Abstract
Background
The quest to cure or to contain the disease in cancer patients leads to new strategies and techniques being added to the armamentarium of oncologists. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) is a recently described surgical technique which is being evaluated at many centers for the management of peritoneal metastasis (PM). The present study is a systematic review to evaluate the current role of PIPAC in the management of gastric cancer associated PM.
Methods
A systematic search was conducted in Pubmed and EMBASE database using relevant keywords and confirming to the PRISMA guidelines to identify the articles describing the role of PIPAC in gastric cancer associated PM. All the studies which were published prior to July 1, 2018 in English literature and reported the role of PIPAC in gastric cancer associated PM were included in the systematic review.
Results
The search yielded 79 articles; there were ten published studies which have reported the use of PIPAC in gastric cancer associated PM. A total of 129 patients with gastric cancer associated PM were treated in the studies. Only two studies had an exclusive cohort of gastric cancer patients while eight other studies had a heterogeneous population with a small proportion of gastric cancer patients. There was only one study highlighting the role of PIPAC in neoadjuvant setting to downgrade the peritoneal carcinomatosis index. All the studies revealed that PIPAC is feasible and has minimal perioperative morbidity, even after repeated applications.
Conclusion
There is a scarcity of English literature related to the role of PIPAC in gastric cancer associated PM. PIPAC is a safe and well-tolerated procedure which has the potential to contain spreading PM. Further studies are warranted to better define the role of PIPAC in gastric cancer associated PM.
Keywords: gastric cancer, peritoneal carcinomatosis, Pressurized IntraPeritoneal Aerosol Chemotherapy
Introduction
Gastric cancer is the fifth most common cancer and the second most common cause of cancer-related death worldwide [1]. The median survival of the patients with gastric cancer associated peritoneal carcinomatosis (PM) remains poor. Even systemic chemotherapy has not been able to provide significant survival benefit to these patients. A cochrane review concluded that systemic chemotherapy prolongs overall survival (OS) by approximately 6.7 months more than best supportive care (BSC) (hazard ratio 0.3, 95 % CI 0.24–0.55). Moreover, a combination chemotherapy adds another one month to the OS (HR 0.84, 95 % CI 0.79–0.89) compared to single agent therapy, which is partly counterbalanced by increased toxicity, though it largely gets offset by the drug-induced adverse events [1]. These patients with gastric cancer associated PM are also likely to have a poor and deteriorating quality of life (QOL) due to associated troublesome pain, ascites, bowel obstruction and fistulae.
PM in any solid cancer represents a disseminated disease and is associated with a dismal prognosis. Various guidelines recommend systemic chemotherapy as a therapeutic option for PM in a well-preserved patient and BSC is the norm in a terminally ill patient [2]. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has generated a considerable interest in the last three decades in the management of PM. Various studies have suggested that CRS/HIPEC may even prove to be a curative treatment in a select group of patients who have isolated low volume peritoneal disease [3]. A retrospective French study of 277 patients with GC related PM indicated a significant survival benefit with CRS/HIPEC compared to CRS alone (median survival – 18.8 vs. 12.1 months) [4]. A recent prospective study of 35 patients with GC related PM (PCI<6) also highlighted a notable median survival of 19 months when they were treated with CRS/HIPEC [5]. Furthermore, a number of phase III randomized controlled trials (RCTs) are being conducted presently across the world in many centers to better identify the selection criteria for CRS/HIPEC [6]. The strict criteria for selecting the patients for CRS/HIPEC is definitely warranted in order to avoid the associated postoperative morbidity and mortality in those patients in whom this procedure will be futile oncologically and will not add to either progression free survival (PFS) or OS [7]. However, these strict criteria leave a large room for a significant number of patients who are not fit for CRS/HIPEC in view of high-volume peritoneal disease where CRS/HIPEC is likely to leave a significant gross residual disease.
Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC), a recently described new surgical technique to administer chemotherapy directly to the peritoneum under pressure, has added a new dimension to the armamentarium of the oncologists to address the PM in those patients who are not suitable candidates for CRS/HIPEC [8]. The first report of successful application of PIPAC in three patients with PM, including one with gastric cancer, was published in 2014 [9]. Since then, a number of articles have described the effectiveness and the safety of PIPAC in PM in patients with cancers of various origins.
Methods
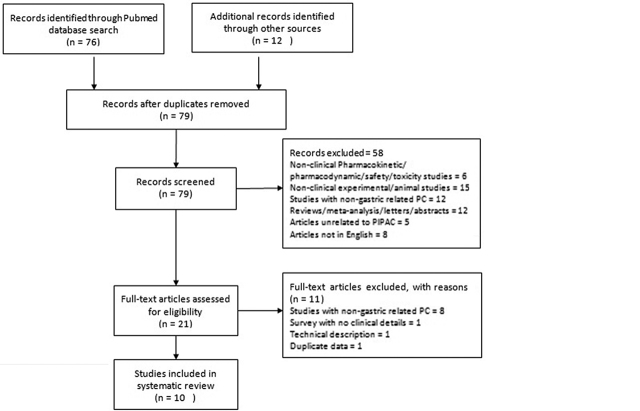
A systematic search was conducted in Pubmed and EMBASE database using keywords PIPAC[All Fields] OR (Pressurized[All Fields] AND intraperitoneal[All Fields] AND (“aerosols”[MeSH Terms] OR “aerosols”[All Fields] OR “aerosol”[All Fields]) AND (“drug therapy”[Subheading] OR (“drug”[All Fields] AND “therapy”[All Fields]) OR “drug therapy”[All Fields] OR “chemotherapy”[All Fields] OR “drug therapy”[MeSH Terms] OR (“drug”[All Fields] AND “therapy”[All Fields]) OR “chemotherapy”[All Fields])) on July 6, 2018. Inclusion criteria were clinical studies reporting the role of PIPAC in gastric cancer associated PM and published in English language prior to July 1, 2018. Exclusion criteria were (a) systematic reviews/meta-analysis/letters/corrections, (b) non-clinical experimental/animal studies, (c) pharmacodynamic/pharmacokinetic/safety studies without clinical details as Figure 1 shows.
Figure 1:
PRISMA flow diagram for the present systematic review.
Results
Ten studies were identified based on inclusion/exclusion criteria, published prior to July 1st, 2018 in English literature, which have reported the use of PIPAC in gastric cancer associated PM [10, 11, 12, 13, 14, 15, 16, 17, 18]. A total of 129 patients with gastric cancer were treated in these studies. There were two studies [10, 11] having an exclusive cohort of gastric cancer patients while eight other studies had a small proportion of gastric cancer patients in their reported cohort of PIPAC. One study highlighted the role of PIPAC in neoadjuvant setting to downgrade the peritoneal carcinomatosis index (PCI) [12]. All but one study reported the use of cisplatin and doxorubicin as preferred chemotherapeutic drugs for PIPAC in patients with gastric cancer. Teixeira Farinha et al. [13] reported oxaliplatin to be used for PIPAC in PM related to colorectal, gastric, small bowel cancer, and pseudomyxoma. PIPAC related adverse events>2 CTCAE (Common Terminology Criteria for Adverse Events) grades, varied from 0 % to 37.5 % in various studies [9, 10, 11, 14, 15, 16, 17]. Six of the ten studies reported QOL data and confirmed that it stabilized or did not deteriorate QOL in the patients who underwent repeated PIPAC procedures [10, 12, 13, 14, 15, 16, 17]. The median survival in the two studies with exclusive cohort of gastric cancer patients was reported to be 13 [11] and 15.4 [10] months. Table 1 highlights the relevant findings of the included studies in the systematic review [9, 10, 11, 12, 13, 14, 15, 16, 17, 19, 20].
