 10 | 81 (6.9%) | 180 (8.1%) | 55 (14.7%) | 316 (8.4%) |
| 10 ≤ HBsAb < 100 | 124 (10.6%) | 834 (37.6%) | 115 (30.8%) | 1,073 (28.6%) |
| 100 ≤ HBsAb < 1,000 | 369 (31.6%) | 983 (44.4%) | 177 (47.5%) | 1,529 (40.7%) |
| HBsAb ≥ 1,000 | 592 (50.8%) | 219 (9.9%) | 26 (7%) | 837 (22.3%) |
Abbreviations: HBsAb, antibodies to hepatitis B surface antigen; PreS2, Pre‐S2 protein.
Among the 3,700 full‐time workers at Showa University Hospital who underwent vaccination against HBV between 1998 and 2015, 447 were vaccinated multiple times as booster injections (Supporting Table S2) because their anti‐HBs became undetectable (<10 mIU/mL) at some point. Booster vaccinations were performed on the same schedule, and acquired anti‐HBs antibody titers were measured as described above.
All retrospective and prospective analyses in this study were approved by the institutional research ethics committees at Showa University and the National Center for Global Health and Medicine (Ichikawa, Japan). We obtained written informed consent from all the participants in the prospective study under protocols approved by the Ethics Committee of Showa University in accordance with the 1975 Declaration of Helsinki and its later amendments or comparable ethical standards.
Long‐Term Follow‐Up of Anti‐HBs Antibody after First‐Time HB Vaccination
We also analyzed the medical check‐up records, including anti‐HBs antibody titers for 2001‐2016, and 392 persons had been followed every year for more than 10 consecutive years. These workers were stratified in three groups based on their initially acquired anti‐HBs antibody titers: low (10 ≤ anti‐HBs < 100 mIU/mL), high (100 ≤ anti‐HBs < 1,000 mIU/mL), and very high (anti‐HBs ≥1,000 mIU/mL). Changes in the percentages of individuals positive for anti‐HBs over time were analyzed with the Kaplan‐Meier method.
Analysis of Immune Reactions to HB Vaccination; their Impact on the Acquisition and Maintenance of Anti‐HBs Antibody
To analyze the immune reactions to HB vaccination, we collected peripheral blood from three groups: first‐time‐vaccinated students, booster‐vaccinated workers, and individuals who had maintained sufficient anti‐HBs antibody for 5 years (Table 2). We collected peripheral blood mononuclear cells and sera before vaccination and 1 month after the third injection. Of the medical students vaccinated for the first time with Heptavax‐Ⅱ in 2016, 47 agreed to be enrolled in the analysis (first‐time vaccination group). We also analyzed 62 booster‐vaccinated workers (30 persons treated with Bimmugen in 2015 and 32 persons treated with Heptavax‐Ⅱ in 2016) who lost anti‐HBs after the initial HB vaccination and agreed to be enrolled in this analysis (booster vaccination group). To clarify the factors that contribute to anti‐HBs maintenance, we recruited 20 individuals who had maintained sufficient levels of anti‐HBs as a control (maintenance group).
Table 2.
Participant Characteristics Used in the Immunologic Analysis
| First‐Time Vaccination | Booster Vaccination | Maintain | |||||
|---|---|---|---|---|---|---|---|
| Failure | Responder | Total (2016) | 2015 | 2016 | Total | Total (2016) | |
| Vaccine | Heptavax‐II | Heptavax‐II | Bimmugen | Heptavax‐II | ‐ | ||
| Number | 11 | 36 | 47 | 30 | 32 | 62 | 20 |
| Male | 9 | 16 | 25 | 15 | 16 | 31 | 9 |
| Female | 2 | 20 | 22 | 15 | 16 | 31 | 11 |
| Age (mean ± SD), years | 21.9 ± 1.21 | 21.8 ± 1.15 | 21.8 ± 1.16 | 35.8 ± 9.87 | 30.2 ± 8.86 | 32.8 ± 9.65 | 38.2 ± 8.14 |
| Health check data | |||||||
| Height (cm) | 170.2 | 163.6 | 165.18 | 164.1 | 165.6 | 164.8 | 165.2 |
| Weight (kg) | 62.0 | 57.7 | 58.7 | 54.6 | 60.7 | 58 | 60.7 |
| BMI (kg/m2) | 21.3 | 21.4 | 21.8 | 20.3 | 22.0 | 21.3 | 22.1 |
| WBC (/μL) | NA | NA | NA | 6.8 | 6.4 | 6.5 | 6.4 |
| RBC (/μL) | NA | NA | NA | 450.5 | 471.9 | 464.7 | 458.3 |
| Hb (g/dL) | NA | NA | NA | 13.4 | 14.2 | 13.8 | 13.8 |
| Ht (mg/dL) | NA | NA | NA | 41.6 | 43.8 | 43.1 | 43.1 |
| Plt (/μL) | NA | NA | NA | 27.4 | 28.1 | 27.8 | 27.9 |
| Neutro (%) | NA | NA | NA | 57.7 | 55.0 | 56.1 | 61.6 |
| Ly (%) | NA | NA | NA | 31.7 | 35.6 | 34 | 29.1 |
| AST (IU/L) | 22.3 | 21.4 | 21.6 | 21.8 | 24.4 | 23.6 | 24.2 |
| ALT (IU/L) | 19.4 | 18.9 | 19 | 15.4 | 22.1 | 20.3 | 23.6 |
| γGT (IU/L) | 25.5 | 25.1 | 25.2 | 24.0 | 21.6 | 22.7 | 24.8 |
| Alb (g/dL) | 4.6 | 4.7 | 4.69 | 4.6 | 4.5 | 4.57 | 4.58 |
| T‐chol (mg/dL) | 176.6 | 186.4 | 184.1 | 184.1 | 187.1 | 187.5 | 198.2 |
| TG (mg/dL) | 118.5 | 87.9 | 95.1 | 74.3 | 79.3 | 79.4 | 83.6 |
Abbreviations: Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; γGT, gamma‐glutamyltransferase; Hb, hemoglobin; Ht, hematocrit; Ly, lymphocytes; Neutro, neutrophils; Plt, platelets; RBC, red blood cell count; T‐chol, total cholesterol; TG, triglyceride; WBC, white blood cell count.
