Abstract
Multiple non-animal-based test methods have never been formally validated. In order to use such new approach methods (NAMs) in a regulatory context, criteria to define their readiness are necessary. The field of developmental neurotoxicity (DNT) testing is used to exemplify the application of readiness criteria. The costs and number of untested chemicals are overwhelming for in vivo DNT testing. Thus, there is a need for inexpensive, high-throughput NAMs to obtain initial information on potential hazards, and to allow prioritization for further testing. A background on the regulatory and scientific status of DNT testing is provided showing different types of test readiness levels, depending on the intended use of data from NAMs. Readiness criteria, compiled during a stakeholder workshop that united scientists from academia, industry and regulatory authorities, are presented. An important step beyond the listing of criteria was the suggestion of a preliminary scoring scheme. On this basis a (semi)-quantitative analysis process was assembled on test readiness of 17 NAMs with respect to various uses (e.g., prioritization/screening, risk assessment). The scoring results suggest that several assays are currently at high readiness levels. Therefore, suggestions are made on how DNT NAMs may be assembled into an integrated approach to testing and assessment (IATA). In parallel, the testing state in these assays was compiled for more than 1000 compounds. Finally, a vision is presented on how further NAM development may be guided by knowledge of signaling pathways necessary for brain development, DNT pathophysiology, and relevant adverse outcome pathways (AOP).
Keywords: developmental neurotoxicity, in vitro testing, zebrafish embryo test, stem cells, regulatory toxicology, toxicity screening, quality assurance
1. Introduction:
1.1. Objectives of the meeting and follow-up activities
A recent OECD/European Food Safety Authority (EFSA) workshop on the use of non-animal test methods for regulatory purposes in the area of developmental neurotoxicity (DNT) proposed to develop a standardized in vitro testing battery that could be used to generate data on the toxic effects of chemicals on the developing nervous system. It was recognized that there is an urgent need for a new alternative testing strategy that supports regulatory decisions with a focus on two specific aims: the first is to use existing alternative test methods to support screening and prioritization of chemicals for future testing, the second aim is to generate data that aid in guiding risk management decisions. The workshop concluded that the task now is to establish performance standards and develop a guidance document for an in vitro DNT testing battery (Fritsche et al., 2017a).
The International Stakeholder Network (ISTNET) on DNT testing is a collaborative effort by groups from academia, industry and regulatory bodies that aims to align the development of alternative (non-animal) testing methods with the needs of regulatory decision making. A first meeting in Zurich in January 2014 explored the potential of applying the adverse outcome pathway (AOP) framework to promote test systems development according to regulatory needs, and to assemble predictive integrated testing strategies (ITS) for DNT (Bal-Price et al., 2015a).
With the outcome of the OECD/EFSA workshop in mind, a second ISTNET Workshop took place in Konstanz in January 2017, focused on practical aspects of such pathway-based testing, and in particular on performance standards that should be applied to alternative DNT tests. The immediate objectives of the meeting and its follow-up activities were:
Define criteria for evaluation for readiness of a given test method.
Evaluate to what extent these criteria are fulfilled.
For the second objective, proof-of-principle examples are given here on how an evaluation may be performed; as information, only historical, published information was used. Therefore, midterm objectives were defined to continue this process:
Establish a standardized evaluation system for assay readiness.
Define a list of suitable test methods based on these criteria.
Establish criteria for a battery of tests for use in a DNT IATA based on readiness scores.
Build an IATA for initial chemical screening and prioritization
The long-term goal is to define a battery of alternative tests based on developmental ontologies (in contrast to the mid-term goal of performance based test definition). Such a battery would include the relevant tests for all biological pathways, processes and domains implicated in DNT.
1.2. Background on the use of existing in vivo test methods: why are alternatives needed?
At present, there is no regulatory requirement for pesticides or other chemicals to be tested for DNT prior to registration. Instead, DNT testing can be triggered based on observed neurotoxic effects in repeat-dose in vivo animal testing, a known neurotoxic mode of action, or a structure-activity alert, in Europe for pesticides, biocides and chemicals, and in the US for pesticides. In these triggered cases, DNT testing is performed as an in vivo higher-tier test as there are no regulatory accepted alternative methods for this purpose. There are two regulatory guidelines for DNT testing, both in rodents: OECD TG426 (Developmental Neurotoxicity Study) which is an update of the 1998 US EPA DNT Guideline, and OECD TG443 (Extended One-Generation. Reproductive Toxicity Study, DNT cohort). Both require neurobehavioral evaluation of cognitive, sensory and motor function, accompanied by histopathological and morphometric evaluation of the brain, but they do not provide detailed guidance on the use of specific behavioral tests, leaving flexibility in the study design and in the interpretation of the results obtained. Moreover, TG426 and TG443 present a number of challenges and limitations (Claudio et al., 2000; Crofton et al., 2004, 2011; Tsuji and Crofton, 2012; Smirnova et al., 2014), including:
They are time- and resource-consuming low throughput assays
A large number of animals is required
Differences in techniques and measures, especially for behavioral endpoints, can make it difficult to compare data between studies
Implementation of the DNT guideline methods in contract laboratories has resulted in datasets with high variability, and low reproducibility, even for positive controls
Measured pathological and behavioral endpoints provide no mechanistic understanding of the underlying effects
The currently required tests do not capture important complex endpoints of relevance for humans, for example higher cognitive functions
The predictivity for protection of the human brain is based on a very limited number of chemicals, and rodent studies may not contain similar toxicodynamic processes, leading in some cases to uncertainties in the relevance of animal outcomes to human DNT.
In reality, TG426 and TG443 are seldom conducted. Studies are currently available for only a relatively limited number of substances (about 120) (van Thriel et al., 2012; Kadereit et al., 2012; Crofton et al., 2012). Therefore, the urgent aim is to develop alternative test methods as part of a test strategy that at least can identify DNT alerts and guide prioritization at a lower-tier level.
A recent review, focused on pesticide active substances, was presented at the DNT OECD/EFSA workshop in Brussels (Fritsche et al., 2017a) by the German Federal Institute for Risk Assessment (BfR). To date, DNT studies have been conducted on only 35 of the 485 pesticide active substances currently approved in the EU. Of these 35, 19 displayed positive in vivo evidence of DNT. It should be noted that a large proportion of these 485 pesticide active substances were classified as adult neurotoxicants (Grandjean and Landrigan, 2006). It is unknown whether a similarly high rate (> 50%) of positive DNT results would be seen for other classes of chemicals that are not enriched in neurotoxicants. Moreover, the DNT testing led to health-based guideline reference values for only 2 of these 19 positive compounds.
An alternative analysis of DNT studies by the USEPA in 2010 demonstrated that of 72 DNT studies, 15 were used to determine the point of departure for one or more risk assessment scenarios, and an additional 13 were determined to have the potential for use as a point of departure for future risk assessments (Raffaele et al., 2010). These assessments are limited to a small number of chemicals that in no way represents the known chemical space of environmental chemicals (Richard et al., 2016). Thus, to clarify the need for DNT testing for regulatory purposes, experimental evidence on the potential for DNT hazard for many more chem-icals is required. However, for this purpose the tests need to be more time- and cost-effective.
The sensitivity of the currently used in vivo DNT test has been questioned (Claudio et al., 2000; Vorhees and Makris, 2015). Some of the issues may be due to toxicodynamics, others may be explained by different toxicokinetics among species (metabolic activity or placental transfer in animals compared to humans as exemplified earlier (reviewed in Aschner et al., 2017)). The issue of sensitivity is, for example, evident regarding the predictivity value of the rat DNT assay for the evaluation of chemicals acting on the hypothalamic-thyroid axis. Despite the human evidence linking developmental hypothyroxinemia with changes in brain development in children (Haddow et al., 1999; Henrichs et al., 2010), several DNT studies investigating rodent offspring from hypothyroid/hypothyroxinemic dams have shown that adverse behavioral outcomes were not always present (York et al., 2005). Although multiple explanations may clarify this issue and should be taken into account (e.g., severity of the effect in the dams, limited milk transfer of the compound, neurobehavioral assess-ment methods not suited for the detection of subtle effects in the brain, presence of compensatory mechanisms), it is evident that design, conduct and interpretation of in vivo DNT studies are complicated. Species differences of developing brain cells in response to thyroid hormones have recently been reported also on the level of pharmacodynamics (Dach et al., 2017).
Due to these issues, the US EPA Office of Pesticide Programs (OPP) suggested to include alternative approaches in the testing paradigm to improve DNT hazard identification in the context of analyzing DNT in vivo studies for 72 pesticide active substances (Raffaele et al., 2010).
Another reason regulatory bodies and authorities support the development of alternative medium- to high-throughput assays s the need for testing large numbers of chemicals for their DNT potential (Crofton et al., 2012; EFSA, 2013; Bal-Price et al., 2015a; Fritsche et al., 2017a).
1.3. Making alternative methodologies for DNT testing acceptable for regulatory purposes
Reliability and human relevance are the two critical require-ments that have to be addressed for regulatory acceptance of alternative test methods. The OECD Adverse Outcome Pathway (AOP) framework (OECD, 2013; Ankley et al., 2010; Bal-Price et al., 2015b; Leist et al., 2017; Terron et al., 2018) is useful in defining the human relevance of data from individual test systems as it takes all available data, including human epidemi-ology and human in vitro data, into consideration. Moreover, it allows development of quantitation and threshold models on the basis of quantified key events (KE) in an established AOP.
The assessment of the readiness and reliability of alternative DNT methods for regulatory purposes is currently lagging be-hind the extremely rapid development of new technologies (e.g., induced pluripotent stem cells, 3D cell co-cultures and organ-oids, high-content omics measurements, bioinformatics tools, etc.) (Leist et al., 2008a, 2014; Marx et al., 2016; Rovida et al., 2015; Smirnova et al., 2016). This is unfortunate, since more guidance on how to ensure reliability of the available and new in vitro DNT assays would help researchers in designing, conduct-ing, and reporting studies. It would also encourage regulators to take NAMs into account.
Therefore, the major focus of this workshop report is to pro-vide a set of readiness criteria that potentially could be accept-able to both regulators and test developers. Moreover, examples are given on how a readiness evaluation of existing in vitroDNT assays could be applied to various regulatory applications. Preliminary scoring by workshop participants of over a dozen methods demonstrates that the field of DNT-NAM is ready to support some regulatory decisions. The readiness criteria will also be helpful to harmonize development of new in vitro tests and to ensure their reliability and relevance.
In addition to data reliability and relevance evaluation, both researchers and regulators will need guidance on data integration from a battery of alternative DNT assays (Behl et al., 2015) in the form of ITS and defined approaches (DA) (OECD, 2016c). This enables a tiered approach, spanning the spectrum from hazard identification/characterization as an input to quantitative risk assessment, aiding the application of human health-related decisions based on data coming from alternative approaches.
Outstanding regulatory challenges for accepting alternative DNT test data are similar for most alternative methods and in-clude uncertainty due to genetic background, cell type and to-pography, life-stage, and exposure temporality in dose-response modeling (Hartung et al., 2017a,b). Some of these issues are addressed in the AOP framework (Bal-Price and Meek, 2017; Leist et al., 2017; Terron et al., 2018), which will thus help in their resolution.
Current hazard identification processes based on in vitro tests accepted by regulatory agencies rely on molecular and cellular KEs within AOPs. Here, the most prominent example is the ap-plication of a testing battery based on KEs identified in the AOP for skin sensitization (OECD 2016b; Delrue et al., 2016; Adel-eye et al., 2015; Urbisch et al., 2015). Transferring this concept to DNT, where currently only a few relevant AOPs are available, and where many more pathways might underlie toxicity for the developing brain (Bal-Price et al., 2015b, 2017; Bal-Price and Meek, 2017), a similar procedure is not yet feasible. Therefore, in vitro assays anchored to key cellular neurodevelopmental pro-cesses should guide the development of an alternative DNT test-ing battery (Fritsche et al., 2017a; Aschner et al., 2017; Schmidt et al., 2017; Bal-Price et al., 2010, 2012; Crofton et al., 2011).
Since 2005, an international community used the CAAT Tox-Smart DNT meetings as a basis to propose alternative approaches for DNT evaluation (Lein et al., 2005; Coecke et al., 2007; Crofton et al., 2011; Bal-Price et al., 2012, 2015a; Smirnova, 2014; Leist et al., 2012). The above-mentioned processes-based alternative DNT testing strategy is a result of this ongoing exchange between basic researchers and regulatory scientists. Such cellular KEs are intermediate to late KEs in an AOP, and examples from existing DNT AOPs include, e.g., “impaired neuronal differentiation” (Bal-Price et al., 2015b; Bal-Price and Meek, 2017), “decreased synaptogenesis” or “decreased neuronal network function” (Bal-Price et al., 2015b; Sachana et al., 2016), see also AOP-Wiki1. However, as the number of available DNT AOPs is small, basic clinical as well as toxicological sciences may inform us on rele-vant and measurable neurodevelopmental KEs, as summarized in Fritsche et al. (2015) and Fritsche (2017b).
Examples from the toxicological side include methylmer-cury-induced inhibition of neural cell migration (Bal-Price et al., 2015b; Moors et al., 2007), arsenic-induced inhibition of neural progenitor cell (NPC) proliferation (Chattopadhyay et al., 2002), valproic acid-induced inhibition of neural crest cell migration (Zimmer et al., 2012), or neuronal differentiation (Foti et al., 2013; Balmer et al., 2012, 2014; Waldmann et al., 2014, 2017). For these examples, the compounds’ modes of action (MoAs) are being elucidated (Bal-Price et al., 2015b).
Knowledge from clinical research on neurodevelopmental dis-orders with genetic alterations as basis for disease are also help-ful in determining human-relevant, cell-based endpoints. Here, for example, diverse receptor tyrosine kinase (RTK) mutations, leading to activation of protein kinase B (AKT, PKB), can cause a variety of morphological disturbances in humans that are based on deregulation of brain cell proliferation and apoptosis (re-viewed in Hevner, 2015). Also, aberrant expression of the brain derived neurotrophic factor (BDNF) and its dependent mole-cules, extracellular signal-regulated kinase (ERK) and cAMP responsive element binding protein (CREB), have been linked to numerous psychiatric disorders, including autism spectrum disorders, mood disorders and schizophrenia. Cellular functions controlled by these pathways are numerous, including brain cell proliferation, dendritogenesis, and synaptogenesis (reviewed in Ehrlich and Josselyn, 2016). These are only examples; a more detailed compilation of relevant neurodevelopmental pathways and cellular functions can be found in Fritsche et al. (2017b).
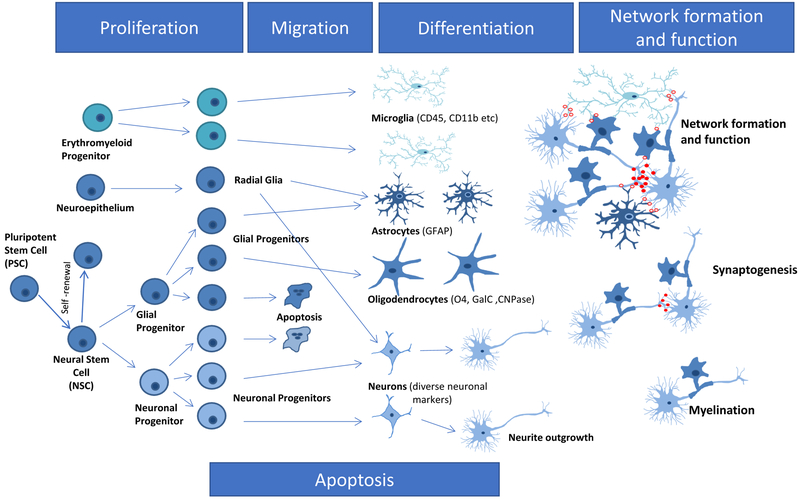
Modelling these key neurodevelopmental processes, from cell division up to neuronal network formation (Fig. 1), in a NAM testing battery will yield information on relative sensitivities of the processes to chemicals. For a small subset of endpoints, the principle of detecting the most sensitive process, and extrapolat-ing from its disturbance in vitro to an in vivo hazard, has been exemplified in Baumann et al. (2016). Thus, information from batteries of tests run in parallel will not only serve as readouts for DNT hazard but will also inform future assay development and design of AOPs. While focusing on all these positive aspects, it will be important to bear in mind that fundamental issues of in vitro assays need to be kept in mind: for instance, the metabolic capacities that may differ from the in vivo situation, the interac-tion of different cell types that may largely affect their response pattern (Gantner et al., 1996), and issues of biological barriers (Leist et al., 2014; Kadereit et al., 2012; Aschner et al., 2017).
Figure 1: Fundamental neurodevelopmental processes relevant for DNT.
Several neurodevelopmental processes are essential for nervous system development. These processes known from in vivo studies can be relatively faithfully modelled in vitro. It is assumed that DNT toxicants exert their toxicity by disturbing at least one of these processes. Therefore, disturbances of the processes depicted here in blue boxes are KEs of AOPs relevant for DNT. The figure gives a short overview of nervous system development from simple precursors (left) to complex functional tissue (with cell-cell interactions) on the right-hand side. For a DNT test battery all these biological processes should be covered by one or more test methods. KE: key event; AOP: adverse outcome pathway; DNT: developmental neurotoxicity.