Table 1:
Relevant characteristics reported in include studies.
| S.No. | Study | Year | Location | Total patients with PM (n) | Patients with GC associated PM (n) | Intent of PIPAC | Chemotherapya | Average PIPAC procedures per patient | Adverse effect>2 CTCAE | QOL following PIPAC | Remark |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Graversen et al. | 2018 | Denmark | 35 | 5 | Palliative | Cisplatin and Doxorubicin | 3.6b | 14.2%b | Stable | Safe and feasible, associated with histological and cytological regression |
| 2 | Teixeira Farinha et al. | 2018 | Switzerland | 42 | 3 | Palliative | Oxaliplatin | 2b | NR | Stable | No significant systemic toxicity even after repeated PIPAC |
| 3 | Alyami et al. | 2017 | France | 73 | 26 | Palliative | Cisplatin and Doxorubicin | 2.2b | 9.7%b | NR | PIPAC is feasible along with systemic chemotherapy. |
| 4 | Robella et al. | 2016 | Italy | 14 | 6 | Palliative | Cisplatin and Doxorubicin | 2.8 | 0 % | No deterioration | No significant hepatic or renal toxicity. |
| 5 | Rezniczek et al. | 2016 | Germany | 63 | 1 | Palliative | Cisplatin and Doxorubicin | NR | NR | NR | Measuring gene expression changes after PIPAC has a predictive and prognostic role. |
| 6 | Girshally et al. | 2016 | Germany | 21 | 3 | Neoadjuvant | Cisplatin and Doxorubicin | NR | NR | Stable | PIPAC as an effective neoadjuvant strategy to lower the PCI for good CRS and HIPEC |
| 7 | Khomyakov et al. | 2016 | Russia | 31 | 31 | Palliative | Cisplatin and Doxorubicin | 1.8 | 3.2 % | NR | Well tolerated procedure, can induce objective tumor regression |
| 8 | Odendahl et al. | 2015 | Germany | 91 | 29 | Palliative | Cisplatin and Doxorubicin | 1.7b | 7.5%b | Stable | Potential to stabilize QOL in patients |
| 9 | Nadiradze et al. | 2015 | Germany | 24 | 24 | Palliative | Cisplatin and Doxorubicin | 2.5 | 37.5 % | NR | Low-dose PIPAC is safe and associated with objective tumor regression. |
| 10 | Solass et al. | 2014 | Germany | 3 | 1 | Palliative | Cisplatin and Doxorubicin | 2 | 0 % | Stable | Complete microscopic peritoneal disease response. |
In patients with gastric cancer.
For the whole cohort and not exclusively to the patients with gastric cancer.
Discussion
The two significant limitations of the intraperitoneal chemotherapy are poor penetration and uniform distribution of the drug over the peritoneum. In 2012, a laparoscopy spray of aerosolized drug under-pressure was reported to address these two problems [21]. The authors sprayed methylene blue intra-abdominally using a spraying device consisting of an injector, a line, and a nozzle, inserted through one of the laparoscopic cannula under a capnoperitoneum of 12 mg of Hg for 30 min. The authors reported that there was a uniform staining of the peritoneum, and more so, even the outer aspect of the peritoneum was found to be stained indicating penetration of the dye under pressure. The first clinical experience of PIPAC in PM was published in 2014 [9]. The authors reported their experience of employing PIPAC in three patients of end-stage advanced PM of different origin (gastric, appendiceal, and ovarian each). The procedure was well-tolerated by the patients with no serious adverse effects noted (absence of any>2 CTCAE adverse events). All the patients responded with a decline in PCI score. Moreover, the authors reported histological regression in the peritoneal metastatic tumors; the patient with gastric cancer showed complete histological resolution of the tumor cells. Following this, many articles were published to confirm the safety, feasibility, and effectiveness of the PIPAC. However, most published studies included a heterogeneous population with PM of different origins and patients with different clinic-pathological parameters.