Immunologic Analysis Using Flow Cytometry and Multiplex Analysis
To evaluate the phenotypes of immune cells, a multicolor flow‐cytometric analysis was performed with an LSR Fortessa cell analyzer (BD, San Jose, CA) and the FlowJo software (Tree Star, Inc., Ashland, OR). The cells analyzed and the gating strategies are shown in Supporting Fig. S1. The concentrations of cytokines and chemokines in serum samples were examined with the Bio‐Plex Pro Human Chemokine Panel, 40‐Plex (Bio‐Rad, Hercules, CA) and a customized Human Magnetic Luminex Assay (R&D Systems, Minneapolis, MN) on the Bio‐Plex 3D Suspension Array System. The reagents are listed in Supporting Table S3.
Statistical Analysis
Values for the clinical and immunologic parameters were compared using a log‐rank test, analysis of variance, Fisher's exact test, the Wilcoxon rank sum test, or a matched‐pair analysis, as appropriate, with Prism7 (GraphPad, La Jolla, CA). P < 0.05 was considered significant.
Results
Retrospective Analysis of Anti‐HBs Antibody Titers Induced with Three Different Vaccines in First‐Time‐Vaccinated Students
Based on vaccination records, we analyzed the response rates and acquired anti‐HBs antibody titers of students who were vaccinated for the first time (Table 1). The response rates to different HB vaccines were similar and, on average, 91.6% of students acquired anti‐HBs. The acquisition rate of anti‐HBs to each HB vaccine was lower in male participants than in female participants. The number of subjects who produced >1,000 mIU/mL anti‐HBs (very high responders) was greater in those given Meinyu than in those vaccinated with the other two vaccines.
Higher Anti‐HBs Antibody Titers in First‐Time Vaccinees LED to Longer Persistence of Anti‐HBs in Healthy Adults
Based on medical health‐check records, we analyzed the changes in subjects positive for anti‐HBs, who were stratified by their initially acquired anti‐HBs antibody titers after first‐time HB vaccination (Fig. 1A). Among the 392 individuals who could be followed more than 10 consecutive years, 75 individuals maintained titers above 10 mIU/mL without booster vaccination. Of these 94 individuals with a very high response (initial anti‐HBs ≥1,000 mIU/mL), 57 maintained detectable anti‐HBs, whereas subjects with low or high responses lost considerable anti‐HBs (P < 0.0001). Although the rate of anti‐HBs acquisition was higher in female than male participants, the maintenance of anti‐HBs did not differ significantly (Fig. 1B).
Figure 1.
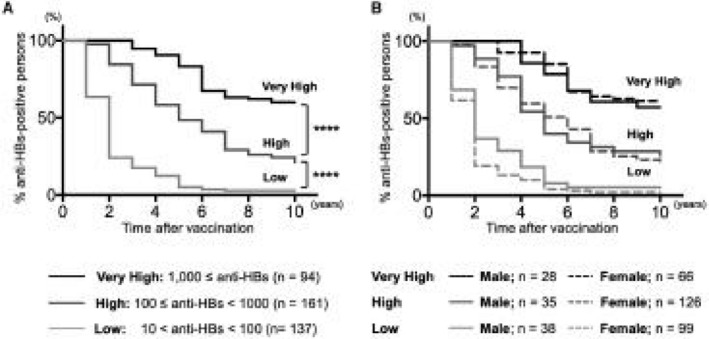
Annual follow‐up of acquired anti‐HBs in first‐time HB vaccinees for 10 years. Anti‐HBs induced by first‐time HB vaccination was monitored regularly in annual health checks. Vaccinees (n = 392) who could be followed for 10 consecutive years were stratified in three groups, depending on their initially acquired anti‐HBs antibody titers: low (10 mIU/mL ≤ anti‐HBs < 100 mIU/mL), high (100 mIU/mL ≤ anti‐HBs < 1,000 mIU/mL), and very high (anti‐HBs ≥1,000 mIU/mL). The y axis expresses the proportion of each group who maintained detectable anti‐HBs antibody titers, and the changes are shown with Kaplan‐Meier curves. (A) Higher acquired anti‐HBs titers led to longer anti‐HBs maintenance (****P < 0.0001). (B) In each group, male participants are shown with a straight line and female participants with a dotted line. No significant difference in any group was observed based on sex.