2. General guidance of quality and performance standards
2.1. OECD guidance on test descriptions and readiness
The rationale for alternative DNT testing is given by the con-sensus between academic, industry and regulatory scientists that chemicals with the potential to trigger DNT should be properly identified and that the current testing paradigm, based on in vivostudies, does not satisfy this need (Fritsche et al., 2017a). For moving alternative DNT tests into action, scientists should focus on defining and applying test specifications (Leist et al., 2010, 2012) and validation paradigms to evaluate their readiness and draw a roadmap for their application in a regulatory context.
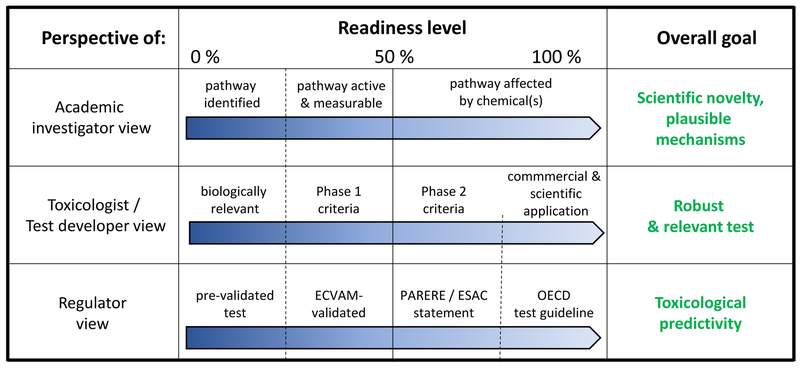
The meaning of the term readiness varies strongly between different interest groups (Fig. 2). For instance, an academic re-searcher uses a cellular model system to investigate pathways of cellular functions and needs a reliable model that mimics human effects. However, this is only the starting point for the work of the test system developer.
Figure 2: Different perspectives of DNT alternative methods readiness evaluation.
In the discussion on “test readiness” it is important to note that different fields and stakeholders have their own perspective. Three of these perspectives are outlined. For each of them, examples for increasing grades of readiness and final goals are given. These perspectives are interdependent to some degree: (i) a test that is 100% ready for an academic investigator in basic science can form the starting point for a toxicological test developer; (ii) a test that is considered ready by the test developer may be at the start of regulatory readiness, e.g. with respect to formal validation; (iii) and a test that is at the highest regulatory readiness level (OECD TG) may provide a starting point for academic researchers who want to unravel key mechanisms and pathways that are essential and that biologically explain the test read outs.
Regulatory acceptance of individual tests will be facilitated by adherence to international regulatory consensus guidance. For instance, the OECD Guidance Document No. 211 (GD 211) provides a template for assay annotations of non-guide-line in vitro methods (OECD, 2014b). GD 211 harmonizes the manner in which non-guideline in vitro methods are described, and thereby facilitates assessment (by the regulator) of the reli-ability and relevance of the produced data. The US EPA Office of Pesticide Programs recommends following this guidance to describe non-guideline in vitro methods for acute toxicity (EPA, 2016). According to this guidance (OECD, 2014b), the method description should include purpose and scope of the assay, meth-od components including protocol and reference chemicals, the stage of development of the assay, the quality/acceptance crite-ria, data interpretation and prediction model(s), and performance metrics including sensitivity and predictivity (i.e., proportion of false negatives for positive controls, and of false positives for negative controls).
A further important document is the guidance document on Good In vitro Method Practices (GIVIMP) for the development and implementation of in vitro methods for regulatory use in hu-man safety assessment (expected release: 2018). This guidance (draft version OECD, 2017a) will be of central importance in regulatory acceptance of the proposed DNT alternative methods. It describes the scientific, technical and quality practices needed at all stages between in vitro method development to implemen-tation for regulatory use. These include roles and responsibilities (of developers, component providers and users), quality consid-erations, facilities, apparatus, material and reagents, test systems, test and reference/control items, standard operating procedures, method performance, and reporting of the results. The GIVIMP document has been written for various users, including GLP test facilities but also research laboratories developing new in vitromethods for regulatory purposes. In the latter case, full compli-ance with GIVIMP may not be realistic, but compliance with as many as possible of the “good practices” will facilitate the acceptance and routine use of the in vitro method in a regulatory environment.
It is understandable that the completeness of the information recommended in the OECD guidance will vary, because the level of development of the DNT alternative methods is different, and this in turn impacts the use of the methods for different regulato-ry applications. However, in all cases, the suggested framework aims to cover some information on 1) a test method definition (including purpose, scientific principle, metabolic competence, quality control criteria, technical limitations and strengths); 2) test method performance (robustness, reference chemicals, per-formance measures/predictive capacity); 3) data interpretation; 4) potential applications; and 5) supporting information avail-able in the existing databases (e.g., DataBase on ALternative Methods DB-ALM of EURL-ECVAM2).
n this context, the consideration of “applicability domains” takes an important and often underestimated role. The test meth-od must be considered like a tool. And like all tools, it has a proper domain of application (e.g., scissors to cut paper), bor-derline domains of application that require case-by-case evalu-ations (e.g., use of scissors to punch holes or to open a bottle), and applications that are physically possible, but usually lead to non-satisfactory results (e.g., use of scissors to open a can or to turn screws). For DNT test methods, several dimensions of “applicability domains” are important.
The three most import-ant ones are:
the type of chemicals to be tested;(
the type of mechanisms explored;
the type of (regulatory) questions addressed.
Thus, a given method may be more ready for certain applications and less ready for others!
2.2. Principles for evaluation of the readiness of test strategies based on multiple test methods
A systematic approach to building a test battery should first de-termine the readiness of individual alternative DNT methods. A general set of readiness criteria has been proposed by OECD (2014b), and these have been clustered in four categories (Tab. 1). Such guidance has been considered here in compiling spe-cific readiness criteria for DNT test methods, and in devising a preliminary scoring system to obtain indications on the readiness status of various published tests (see chapters below). Currently, none of the proposed DNT alternative methods are stand-alone methods, thus a battery of the assays that capture essential infor-mation across neurodevelopmental processes and developmental timing is considered important for a comprehensive hazard as-sessment. Here, we discuss briefly the evaluation of ITS.
Table 1: Example for ranking parameters for in vitro methods for detection of chemicals that disturb the thyroid hormone axis.
Ranking parameters were established by OECD for thyroid-disrupting chemicals to determine readiness of tests for validation (OECD, 2014). The criteria in Category 1 are considered of highest priority. Each criterion within this category is considered to have equal weight, and all are essential to demonstrate the readiness of the assay. For instance, the assessment of the biological plausibility is considered very important in defining readiness of the method for validation. However, criteria in this category are hard to quantify. Moreover, many DNT tests cover multiple mechanisms and processes with varying levels of plausibility and data on their in vivo relationship. Thus, the practical value of such criteria for DNT methods needs to be considered case-by-case. The criteria for Category 2 are better defined and quantifiable. They relate to the evaluation of reliability and efficacy of the method. Sufficient positive and negative compounds should be included to assess specificity and sensitivity, and focus should be given to the robustness of the assay. Regarding Category 3, the criteria are also relevant to assay performance evaluation. However, the particular performance issues described under this category are considered to be of less significance during initial phases of test development and evaluation. Category 4 contains criteria for the methods that are considered as good to meet, in order to gain broad acceptance.
| CATEGORY 1 Initial High Priority Considerations |
CATEGORY 2 Method performance considerations |
|---|---|
| Biological plausibility Extrapolation to humans or broadly applicable across vertebrates/phyla Availability of resources Reference chemicals |
Within-laboratory reproducibility
Between-laboratory reproducibility Assay variability Accuracy Assay specificity/assay sensitivity |
| CATEGORY 3 Technical capability |
CATEGORY 4 Other practical considerations |
| Dynamic range/concentration test
range Detection/adjustment of confounding factor and/or Incorrect/inconclusive measurements and/or other bias Response characterization |
Technological transferability/proprietary
elements Transparency of the method Documentation of development and utility of the method |
he evaluation of ITS could be based on the principles devel-oped for the reporting of DAs to testing and assessment based on multiple information sources (OECD, 2016b). A DA can be built in various ways and may take the form of a sequential testing strategy (STS) or an ITS. The fixed data interpretation procedure is then used to interpret data generated with a defined set of alter-native methods that can either be used on its own or together with other methods and existing information within an IATA (OECD, 2016a). In this case, the template for data reporting of individual information sources used in a DA that was published in an OECD guidance (OECD, 2016b) will ensure a transparent and accurate documentation of the methods used within a DA. Within such a DA, information has to be documented properly to ensure trans-parency of the methods used. The description should include the chemical and/or biological mechanism addressed by the meth-ods and provide some indications of the plausible linkage of the modelled mechanisms or neurodevelopmental processes to the apical endpoint being predicted. Known scientific confidence and limitations of methods should also be reported, including a com-parison to existing similar non-testing or testing methods.
Principle 1 aims to ensure clarity in the endpoint addressed, by defining it. From this perspective, a relationship between the combination of the alternative test methods’ endpoint(s) and the biological phenomena of interest should be explored. The limitations (e.g., inability to determine DNT effects secondary to systemic effects like hormonal imbalance) are to be clearly identified. The scientific validation of the testing strategy should be based on a mechanistic ground with the assumption that a de-rangement of fundamental processes in neurodevelopment will lead to an adverse effect.
Principle 2 aims to ensure clarity in the purpose for which the combination of the alternative methods is proposed. Considering that a test method should fit for a specific purpose, the problem formulation should be defined at the beginning of the process. This would not only include the regulatory purpose, i.e., screen-ing and prioritization vs. single chemical hazard identification, but it would also specify the target performance values (predic-tive capacity required).
Principle 3 intends to provide transparency on the rationale used for applying DAs. The rationale may be based on an exist-ing AOP or network of AOPs or other mechanistic information relevant to the endpoint. In the case of DNT, due to the lim-ited number of available AOPs, mechanistic information de-rived from studies exploring disturbance of brain development processes by well-established DNT compounds can form the rationale for constructing a DNT testing strategy that relies on alternative methods (Fritsche, 2017a,b).
Principle 4 deals with data generated by the different infor-mation sources and how it is used within the DA to derive a prediction/assessment and aims to provide transparency on this aspect. The description should ideally include a schematic representation (e.g., flowchart or decision tree) to illustrate the procedure. The approach followed to provide prediction needs to be documented and understandable by the regulators.
Principle 5 allows the capture of the sources of uncertainty in predictions. Of particular interest would be to define if the proposed DNT testing strategy is reliable only for positive predictions or only for specific pathways or mechanisms of action. Additionally, the level of confidence (reliability of prediction) associated with the application of the testing strategy to different chemicals is needed. It is relevant to include as many chemicals as feasible as the determination of the applicability domain is expected to be correlated with the number and diversity of chem-icals tested. More importantly, this principle aims to capture the variability of the data produced by the alternative methods as well as the variability of the output data (i.e., from the DA) asso-ciated with the reference data (e.g., animal or human DNT data) used as benchmark data. In other words, the prediction of a DA aims to capture the variability and uncertainty of the alternative approach and the reliability of the gold standard data by applying appropriate statistical concepts and qualitative approaches.The application of these criteria and principles helps to estab-lish the overall relevance of the alternative methods and of the testing strategy.
3. Evaluation of in vitro DNT assays against defined readiness criteria
3.1. Compilation of readiness criteria
The development and application of in vitro test methods is driven by various stakeholders: basic academic researchers, test developers in industry and public institutions, and regulatory decision makers. As shown and discussed in Fig. 2, these three groups may have different points of views regarding the readiness of a test method. Moreover, readiness depends on the application of a method, in the field of toxicology in question (here DNT), and on the quality of animal experiments in the given field (Hartung and Leist, 2008). To take all this into account, a 2-step consensus process has been organised to establish a practical set of readiness criteria. They were first suggested and discussed during a workshop with different stakeholders, and then assembled for this report by a working group. A third step (described below) involved testing of the applicability of the criteria for actual scoring.
The criteria were clustered in 13 groups, e.g. concerning the test system, the prediction model, or the applicability for screening. For each of the criteria, a short heading was defined (e.g. critical components of the cell system). Then, the criterion was described in more detail. To do this, often specifying or guiding questions have been defined that need to be answered to provide information on the respective criterion. For instance, for the ‘critical components of the cell system’, this is “Have critical components and handling steps been identified and have they been clearly and explicitly described? Are examples for normal performance and morphology given? Are there examples for alerts?”. Finally, examples on the type of information required are given. In the chosen example: “E.g. cell density on a specific day of differentiation could be a critical step; wrong, strange morphology of cells could be an alert”. In this way, a compromise was reached between length (and clarity) of the document, and the information needed to perform a readiness evaluation (Table 2).
Table 2: Performance criteria to define the readiness of test methods for hazard evaluation.
This set of criteria was developed with the special need of developers of toxicological tests in mind. It should help them to prepare their assay for priority screening as well as for incorporation in an ITS. In the first column, the criteria are listed in their short form, the second column gives a definition or short description of each criterion (with some supporting and guiding questions), and the third column provides examples or further explanations for each of the criteria. In the fourth column the maximum score that can be reached is given. There are 13 main categories of criteria, with different numbers of sub-items. Within each main category, the sub-item can be scored for a readiness evaluation, and the sum of these scores results in the score for the main category. The fourth column indicates the maximum score that can be given for each category. Furthermore, the main criteria can be assigned to three different phases of test method development (phase I in green, phase II in blue, phase III in yellow). The topics printed in italics (e.g. 1j, 3a, 3c, 4d, 5d, 5e, 5i) may not apply to each test method. If they do not apply, the score is automatically set to 1 for these sub-items. Abbreviations: KE: key event; MIE: molecular initiating event; AOP: adverse outcome pathway; SOP: standard operation procedure; BMCL: benchmark concentration lower bound.