Nadiradze et al. [10] published a study to share their experience of PIPAC in an exclusive cohort of 25 patients with gastric cancer associated PM. One patient could not have even one PIPAC procedure due to inability to access peritoneal cavity in view of extensive peritoneal adhesions. Out of 60 PIPAC procedures in 24 patients (with a mean PCI of 16), the authors reported that 50 % of the patients (12/24) had an objective tumor response with an impressive median survival of 15.4 months. Repeat peritoneal biopsy after PIPAC did not show any visible tumor cell in six patients (complete pathological response). However, the authors admitted that these impressive results should be viewed with caution as a repeat PIPAC was done in those patients who had an objective clinical response (selection bias). Seven patients (7/24) did not have second PIPAC procedure due to progressive disease. The study had a high number of postoperative adverse events>2 CTCAE (9/24, 37.5 %); this can, however, be explained due to inclusion of high risk patients – with extra-abdominal metastasis, with a poor ECOG sore of ≥3, very high PCI, gross ascites, and bowel obstruction.
Another phase 2 prospective trial [11] with an exclusive cohort of gastric cancer patients also reported that PIPAC leads to pathological response (including complete and partial) in almost one third of the patients (60 %) and a median survival of 13 months. It must be noted here that pathological response could be assessed in 15 of the 31 patients who were enrolled in the study and could have had more than one PIPAC procedure.
There have been large variations in the reported adverse events in various studies varying from 0 % to 37.5 %. However, this is largely due to inclusion of a heterogeneous population of patients with PM of different origins and with varying risk factors. Most of the studies involved a few patients of gastric cancer related PM and did not report procedure related adverse events. Alyami et al. [14] reported grades 3 and 4 CTCAE adverse events in 9.7 % and a mortality rate of 6.8 % in their patient cohort. Wound infection, wound dehiscence, and intestinal obstruction were commonly seen adverse event. The common causes of mortality were reported to be progressive disease, aspiration pneumonia, and intestinal obstruction.
The feasibility of PIPAC has been described differently in various studies. Graversen et al. [17] defined PIPAC procedure to be feasible if – (a) a laparoscopic access was possible in 80 % of the patients, (b) the procedure could be completed successfully in 80 % of the patients without any CTCAE grade 4 or 5 events, and (c) if 80 % of the patients could be discharged within 2 days of PIPAC procedure. They reported that PIPAC was feasible for all the patients (35 patients, 129 PIPAC procedures). Nadiradze et al. [10] reported that mere 3 out of the 24 patients could not undergo repeated PIPAC due to non-access to the abdominal cavity in view of severe adhesions.
Whenever any intervention is performed with a palliative intent in any patient with an advanced cancer, it must not deteriorate the current QOL. Even a stabilization of QOL in a terminally ill patient can be considered as a success of a palliative procedure. Six of the nine studies confirmed that repeated PIPAC helps stabilize the QOL and prevent its further deterioration (Table 1).
The next natural question is what the indications of PIPAC are? Almost all studies except one performed PIPAC in patients with high PCI or co-morbid conditions which made them unsuitable for CRS/HIPEC. Obviously, PIPAC in this setting can be regarded as a palliative procedure to improve the quality of life, or at least being able to stabilize it from further deterioration. The ‘one shoe fits all’ approach is not possible in medicine and so, it is of utmost importance to identify patients suitable for a particular procedure to have optimum results. In the time line of the disease progression, a window needs to be identified for PIPAC to be brought in, when the patient does not respond to systemic chemotherapy but still has a reasonable performance status and not having gross ascites, bowel obstruction, or extra-abdominal disease [10].
Can one expect the PCI to drop to that level with repeated PIPAC procedures where one can think of performing secondary CRS/HIPEC; or in other words, can PIPAC be used as a neoadjuvant procedure in patients with high PCI? Girshally et al. reported that neoadjuvant PIPAC is feasible and has the potential to consider secondary CRS/HIPEC in a select group of patients with diffuse small bowel involvement to reduce the extent of CRS [12]. In their patient cohort, twelve of 21 patients had a low PCI (mean 5.8±5.6) and the remaining nine patients were having an advanced peritoneal disease (mean PCI 14.3±5.3) at initial laparoscopy. Repeated PIPAC (3–4 cycles per patient) led to radiological tumor regression in 7/9 patients while major histological regression was made out in 8/9 patients. Though, there were only three patients with gastric cancer in their cohort of 21 patients and none was in the cohort of advanced PM, this study suggests expanding indications of PIPAC in PM.