Optimal Increase in Follicular Helper T Cells and Induction of Activated Antibody‐Secreting Cells are Required for Favorable Response to the HB Vaccine in First‐Time Vaccinees
We analyzed the immune reactions of first‐time‐vaccinated students. Among 47 students, 36 produced anti‐HBs ≥ mIU/mL (responders) and 11 failed to do so (failures) (Table 2). We used flow cytometry to analyze the differences between responders and failures in the phenotypes and frequencies of various immune cells.
HB vaccination significantly increased the frequency of bulk T cells in the responders, with a relative reduction in B cells, whereas no other immune cells differed between the groups, including monocytes, dendritic cells, natural killer cells, and natural killer T cells (Fig. 2A; Supporting Fig. S2A). In a subpopulation analysis of T cells, the frequency of clusters of differentitation (CD)4+ or CD8+ T cells among the total CD3+ T cells did not change significantly (Supporting Fig. S2B). However, T cells with a CD45RA− memory phenotype, especially CD45RA− chemokine (C‐C motif) receptor 7 (CCR7)+ central memory T cells in addition to effector memory T cells (CD45RA−CCR7−), were elevated in responders (Supporting Fig. S2C). These memory T cells displayed increased surface expression of CD40L (Supporting Fig. S2D). In parallel with the increases in these cells, the frequency of naive T cells (CD45RA+CCR7+) decreased, suggesting that the HB vaccine induced differentiation from the naive T‐cell phenotype to the memory T‐cell phenotype.
Figure 2.
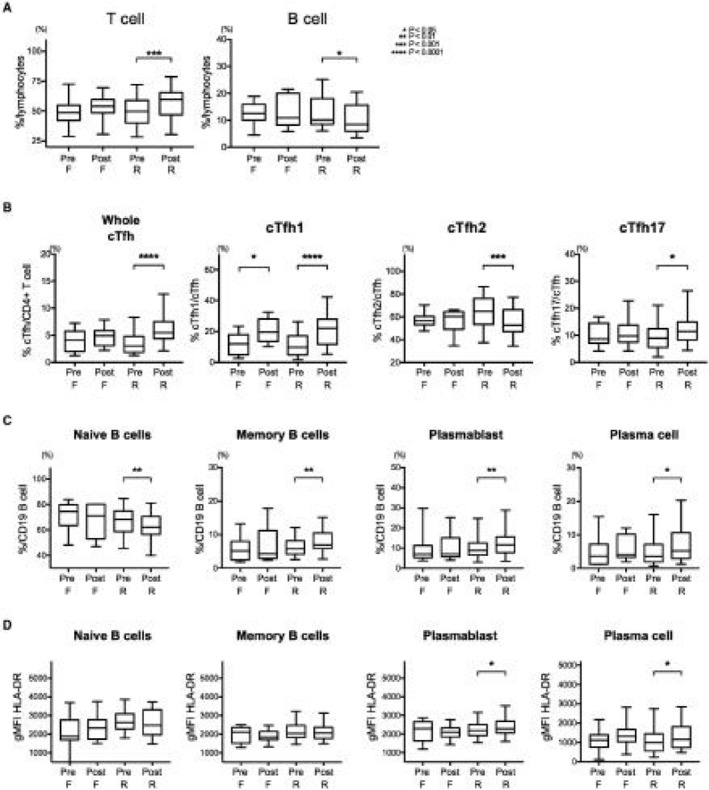
Comprehensive analysis of changes in peripheral immune cells after first‐time vaccination. PBMCs were stained with a multiple staining panel, and the data before and 7 months after the initial HB vaccination are shown, categorized by the vaccine response (failure, n = 11; responder, n = 36). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (A) Among the peripheral immune cells, T‐cell frequency increased significantly (P < 0.0001), accompanied by B‐cell reduction (P = 0.0152), in responders, but no other cells showed significant differences by HB vaccination. (B) In responders, among the CD4+ cells, cTfh cells were significantly increased (P < 0.0001). Among the cTfh cells, the proportion of cTfh1 cells (P < 0.0001) and cTfh17 cells (P = 0.0144) increased significantly, whereas cTfh2 cells decreased (P = 0.0013). (C) Bmem cells (P = 0.0021), PBs (P = 0.0014), and PCs (P = 0.0175) were significantly increased in responders, whereas naive B cells decreased (P = 0.0045). (D) HB vaccination also induced significant activation of PBs (P = 0.0393) and PCs (P = 0.0210). Boxplot shows median ± interquartile range and whisker shows min to max. Abbreviations: F, failure; gMFI, geometric mean fluorescence intensity; PBMC, peripheral blood mononuclear cell; Post, after vaccination; Pre, prevaccination; R, responder.