| Criteria | Description | Examples / Why is it important | Max. score |
|---|---|---|---|
| 1 Test system | Note: here scoring not for ‘test method’ | 10 | |
| 1a What is modelled | Is there a clear rationale given for what target organ/tissue relevant for human poisoning/pathology the test systems should reflect | Here: question is not for relevance, but whether there is documentation and a rationale at all. | 1 |
| 1b Relevance | Is the chosen test system known to be a key component in pathogenesis, or why is it thought to reflect a key component, mechanism or tissue | Here: is the tissue/organ modelled important for regulatory toxicology or biomedical research purposes. Is evidence given for the relevance of the model by morphological comparison, gene expression or functional criteria? Are all/sufficient cell types included in the model? | 1 |
| 1c System uncertainties and human correlate (HC) | (i) Is there a discussion on where the test system differs from the mimicked human tissue, and which gaps of analogy need to be considered? (ii) Do toxicant-altered genes (or other biomarkers) correspond to changes in mimicked human tissue (after poisoning or in relevant pathologies) | (i) E.g. a differentiated cell or a cell line (such as HepG2) does not necessarily reflect all features of the corresponding in vivo tissue/conditions. (ii) This is an additional measure to increase confidence in the test; not mandatory, but helpful. | 1 |
| 1d Definition of cells | Is the test system sufficiently characterized (source; multiple positive and negative markers for cell identity, number, quality, composition, differentiation state, viability, usual morphology, basic function, basic reaction to stimuli, STR… [“STR”=?]) | This is especially important for cells that have to be produced regularly, e.g. by differentiation or primary cell isolation. | 1 |
| 1e Cell composition | For multi-component systems: information on all cellular subpopulations. What is the percentage of contaminating cells or in co-cultures what is the percentage of all subpopulations. | This is important for the test endpoints as it could be that only one cell type may be affected by a toxicant. For primary cells: have cells from different sources (suppliers) been tested (e.g. hepatocytes from different suppliers may differ in purity and quality)? For routine use it would be beneficial to have pre-set acceptance criteria for each cell type | 1 |
| 1f Cellular environment | Information on structuring components of the test system: coating, scaffolds, matrix description, medium (supplements), microfluidic effects, supportive cells, dimensions and positioning/handling of 3D constructs,.... | This means a very detailed description of the culture conditions, including temporal and spatial aspects. Cell differentiation and response (quality, quantity, kinetics) may depend on multiple external factors and on the 3D arrangement | 1 |
| 1g Biological consistency | (i) Has the variation of the test system been assessed, influencing factors identified? (ii) Have acceptance criteria and performance standards for the test system been defined (different from the test!)? | (i) E.g. do medium supplements have an influence on the outcome of the cells; such as batch effects of FCS or serum replacement additives? (ii) e.g. a range of marker expression levels, of biological function (proliferation, protein production,…), of structural features (cell number, organoid size,…),… For lines: what is the optimum passage number of cells?. For routine use it would be beneficial to have pre-set acceptance criteria for the whole model/test system | 1 |
| 1h Critical components | Have critical components and handling steps been identified and described? Are examples for normal performance and morphology given; are there examples for alerts? | E.g. cell density on a specific day of differentiation could be a critical step; wrong, strange morphology of cells could be an alert.). For routine use it would be beneficial to have pre-set acceptance criteria. | |
| 1i Cell stability | Stability proven over multiple doublings; genetic stability shown; pluripotency/multipotency (for stem cells) shown, cell identity shown | For stem cells, stability needs to be shown over many passages (≥10). For primary cells: stability and identity of supply needs to be shown; stability of function (e.g. xenobiotic metabolism) shown. | 1 |
| 1j Transgenic cells | Transgene characterized (source, sequence, regulation); insertion characterized; stability of function shown and quantified; cell identity and function related to wt [weight?]; clonality documented. | 1 | |
| 2 Exposure scheme | 3 | ||
| 2a Description | Complete, detailed, unambiguous. | Medium changes, re-additions, coating, treatment period and timing, incubation conditions (temp. gassing,..) | 1 |
| 2b Unique identity | Tests with multiple variants of a test need to define very transparently, which variant the data come from | E.g. from which cell type/clone; which time; which plate format; which medium additives… | 1 |
| 2c Graphical scheme | Complete sequence of events, including endpoint assessment | Supports clarity and data assignment to test variants | 1 |
| 3 Documentation / SOP | 5 | ||
|
3a
Availability |
Method description for test system, test procedure, analytical endpoints and prediction model; public availability of SOP (data bank or test developer upon request) | Normal scientific publications are usually not sufficient, unless it is a specific methods paper. For transferability of the test method it is beneficial to have SOPs or other documents covering each component of test method and the whole testing process | 1 |
| 3b Stage of development | Version history; updated | 1 | |
| 3c For CRO tests | Are full performance standards and corresponding data delivered by the CRO along with test data (in case SOP details are not disclosed) | Non-disclosure of SOP is acceptable, if full performance/readiness criteria are given. | 1 |
| 3d Test components | Documented and available (receipt, storage, handling and disposal documents); quality criteria and checking procedure established | E.g. for media, plates, coating it should be defined, what is acceptable/non-acceptable and how this is controlled. Test chemical identity and purity (certificate of analysis) and safety data sheets for chemicals | 1 |
| 3e Stocks | Procedure for preparation, storage and quality control of stocks established | 1 | |
| 4 Main endpoint(s) | Mainly referring to specific/functional endpoints | 4 | |
| 4a Biol. relevance | Is there a rationale given why test endpoint is relevant to adverse outcomes | Helps to interpret the results obtained. | 1 |
| 4b Toxicological relevance | Are toxicants (≥ 3) known to affect the endpoint | Helps to interpret the results obtained. | 1 |
| 4c Analytical methods | Methods defined, rationale given; positive controls and acceptability criteria | Positive controls for analytical method may differ from controls for test/endpoint | 1 |
| 4d Multiple endpoints | Are all endpoints and their relation to one another (priority, preference) defined | E.g. neurite outgrowth / cytotoxicity | 1 |
| 5 Cytotoxicity | Here: if cytotoxicity is not main endpoint | 5 | |
| 5a Cytotoxicity within test | Cytotoxicity is preferentially determined within same test compartment as the major endpoint; second choice is under same conditions in parallel | Control of cytotoxicity in a different format (e.g. other types of plates; other time are very problematic). Measuring cytotoxicity under the same test conditions as the main end point help to interpret the mechanism related to the adverse effects for the main end point (specific or cytotoxicity driven mechanism) | 1 |
| 5b Subpopulation effects | Are subpopulations detected by measure for cytotoxicity or proliferation; are minor changes detected? Has sensitivity been shown? | Usually at least three types of assay required (measurement of viability, measurement of cell death, single cell analysis) | 0.5 |
| 5c Specificity (compared to cytotox) | A measure needs to be established to distinguish a specific/functional endpoint from cytotoxicity | E.g. neurite outgrowth, migration inhibition in non-cytotoxic concentration ranges | 0.5 |
| 5d Timing within test | For repeated/prolonged dosing, early death and compensatory growth need to be considered | The test of cytotoxicity only at the end may give false negative data, if cells die early and this is not detectable late, because of compensatory proliferation. | 0.5 |
| 5e Timing after test | For very short endpoints, e.g. electrophysiology measured 30 min after toxicant exposure, delayed measure of cytotoxicity is necessary | Cells cannot die in very short time, even though compound triggers lethal changes. Data for 24h exposure should be given. | 0.5 |
| 5f Curve fitting | Sufficient non-toxic data points (baseline); at least 40% toxicity / change to allow fitting | 0.5 | |
| 5g Non-cytotoxicity | Absence of ‘cytotoxicity’ does not mean non-cytotoxicity (question of power): has data variation been considered; is a measure of uncertainty given for non-cytotoxicity (e.g. BMCL calculation)? | 0.5 | |
| 5h Bench mark response | Has a rationale been given for setting a threshold value for cytotoxicity (statistical or biological significance) | E.g. statistical: 3x standard deviation; biological: 90% viability; see also: http://invitrotox.uni-konstanz.de/ | 0.5 |
| 5i Apoptosis/ Proliferation | If natural feature of the test system: measure for normal rate required | 0.5 | |
| 6 Test method controls | 4 | ||
| 6a Positive controls (PC) | ≥ 3 toxicants required for test definition; preferentially of different mechanisms; preferentially human-relevant toxicants; indicate variation of PC within and across assays | Used to define acceptability criteria, S/N ratio or z’-value of screen | 1 |
| 6b Negative controls (NC) | ≥ 5 negative controls are required to define specificity at ±20% level; concentration of negatives needs to be defined and rationalized | Ways to define negatives: (i) e.g. compound only acting when metabolized, (ii) acting on another organ, (iii) known to be safe for pregnant women, (iv) being selective for another assay, (v) pairs/matches of a specific positive control (e.g. inactive metabolite) | 1 |
| 6c Unspecific controls (UC) | A type of negative control for functional assays: not inactive, but only cytotoxic | Absolutely essential to define baseline variation and thus the relevant benchmark response for positive hits | 1 |
| 6d Endpoint-specific controls (EC) | To provide plausibility, and to help initial test setup: EC show that pathways considered to be relevant for test endpoint are indeed affecting the test endpoint. EC help to correlate (by concentration and time) compound effect on pathway (activity measure to be established) and on test endpoint (standard test readout). EC may be chemicals or siRNA; pathways may be defined from literature or experimentally (gene expression) | Example: actin is required for migration, thus an actin inhibitor should affect migration endpoint | 1 |
| 7 Data evaluation | Here: referring to main endpoint(s) | 4 | |
| 7a Outliers | Procedure for handling and documentation should be established | 1 | |
| 7b Concentration -dependence | Higher confidence in concentration-dependent data; no-effect concentrations must be included (full range curve); data need sufficiently dense spacing around benchmark concentration; preferably provide statistical significance for key data points | 1 | |
| 7c Benchmark response | Give rationale for definition (statistical (after FDR correction) or biological). Provide power estimate if conclusions are drawn from negatives. | 1 | |
| 7d Curve fitting | Indicate detailed procedure used for curve fitting; preferentially force fitted curve through 100% at negative control conditions (full function) | E.g. sigmoidal, linear or exponential curve fit | 1 |
| 8 Testing strategy | 4 | ||
| 8a Hazard prediction | Which hazard is assessed; which question does the test method answer? | 1 | |
| 8b Link to an AOP | Does the test give input to a mechanistic concept, e.g. an AOP? | Helps to position in battery; helps to interpret results | 1 |
| 8c Role in battery | Full score for stand alone tests. For tests that are not stand alone, information on their relation to other tests in a battery is required. | Information is required on how the test data would be used in a battery and under which conditions this is possible. | 1 |
| 8d Comparison to similar tests | Does the test fill a gap in a battery? Is it providing advantages compared to another test for the same hazard? | Avoid overlapping tests to be performed. Ensure adequate testing battery/strategy | 1 |
| 9 Robustness | 4 | ||
| 9a Reproducibility | Data available on normal variation; Information on factors affecting test variation is given | Historic control data on positive controls show normal range; known artefacts and shortcomings | 1 |
| 9b Intra-lab | Data available from different operators, different test runs over longer time | 1 | |
| 9c Inter-lab | Data available on transferability / reproducibility in another lab | 1 | |
| 9d Historical controls | Data for PC and NC over time | 1 | |
| 10 Test benchmarks | 4 | ||
| 10a Sensitivity (of the test) | Signal noise ratio (S/N) defined. Sensitivity information available | S/N based on adequate data sets. The S/N is used to determine the limit of detection. Additional measures: True positive rate, hit rate; sensitivity to detect a panel of positive controls, etc… | 1 |
| 10b Specificity (of the test) | Tested with sufficient number and quality of negative controls | Additional measures: true negative rate, etc. | 1 |
| 10c Acceptance criteria | Clearly defined and documented. Normal range of variation known | E.g. a given positive control has to reduce the main endpoint by at least 25%, otherwise test plate is discarded. | 1 |
| 10d Response characteristics | Should the response be linear? What are the upper and lower limits? | Additional measures: mono-directional or bi-directional deviation defined; Info on accuracy, precision, limit of quantification, etc. | 1 |
| 11 Prediction model | 4 | ||
| 11a Definition | Information should be available and clear
(including rationale for model, i.e. its particular strengths). Information and rationale should be given for use of sharp thresholds or probabilistic approach. |
Information on how many classes of toxicants
are predicted. Positives and non-positives; or strong, medium, weak
positives. Information on uncertainty of prediction should be given, at
least for positives (note that uncertainty of negatives is often not
defined). E.g. you can define a sharp threshold all above 4 is positive or you can define above 4 has a 70% likelihood to be positive |
1 |
| 11b Rationale | Reason, and mathematical basis / plausibility for prediction model given | Reason for the choice and value of thresholds | 1 |
| 11c Confirmation | Experimental testing of prediction model; confirmation of function/predictivity | 1 | |
| 11d Limitations | Information on limitations of prediction model, and on how exceptions and special cases are to be handled | Strange curve shapes, solubility issues, assay
interferences, … How special chemical classes are handled |
1 |
| 12 Applicability domains | 3 | ||
| 12a Chemicals | Is information on the types of chemicals that fall into the prediction model / testing range available? | 1 | |
| 12b Pathways | The type of pathways that are relevant for the test (to be disturbed or to be detected) | 1 | |
| 12c AOP | Information contributed to an AOP KE/MIE; element of a KE testing battery | 1 | |
| 13 Screening hits | 4 | ||
| 13a Hit definition | Transparent, pre-defined criteria (including curve-fitting/statistical procedure) | Usually, non-hits are discarded. If statements of non-hits are made, they need definition and power calculation. | 1 |
| 13b Hit confirmation (prim.) | Independent test run(s) in “same” test method; full concentration-response | Often loose (soft) criteria for hits, and no correction for false discovery rate. Confirmation assays can counteract such problems; use of new cells and new compound stocks provides additional robustness. | 1 |
| 13c Hit confirmation (sec.) | Additional test (different from primary test method) confirming hit on same endpoint as screen | E.g. migration may be measured by tracking cells (primary test) and then (secondary test) by a Boyden chamber method. | 1 |
| 13d Screen documentation | Acceptability criteria, performance of positive controls, internal robustness controls | 1 |
Our criteria list is meant to provide an easy-to-use tool for test developers and users in order to provide a quick and fast overview for them to judge how far the method is developed and what important points need to be addressed. Moreover, the semi-quantitative or quantitative scoring might help regulators to identify the strengths and weakness of a given test method. This could help them to decide to what extent the data generated by a given test method could be used. Notably, the tool may also be useful to identify and exclude data from non-ready methods from regulatory use or to prevent scientifically unsound data from creating anxiety in the general public.
3.2. Scoring system for readiness criteria
According to the OECD GD211 (OECD, 2014b), the new generation of in vitro test methods may be very useful for some regulatory purposes, even if they are not officially validated. For instance, they may be used to provide additional/supplemental mechanistic information on top of standard testing results. Moreover, such tests may be used in companies or regulatory authorities for internal decision making, or for screening programs with the aim of prioritization for further testing (Browne et al., 2017). Although there is guidance on what needs to be considered for test method validation, not many tools are available that provide an actual measure of readiness.
Since readiness needs to be quantified to a certain extent, a simple scoring system was established with the intention of pro-viding a rough quantification of readiness levels. In the future, such a system may be further refined, concerning the criteria considered, the weight given to the criteria, and especially by providing guidance on how the scoring is performed. Here, the system was kept simple, by assigning a maximum score to each criterion (see fourth column in Tab. 2), and by establishing a simple tool for clustering of scores (Fig. 3). The scores were assigned on the basis of publicly available information extracted from publications. The process may be facilitated in the future by a process that assembles all relevant information in a “read-iness dossier”, including data not easily found in publications, e.g., provided by test developers and applicants.
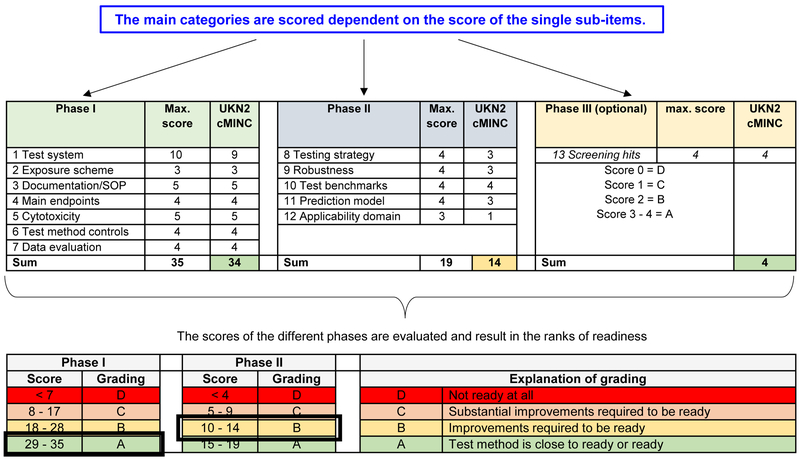
Figure 3: Scoring system for readiness criteria.
Overview of the scoring system for the readiness criteria. The 13 criteria are sorted into three phases. Each areas has various sub-items and the number of points that can be obtained is indicated in Table 2. Phase I (green) includes the basic features of the test method as they would be provided by academic researchers. They include biological plausibility of the test method, features of the test system, and the availability of controls. A high number of points can be obtained for test system description (10 out of 35), as this is very important at early stages of test development. However, still two thirds of the points come from other areas not to be neglected. The second phase (blue) relates to the implementation of a test for practical applications in industry or for regulatory purposes. Here, the relation to a testing strategy, good robustness, and the availability of a prediction model are important. The third phase (yellow) is optional as not each test method is used for a screening approach. Notably, not all points apply to all tests. In the preliminary rating scheme suggested here, these items are then scored positive automatically (labelled in italics in Table 2). Each phase if evaluated independently, and then categorized into one of four readiness classes (A-D). In the figure, an example is given for the rating of the cMINC (UKN2) test method. It would score as ‘A’ (largely ready) in phase I, and as ‘B’ in phase II. For phase III, it would score as ‘A’.
This clustering of scoring criteria is an important concept, as it provides individual scores for phases of test development. Phase I concerns all criteria that can be fulfilled during initial test method development. Phase II criteria refer to the test method performance based on, e.g., evaluation of replicates to conclude on robustness and reproducibility. Phase III is optional as a prop-er screening is not always feasible for each test method, i.e., 2ndand 3rd tier methods. This allows a distinction of readiness for, e.g., academic research purposes, screening and prioritization, or regulatory risk assessment. The example of the UKN2 test shows that a method can have a high readiness level for screen-ing, but still needs further improvement of hazard assessment of individual compounds in the context of a risk assessment process (Fig. 3).
3.3. Exemplary DNT test methods and their preliminary evaluation for readiness
To demonstrate the application of readiness scoring for DNT assays, a set of 17 test methods was selected, and the scoring was performed. Notably, the information used had to be extracted from the published literature, and thus, some information may have been missed or may not have been taken into account. It is also important to note that some methods were not developed specifically for regulatory use. In such cases, information retrieval was from multiple publications, and there were un-certainties and ambiguities concerning several criteria. A more formalized process of information retrieval might lead to higher scores. The selection of scored test methods was meant to give a representative overview of what is available to test interference of chemicals with various neurodevelopmental processes. The selection does not purport to be complete.
The individual scoring information can be found in supplementary Table 1. A summary overview is given in Table 3. In the following, some additional details are given on the test methods that have been considered here:
Table 3: Overview of the readiness levels of exemplary DNT test methods.