The present review highlights the paucity of the data related to the role of the PIPAC in gastric cancer related PM. The PIPAC procedure is still in its infancy. Most of the studies had a heterogeneous cohort of the patients of PM of different origins making it difficult to evaluate the true benefit of the procedure in a specific condition. Furthermore, none of the published study has reported the survival benefit of PIPAC in gastric cancer.
As all the studies conducted so far have, at least, established the safety, feasibility, and potential to stabilize the QOL, multiple trials are currently being undertaken at various centers to evaluate the role of PIPAC in gastric cancer associated PM (Table 2). However, one may note that only two out of five trials have exclusive cohort of gastric cancer patients, other trials have a largely heterogeneous population of patients with PM of varying origin.
Table 2:
Various ongoing trials to evaluate the role of PIPAC in eritoneal carcinomatosis of various origins including gastric cancer (www.clinicaltrials.gov.in as accessed on 12-07-2018).
| S.No. | Study title | Peritoneal origin of peritoneal carcinomatosis | Location | Study to be completed |
|---|---|---|---|---|
| 1 | IntraPeritoneal Aerosol Chemotherapy in Gastric Cancer | Gastric cancer | Ruhr University of Bochum, Herne, North Rhine Westphalia, Germany | Completed, results not yet published |
| 2 | Study of Efficacy and Safety of Laparoscopic Intraabdominal Chemotherapy (PIPAC) Performed in Patients With Peritoneal Carcinomatosis From Colorectal, Ovarian, Gastric Cancer and Primary Peritoneal Tumors | Colorectal, ovarian, gastric, and primary peritoneal tumors | FPO-IRCCS Institute for Cancer Research and Treatment, Candiolo, Turin, Italy | October, 2018 |
| 3 | PIPAC Nab-pac for Stomach, Pancreas, Breast and Ovarian Cancer | Stomach, pancreas, breast, and ovarian cancer | UZ Ghent, Ghent, East-Flanders, Belgium | December, 2020 |
| 4 | International Registry of Patients Treated With Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) | Colorectal, ovarian, gastric, appendical, pancreatic, gallbladder, small bowel, pseudomyxoma peritonei, and malignant mesothelioma | Multicentric study | May, 2019 |
| 5 | Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) With Oxaliplatin In Patients With Peritoneal Carcinomatosis | Gastric cancer | National University Hospital Singapore, Singapore | January, 2019 |
Conclusion
The present systematic review clearly highlights the scarcity of English literature to support the role of PIPAC in gastric cancer associated PM. PIPAC is a safe and well-tolerated procedure which has the potential to contain spreading PM. Large studies with an exclusive cohort of gastric cancers are warranted to better define the role of PIPAC in gastric cancer associated PM.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Contributor Information
Pankaj Kumar Garg, Email: dr.pankajgarg@gmail.com.
Maximilian Jara, Email: maximilian.jara@charite.de.
Miguel Alberto, Email: miguel.alberto@charite.de.
Beate Rau, Email: beate.rau@charite.de.