An essential player in the antigen‐specific antibody response is CD4+ chemokine (C‐X‐C motif) receptor 5 (CXCR5)+ follicular helper T (Tfh) cells. Although the interaction between Tfh and B cells occurs in germinal centers (GCs) located in secondary lymph nodes, Tfh cells are also detected in the peripheral blood as circulating Tfh (cTfh) cells10 (Supporting Fig. S2E). cTfh cells were only significantly increased in responders (Fig. 2B; Supporting Fig. S2E). Among the three cTfh subpopulations, the proportions of cTfh1 (CXCR3+CCR6–) and cTfh17 (CXCR3−CCR6+) cells were significantly increased by the HB vaccine in responders, whereas cTfh2 (CXCR3−CCR6−) cells were reduced.
B‐cell differentiation produces antibody‐secreting cells (ASCs), such as plasmablasts (PBs) and plasma cells (PCs). Among the CD19+ B‐cell population, only naive B cells (IgD+CD27−) decreased, whereas class‐switched memory B cells (Bmems) (IgG+CD27+), CD27+CD38+ PBs, and CD138+ PCs were significantly increased in responders (Fig. 2C; Supporting Fig. S2F). The increased expression of the activation marker human leukocyte antigen DR isotype (HLA‐DR) on PBs and PCs but not on naive B cells or Bmem cells was observed in responders (Fig. 2D). Serum levels of cytokines or chemokines before vaccination did not differ significantly between responders and nonresponders (Supporting Table S4).
Interferon‐γ, Chemokine (C‐X‐C Motif) Ligand 9, 10, and 12, and Interleukin‐6 Levels in Prevaccine Sera were Associated with Acquired Anti‐HBs Antibody Titers
To elucidate the immunologic factors that are important for acquiring higher anti‐HBs, we analyzed the immune response in primary vaccine responders. Although HB vaccination increased the frequencies of cTfh cells and ASCs, these did not differ according to the acquired anti‐HBs antibody titer (data not shown). In contrast, among the panel of cytokines and chemokines shown in Supporting Table S4, interferon‐γ (IFN‐γ), chemokine (C‐X‐C motif) ligand 9 (CXCL9), CXCL10, CXCL12, and interleukin‐6 (IL‐6) levels before HB vaccination correlated positively with the vaccine‐induced anti‐HBs antibody titer (Fig. 3A,B; Supporting Fig. S3A). These factors also correlated strongly with each other (Supporting Fig. S3B). No other soluble factors showed a significant association with the acquired anti‐HBs antibody titer.
Figure 3.
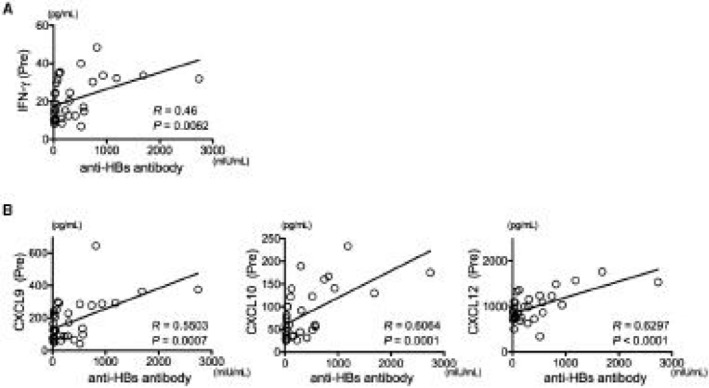
Preexisting IFN‐γ regulated the anti‐HBs antibody titer induced by HB vaccination. The relationships between immunologic factors and the acquired anti‐HBs antibody titers were investigated. (A) Preexisting IFN‐γ concentration was significantly associated with the acquired anti‐HBs antibody titer. (B) IFN‐γ ‐associated chemokines (CXCL9, CXCL10, and CXCL12) were significantly associated with the acquired anti‐HBs antibody titer. Abbreviation: Pre, prevaccination.
Higher Frequencies of Memory Bmem Cells and ASCs are Associated with the Persistence of Vaccine‐Induced Anti‐HBs Antibody
To identify the factors essential for anti‐HBs persistence, we next compared the immune cells and serum proteins in individuals who retained their anti‐HBs for more than 5 years (Maintenance group) with those in individuals who lost detectable level of anti‐HBs (Lost group; also booster vaccination group) before their booster vaccination. We analyzed the immune cells and serum proteins using the strategy described above. The frequencies of Bmem cells, PBs, and PCs were lower in the Lost group than in the Maintenance group, whereas no other immune cells differed significantly between these groups (Fig. 4A). Among the B‐cell lineage, only the frequency of PCs was strongly associated with the anti‐HBs antibody titers in the Maintenance group (Fig. 4B). These results indicate that Bmem cells and ASCs are involved in the persistence of acquired anti‐HBs.
Figure 4.