Different DNT test methods were scored according to the criteria presented in Table I and Figure 3. The readiness levels for Phase I –III varied from D (not at all ready, red), over C (orange), B (yellow) to A (largely ready, green). Detailed data are found in supplementary table 1. The overall readiness was estimated semi-quantitatively from the pattern of readiness in the different phases. Notably, the term overall readiness has to be used with care, as readiness depends on the purpose, and it is given here only to provide an orientation on the availability of methods in the field. This is exemplified by the cMINC (UKN2 method) which scores A in phase I and B in phase II. According to this, the method is not ready for regulatory risk assessment. However, it scores A for screening, and is thus ready for initial prioritization of compounds. List of abbreviations: UKN1 - PSC differentiation into NPC/NSC embryonic phase differentiation, NPC1 – hNPC proliferation, NPC2 – hNPC migration, NPC3 – hNPC neuronal differentiation, NPC4 – hNPC differentiated neurons, NPC5 – hNPC oligodendrocyte differentiation, NPC6 – hNPC oligodendrocyte maturation and TH disruption, UKN2 - NCC proliferation and migration, MESn - Morphological ESC to neurons, 3Dr - astrocytes, oligodendrocytes, myelination, microglia in 3D rat, 3Dh - astrocytes, oligodendrocytes, myelination, microglia in 3D human, foetal phase: UKN4 (NeuriTox) –neurite outgrowth of central neurons, UKN5 (PeriTox) – neurite outgrowth peripheral neurons, NSR neuronal subtype ratio, neuronal maturation: Syn – synaptogenesis, Nnff – neuronal network formation and function, ZFE – zebrafish
| Readiness/ Test method |
Phase I | Phase II | Phase III | Overall readiness |
|---|---|---|---|---|
| UKN1 | A | B | B | B+ |
| NPC1 | A | A | A | A |
| NPC2 | A | A | A | A |
| NPC3 | A | A | B | A- |
| NPC4 | A | B | C | B |
| NPC5 | A | A | B | A- |
| NPC6 | A | B | B | B+ |
| UKN2 (cMINC) | A | B | A | A- |
| MESn | C | D | D | D+ |
| UKN4 (NeuriTox) | A | A | A | A |
| UKN5 (PeriTox) | A | B | A | A- |
| NSR | C | D | D | D+ |
| SYN | B | B | B | B |
| Nnff | B | A | B | B+ |
| 3Dr | A | A | A | A |
| 3Dh | B | C | C | C+ |
| ZFE | B | B | A | B+ |
Differentiation of pluripotent stem cells into neural precursor cells (UKN1 test)
This test is exemplary for tests examining processes in the em-bryonic (very early) phase of brain development. A very early step in embryonic development is the lineage specification of the cells of the inner cell mass into the three germ layers, endoderm, mesoderm and ectoderm (Leist et al., 2008b). The ectoderm is further divided into neural ectoderm, which gives rise to the cen-tral nervous system, and the non-neural ectoderm.
The UKN1 test method mimics this early neuroectoderm lin-eage specification. Human pluripotent or embryonic stem cells (hPSC or hESC) are differentiated into early neuroectoderm pro-genitor cells. This stage is reached after 6 days under the given assay conditions (prevention of SMAD signaling) (Balmer et al., 2012; Balmer and Leist, 2014). The differentiation is extensively characterized by whole transcriptome analysis, showing that the differentiation protocol results in a homogenous neuroepithelial progenitor (NEP) cell population with an anterior gene expres-sion patterning. The process has been extremely well character-ized on the level of transcriptome and epigenetic changes (Shin-de et al., 2015, 2016; Rempel et al., 2015; Balmer and Leist, 2014; Weng et al., 2012, 2014). A change in this gene expression pattern indicates a wrong differentiation track and may help to measure KEs such as neural tube patterning or neural differenti-ation (Rempel et al., 2015; Tonk et al., 2015; Krug et al., 2013).
The evaluation of UKN1 with our suggested criteria list re-vealed that the system is ready concerning phase I. For phase II, the transferability to another laboratory is missing, as well as a final confirmed prediction model. It is a challenge to set up a pre-diction model based on gene expression data alone. Therefore, anchoring of data to a functional endpoint (rosette formation) will be included (Waldmann et al., 2017). Regarding the screen-ing issue of phase III, this test method reaches a readiness level of “B”, which means improvements are required.
Neuronal differentiation of pluripotent stem cells (various publications, MESn)
The UKN1 test method models early stages of embryonic neurodevelopment by the differentiation of early anterior determined NPC. However, there are more and more differentiation protocols published that enable differentiation of hESC or iPSC into other neuronal cell types. Each of these cellular systems is ready in terms of academic research and could serve as a starting point to develop new toxicological test methods.
In these test systems, human ESC are differentiated directly to neurons. It is important that this stage of brain development is covered by a DNT test battery as several compounds such as ethanol, methylmercury and lead have shown to induce perturbations at this time window. The most common approach to assess morphological neuronal differentiation is by immunohistochemistry for neuronal specific proteins such as neurofilaments, β-tubulin III and Map2. Most studies combine the imaging approach with other quantitative measurements e.g. by western blot (protein detection) or RT-PCR (mRNA expression). Several groups have developed protocols for the differentiation process; however, there is no harmonization between these different protocols. Furthermore, very few groups have tested more than one compound or generated concentration-dependent data. The main endpoints often show effects at cytotoxic concentrations. The performance criteria have been scored as the mean of five studies from different academic labs (He et al., 2012; Pal et al., 2011; Senut et al., 2014; Stummann et al., 2009; Talens-Visconti et al., 2011). Publications that described a promising test system but did not test any compounds were not included. The score for phase I (C) indicates that the test method needs substantial improvements to be ready; the score of phase II (D) and III (D) shows that the test method is not ready at all for direct application. The main shortcoming of this test method is the few compounds tested, while the test system itself is promising and relevant for DNT. Once data is generated from reference compounds this test method would likely be useful in a DNT testing battery. Similar tests have also been developed for murine ESC (Zimmer et al., 2011a,b; Kuegler et al., 2010), and may be used for species comparison. An interesting development is also the use of a 3D hiPSC-based system that has promising toxicological performance parameters (Schwartz et al., 2015)
Primary hNPC proliferation assay (NPC1)
Various assays are available to study KEs belonging to the fetal phase of brain development. Exemplary are the NPC tests, the PeriTox and the NeuriTox assay. NPC proliferation is a fundamental neurodevelopmental KE that, when disturbed, like in Zika virus infected primary NPC, leads to microcephaly in children (Tang et al., 2016; Devakumar et al., 2018). Proliferation of primary hNPC of fetal origin (Lonza) grown as neurospheres in 3D is studied by measuring increase in sphere size over 14 days using phase contrast microscopy (Baumann et al., 2014, 2015; Gassmann et al., 2010, 2012; Moors et al., 2009; Schreiber et al., 2010; Tofighi et al., 2011) and/or by measuring BrdU incorporation after 3 days in vitro (DIV) using a luminescence-based BrdU Assay (Roche) and a luminometer (Baumann et al., 2014, 2015). Briefly, neurospheres with a diameter of 300 μm are plated one sphere/well in a 96-well plate with or without chemical in EGF and FGF containing defined medium. For neurosphere diameter assessment, phase-contrast microscopic images are taken on plating day (day 0) as well as on day 7 and 14. Sphere diameter is measured with ImageJ and change in diameter monitored for each individual sphere. The same set-up is used for the BrdU assay, where BrdU incorporation into the DNA of hNPC is measured by using a luminometer. The endpoint-specific control for this assay is withdrawal of growth factors significantly reducing hNPC proliferation. This assay is part of a ‘high content DNT test’, the ‘Neurosphere Assay’ (NPC1–6), and is also set up with hiPSC-derived neurospheres as well as with spheres generated from prepared rat, mice or rabbit brains (Baumann et al., 2016; Barenys et al., 2017, unpublished data)
Scoring of the assay with our suggested criteria list revealed that the system is ready (scoring A) concerning phase I. For phase II the assay also scored A, although the transferability to another laboratory is missing and the prediction model needs finalization. This is currently under development with a large data set. Concerning the screening issue of phase III, this test method also reaches A.
The cMINC neural crest cell migration assay (UKN2 test)
100 different cell types in the human body, including the peripheral nervous system, melanocytes, cardiomyocytes or facial connective tissue (Huang and Saint-Jeannet, 2004). One major feature of neural crest cells is that they migrate to the different parts of the developing embryo and once they arrived at their final destination they differentiate to the according cell type. A large percentage of developmental disorders (e.g. congenital heart defects, orofacial clefts, Hirschsprung’s disease) are caused by NC cells (NCC) deficit. These kinds of alterations can be induced by genetic factors (Lee et al., 2009) or exposure to pharmaceuticals (e.g. valproic acid, Fuller et al., 2002) and pesticides (e.g. triadimefon, Menegola et al., 2005).
For the migration inhibition of neural crest cells (cMINC assay), human pluripotent stem cells are differentiated into HNK-1+/DLL-neural crest cells. The cells are then further expanded for up to 30 days before freezing. For testing the effect of chemicals on neural crest migration, the differentiated cells are thawed and seeded in 96-well plates supplemented with a silicon stopper that creates a 2 mm cell-free area. Migration is initiated by removal of the stoppers and the number of viable cells is measured after 48 hours (Nyffeler et al., 2017a).
The evaluation of the MINC assay revealed an A-score for readiness for phase I and III as an extensive screen including screen confirmation was performed using the NTP library of chemicals (Nyffeler et al., 2017b). For full readiness in phase II, the transferability into other laboratories has to be shown and further responsible pathways and AOPs are missing.
An additional feature of the assay is that other endpoints such as proliferation have been established and may be easily incorporated in standard testing.
ReNcell CX-based proliferation assay
ReNcell CX cells (Millipore, Temecula, CA) are a myc-immortalized cell line derived from a 14-week gestation human fetal cortex growing as a monolayer. For the proliferation assay, cells are plated in laminin-coated 96-well plates. ReNcell CX cell proliferation is determined by quantifying DNA replication using the Cellomics BrdU Cell Proliferation Kit for high-content screening (Thermo-Fisher Scientific, Pittsburgh, PA) by using the Cellomics ArrayScan. Proliferation is assessed after 4, 24, and 48 hours of compound treatment in a high content format (Breier et al., 2008; Radio et al., 2015).
Primary hNPC migration assay (NPC2):
Cortex development takes place during the fetal phase of development. It involves radial glia migration leading to the development of a scaffold that is subsequently used by neurons as ‘highways’ to migrate and reach their final cortical destination. In contrast to rodents, human brain is gyrencephalic and radial glia composition of gyrencephalic species differs from non-folded brain surface species and determines gyrencephaly (Borrell et al., 2014). Thus, NPC migration is a fundamental neurodevelopmental KE that, when disturbed, e.g. in methylmercury exposed children, leads to alterations in cortex development (Choi et al., 1989).
Primary hNPCs of fetal origin (Lonza) grow as neurospheres in 3D (see NPC1). Plating of size-defined (300 μm diameter) spheres on a poly D-Lysin/laminin matrix in a 96-well plate or 8-chamber slide format in absence of growth factors initiates radial NPC migration out of the sphere. First out of the neurosphere migrating cells display radial glia morphology and are NESTIN, SOX-2 and PAX-6 positive (Moors et al., 2007, 2009, 2012; Edoff et al., 2017). Their migration is dependent on laminin-integrin interaction (Barenys et al., 2017) that is also known to be crucial for radial glia migration in vivo (Belvindrah et al., 2007). Moreover, treatment with bone morphogenic proteins (BMPs) causes Glial Fibrillary Acidic Protein (GFAP) enrichment accompanied by morphological changes towards star-like, astrocyte cell shapes (Baumann et al., 2015). These data support the concept that these cells are radial glia cells (Moors et al., 2007, 2009; Baumann et al., 2016; Edoff et al., 2017).
Secondarily, neurons and oligodendrocytes arise, the former migrating on the glia carpet (Schmuck et al., 2017). Hence, this multicellular secondary 3D model (Alépée et al., 2014) can be used for measuring a) radial glia cell, b) early neuronal and c) oligodendrocyte migration. Radial glia cell migration is measured after 24 and/or 72 hours by determination of the distance the cells cover from the sphere core to the furthest migrated cell using phase contrast microscopy (Baumann et al., 2015, 2016; Gassmann et al., 2010, 2012; Moors et al., 2007, 2009; Schmuck et al., 2017; Barenys et al., 2017; Tofighi et al., 2011; Edoff et al., 2017) or applying high content image analyses (HCIA) and the Omnisphero program (Schmuck et al., 2017; 4) to DAPI-stained spheres (Baumann et al., 2016; Schmuck et al., 2017). When the latter approach is multiplexed with β(III)tubulin-stained neurons or O4-stained oligodendrocytes, the Omnisphero program (Schmuck et al., 2017) quantifies not only radial glia cell, but also neuronal and oligodendrocyte migration simultaneously. Migration cues differ between radial glia cells and neurons, as epidermal growth factor (EGF) stimulates radial glia- and does not affect neuronal migration at very low concentrations, while at higher exposure levels both cells types are responsive to the EGF cue. EGF also stimulates migration in vivo (Puehringer et al., 2013). This assay assesses early fetal neuronal and oligodendrocyte differentiation at the same time, yet these are described as separate assays (NPC3 and NPC5) as they can also be studied without migration measures. The NPC2 assay for total cell migration is also established for NPC prepared from rat, mice or rabbit brains (Baumann et al., 2016; Barenys, unpublished data).
Scoring of the assay with our suggested criteria list revealed that the NPC2 assay is ready (scoring A) concerning phase I. For phase II the assay also scored A, only the prediction model needs finalization. This is currently under development with a large data set. Concerning the screening issue of phase III, this test method also scores A.
Primary hNPC neuronal differentiation assay (NPC3)
Primary hNPC of fetal origin (Lonza) grow as neurospheres in 3D (see NPC1). Plating of size-defined (300 μm diameter) spheres on a poly D-Lysin/laminin matrix in a 96-well plate or 8-chamber slide format in the absence of growth factors initiates radial NPC migration out of the plated sphere (NPC2) accompanied by consecutive cell differentiation into nestin+ radial glia, β(III)tubulin+ neurons and O4+ oligodendrocytes (Moors et al., 2012; Edoff et al., 2017; Baumann et al., 2014, 2015) over a period of one to five days (Schmuck et al., 2017). Neuronal cells are identified by positive β(III)tubulin staining within the migration area of each neurosphere three or five days after plating either manually or by using the Omnisphero platform (Schmuck et al., 2017, 3). With this program, DAPI-stained nuclei are identified. An algorithm specifically created for small, young neurons with short neurites identifies β (III)tubulin+ neurites and secondarily finds the belonging nucleus by its association with the skeletonized neurite. By comparing this Omnisphero algorithm to the Neuronal Profiler Bioapplication (NPBA), the program that is customised for studying neuronal morphology with the Cellomics Array Scan (Thermo Scientific), we reduced the false-positive neuronal identification rate from 40% to <10%. NPC3 can be multiplexed with NPC4 (neuronal morphology, see below) or NPC2 (radial glia and neuronal migration); in the latter, information on neuronal (β(III)tubulin+ cell) positioning is further processed to values of neuronal migration (Schmuck et al., 2017). In addition, multiplexing of NPC3 with NPC2 and NPC5 (oligodendrocyte differentiation and positioning, see below) after five days in vitro reveals information on neuronal and oligodendrocyte differentiation and migration within one assay (Schmuck, unpublished data).
Scoring of the NPC3 assay with our suggested criteria list revealed that the assay is ready (scoring A) concerning phase I. For phase II the assay also scored A, only the prediction model needs finalization. This is currently under development with a large data set. Concerning the screening issue of phase III, this test method reaches B level of readiness.
Neuronal morphology (neurite number, average and total neurite length, neurite branching) of young neurons differentiated from fetal hNPC (NPC4)
The outgrowth of neurites is a major process during brain development. It is needed for the formation of dendrites and axons and is therefore a pre-requisite for cell connectivity of neurons. A disturbed or impaired neurite outgrowth during human brain development is thought to be one reason for the development of autism spectrum disorders. Therefore, this test method was developed in order to more rapidly assess chemical toxicity on the growth of neurites.
The NPC4 assay is an extension of the NPC3 assay when NPC3 is evaluated with the Omnisphero software (see above) because it quantifies morphological measures of stained, human fetal NPC differentiated, young β(III)tubulin+ neurons.
Skeletonized neurites are evaluated for their number, length and branching (Schmuck et al., 2017). The test is a HCIA assay, which has been extensively characterized with two individual software programs versus manual evaluation of all endpoints and thus there is high confidence in the outcome.
Scoring of the assay NPC4 with our suggested criteria list revealed that the assay is ready (scoring A) concerning phase I. For phase II the assay scored B, and in phase III this test method reaches C level of readiness.
The NeuriTox neurite outgrowth of CNS neurons test (UKN4)
For the establishment of this test method, immortalized primary cells derived from an 8-week old mesencephalon were used (Scholz et al., 2011). These cells are kept in a progenitor status by overexpression of v-myc under the control of a TET-off promotor. Upon silencing of the v-myc expression the neuronal progenitors differentiate into mature post-mitotic neurons in 6 days. In order to assess effects of chemicals on neurite outgrowth the differentiating cells are plated after two days of differentiation into 96-well plates and are treated for 24 hours (Krug et al., 2013b). Then the cells are stained with Hoechst and calcein and imaged with an automated microscope. The viable cells and the neurite area are determined by double positivity and measurement of calcein-positive pixels by the software of the microscope.
The evaluation of the UKN4 test method revealed a full readiness for phase I criteria (scoring A) and for phase II the transferability of the method needs to be shown (scoring A). Nevertheless, the cellular system including the differentiation has already been transferred into many different laboratories. A first screening was performed with the 80 compounds of the NTP library and will be published soon. In phase III this test also reached level A of readiness.
The PeriTox neurite outgrowth of PNS neurons test (UKN5)
Besides the neurite outgrowth of CNS neurons, also the neurites of PNS neurons are sensitive targets of chemicals. A prominent example is the development of neuropathies during chemo therapy after treatment with platinum compounds (Quasthoff and Hartung, 2002). In addition, acrylamide is a known toxicant that induces neuropathies in humans.