References
- 1.Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai B-C, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;29:CD004064. [DOI] [PMC free article] [PubMed]
- 2.Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. 2016;14:1286–312. [DOI] [PubMed]
- 3.Morales-Soriano R, Esteve-Pérez N, Segura-Sampedro JJ, Cascales-Campos P, Barrios P. Spanish Group of Peritoneal Malignancy Surface (GECOP). Current practice in cytoreductive surgery and HIPEC for metastatic peritoneal disease: Spanish multicentric survey. Eur J Surg Oncol. 2018;44:228–36. [DOI] [PubMed]
- 4.Bonnot PE, Piessen G, Pocard M, Meunier B, Bereder JM, Abboud K, et al. CYTO-CHIP: cytoreductive surgery versus cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis: a propensity-score analysis from BIG RENAPE and FREGAT working groups. J Clin Oncol. 2018;36:8. (suppl 4S; abstr 8). [DOI] [PubMed]
- 5.Rihuete Caro C, Manzanedo I, Pereira F, Carrion-Alvarez L, Serrano Á, Pérez-Viejo E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur J Surg Oncol. 2018;44:1805–10. [DOI] [PubMed]
- 6.Ji Z-H, Peng K-W, Yu Y, Li X-B, Yonemura Y, Liu Y, et al. Current status and future prospects of clinical trials on CRS+HIPEC for gastric cancer peritoneal metastases. Int J Hyperth. 2017;33:562–70. [DOI] [PubMed]
- 7.Garg PK, Brandl A, Rau B. Hyperthermic intraperitoneal chemotherapy – fading perspective in the light of modern systemic chemotherapy? Visc Med. 2018;34:412–6. [DOI] [PMC free article] [PubMed]
- 8.Alberto M, Brandl A, Garg PK, Gül-Klein S, Dahlmann M, Stein U, et al. Pressurized IntraPeritoneal Aerosol Chemotherapy and its effect on gastric-cancer-derived peritoneal metastases: an overview. Clin Exp Metastasis. 2019. DOI: 10.1007/s10585-019-09955-4. [DOI] [PubMed]
- 9.Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21:553–9. [DOI] [PMC free article] [PubMed]
- 10.Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond M-A. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg. 2016;20:367–73. [DOI] [PMC free article] [PubMed]
- 11.Khomyakov V, Ryabov A, Ivanov A, Bolotina L, Utkina A, Volchenko N, et al. Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and Doxorubicin administered as a pressurized aerosol: an open-label, Phase-2 study (PIPAC-GA2). Pleura Peritoneum. 2016;1:159–66. [DOI] [PMC free article] [PubMed]
- 12.Girshally R, Demtröder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2016;14:253. [DOI] [PMC free article] [PubMed]
- 13.Teixeira Farinha H, Grass F, Kefleyesus A, Achtari C, Romain B, Montemurro M, et al. Impact of Pressurized IntraPeritoneal Aerosol Chemotherapy on quality of life and symptoms in patients with peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract. 2017;2017:4596176. [DOI] [PMC free article] [PubMed]
- 14.Alyami M, Gagniere J, Sgarbura O, Cabelguenne D, Villeneuve L, Pezet D, et al. Multicentric initial experience with the use of the Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur J Surg Oncol. 2017;43:2178–83. [DOI] [PubMed]
- 15.Odendahl K, Solass W, Demtröder C, Giger-Pabst U, Zieren J, Tempfer C, et al. Quality of life of patients with end-stage peritoneal metastasis treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC). Eur J Surg Oncol J. 2015;41:1379–85. [DOI] [PubMed]
- 16.Robella M, Vaira M, De Simone M. Safety and feasibility of Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol. 2016;14:128. [DOI] [PMC free article] [PubMed]
- 17.Graversen M, Detlefsen S, Bjerregaard JK, Fristrup CW, Pfeiffer P, Mortensen MB. Prospective, single-center implementation and response evaluation of Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) for peritoneal metastasis. Ther Adv Med Oncol. 2018;10:1–11.:1758835918777036. [DOI] [PMC free article] [PubMed]
- 18.Rezniczek GA, Jüngst F, Jütte H, Tannapfel A, Hilal Z, Hefler LA, et al. Dynamic changes of tumor gene expression during repeated Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in women with peritoneal cancer. BMC Cancer. 2016;16:654. [DOI] [PMC free article] [PubMed]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. [DOI] [PubMed]
- 20.Hübner M, Teixeira Farinha H, Grass F, Wolfer A, Mathevet P, Hahnloser D, et al. Feasibility and safety of Pressurized IntraPeritoneal Aerosol Chemotherapy for peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract. 2017;2017:6852749. [DOI] [PMC free article] [PubMed]
- 21.Solaß W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc. 2012;26:1849–55. [DOI] [PubMed]