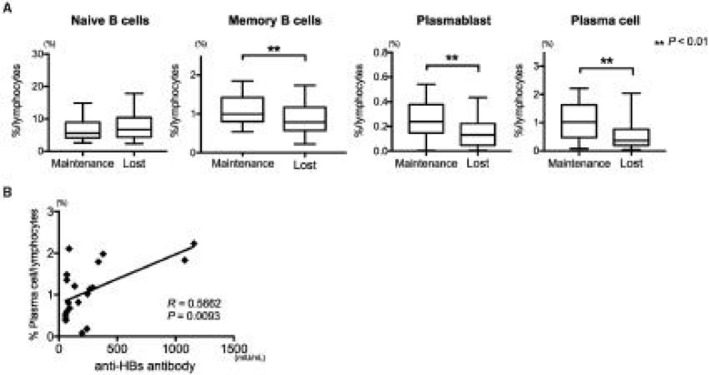
Significantly lower frequency of class‐switched B cells in vaccinees who lost anti‐HBs. Maintenance, individuals who maintained anti‐HBs antibody titers (≥50 mIU/mL) for more than 5 years; n = 20. Lost, individuals who lost their anti‐HBs antibody titers; n = 62. (A) In the Lost group, the frequencies of Bmem cells (P = 0.0095), PBs (P = 0.0092), and PCs (P = 0.0017) were significantly lower than those in the Maintenance group. (B) Plasma‐cell frequency was associated with the anti‐HBs antibody titer in individuals in the Maintenance group (R = 0.5662; P = 0.0093). Boxplot shows median ± interquartile range and whisker shows min to max.
Anti‐HBs Antibody Response was Augmented by Consecutive HB Vaccinations
We lastly performed immunologic analysis in booster‐vaccinated medical staff who lost detectable anti‐HBs. We prospectively analyzed the immune responses to booster vaccination based on the same strategy as the initially vaccinated students. Although the vaccines used were different (Bimmugen in 2015 and Heptavax‐Ⅱ in 2016), acquired anti‐HBs antibody titer and immune reactions were similar in both groups (Fig. 5A‐C). Interestingly, the pivotal immune cells, such as cTfh or ASCs, in anti‐HBs acquisition in first‐time‐vaccinated persons were not increased in booster‐vaccinated persons. No significant association between serum proteins and acquired anti‐HBs in booster‐vaccinated persons existed. To analyze the immunologic factors associated with anti‐HBs decline, we followed some individuals who acquired anti‐HBs from booster vaccinations in 2015 (n = 17) and 2016 (n = 12). We examined the relationship between the immune cells or serum proteins present before boosting and the degree of reduction in anti‐HBs antibody titers during 1 year (percentage reduction of anti‐HBs). The frequency of Bmem cells before booster vaccination correlated inversely with the reduction in anti‐HBs (Fig. 5D). No serum cytokine or chemokine was associated with the persistence of anti‐HBs.
Figure 5.
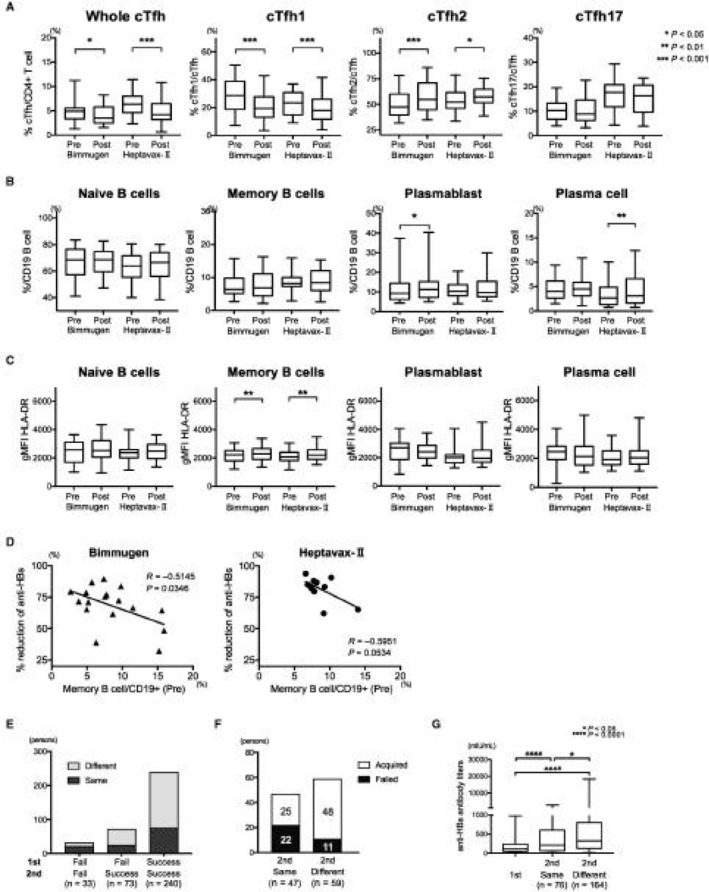
Prospective and retrospective analysis of responses to booster vaccination. We analyzed 62 booster‐vaccinated workers: 30 persons treated with Bimmugen in 2015 and 32 persons treated with Heptavax‐Ⅱ in 2016. No significant difference was observed in the acquired anti‐HBs antibody titers. (A) The frequencies of total cTfh cells, cTfh1 cells, and cTfh17 cells were significantly reduced by HB booster vaccination, whereas the frequency of cTfh2 cells increased. (B) Only modest increases in ASCs were observed after HB booster vaccination. (C) Significant activation, measured as HLA‐DR expression, was observed among Bmem cells but not among ASCs after booster vaccination. (D) In a proportion of booster‐vaccinated individuals, the reduction in anti‐HBs was inversely associated with the preexisting Bmem cell frequency 1 year after booster vaccinations by both Bimmugen (n = 17) and Heptavax‐Ⅱ (n = 12). Percentage reduction in anti‐HBs = 100 × (initially acquired anti‐HBs − anti‐HBs antibody titer 1 year after vaccination)/initially acquired anti‐HBs. (E) Among the 346 individuals who were vaccinated twice, 33 never acquired anti‐HBs (Fail/Fail), 73 acquired anti‐HBs after the second vaccination (Fail/Success), and 240 acquired anti‐HBs after both vaccinations (Success/Success). (F) Among the 106 individuals who failed to acquire anti‐HBs after their first series of vaccinations, 47 were vaccinated with the same vaccine and 59 with a different vaccine. Different vaccines induced detectable anti‐HBs more effectively (48/59, 81.4%) than the same vaccines (25/47, 53.2%) (P = 0.0029). (G) The first and second anti‐HBs antibody titers in 240 individuals who acquired anti‐HBs twice were compared. The second vaccination induced significantly higher anti‐HBs antibody titers than the first (P < 0.0001), especially when different vaccines were used (P = 0.0266). Boxplot shows median ± interquartile range and whisker shows min to max. Abbreviations: gMFI, geometric mean fluorescence intensity; Post, after vaccination; Pre, prevaccination.