In order to differentiate immature human dorsal root ganglia cells, human pluripotent stem cells are used, differentiated for 8 days resulting in neural crest cells. These progenitor cells can then be frozen in liquid nitrogen. After thawing the neural crest cells immediately start to grow neurites. Therefore, one hour after thawing the cells are treated for 24 hours with different chemicals and stained with Hoechst and calcein. For imaging and quantification of viable cells and neurite area the principle is the same as in the UKN4 test method (see above) (Hoelting et al., 2016).
The evaluation revealed that the PeriTox test has a full readiness score for phase I (scoring A), whereas for phase II the transferability to another laboratory has to be shown and a final prediction model needs to be developed and confirmed (scoring B). A first screening was performed with the 80 compounds of the NTP library and will be published soon. In phase III this test received scoring A.
Neuronal maturation/neuronal network formation – Synaptogenesis (Syn)
The synapse formation assay allows to measure changes in number of synapses induced by an exposure to a compound that occurs during synaptogenesis process. Impairment of synaptogenesis is an important KE in the existing AOPs relevant to DNT (Bal-Price and Meek, 2017; Bal-Price et al., 2017) since this key neurodevelopmental process is affected by different classes of chemicals (e.g. Shi et al., 2011; Viberg, 2009; Harrill et al., 2011a,b, 2015a,b). Several approaches exist to measure synaptogenesis in vitro including (i) a commercially available Kit based on High Content Image Analysis (HCIA) (Thermo Fisher Scientific), referring to previously published data (e.g. Harrill et al., 2015a, b); (ii) synapse microarrays (Shi et al., 2011) and (iii) protein (Viberg, 2009; Kim and Lee, 2012) or mRNA analyses (Laurenza et al., 2013).
These assays allow quantification of presynaptic (e.g. synaptophysin, synapsin1, synaptobrevin, synaptogamin) and postsynaptic markers (PSD95, gephyrin, drebrin) at protein or mRNA levels as well as evaluation of their co-localization (HCIA).
The effects of chemicals on synapse function are routinely evaluated using whole-cell patch-clamp recording (Bal et al., 2010) or microelectrode arrays (MEA) applied to neuronal networks (e.g. Hogberg et al., 2011a; Vassallo et al., 2017) as described in this report (see Neuronal network formation and function).
However, to apply a synaptogenesis assay for a routine chemical screening, it needs further development of the performance criteria, i.e. threshold for hits and data interpretation procedure.
The score for phase I (B) and the score of phase II (B) and III (B) indicates that the test method is already well developed and standardized, however it still needs further optimization to fully satisfy the regulatory requirements. The test system itself is critical to be included in a DNT in vitro testing battery.
Development of neuronal subtypes (e.g. different neurotransmitters, NSR)
Perinatal exposure to low doses of toxicants such as lead and methylmercury can alter neuronal functions rather than leading to morphological alterations or to a net cell loss (Neal and Guillarte, 2010; Gimenez-Llort et al., 2001; Zimmer et al., 2011a,b). This effect may precede neurobehavioral and neurophysiological abnormalities that may also manifest long-term after exposure to the toxicant in later life (Tamm and Ceccatelli, 2017; Heyer and Meredith, 2017). Possible explanations concerning the molecular mechanisms are that such toxicants may interfere with expression of functionally relevant genes. Also, missregulation of genes involved in the neurotransmitter metabolism can lead e.g. to an altered ratio of neuronal subtypes. This might affect the patterning of the body axis or later on the homeostasis of the neurotransmitter system and eventually may affect neuronal function and connectivity, which could have implications in the adult organism.
Approaches used to evaluate different neuronal subtypes, are based on gene and protein expression of specific marker enzymes involved in the synthesis of specific neurotransmitters (i.e. glutamate decarboxylases (GAD1), tyrosine hydroxylases (TH), neurotransmitters transporters such as dopamine transporter (DAT), glutamate aspartate transporter (GLT) or the serotonin transporter (5-HTT)). Further, a toxicant may affect the expression of receptors of specific neurotransmitters. Profiling of relevant genes and/or proteins associated with neurotransmitters signaling have been performed on biased candidate genes by RT-qPCR (Zimmer et al., 2011a, b) and on whole transcriptome level during the maturation of neurons (Zimmer et al., 2011a, b). Together with functional endpoints, i.e. measurements of calcium flux, whole patch clamp or microelectrode arrays, (see Neuronal network formation and function), this provides further indication on the ability of toxicants to disrupt neuronal activity due to previously altered gene expression.
Differentiating mESC have shown some potentiality to address this issue at a stage where most neuronal precursors are formed and maturation of neuronal subtypes take place. The main endpoints addressed have been differentiation of neuronal subtypes and expression of specific neurotransmitters receptors and transporters (Zimmer et al., 2011a, b; Sanchez-Martin et al., 2013). Importantly, adverse effects of tested toxicants (MeHg, Pb) on these endpoints were not related to growth inhibition or cytotoxicity (Zimmer at al., 2011a, b; Sanchez-Martin et al., 2013). Although these test systems are of murine origin, they are very useful and helpful to investigate such toxic mechanisms, especially because human systems are rare. The test system as described in Zimmer et al., (2011a, b) (NSR: neuronal subtype ratio) and in Sanchez-Martin et al., (2013) was initially not developed as a test method and therefore would need further development to fulfill the readiness criteria as suggested here. The NSR test system reached scoring C for phase I and scoring D for phase II and III.
The modern trend in toxicology is to use human cellular systems to investigate such toxic effects (Daneshian et at., 2016). So far, protocols to obtain glutamatergic, GABAergic, dopaminergic or region-specific neuronal subtypes from human embryonic stem cells and human pluripotent stem cells have been published (Daadi et al., 2012; Gut et al., 2013; Begum et al., 2015), although no compounds have been tested for an effect on the differentiation process.
A further trend in toxicology is to use 3-dimensional models (3D) to investigate the more complex cellular structure of the nervous system. These models are of high interest in neurotoxicology and may be an opportunity to investigate possible shifts in neuronal subtypes. Moreover, they might be good test systems for investigations of cellular composition of neural cells, including neurons and glia cells. There have been several human models developed recently using various techniques and with different cell sources such as cell lines (Smirnova et al., 2016; Simão et al., 2016), ESCs (Lancaster et al., 2013; Sandström von Tobel et al., 2014; Sandström et al., 2017a,b) and iPSCs (Pamies, 2017a,b,c; Dang, 2016). These models have the capacity to differentiate into various neuronal subtypes and different glial cells (see Glial cell differentiation and maturation) making them suitable test systems for neurotoxicity and DNT. However, very few compounds have been tested in these systems, and previous developed assays generally need to be optimized to the 3D condition. Therefore, there is currently no well-developed DNT test available using these human models. There will likely be a rapid increase to use these systems for DNT in the near future, especially as many groups have already showed the relevance of using these systems to study neurological diseases and pathologies e.g. Alzheimer (Choi et al., 2014; 2016), microcephaly (Lancaster et al., 2013) and Zika infections (Dang et al., 2016; Qian et al., 2016).
Neuronal network formation and function (Nnff)
This method resembles early phases of brain development during which neuronal contacts are formed and become active. A few groups used these methods to establish effects of developmental exposures to several compounds (including MeHg, several insecticides and domoic acid) on the development of neuronal activity (Brown et al., 2016; Dingemans et al., 2016; Hogberg et al., 2011a; Robinette et al., 2011). Primary cortical culture from rat neonates grown on microelectrode arrays (MEAs) that develop into spontaneously active neuronal networks over time (Cotterill et al., 2016; Brown et al., 2016; Dingemans et al., 2016; Wagenaar et al., 2006) is the most established cell model for such measurements.
However, there is not yet much harmonization between these different protocols in terms of exposure window or exposure duration. However, for at least one of these protocols, the procedure has been published with a small set of assay positive controls (Brown et al., 2016), and a set of 86 compounds has been screened that included 60 compounds known to cause DNT in mammals, of which nearly 82% altered at least one parameter of network formation (Frank et al., 2017). In addition to chronic/developmental exposure, neuronal networks grown on MEAs are routinely used for acute exposure studies to determine effects on neuronal network function, which by now has been done for >1000 compounds (Strickland et al., 2017) using multiwell MEAs (mwMEAs). More recently, human iPSC-derived neuronal networks have been grown on MEAs (Tukker et al., 2016; Pamies et al., 2017a), although the degree of characterization of these human-based models and the number of compounds tested is currently limited. Regardless of the cell model used, MEAs can be multiplexed with cell viability assays such as LDH leakage, MTT and CellTiter Blue assays to distinguish neurotoxicity from cytotoxicity (Wallace et al., 2015). The scores for phase I (B), phase II (A) and phase III (B) indicates that improvements are still required to be ready, mainly regarding controls and harmonization of exposure paradigms and methods of analysis. Once done, this test method would be a useful inclusion in a DNT testing battery.
Glial cell differentiation and maturation: assays to evaluate the potential role of astrocytes, oligodendrocytes, myelination, microglia, and neuroinflammation
Regarding glial cells, two types of disturbances may occur: (a) impaired development of the respective cell type; (b) inflammatory over-activation of glial cells during the developmental period. The latter disturbance may have long-term consequences for brain structure and function: for instance, chronic neuroinflammation triggered during brain development was shown to be associated with Alzheimer’s pathology when aging (Krstic et al., 2012; Krstic and Knuesel, 2013), suggesting that the consequences of such DNT effects may only be revealed after a long asymptomatic delay (AOP-125).
Assays to evaluate glial differentiation (astrocytes and oligodendrocytes) can be performed in 2D or 3D rodent models.
Alternatively, cells may be differentiated from human ESC or IPS (this chapter), or from neural progenitor cells (following chapter) (Alépée et al., 2014).
Microglial cells in the brain are derived from yolk sack myeloid progenitors (Gomez Perdiguero et al., 2013). Microglial differentiation per se, in the brain, has not been studied as a DNT endpoint but, since microglia have an essential role in the neuroinflammatory process, and in the removal of other dying cells (Hirt et al., 2000), their reactive potential may differ depending on their maturation state or tissue environment (Sandström et al., 2017a; Lund et al., 2006).
Maturation of astrocytes can be assessed by a progressive decrease of vimentin expression and a progressively increased expression of GFAP and glutamine synthase (GS), as specific markers of astrocytes (Molofsky and Deneen, 2015). Toxicity to differentiating astrocytes would lead to a decrease of GFAP or GS levels, but it could also manifest by a re-expression of vimentin and mainly by an increased expression of GFAP over control level, as a sign of astrocyte reactivity (astrocyte activation is a typical sign of neuro-inflammation).
Oligodendrocyte differentiation and maturation can be evaluated by measuring the sequential expression of markers of different stages of differentiation (i.e. fist SOX10, followed by NG2 and O4, Gal-C, CNP, then MBP and finally MOG) (Rowitch, 2004). In mixed cultures, oligodendrocyte maturation can also be quantified by studying MBP expression. Completion of the myelination process can be assessed by the presence of compact myelin sheets visualized by electron microscopy (Pamies et al., 2017a).
Neuro-inflammation is mainly measured by glial reactivity, evidenced by increased expression of microglial and astrocyte specific markers (CD11b, Iba1, Isolectin B4, GFAP) and morphological changes, accompanied by increased expression and release of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6). Reactive glial cells can acquire neurotoxic (M1, A1) or neuroprotective (M2, A2) phenotypes (Kigerl et al., 2009; Liddelow et al., 2017; Shinozaki et al., 2017). Development-dependent changes in the expression of M1/2 phenotype markers of microglial cells have been observed upon toxicant-exposure (Sandström et al., 2017a).
Various test systems for glial differentiation (3Dr, 3Dh) have been evaluated for their readiness (see Table 3 and below).
The more complex 3D culture systems are required for measurements of myelination and neuro-inflammation, processes depending on complex cell-cell interactions. Using the suggested criteria list, the 3D culture systems derived from human ESC or iPS (Sandström et al., 2017b; Hogberg et al., 2013; Pamies et al., 2017 a,c) were scored ‘B’ for phase I and ‘C’ for phase II and III. High readiness (A for phase I and II, B for phase III) was achieved by the 3D rat brain cell culture system (Monnet-Tschudi et al., 1993, 1996, 1999, 2000; Zurich et al., 2000, 2002, 2012).
Oligodendrocyte differentiation (NPC5)
Primary hNPCs of fetal origin (Lonza) grow as neurospheres in 3D (see NPC1). Plating of size-defined (300 μm diameter) spheres on a poly D-Lysin/laminin matrix in a 96-well plate or 8-chamber slide format in the absence of growth factors initiates radial NPC migration out of the plated sphere (NPC2) accompanied by consecutive cell differentiation into nestin+ radial glia, β(III)tubulin+ neurons and O4+ oligodendrocytes (Moors et al., 2012; Edoff et al., 2017; Baumann et al., 2014, 2015) over a period of one to five days (Schmuck et al., 2017). Oligodendrocytes are identified by positive O4 staining within the migration area of each neurosphere five days after plating either manually or by using the Omnisphero platform (Schmuck et al., 2017,4). Thus, DAPI-stained nuclei that co-localize for the epitope O4 are identified. The number of identified O4+ oligodendrocytes divided by the number of total nuclei in the migration area reveals % of differentiated oligodendrocytes (Baumann et al., 2016; Barenys et al., 2017; Dach et al., 2017; Schmuck et al., 2017). The endpoint-specific control bone morphogenic protein (BMP) reduces oligodendrocyte differentiation and accelerates astrocyte maturation in hNPC (Baumann et al., 2016) similar to its effects in vivo (Bond et al., 2012). NPC5 can be multiplexed with NPC2 (migration), NPC3 (neuronal differentiation) and NPC4 (neurite morphology).
Scoring of the assay NPC5 with our suggested criteria list revealed that the assay is ready (scoring A) for phase I. For phase II the assay scored A, and in phase III this test method reaches B level of readiness.
Oligodendrocyte maturation – Thyroid hormone (TH) disruption assay (NPC6)
Maturation of O4+ oligodendrocytes differentiated from hNPC is studied by quantifying myelin basic protein (MBP) mRNA expression divided by the % O4+ cells as assessed within NPC5. This ratio is defined as the oligodendrocyte maturation quotient (QM). During NPC development, QM strongly increases upon treatment of cultures with the TH triiodothyronine (T3; Dach et al., 2017). Human TH disruptors are identified by interfering with this process, i.e. when QM solvent control < QM TH + compound < QM TH. Oligodendrocyte toxicants can be distinguished from TH disruptors when % oligodendrocyte decreases accompanied by no change or reduction in QM TH + compound in a concentration-dependent manner, respectively. This assay can also be performed in mouse and rat NPC, but the MoA of TH and its disruptors is different in rodent compared to human NPC (Dach et al., 2017).
Scoring of the assay NPC6 with our suggested criteria list revealed that the assay is ready (scoring A) concerning phase I. For phase II the assay scored B, and in phase III this test method also reaches B level of readiness.
Zebrafish assays
The zebrafish behavioral assays at early developmental stages (0–5 days post fertilisation (dpf), considered non-animal testing according to EU legislation) have shown their potential as a whole organism approach to predict human DNT, complementary to in vitro assays (Nishimura et al., 2015; Padilla et al., 2011; Garcia et al., 2016; Fritsche et al., 2015). These tests may be incorporated in a test battery in different ways (Fig. 4). The behavioral endpoints are readouts that integrate early events of central nervous system (CNS) development and functioning in a metabolically competent in vivo model system. Zebrafish brain development, anatomical features such as the blood-brain barrier, and physiology of early life stages are well described (Fleming et al., 2013; Mueller and Wullimann, 2016; Schmidt et al., 2013), while genetic and functional homology with human has been demonstrated (Howe et al., 2013; Khan et al., 2017; Parker et al., 2013). Many brain subdivisions found in the developing mammalian brain are identifiable in the developing zebrafish, and neurotransmitters including γ-aminobutyric acid (GABA), glutamate, serotonin, dopamine, noradrenalin, and acetylcholine are found in the neurons of zebrafish at 1–5 dpf with spatio- temporal expression highly consistent with those in the mouse. (Panula et al., 2010).
Figure 4. Incorporation of ZFE model in a low- and high throughput mode battery of tests.
The zebra fish embryo (ZFE) test may be incorporated in various ways into a DNT test battery, depending on resources, lab automation and the purpose of testing. If ZFE testing allows only low throughput, it may be used as second tier to further examine hits from other in vitro tests by a more complex whole-animal based test. Conversely, ZFE testing available as high-throughput system may be used to identify primary hits that are further characterized and/or confirmed for human relevance by human cell-based in vitro tests. As a third approach, ZFE testing may be run in parallel with in vitro tests to feed data into an overall decision model.
The zebrafish genome has been mapped and approximately 70%–80% of zebrafish genes share homology with the human genome, and 82% of genes associated with disease in humans can be related to at least one zebrafish orthologue (Howe et al., 2013).