To clarify the impact of booster vaccination, we retrospectively examined subjects who had lost their anti‐HBs and received consecutive vaccinations. Based on our records, 346 individuals were vaccinated twice (Supporting Table S5). We paid attention to the vaccine on both occasions; the response patterns of these individuals to the first and second vaccinations are shown in Fig. 5E. Thirty‐three persons were primary nonresponders (Fail/Fail). Of the 106 subjects who failed to produce anti‐HBs after the first vaccination, 73 successfully produced anti‐HBs after the second series (Fig. 5E; Fail/Success). When we examined the effects of the types of vaccines used, 25 of 47 (53%) individuals who received the same vaccine on both occasions acquired anti‐HBs whereas 48 of the 59 (81.4%) individuals who received different types of vaccines on the two occasions acquired anti‐HBs (Fig. 5F). Among the 240 consecutive responders in both series, the anti‐HBs antibody titers were higher after the second vaccination (booster) than after the first, especially when a different type of vaccine was used as the booster (Fig. 5G).
Discussion
The HB vaccine is one of the safest immunizations for recipients, preventing infection and thereby reducing the future risk of liver cancer.11 In this study, we analyzed adult populations that had not been given the birth‐dose HB vaccine. Because the vaccination records of thousands of people have been archived in our Health Service Center, we were able to track their anti‐HBs responses with information from their periodic health checks. We also analyzed their vaccine‐induced immune responses in various phases: (1) the acquisition of anti‐HBs; (2) the maintenance of anti‐HBs; and (3) the memory response induced by booster vaccination.
To provide high anti‐HBs antibody titers to vaccinated subjects, the selection of the vaccine type is a practical option. Although the anti‐HBs acquisition rates were approximately 90% in the HB vaccine‐naive young adults regardless of the type of vaccine used in this study, Meinyu, which contains the Pre‐S2 protein in addition to the adr type of HBsAg, induced very high anti‐HBs antibody titers (>1,000 mIU/mL) in 50.8% of vaccinees. Although we could not perform the immunologic analysis in detail because the vaccine is currently unavailable in Japan, the HB vaccines that contain Pre‐S1 or Pre‐S2 protein have been reported to be more immunogenic than the small HBsAg alone.12, 13 One of the proposed mechanisms of the prominent vaccine response is the Pre‐S proteins’ help for circumventing nonresponsiveness to HBsAg, which is partly controlled by HLA class II alleles.14 Therefore, the ethnic variations in HLA class‐II allele frequency and the employed vaccine formulation in each country could impact the response of HB vaccination.15, 16 We also identified primary nonresponders to multiple series of vaccinations. The mechanism of nonresponse may involve specific HLA alleles or single‐nucleotide polymorphisms of immune‐related genes. Further research into their genetic backgrounds is required to improve the efficacy. However, the use of HB vaccines that contain Pre‐S protein could be an attractive strategy to improve efficacy, especially in nonresponders or low responders, including individuals who are immunodeficient.