The stereotypic motor activity of the developing zebrafish, includes three sequentially appearing behaviors which are in line with neurodevelopment: a transient period of alternating tail coiling, followed by responses to touch and the appearance of organized free swimming of larvae (Nishimura et al., 2015). Behavioral assays for DNT in zebrafish exposed to a diversity of compounds or drugs include one or more of these 3 basic behaviors (Chen et al., 2012b; He et al., 2016; Selderslaghs et al., 2010; 2013; Jin et al., 2016) or some variants including a light stimulus in the photomotor response test (PMR) or light/dark challenge (Ali et al., 2012; Jarema et al., 2015; Noyes et al., 2015). These behaviors appear comparable at a functional level with human behavior, with links to neural circuitry underlying the basic form of behavioral regulation. Consistent with mammals, neural networks generate e.g. periodic motor commands for rhythmic movements, and visual challenges can result into anxiety-like behavioral effects (Nishimura et al., 2015).
Many different zebrafish behavioral assays were reviewed by Legradi et al. (2015), concluding that there is a need for a harmonized protocol with recommendations for e.g. inclusion of embryo teratogenic endpoints, positive and negative controls, and standard exposure scenario. Nevertheless, the robustness of the behavioral endpoints has been demonstrated through comparison among different assays for a small number of chemical (i.e., three compounds: ethanol, valproate and pentylenetetrazole) in respectively 7, 3 and 4 studies respectively giving similar results (Legradi et al., 2015). The scoring for readiness considered aforementioned publications, covering screening for between 1 up to 60 compounds, demonstrating compliance for a majority of performance criteria with B for phase I and phase II and A for phase III. Zebrafish behavioral analyses are promising tools, complementary to cellular assays which will benefit from further protocol harmonization and defining screening hits. The behavioral assays might be strengthened through inclusion of mechanism-based assays (axon growth, gene expression profiles, neurotransmitter activity) in relation to observed adverse outcomes (Chen et al., 2012a; He et al., 2016; Jin et al., 2016) and link to other human-based cellular model systems within the DNT battery.
4. Key neurodevelopmental processes covered by a battery of DNT in vitro assays
Over the last decade, there has been a thorough effort from neurotoxicologists to identify neurodevelopmental KEs that are essential for brain development (Fig. 1) and can reliably be tested in an in vitro assay format. This task is complex as the developmental period of the brain is the longest compared to other organs - spanning from post-conceptual week four until the mid-20 years of age - and during the different phases of neurodevelopment various brain cell types perform distinct yet coordinated tasks. Neurodevelopmental processes in the context of timing and with a focus on human brain development are summarized in Silbereis et al. (2016), which serves as the basis for this chapter. These processes are laid out here and corresponding in vitro assays that have the ability to detect changes in such are identified. The list of assays comprising a possible future testing battery can be found in Chapter 3.3 of this paper and is not repeated here. However, missing assays for certain neurodevelopmental processes are identified.
During early embryogenesis, embryonic stem cells commit to the neural lineage by becoming neural precursor cells (NPCs). These cells migrate and form the neural plate and subsequently the neural tube as the first defined structures of the brain. Later during development, the neural tube is called the subventricular zone, the area of cell origin (Kolb and Gibb, 2011). Assays capturing effects of chemical exposure on these endpoints include development of NPCs from hESC or hiPSC and stem cell- derived rosette formation. At this time, the rosettes resemble the neuronal tube structure in a two-dimensional (2D) format (Stummann et al., 2009; Colleoni et al., 2011, 2012; Senut et al., 2014; Waldmann et al., 2017). Readouts are either morphological features of rosette formation or changes in gene expression levels below the cytotoxic threshold. On this basis, the transcriptomics-based teratogenicity index was established (Waldmann et al., 2014, Shinde et al., 2016).
During a phase of exponential growth, the neural tube expands to form the critical brain processes that establish the primary organization of the central nervous system. This involves proliferation of NPC, that can be measured with different cell systems in 2D, i.e. hESC (Talens-Visconti et al., 2011; Bai et al., 2013), hiPSC (Souza et al., 2016), myc-immortalized ReNcell CX (Breier et al., 2008; Radio et al., 2015) or 3D, i.e. NPC (Gassmann et al., 2010; Schreiber et al., 2010; Baumann et al., 2015, 2016; Barenys et al., 2017). In the neurulating embryo during neural plate formation, neural crest cells (NCCs) emerge that will later develop into cell types of various tissues (e.g. bone, cartilage, neurons, and melanocytes). For terminal specification, NCCs migrate to their loci of function (Dupin and Sommer, 2012). Disturbance in NCCs migration might lead to e.g. Wardenburg’s syndrome, Hirschsprung’s disease, craniofacial abnormalities like frontonasal dysplasia and others. Thus, development of the Neural Crest Cell Migration (MINC) assay is an important tool to study effects of chemicals on this endpoint (Dreser et al., 2015; Pallocca et al., 2016; Zimmer et al., 2012, 2014; Hirsch et al., 2017).
For development of individual brain regions and connections between parts, distinct signaling is necessary, as illustrated by brain region-specific transcriptome profiles in developing human brains (Miller et al., 2014). For human cortical development, differences from other species like rodents include the appearance of a secondary proliferative zone that allows the massive expansion of the human cortex (Kriegstein et al., 2006; Hansen et al., 2010). Outer radial glia (oRG, or basic radial glia (bRG)) cells, which contribute the majority of human radial glia cells and reside in this outer subventricular zone, are thought to produce the greater part of human cortical neurons (Smart et al., 2002; Lewitus et al., 2013). Lack of oRG cells causes lissencephaly, a normal condition in, for example, mice, but a rare, severe brain malformation in humans. An assay where RG cell migration can be addressed is the human NPC2 assay (see above; Moors et al., 2007, 2009; Barenys et al. 2017; Schmuck et al., 2017). Initially migrating cells show a RG cell morphology and express NESTIN and GFAP. Upon BMP treatment, they develop into star-shaped, GFAP expressing astrocytes. More detailed molecular knowledge on the specific type of RG cell differentiated in these cultures will be helpful in development of brain region-specific in vitro models.
The first neurons that develop as early as in human gestation week (GW) 4 are motoneurons (Bayer and Altman, 2007; O’Rahilly and Muller, 2006). Several methods for the generation of motor neurons from embryonic stem cells have been established and characterized. With regard to neurogenesis in the context of cortical development, neocortical neurons start to arise from GW7 and with some exceptions the majority of neurons are formed prenatally, i.e. neocortical excitatory neuron generation ceases at GW27 (Workman et al., 2013;6). Here, one can distinguish between early neurogenesis creating the most essential neuronal circuits mainly from hindbrain rhombomeres (Kiecker and Lumsden, 2005) and later neurogenesis during cortex formation from RG cell populations (Borrell and Götz, 2014). As for the early neurogenesis, methods for in vitro neuronal differentiation from hESC or lately hiPSC are established (Stummann et al., 2009; He et al., 2012; Nash et al., 2012; Druwe et al., 2016; Pistollato et al., 2017; Zagoura et al., 2017). For later neurogenesis during corticogenesis, it seems advantageous to employ fetal cells that arise from the 2nd trimester of gestation (Hansson et al., 2000) and which form neurons from RG neural precursors as in 3D neurospheres from primary human fetal NPC, as described in the NPC3 assay (Moors et al., 2009; Baumann et al., 2015; Barenys et al., 2017) or equivalent stem cell-derived neurons with cortical features (Rigamonti et al., 2016).
During brain development, more neurons are generated than needed, and final circuits are shaped by programmed death of surplus neurons that do not reach their target area. This has been modelled in primary neurons by conditions favouring hypo-polarization (Gerhardt et al., 2001; Volbracht et al., 1999), and similarly dedicated test methods may need to be devised for human neurons (Druwe et al., 2015).
In addition to neurogenesis, neuronal migration is a hallmark of cortex formation. Neuronal migration can also be measured with the ‘Neurosphere in vitro Assay’ by using a specific software, Omnisphero, in which the NPC2 and the NPC3 assay are multiplexed (Schmuck et al., 2017). After birth, newly formed and migrated neurons develop further by massively growing out neurites, dendrites and axons, followed by synaptogenesis. These processes are indispensable for neuronal network formation. Different neuronal in vitro systems allow measurements of these endpoints ranging from hESC- or hiPSC-derived neuronal monoculture (Harrill et al., 2011a; Druwe et al., 2016) or mixed cultures (Zagoura et al., 2017; Pistollato et al., 2017) to 3D hESC- or hiPSC-derived mixed cultures (He et al., 2012; Rigamonti et al., 2016), LUHMES dopaminergic neuronal monocultures as in the UKN4 assay (Scholz et al., 2011) or primary hNPC-derived mixed cultures using the Omnisphero software by using the NPC4 assay (Schmuck et al., 2017). Synaptogenesis, however, has been quantitatively assessed in rat neurons via HCIA (Harrill et al., 2011b). As already mentioned in the endpoints evaluation section, several different methods exist to measure synaptogenesis in vitro quantitatively, including a commercially available kit based on HCIA (Thermo Fisher Scientific).
Recently, synapsin staining as a pre-synaptic vesicles protein was detected in hiPSC-derived mixed cultures that contain GABAergic, glutamatergic and dopaminergic neurons (Zagoura et al., 2017), however, synapse number or protein expression was not quantified. Functionality of synapses in these cultures was displayed by electrical activity on microelectrode arrays (MEA),
i.e. spikes and bursts, but do not seem to present synchronized bursting as seen for rat primary cortical cultures-derived networks (Brown et al., 2016; 2017) or hESC-derived cultures on MEA chips (Kapucu et al., 2012; Kiiski et al., 2013). Nonetheless, MEA measurements were already successfully applied for in vitro DNT testing during chronic exposure to domoic acid (Hogberg et al., 2011a), including evaluation of different receptor subtypes involvement (Hogberg et al., 2011b), MeHg (Dingemans et al., 2016) and several insecticides (Dingemans et al., 2016), and recently a set of 86 environmentally relevant chemicals (Frank et al., 2017). As briefly described in Chapter 3.3, neuronal morphological and functional maturation (including expression of functional receptors, ion channels, pathways involved in various range of cellular responses and defence mechanisms etc.) can be evaluated by immunocytochemistry specific protein staining, mRNA expression or pathways specific responses measurements using specific agonists or antagonists.
Human stem cell-based protocols need further optimization to improve neuronal and glial maturation in mixed cultures derived from hiPSCs which will be able to generate reliable and reproducible neuronal network activity. Such cultures should contain various cell types, as in vivo, of excitatory and inhibitory synapses originating from different neuronal subtypes grown in the presence of glial cells (astrocytes, oligodendrocytes and microglia).
Indeed, besides neurons, glia cells are integral parts of the CNS representing 50% of cells in the adult brain (Kuegler et al., 2012). Glia cell (astrocytes and oligodendrocytes) generation from RG by producing astrocyte and oligodendrocyte precursor cells generally follows neurogenesis and continues until after birth (Kleiderman et al., 2016a,b).
Astroglia differentiation is a crucial event during brain development because astrocytes create the brain environment, build up the micro-architecture of the brain parenchyma, maintain brain homeostasis, store and distribute energy substrates, control the development of neural cells, synaptogenesis and synaptic maintenance and provide defence strategies for the brain. There are different astrocyte types with different functions in the brain (Hu et al., 2016). Some in vitro systems recapitulate astrocyte development from hESC, hiPSC or hNPC (Talens-Visconti et al., 2011; Zagoura et al., 2017; Moors et al., 2009). There is, however, a lack of precise astrocyte molecular characterization besides the expression of GFAP or vimentin that allows understanding of the astrocyte subtypes role in such systems. Compound effects on astrocyte reactivity (Zagoura et al., 2017; Sandström et al., 2017b), development (Moors et al., 2010; Baumann et al., 2015) or susceptibility (Talens-Visconti et al., 2011) are just beginning to contribute to the understanding of different astrocyte subtypes and functions in human cultures in vitro.
Much more information is available on murine primary astrocytes (Falsig et al., 2006), or the combination of murine astrocytes with human neurons (Efremova et al., 2015, 2017), and fully humanized systems can be optimized to yield similar data.
Compared to other glial subtypes, oligodendrocyte myelin production is protracted in humans (Bradl and Lassmann, 2010). Given the inhibitory action of myelin on synapse formation and neuronal network plasticity, delayed myelination prolongs the development of learning activities, memory, and complex sensory perception. This species difference in timing highlights the importance of using human cells for complex oligodendrocyte or myelination in vitro models. Some of the recently developed methods for multiple sclerosis research referring to oligodendrocytes are summarized in Madill et al., (2016). In addition, O4+ cells generated from human fetal NPC neurospheres can be used for oligodendrocyte formation in the NPC5 assay (Moors et al., 2009; Schreiber et al., 2010) and TH-dependent maturation evaluation in the NPC6 assay (Dach et al., 2017) as described in chapter 3.3. The formation of mature myelin sheets is still challenging to obtain in vitro and the 3D structure is crucial for this process. The 3D rat brain cell system has one of the best-developed tests for this process (Monnet-Tschudi et al., 1999), however, the species difference is of concern. A few human models have recently been developed showing characteristic myelin sheet morphology, but the test method needs to be further developed to fulfill the criteria of the DNT test battery (Sandström et al., 2017b; Pamies et al., 2017a).
5. The status of in vitro testing in the field of DNT
5.1. Which chemicals have already been tested in assays that can contribute to a DNT test battery?
An alternative approach towards evaluation of test readiness would be to examine which compounds known to be associated with a DNT hazard have been correctly or incorrectly identified by NAMs. This question can only be answered conclusively by data from an entire test battery, as no single in vitro method covers the whole spectrum of DNT-relevant processes. A small step towards this ultimate goal would be taking stock of the available data to see which chemicals have been tested, and which gaps in chemical and biological space would need to be filled. In a subsequent step, generally applicable prediction models would need to be established in order to eventually compare the outcome of in vitro testing with knowledge on in vivo hazard.
We conducted a literature search investigating which of the 32 compounds listed by Aschner et al., (2017) as DNT toxicants have been tested in vitro. The outcome of our survey shows (Table 4) that only a few compounds (e.g. methylmercury) have been tested broadly, while for others (e.g. heroin) only limited in vitro data are available. However, testing this small subset of compounds will not be sufficient. There are other positive controls, and, even more importantly, large numbers of negative controls need to be identified and tested to establish good prediction models. Thus, an important task for future research activities would be to close such data gaps by encouraging the development and use of a larger test set of chemicals to be used widely within the DNT in vitro field.
Table 4. Overview of testing status of DNT reference compounds, with respect to NAMs.
A subset of chemicals with strong evidence for a DNT hazard in vivo (as described in Aschner et al., 2017) was selected. A literature search was performed to retrieve data on in vitro testing of these compounds. Data for 11 assays have been compiled here. Grey fields indicate that no clear test data have been retrieved. Green fields indicate that the compound has been examined in the respective test method, and was found to show a positive effect. Orange fields indicate that the compound has been tested, but did not show any effect specific of DNT. In the latter two cases, the literature evidence is indicated. Each assay allows testing of specific DNT endpoints as indicated below: NEP differentiation: neural tube formation. Neurospheres: NPC proliferation; radial glia migration; neuron and glia differentiation; neurite outgrowth. ReNcell: NPC proliferation. MINC: NCC migration. hESC/hiPS: neuron, astrocyte and oligodendrocyte differentiation; neurite outgrowth. UKN4 (NeuriTox): DA neuron differentiation; neurite outgrowth. 3D human: neuron, astrocyte and oligodendrocyte differentiation,; synaptogenesis; myelination; neuronal network formation. 3D rat: neuron, astrocyte and oligodendrocyte differentiation; synaptogenesis; myelination; neuronal network formation; neuroinflammation. 2D murine: neuron and glia differentiation; synaptogenesis; neuronal network formation. UKN5 (PeriTox): neurogenesis. Zebra fish: brain development. Literature as indicated by numbers in the orange and green fields: 1. Zimmer et al., 2014; 2. Dreser et al., 2015; 3. Pallocca et al., 2016; 4. Zhou et al., 2015; 5. Chattopadhyay et al., 2002; 6. Breier et al., 2008; 7. Culbreth et al., 2012; 8. Gulisano et al., 2009; 9. Monnet-Tschudi et al., 1993; 10. Tasneem et al., 2016; 11. Chow et al., 2008; 12. Selderslaghs et al., 2013; 13. Baumann et al., 2016; 14. Lee et al., 2014; 15. Krug et al., 2013b; 16. Monnet-Tschudi et al., 2000; 17. Slotkin et al., 2012; 18. Crumpton et al., 2000; 19. Visan et al., 2012; 20. Dingemans et al., 2016; 21. Lee et al., 2017; 22. McCarthy et al., 2011; 23. Shang et al., 2007; 24. Harrill et al., 2011; 25. Moors et al., 2012; 26. Ninomiya et al., 2014; 27. Bramanti et al., 2010; 28. Khor et al., 2013; 29. Perez-Gomez et al., 2012; 30. Hogberg et al., 2011; 31. Tiedeken et al., 2005; 32. Palmer et al., 2012; 33. Talens-Visconti et al., 2011; 34. Nash et al., 2012; 35. Guadagnoli et al., 2016; 36. Parker et al., 2014; 37. Benninghoff et al., 2013; 38. Bai et al., 2013; 39. Slikker et al., 2015; 40. Hondebrink et al., 2017; 41. Zimmer et al., 2012; 42. Senut et al., 2014; 43. Zurich et al., 2002; 44. Monnet-Tschudi et al., 1999; 45. Dou et al., 2011; 46. Chen et al., 2012; 47. Hoareau et al., 2006; 48. Suarez-Isla et al., 1984; 49. Kindlundh-Hogberg et al., 2010; 50. Hondebrink et al., 2016; 51. Santos-Fandila et al., 2015; 52. Stummann et al., 2009; 53. Schmuck et al., 2016; 54. Moors et al., 2009; 55. Wilson et al., 2014; 56. Pallocca et al., 2013; 57. Stiegler et al., 2011; 58. Sandström et al., 2017; 59. Hoelting et al., 2013; 60. He et al., 2012; 61. Monnet-Tschudi et al., 1996; 62. Popova et al, 2014; 63. Yao et al., 2017; 64. Coronas et al., 2000; 65. Sandström von Tobel et al., 2014; 66. Schreiber et al., 2010; 67. Hirsch et al., 2017; 68. Xiong et al., 2012; 69. Tofighi et al., 2011; 70. Yang et al., 2014; 71. Markus et al., 2010; 72. Colleoni et al., 2011; 73. Orsolits et al., 2013; 74. Addae et al., 2012; 75. Colleoni et al., 2012; 76. Wang et al., 2015; 77. Zimmermann et al., 2015.