In addition to B‐cell lineages, T‐cell lineages are required to induce the optimal response and generate a protective level of anti‐HBs by the HB vaccine.17 In responders, we observed significant increases in cTfh cells and CD45RA− memory T cells with elevated CD40L expression, which are important for anti‐HBs acquisition after vaccination.18 Furthermore, a reduction in naive B cells and an increase of PBs or PCs were also observed in the responders, which indicate the differentiation of the B‐cell lineage. Tfh cells, which are essential for the induction of antigen‐specific B cells in the GC,19 circulate in the peripheral vasculature.20, 21 Among the cTfh cells, we observed significant increases in cTfh1 and cTfh17 cells but a reduction in cTfh2 cells in responders after first‐time HB vaccination. The key population of immune cells that is associated with a favorable response may differ depending on the kind of vaccine. For instance, cTfh1 cells are reportedly important in the response to influenza vaccines,22 whereas T helper type 2 (Th2) cells are important in the response to the papilloma virus vaccine23 and Th17 cells in the response to the Ebola vaccine.24
Anti‐HBs acquired with vaccination gradually decrease over time, and low anti‐HBs antibody titer may be insufficient to confer reliable protection.9 Individuals who maintained their anti‐HBs for a long time had more Bmem cells and ASCs than those who lost their anti‐HBs (Fig. 4A); the impaired maintenance of anti‐HBs can be partly attributed to a reduction in Bmem cells (Fig. 5D), which has also been reported in older persons.25
Based on our retrospective analysis shown in Fig. 1, acquiring a higher anti‐HBs antibody titer in the initial vaccination is of great importance to maintain the neutralizing antibody for a long time without distinction of sex. Interestingly, we found that the prevaccination levels of serum IFN‐γ, CXCL9, CXCL10, CXCL12, and IL‐6 correlated with their vaccine‐induced anti‐HBs antibody titers, whereas the frequencies or phenotypes of their immune cells were not significantly associated. Therefore, the basal immune status of the recipients contributes to their vaccination response. Because the early IFN‐γ response to vaccination is reported to be a predictor of immunologic memory,26 IFN‐γ could be used as an adjuvant, as is IFN‐α.27 It has also been reported that patients with atopic dermatitis show a reduced response to HB vaccination, suggesting that a Th2‐prone immune environment in the host inhibits this response.28 In a recent study, transcriptional and cytometric profiling showed that systemic inflammation before vaccination is associated with the age‐related response to the HB vaccine.17 Therefore, the prevaccination immune status of the recipient could be tailored to improve the vaccine response, for example, by modifying the vaccine with adjuvants or using multivalent vaccines.
As recommended in birth‐dose‐vaccinated persons in early adolescence,29 our retrospective analysis revealed that booster vaccination, preferably with a different kind of vaccine from the previous vaccine, is useful in providing higher anti‐HBs antibody titers, which are expected to result in longer anti‐HBs maintenance. The observed immune reactions in booster vaccination were different from those observed in first‐time vaccination. This appears to be reasonable because the immune response to booster vaccination originated from the immunologic memory and can bypass the steps of antigen‐specific acquisition that requires the help of Tfh cells. Although we did not observe a significant association between immunologic factors and anti‐HBs antibody titers in booster‐vaccinated persons, this is potentially because of the small sample size or the variation in age in the booster‐vaccinated group compared to initially vaccinated persons. Further study is indispensable to elucidate the mechanism.
We need to consider some substantial limitations of this study. First, as the mean age of the vaccinees in our analysis was more than 20 years old, the finding of the study might be biased. Second, our immunologic analyses were based only on samples collected at designated time points. Detectable peripheral immune reactions probably differ depending on the timing of the analysis and the kind of vaccine used. In fact, we were unable to detect the increase of CD71+IgD−CD38+ B cells that was reported in influenza vaccine recipients.30 Third, we were unable to assess the antigen‐specific cellular immune response to HB vaccination. Further study is warranted to evaluate the dynamics of HBsAg‐specific B cells and anti‐HBs.
In conclusion, the acquisition of high anti‐HBs antibody titer at vaccination is important for the long‐term maintenance of anti‐HBs. We demonstrated the vaccine‐induced anti‐HBs antibody titer is influenced by the prevaccination immune status of the recipient, and conditioning the immunologic milieu before vaccination can be a strategy to improve the impact of HB vaccination.
Supporting information
Acknowledgment
We thank the participants in the Showa University HB vaccination study for their valuable contributions. We also thank the staff of Showa University and the National Center for Global Health and Medicine, especially Hisako Nozawa, Chizu Kanokoda, and Hiromi Tamada for technical assistance; Yoko Nakajima and Shinya Nakatani for collecting samples; Sachiko Akaogi and Nanae Yagisawa for coordinating the schedules; and Ikuta Nakano for constructing the recording system at the Showa University Health Service Center.
Supported by a Grant‐in‐Aid for Young Scientists (B) (15K19352 to H.D.), the National Center for Global Health and Medicine (grants 29‐shi‐2 and 29‐shi‐1007 to T.K.), a Grant‐in‐Aid for Scientific Research (B) (17H04168 to T.K.), and the Japan Agency for Medical Research and Development, Program for Basic and Clinical Research on Hepatitis (grants JP17fk0310106h0001 and JP18fk0310106h0002 to T.K.).
Potential conflict of interest: Dr. Kanto is on the speakers’ bureau for MSD and Gilead. The other authors have nothing to report.
Contributor Information
Hiroyoshi Doi, Email: hachivan.qb@gmail.com.
Tatsuya Kanto, Email: kantot@hospk.ncgm.go.jp.
References
- 1. Committee on Infectious Diseases ; Committee on Fetus and Newborn . Elimination of perinatal hepatitis B: providing the first vaccine dose within 24 hours of birth. Pediatrics 2017;140:pii:e20171870. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Hepatitis B In Epidemiology and Prevention of vaccine‐preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html. Updated March 16, 2017. Accessed March 2019. [Google Scholar]
- 3. World Health Organization . Global hepatitis report 2017. https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Published April 2017. Accessed March 2019.
- 4. World Health Organization , Western Pacific Region . Hepatitis B control through immunization: a reference guide. https://www.who.int/immunization/sage/meetings/2015/october/8_WPRO_Hepatitis_B_Prevention_Through_Immunization_Regional_Reference_Guide.pdf. Accessed March 2019.