| Cellular system | NEP diff. | Neuro- spheres |
ReNcell | Neural crest migration | hESC
/ hiPS based diff. |
CNS neurons |
3D human cell culture | 3D rat cell culture | 2D murine cell culture | PNS neurons |
Zebra fish |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name of assay | NEP diff. | NPC 1–6 | ReNcell | UKN2 (cMINC) |
UKN1 | UKN4 (NeuriTox) |
3Dh | 3Dr | 2Dm | UKN5 (PeriTox) |
ZFE |
| COMPOUNDS | |||||||||||
| Arsenic | 1–3 | 4,5 | |||||||||
| Cadmium | 6,7 | 2 | 8 | 9 | 10 | 11 | |||||
| Chlorpromazine | 1 | 12 | |||||||||
| Chlorpyrifos | 13 | 7 | 14 | 15 | 16 | 17–20 | |||||
| Cocaine | 21 | 22 | 23 | ||||||||
| Dexamethasone | 6 | 24–26 | 27 | 28 | |||||||
| Diphenyldantoin | |||||||||||
| Domoic acid | 29,30 | 31 | |||||||||
| Ethanol | 32–34 | 35 | 36 | ||||||||
| Haloperidol | 15 | 37 | |||||||||
| Heroin | |||||||||||
| Hexachlorophene | |||||||||||
| Ketamine | 38 | 39,40 | |||||||||
| Lead | 6 | 41 | 24,42 | 43,44 | 10,19 | 45,46 | |||||
| Lindane | |||||||||||
| MAM | 13 | 47 | |||||||||
| Maneb | |||||||||||
| Manganese | 48 | ||||||||||
| MDMA | 49,50 | ||||||||||
| Methanol | 51 | ||||||||||
| Methyl mercury | 52 | 13,53,54 | 6 | 41 | 24,55,56 | 15,57 | 58–60 | 61 | 10,19,20,62 | 12 | |
| MPTP | 63 | ||||||||||
| Nicotine | 50,64 | ||||||||||
| Paraquat | 15 | 58 | 65 | ||||||||
| PBDE | 66 | 3,67 | 68 | ||||||||
| PCB | 2 | 69 | 70 | ||||||||
| Perfluorate-PFOA | |||||||||||
| Perfluorate-PFOS | |||||||||||
| Terbutaline | 71 | ||||||||||
| Toluene | |||||||||||
| Trans retinoic acid | 72 | 6,7 | 24 | 73,74 | |||||||
| Triethyl tin | |||||||||||
| Valproic acid | 75 | 13 | 2,3,41 | 76 | 77 |
One step in this direction would be development of a database of all compounds tested to date in DNT alternative assays. So far, a summary list of chemicals tested has been generated (Figure 5). This figure illustrates the current status of chemical testing in assays that could be used as part of an IATA for DNT. The table was compiled based on publications describing various assays and requesting that the lead authors of those publications report which compounds they have tested. All chemicals were mapped based on conversion of CAS#s to DSSTOX ID numbers using the EPA Chemistry Dashboard7.
Figure 5. The current chemical landscape of in vitro DNT testing.
The heatmap plots chemicals as rows and test status as columns. The first 5 columns provide evidence of the class of chemicals relative to evidence of DNT or priority for testing (see main text chapter 5.1). The other columns list assays grouped by neurodevelopmental processes. A brief description of each column is provided below, along with a reference or references, if available. Compounds from columns A-E that have been tested in different assays (columns 1–31), are indicated by a blue (human), red (rodent), or green (alternative species) horizontal line. It should be noted that the information on what compounds have been tested was provided by the laboratories engaged in testing, and that not all of the data for each compound/assay pair have been published. Chemical class columns: A Compounds with evidence of developmental neurotoxicity from multiple laboratories (Mundy et al., 2015); B Compounds with evidence of developmental neurotoxicity from only 1 laboratory (Mundy et al., 2015); C Compounds in the 87 chemical library supplied by the National Toxicology Program; D Compounds subjected to the literature search in Mundy et al., 2015 that did not have evidence of developmental neurotoxicity; E Other compounds. This consists primarily of ToxCast compounds, but also assay positive controls and other miscellaneous compounds. Assay columns: 1 Proliferation in human neurospheres (Baumann et al., 2016); 2 Proliferation in hNP1 neuroprogenitor cells (Mundy et al., 2010); 3 Proliferation in ReNcellCX human neuroprogenitors (Breier et al., 2008; Radio et al., 2015); 4 Proliferation in mouse neurospheres (Fritsche et al., unpublished data); 5 Proliferation in rat neurospheres (Baumann et al., 2016); 6 Neuronal differentiation in human neurospheres (Baumann et al., 2016); 7 Oligodendrocyte differentiation in human neurospheres (Fritsche et al., unpublished data); 8 Differentiation in mouse neurospheres (Fritsche et al., unpublished data); 9 Neuronal differentiation in mouse neurosphere (Fritsche et al., unpublished data)10 Oligodendrocyte differentiation in mouse neurospheres (Fritsche et al., unpublished data); 11 Neuronal differentiation in rat neurospheres (Baumann et al., 2016); 12 Oligodendrocyte differentiation in rat neurospheres (Fritsche et al., unpublished data); 13 Apoptosis in human NP1 neural precursors (Druwe et al., 2015); 14 Migration of human neuroprogenitor cells; 15 Migration in human neurospheres (Baumann et al., 2016); 16 Migration in human neural crest cells (Nyeffler et al., 2017a, Nyeffler et al., 2017b); 17 Migration in mouse neurospheres (Fritsche et al., unpublished data); 18 Migration in rat neurospheres (Baumann et al.,2016); 19 Neurite outgrowth in human hN2 neurons. (Harrill et al., 2010); 20 Neurite outgrowth in human peripheral neuroprecursors (Hoelting et al., 2016); 21 Neurite outgrowth in LUHMES neurons (Krug et al., 2013b); 22 Neurite outgrowth in human iPS-derived neurons. (Ryan et al., 2016); 23 Neurite outgrowth in PC12 cells (Radio et al., 2015); 24 Neurite outgrowth in rat cortical neurons (Harrill et al., 2011a); 25 Maturation of neurites in rat cortical neurons (Harrill et al., 2011b); 26 Synaptogenesis in primary cortical neurons (Harrill et al., 2011b); 27 Neuronal Network Function- Acute (Strickland et al., 2017, in press); 28 Neuronal Network Formation- Developmental (Brown et al.,2016); 29 Feeding, larval development and reproduction in C. elegans (Behl et al., 2016.); 30 Zebrafish behavior- (Cowden et al., 2012: Padilla et al., 2011); 31 Zebrafish behavior 24 hr post-fertilization (Reif et al., 2016).
Chemicals were not considered desalted, so there may be similar desalted chemicals mapped in more than one place (e.g., amphetamine sulfate will be in a separate row from amphetamine hydrochloride). The first set of columns A – E, provides an idea of the compound space that has been tested. Column A lists compounds identified as having in vivo studies from two or more laboratories indicating the ability to cause DNT in mammals, and column B for chemicals with only one laboratory (Mundy et al., 2015). Column C is a set of ~91 high priority chemicals provided to investigators by the NIEHS National Toxicology Program.
Column D is a list of chemicals from Mundy et al. (2015) for which there was no evidence found for development neurotoxicity. In most cases the lack of evidence of DNT was likely due to a lack of any test data, so false positives may be likely for the chemicals in Column D. The remainder of the compounds in column E were primarily from ToxCast testing and/or assay-specific positive controls. The remaining columns group assays run by different laboratories in a manner consistent with KEs in the development of the nervous system; e.g. proliferation assays, differentiation assays, etc. If an investigator reported that a chemical has been tested in a particular assay, then it is indicated by a colored horizontal bar in the appropriate column. Note that this is an indication that the compound has been tested in a particular assay, not a determination of whether that compound was positive or negative in the assay, and that data may be published or unpublished at this time. Clearly future work to populate a database with hit-call for these chemicals is needed.
Several important observations are immediately evident from Figure 5. First, for most assays, the total number of compounds tested is small and ranges from 25–100 for most assays. A larger number of chemicals have been tested only in a smaller number of assays. Examples include: ~2000 chemicals for acute network function (column 27) and zebrafish behavior (column 31); ~1000 chemicals for neural cell proliferation (column 3) and neurite outgrowth (column 23).
Importantly, there are many data gaps in the testing of compounds where there is information about their ability to cause DNT (compounds above the dashed line). Of the compounds with evidence for DNT, there are two subsets that have not been tested in any in vitro assay. The first consists of a variety of compounds which could be tested, but to date have not been, including some pesticides (e.g. fenvalerate, cyhalothrin, ivermectin), metals (e.g. arsenic, manganese dioxide) and pharmaceuticals (e.g. naloxone, naltrexone, propranolol). The second untested set includes compounds that currently would be difficult to test in vitro, including gases (carbon monoxide, carbon disulfide), volatiles (e.g., xylenes, trichloroethylene, tetrachloroethylene) or semi-volatiles (e.g., methanol, xylenes). This latter group highlights a need for the optimisation of the experimental set up of available in vitro DNT test systems for reliable exposure to volatile chemicals.
Also apparent from the Fig. 5 heatmap is that among the different key neurodevelopmental events, data are particularly lacking for differentiation and migration assays, while proliferation, network function and behavioral assays in zebrafish have broader coverage of compound space. Finally, of the currently available assays, none of them focus on glial endpoints, so there is clearly a need to develop glial-specific assays (see discussion above).
6. How can the field of NAM-based approaches to DNT testing develop in the short-term versus mid-term / long-term?
Here examples are given for different types of approaches. The examples define knowledge gaps and research needs of areas that are not yet ready, but have large potential.
6.1. How ready is the pathway concept for immediate use?
It is well documented that DNT compounds impair key neurodevelopmental processes leading to diverse pathologies through impairment of certain signaling pathways. As described in Fritsche et al. (2017b) signaling pathways are known to be involved in fundamental neurodevelopmental processes including NPC proliferation (e.g. BDNF-ERK-CREB, RTK-PI3K-AKT), NPC apoptosis (e.g. RXR activation, PGE2, RXR), radial glia proliferation (e.g. miRNA-17–92), neuronal and glial migration (e.g.
MAP kinase, PI3K, BDNF/TrkB, Reelin-Dab, PLCγ1), astrocyte differentiation (e.g. mTORC1-STAT3, Notch signaling), oligodendrocyte differentiation and myelin formation (TH), neuronal differentiation (e.g. mTORC1, BDNF-ERK-CREB, TH, PKC), synaptogenesis (e.g. NMDA receptor activation, calcium signaling, BDNF-Trk, BDNF-ERK-CREB), and neuronal network formation (e.g. PIP metabolism, TH, BDNF-TrkB, BDNF-ERK-CREB).
These pathways, if disturbed sufficiently, will lead to adverse neurodevelopmental outcomes and are therefore thought to serve as anchors for DNT in vitro assay development. In combination with basic information on chemical effects on signaling pathways (e.g. via ToxPi; Reif et al., 2010, 2013), DNT in vitro testing results concerning key neurodevelopmental processes can be used to inform AOPs on the cellular level, and will thus be fundamental for the establishment of DNT AOP networks. Some of them, such as impaired neuronal differentiation, increased neuronal apoptosis, decreased synaptogenesis, or altered neuronal network formation, have already been identified as KEs in the existing DNT AOPs (Table 2A in Bal-Price and Meek, 2017).
Selected signaling pathways involved in a variety of neurodevelopmental processes are described below (Table 5).
Table 5: Examples of signaling pathways and disturbed neurodevelopmental processes involved in diverse neurodevelopmental pathologies.
Exposure to compounds which disrupt certain signaling pathways during brain development may impair key neurodevelopmental processes resulting in diverse neurodevelopmental pathologies. This table presents a few selected examples of signaling pathways dysfunction (CREB - cAMP Responsive Element Binding protein; TH- Thyroid Hormone; BDNF – Brain Derived Neurotrophic Factor, AKT - Protein Kinase B: PKB) based on in vivo studies cited in Chapter 6.1 How ready is the pathway concept for immediate use. (PSC - Pluripotent Stem Cells; NPC-Neural Precursor Cells, NCC - Neural Crest Cells; ESC: Embryonic Stem Cells.
| Exemplary signaling pathways important for normal brain development | Neurodevelopmental pathologies associated with signalling pathway dysfunction | Disturbed neurodevelopmental processes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Differentiation of PSC into NPC NPC proliferation | NCC proliferation and migration | Differentiation of ESCs towards neurons | Radial glia migration | Neurite outgrowth | Neuronal differentiation | Synapto-genesis | Neuronal network formation and function | Glial cells differentiation Myelination | ||
| CREB | Decreased
activity: involved in deficit of cognitive function (AOP 13, 54, 42) |
X Proliferation | X | X | X | |||||
|
TH
|
Decreased
levels: deficit in cognitive function (AOP 54 &42) |
X | X | X | X |
X Oligoden-drocytes, myelination |
||||
| BDNF | Decreased
levels: impairment of learning and memory (AOP, 12,13, 54) |
X | X | X | ||||||
| AKT | Pathway activation: brain overgrowth syndromes in humans |
X NPC proliferation |
X | X | X | X | ||||
CREB (cAMP responsive element binding protein) signaling pathway
The CREB pathway is crucial in the development of the central nervous system (CNS), including neuronal survival, neurite outgrowth, precursor proliferation and neuronal differentiation (Lonze and Ginty, 2002; Lesiak et al., 2014) during brain development. It regulates cell density, neuronal morphology, synaptic connectivity (e.g. potentiates transmitter release, promotes dendrogenesis), neuronal excitability, glutamatergic and gabaergic neurotransmission.
It also plays an important role in learning and memory formation through converging BDNF-ERK CREB signaling cascades in brain development, especially amygdala depending learning and neuronal plasticity (Ehrlich and Josselyn, 2016). CREB acts as an effector of multiple signaling cascades to transmit signals from synapse to the nucleus, affecting transcription of plasticity-regulated genes.
A wide range of stimuli can activate CREB signaling in neurons, including hormones, neurotransmitters, growth factors and Ca2 +, but also stress (Lonze and Ginty, 2002). In addition, CREB is a phosphorylation target of Akt which is activated by BDNF and TrkB receptors via the PI3K pathway. Phosphorylation of CREB allows it to interact with transcriptional coactivators to promote transcription of genes enabling structural and functional plasticity of neurons (Ehrlich and Josselyn, 2016).
Due to the variety of their functions, CREB as well as BDNF and ERK have been linked to a range of psychiatric disorders including autism spectrum disorders. The relevance of the CREB pathway for neurotoxicity has been demonstrated, showing that perturbation of the CREB signaling pathway leads to neurotoxicity (Schuh et al., 2002; Zuo et al., 2009; Brunelli et al., 2012) including DNT in vivo upon exposure to fluoride and arsenic (Zhu et al., 2017), lead (Toscano et al., 2002), paraquat+maneb (Li et al., 2016) and using human pluripotent stem cells-derived mixed neuronal/glial cultures (Pistollato et al., 2014).
BDNF (Brain-derived neurotrophic factor) signaling pathway
The neurotrophin BDNF plays an important role during brain development. BDNF is critical for the formation of appropriate synaptic connections in the brain since it regulates dendritic morphogenesis and axon guidance and its growth (reviewed in Park and Poo, 2013). Responses of growing axons to extracellular gradients of BDNF trigger activation of the phosphatidylinositol-3 (PI3) kinase, mitogen-activated protein (MAP) kinase and phospholipase C-γ (PLC-γ) (for review see Huang and Reichardt, 2003; Huber et al., 2003).