- 5. World Health Organization . Preventing perinatal hepatitis B virus transmission: a guide for introducing and strengthening hepatitis B birth dose vaccination. http://www.who.int/iris/handle/10665/208278. Published December 2015. Accessed March 2019.
- 6. Schillie S, Murphy TV, Sawyer M, Ly K, Hughes E, Jiles R, et al. Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health‐care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013;62:1‐19. [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . Hepatitis B questions and answers for health professionals. Hepatitis B information. https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm#vaccFAQ. Last updated October 31, 2018. Accessed March 2019.
- 8. Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30‐year follow‐up study and response to a booster dose. J Infect Dis 2016;214:16‐22. [DOI] [PubMed] [Google Scholar]
- 9. Stramer SL, Wend U, Candotti D, Foster GA, Hollinger FB, Dodd RY, et al. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med 2011;364:236‐247. [DOI] [PubMed] [Google Scholar]
- 10. Schmitt N, Bentebibel S‐E, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 2014;35:436‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of hepatitis B virus infection and impact of vaccination on disease. Clin Liver Dis 2016;20:607‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bertino JS, Tirrell P, Greenberg RN, Keyserling HL, Poland GA, Gump D, et al. A comparative trial of standard or high‐dose S subunit recombinant hepatitis B vaccine versus a vaccine containing S subunit, pre‐S1, and pre‐S2 particles for revaccination of healthy adult nonresponders. J Infect Dis 1997;175:678‐681. [DOI] [PubMed] [Google Scholar]
- 13. Krawczyk A, Ludwig C, Jochum C, Fiedler M, Heinemann FM, Shouval D, et al. Induction of a robust T‐ and B‐cell immune response in non‐ and low‐responders to conventional vaccination against hepatitis B by using a third generation PreS/S vaccine. Vaccine 2014;32:5077‐5082. [DOI] [PubMed] [Google Scholar]
- 14. Shouval D, Ilan Y, Adler R, Deepen R, Panet A, Even‐Chen Z, et al. Improved immunogenicity in mice of a mammalian cell‐derived recombinant hepatitis B vaccine containing pre‐S1 and pre‐S2 antigens as compared with conventional yeast‐derived vaccines. Vaccine 1994;12:1453‐1459. [DOI] [PubMed] [Google Scholar]
- 15. Wiesen E, Diorditsa S, Li X. Progress towards hepatitis B prevention through vaccination in the Western Pacific, 1990–2014. Vaccine 2016;34:2855‐2862. [DOI] [PubMed] [Google Scholar]
- 16. Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the South‐East Asia Region, 1992–2015. Vaccine 2018;36:6‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol 2012;12:479‐491. [DOI] [PubMed] [Google Scholar]
- 18. Goncalves L, Albarran B, Salmen S, Borges L, Fields H, Montes H, et al. The nonresponse to hepatitis B vaccination is associated with impaired lymphocyte activation. Virology 2004;326:20‐28. [DOI] [PubMed] [Google Scholar]
- 19. Fazilleau N, Mark L, Mcheyzer‐Williams LJ, Mcheyzer‐Williams MG. Follicular helper T cells: lineage and location. Immunity 2009;30:324‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bentebibel S‐E, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, et al. Induction of ICOS+CXCR20+CXCR20+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 2013;5:176ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morita R, Schmitt N, Bentebibel S‐E, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR21+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011;34:108‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lüthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, et al. The development and fate of follicular helper T cells defined by an IL‐21 reporter mouse. Nat Immunol 2012;13:491‐498. [DOI] [PubMed] [Google Scholar]
- 23. Matsui K, Adelsberger JW, Kemp TJ, Baseler MW, Ledgerwood JE, Pinto LA. Circulating CXCR23⁺CD4⁺ T follicular‐like helper cell and memory B cell responses to human papillomavirus vaccines. PLoS ONE 2015;10:e0137195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farooq F, Beck K, Paolino KM, Phillips R, Waters NC, Regules JA, et al. Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV‐ZEBOV Ebola vaccine. Sci Rep 2016;6:27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ademokun A, Wu Y‐C, Dunn‐Walters D. The ageing B cell population: composition and function. Biogerontology 2010;11:125‐137. [DOI] [PubMed] [Google Scholar]
- 26. Bejon P, Keating S, Mwacharo J, Kai OK, Dunachie S, Walther M, et al. Early gamma interferon and interleukin‐2 responses to vaccination predict the late resting memory in malaria‐naïve and malaria‐exposed individuals. Infect Immun 2006;74:6331‐6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, et al. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol 2002;169:375‐383. [DOI] [PubMed] [Google Scholar]
- 28. Patel DP, Treat JR, Castelo‐Socio L. Decreased hepatitis B vaccine response in pediatric patients with atopic dermatitis, psoriasis, and morphea. Vaccine 2017;35:4499‐4500. [DOI] [PubMed] [Google Scholar]
- 29. Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine 2017;35:6302‐6307. [DOI] [PubMed] [Google Scholar]
- 30. Ellebedy AH, Jackson KJ, Kissick HT, Nakaya HI, Davis CW, Roskin KM, et al. Defining antigen‐specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol 2016;17:1226‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