The biological functions of BDNF are mediated by binding to tyrosine kinase B (TrkB) receptor that leads to the activation of three major intracellular signaling pathways, including MAPK, PI3K and PLCγ1 (Soulé et al., 2006). TrkB-mediated signaling regulates gene transcription in the nucleus through the activation of several transcription factors that regulate neurite outgrowth, synaptogenesis, synapse maturation, stabilization (Nelson and Alkon, 2015; Nagappan and Lu, 2005) and synaptic plasticity. Experimental evidence showing that loss of BDNF through transgenic models or pharmacological manipulation leads to impaired long-term potentiation (LTP) (Monteggia et al., 2004) and decreased learning and memory (Lu et al., 2008). The important role for BDNF in LTP and learning and memory is suggested from numerous studies in rodents. Hippocampal LTP is impaired in mice lacking BDNF in their neurons, and BDNF enhances LTP in the hippocampus and visual cortex (reviewed in Mattson, 2008).
In humans, a common single-nucleotide polymorphism in the BDNF gene results in poor performance on learning and memory tasks and contributes to the pathogenesis of depression and anxiety disorders (reviewed in Cohen and Greenberg, 2008). Similarly, transgenic mice with this mutation display deficits in learning and memory tasks as well as anxiety-related behaviors (reviewed in Cohen and Greenberg, 2008).
BDNF has also been shown to play a pivotal role in a variety of learning paradigms in a variety of animal models such as mice, monkeys, zebra finches and chicks (reviewed in Tyler et al., 2002). It is suggested that BDNF, ERK and CREB are playing an important role in neuronal plasticity through regulation of gene expression to adapt to environmental changes.
As documented in DNT AOP 13 (AOP-Wiki: Chronic binding of antagonist to N-methyl-D-aspartate receptors (NMDARs) during brain development induces impairment of learning and memory abilities) and AOP 54 (Inhibition of Na+/I-symporter (NIS) leads to learning and memory impairment), a reduced level of BDNF has been defined as the upstream KE that triggers downstream KEs such as reduced presynaptic glutamate release, increased neuronal cell death and aberrant dendritic morphology leading to decreased synaptogenesis and decreased neuronal network function resulting in impairment of learning and memory in children, the adverse outcome (AO) in these two AOPs.
Experimental support for relationship between reduced BDNF levels and affected downstream Key Event downstream can be triggered by lead exposure as described in detail in the AOP-Wiki5.
TH (thyroid hormone) signaling pathway
The thyroid hormones (TH), triiodothyronine (T3) and thyroxine (T4) are essential for brain development, maturation, and function as they regulate the early key developmental processes such as neurogenesis, neuronal migration, proliferation, myelination and neuronal and glial differentiation and maturation (de Escobar et al., 2004; Bernal, 2015). Normal human brain development and thus cognitive function rely on sufficient TH presence during the perinatal period.
Thyroid hormone developing brain depresses neurogenesis, and thyroid hormone administration stimulates it. T3 acts through TR alpha1 nuclear receptor to increase the commitment of neural stem cells to migrating neuroblasts. Neuronal migration in the cerebral cortex, hippocampus and cerebellum is extremely sensitive to thyroid hormones, and even minor deficiencies are associated with migration defects (Berbel et al., 2001). Among possible mechanisms is the action on the radial glia. The radial glia extend long processes to the cerebral wall, providing a scaffold that serves for cell migration. Maturation of radial glia in the foetal rat brain is delayed in the hippocampus of hypothyroid rats. Thyroid hormones may influence neuronal migration in the cerebral cortex is through the regulation of the expression of the Reln gene in interneurons.
Thyroid hormone also controls the expression of many genes encoding proteins with roles on terminal neuronal and glial differentiation (Morte et al., 2010). Among them there are cell cycle regulators, cytoskeletal proteins, neurotrophins and neurotrophin receptors and extracellular matrix proteins. A striking phenotype in the hypothyroid neonatal brain is the reduction in myelination (Adamo et al., 1990) as TH is involved in oligodendrocytes differentiation (Nygard et al., 2003). After prolonged neonatal hypothyroidism, the number of myelinated axons in adult rats is abnormally low, which corresponds with decreased expression of the major constituents of myelin (Myelin Basic Protein (MBP), Proteolipid protein (Plp), 2’, 3’-cyclic nucleotide 3’- phosphodiesterase (CNPase) and Myelin Associated Glycoprotein (MAG) (Bernal, 2015).
In humans, developing brain hypothyroidism based on TH transporter mutations that cause a lack of TH uptake through the blood-brain-barrier into the developing brain causes severe neurodevelopmental deficits as seen in the Allan-Herndon-Dudley Syndrome. These patients show delayed myelination due to less oligodendrocyte formation or maturation or a combination of both (Tonduti et al., 2013; López-Espíndola et al., 2014). Hence, neurodevelopmental effects due to disturbance of TH homeostasis can be due to either systemic TH disruption, i.e. due to thyroid dysfunction or altered TH metabolism, or both. These differences in modes-of-action need consideration when studying TH disruption in vitro.
With regards to the latter (i.e. local TH disrupting effects on developing brain cells), TH effects on O4+ oligodendrocyte formation and maturation was recently studied in human and mouse NPC differentiating into three major brain cell types, neurons, oligodendrocytes and astrocytes. While TH stimulates formation and maturation of mouse NPC-derived O4+ cells in vitro, TH guides only oligodendrocyte maturation in the human in vitro system. The suspected TH disruptor BDE-99 disrupted TH-dependent O4+ cell maturation only in mouse NPC, while it reduced generation of human O4+ cells independent of TH signaling in human NPC (Dach et al., 2017). This work proposed the ‘oligodendrocyte maturation assay’ as a test for distinguishing between human neural TH disruptors and oligodendrocyte toxicants (Dach et al., 2017).
As described in DNT AOPs (AOP 54: Inhibition of Na+/I- symporter (NIS) leads to learning and memory impairment1 and AOP 42: Inhibition of Thyroperoxidase (TPO) and Subsequent Adverse Neurodevelopmental Outcomes in Mammals8), reduced level of TH in the blood results in lower TH levels in the brain that lead to alterations in gene expression and subsequent protein levels (e.g., decreased levels of BDNF) that are associated with alterations in neuroanatomical structures and physiological functions, that ultimately lead to impairment of cognitive function (AO). This has been shown for chemicals that inhibit NIS (e.g., perchlorate) or TPO (e.g., propylthiouracil, methimazole). Experimental support for a relationship between decreased TH levels and KEs that lead to this AO is described in detail in the AOP-Wiki9. Recently the OECD published a scoping document where currently available in vitro and ex vivo assays for evaluation of disturbance of thyroid functions, including TH signaling pathways are characterized (OECD, 2014a).
AKT (Protein kinase B: PKB) signaling pathway
AKT regulates a variety of general cellular processes, including cell proliferation and growth, autophagy, apoptosis and migration. AKT activity is hereby steered by receptor tyrosine kinase (RTK)-phosphatidylinositol-3-OH kinase (PI3K)- stimulation, with RTK-PI3K-AKT further activating mammalian target of rapamycin (mTOR; Hennessy et al., 2005; Yu and Cui, 2016; Zheng et al., 2011), glycogen synthase kinase 3β (GSK3β), and β-catenin (Manning and Toker, 2017; Fang et al., 2007).
The pivotal role of this RTK-PI3K-AKT signaling pathway in brain development is well established because dysregulation of this assembly in either direction leads to several neurodevelopmental diseases, such as megalocephaly, microcephaly, autism spectrum disorders, intellectual disability, schizophrenia, and epilepsy (reviewed in Hevner et al., 2015; Wang et al., 2017). On the cellular level, elevation of the PI3K-AKT-mTOR signaling pathway stimulates NPC proliferation, neuronal hypertrophy, and excessive dendritic branching, whereas suppression has the opposite consequences (Costa-Mattioli and Monteggia 2013; Huber et al., 2015; Lipton and Sahin, 2014; Zhou and Parada, 2012).
In the organism, AKT is represented by three isoforms, AKT1, AKT2 and AKT3 in a tissue-specific manner. The effects of altered AKT1–3 abundance in mouse brains (Easton et al., 2005) as well as transgenic modulations of AKT1 and 3 in mice (Easton et al., 2005; Tschopp et al., 2005; Tokuda et al., 2011) indicate that AKT1 and 3 are the isoforms mainly responsible for guidance of neurodevelopmental processes. AKT3 knockout mice display a selective reduction in brain size (Easton et al., 2005; Tschopp et al., 2005), whereas mice with an activating AKT3 mutation have larger brains and a thicker corpus callosum.
AKT1 deficiency also leads to decreased brain size, however, by a distinct mechanism: while Akt3 −/− mutants display a reduction in both cell size and cell number, Akt1 −/− mice only show reduced cell numbers (Easton et al., 2005).
In human fetal brains, AKT3 expression is by far overrepresented compared to the two other isoforms (Wu et al., 2009) pointing to a major involvement of AKT3 in human brain development. The significance of this RTK-PI3K-AKT pathway for human brain development in vivo is demonstrated by the neurodevelopmental effects of mutations overstimulating its signaling. These can be grouped into mutations causing overstimulation of RTK (Cohen and Kreiborg, 1990; Faivre et al., 2002; Hevner 2005; D’Ercole and Ye 2008), PI3K and AKT (Flores-Sarnat et al. 2003; Salamon et al., 2006), or AKT downstream signaling (Fraser et al., 2004; Li et al., 2002) that are responsible for diverse brain overgrowth disorders. These data strongly support the notion that compounds interfering with the RTK-PI3K-AKT-mTOR signaling cascade by stimulation or inhibition will lead to an adverse neurodevelopmental outcome.
Because of the function of AKT in regulation of brain size, NPC might be a useful cell method for studying functional effects of impaired AKT signaling. That the AKT signaling machinery is functional in human NPC was recently shown (Iaconelli et al., 2017). In addition, neuronal differentiation models might be adequate to study AKT effects on neuronal mass and dendrite branching.
Exemplary signaling pathways and disturbed neurodevelopmental processes involved in neurodevelopmental pathologies
Based on the in vivo data cited above a few examples of neurodevelopmental pathologies associated with specific pathway dysfunction that are involved in deregulation of certain neurodevelopmental processes, are illustrated in Table 5. These neurodevelopmental pathologies are correlated to environmental chemical exposures as described in the relevant DNT AOPs. As shown, cognitive functional deficits (including impairment of learning and memory) in children is the most frequent adverse outcome associated with the disturbance of these selected signaling pathways and damaged neurodevelopmental processes. Most of these dysregulated neurodevelopmental processes could also be studied using in vitro test methods, evaluated in this manuscript.
6.2. Towards an ontology-based concept of future DNT testing
Individual alternative tests should obviously be characterized for their variability, reproducibility and transferability (Hartung et al., 2004). In addition, the biological domain of the assay and its chemical applicability domain are crucial aspects for characterization of the range and limitations of use of each assay. These technical characteristics are paramount to allow interpretation of results of any assay in any context.
However, the classical approach of assessing predictive performance (predictivity, sensitivity, specificity) on the level of individual test systems needs reconsideration in view of innovative approaches that employ testing strategies involving combinations of tests rather than a single individual assay replacing an animal study (Piersma et al., 2013; Leist et al., 2014).
The concept of ontologies provides a basis for transition to a biology-based system of animal-free hazard and risk assessment (Brinkley et al., 2013). For computational toxicology, ontologies can be defined as networks of factors which are connected by their quantitative relationships. They can for example be used as a matrix to describe physiology from the molecular, via cellular, tissue and organ to the organism level. For toxicological application, only part of this physiological interaction network needs to be described, and the level of detail can be limited to essentials. Thus, the ontology is fit for purpose if it covers the subnetwork of adverse outcome pathways (AOP) that can be triggered by toxicant exposures (Vinken, 2013). An AOP is defined as the linear, one-directional route from molecular initiating event triggered by a compound, via a number of causally linked KEs steps from the molecular, via the cellular and tissue to the organism level, leading to a defined adverse outcome. The toxicity pathway network can be understood as a compilation of all AOPs, including their interrelationships. This may include stimulating and repressing interactions and feedback information, together describing the pathway from compound exposure to adverse effects at the organism level (Tonk et al., 2015). From this AOP network, it should be possible to select a limited number of rate-limiting KEs in the network which are sufficient to predict all toxicant-induced adverse health effects.
These KEs need then be represented in a limited combination of animal-free assays. The challenge then remains to develop a computational model which combines the outcomes of these assays and translates them into a predictor of toxicity. In developmental toxicity, such models are emerging, so far describing individual developmental processes and their perturbation by chemical exposures (Kleinstreuer et al., 2013; Hutson et al., 2017; Leung et al., 2016). Thus, first steps are being taken on the way to full coverage of toxicity pathways in computational systems toxicology.
The above ontology-derived selection of in vitro assays can be employed in different ways, dependent on available knowledge on the chemicals of interest. This information may include biological activity, physicochemical properties, structure- activity relationships of related compounds, and expected use patterns. Case by case, relevant assays can be selected and carried out in battery or tiered approaches, to optimally and pragmatically collect the necessary information about AOPs affected and its consequences for hazard and risk assessment. Such flexible approaches can be described in Integrated Approaches to Testing and Assessment (IATA), as formulated by OECD (Tollefsen et al., 2014). IATA-based approaches are inherently flexible, are designed on the principles of ‘fit-for-purpose’ and ‘case by case’, and require scientific justification based on all available knowledge. It is therefore paramount that the ontology underlying these approaches be comprehensive as to monitoring all possible toxicity pathways, and be fine-tuned to model the human situation. In vitro assays included in IATA should ideally be based on human derived cell cultures to avoid interspecies differences (Fritsche et al., 2017a). This will increase scientific confidence in the reliability of the system as a whole as to sufficient coverage of the entire spectrum of toxicology.
The validation of such testing strategies for DNT or combinations of assays, requires a novel approach. Validation studies on the predictivity of individual assays in the past were based on limited numbers of compounds, and have shown limited relevance for alternative groups of chemicals (Marx-Stoelting et al., 2009). In addition, the notion that reductionist in vitro assays cannot represent the complexity of the intact organism has also hampered acceptance of alternative methods (Piersma et al., 2014). In contrast, the animal study protocols which were introduced half a century ago as models for human hazard and risk assessment have been accepted without validation, but their introduction was based on general agreement in the scientific arena that these were the best possible models for the human situation. Likewise, one could contemplate introducing ontology-based testing strategies without the validation procedure as regards predictivity that is currently common practice for individual alternative assays. Given that ontology-based testing strategies are designed to cover the entire network of toxicological mechanisms, and moreover can be fine-tuned to human physiology, these strategies should be considered inherently superior to animal testing procedures, based on their sufficient coverage of the human biology that is targeted by toxicant exposures. Of course, these approaches are still in their infancy and need considerable further development. However, as proofs of principle emerge for defined aspects of the toxicological spectrum, these approaches merit further development in the interest of improved chemical hazard and risk assessment, using animal-free methods fine-tuned to the species of interest, which is human.
6.3. Towards the development of Integrated Approaches to Testing and Assessment (IATA)
IATA are structured strategies that integrate and weight different types of data, based on “fit-for-purpose” principle to address questions of hazard, safety or risk assessment within a specific regulatory decision context (Tollefsen, et al., 2014). It incorporates multiple sources of information, including that from different levels of biological organization, obtained by a variety of methods [(Q)SAR, read-across, in chemico, in vitro but also human data, ex vivo, in vivo etc] or OMICs technologies (e.g. proteomics, toxicogenomics, metabolomics)] (Tollefsen, et al., 2014; OECD, 2016a) to assess whether the existing information is sufficient to address the purpose-specific regulatory decision. To begin, problem formulation should be clearly defined as it will influence the IATA construction in terms of data requirements, types of testing (e.g. in vitro, in chemico, in vivo), non-testing methods (QSAR, read-across), data integration approaches and acceptable level of uncertainty (e.g., screening and prioritization versus hazard or risk assessment). Taking into consideration the huge gap of knowledge (only 19 known human DNT compounds identified so far; Evans et al., 2016) the most urgent issue is to develop IATA for chemical screening and prioritization purposes (the problem formulation) that could serve as a promising tool, permitting initial identification of substances with DNT potential, among thousands of non-tested chemicals to which humans are exposed. Having such data that sufficiently cover the biology of the system (especially as to toxicity pathways) will improve confidence that IATA is useful in identification of DNT compounds.
IATA construction should be initiated by gathering all existing information (human data, in vivo, in vitro, non-testing data) on a chemical that is evaluated through weight of evidence assessment based on expert judgment. However, if the existing information is not adequate for addressing the regulatory need (problem formulation), the IATA will identify data gaps that can be used to guide the generation of new data.
It is strongly advised that an IATA should be mechanistically informed (Tollefsen et al., 2014; Worth and Patlewicz, 2016; OECD 2016a,b), referring to the pathways of toxicity through which chemicals trigger the cascade of KEs resulting in an adverse outcome. This information can be captured using the AOP framework. For some human adverse outcomes (e.g., skin sensitisation), various mechanistically informed DAs have already been developed based on AOPs (AOP-informed IATA). AOP- informed IATA for skin sensitization incorporates methods, anchored against KEs identified in the published AOP in conjunction with non-testing approaches (QSARS and read across) (Patlewicz et al., 2014; Fitzpatrick and Patlewicz 2017; OECD, 2016b).
Currently, in the area of DNT, there are only a few DNT AOPs available. Notably, these differ clearly from adult neurotoxicity AOP (e.g. Schildknecht et al., 2017), which supports the notion that DNT assessment requires very different approaches and concepts compared to the evaluation of toxic hazard for the adult nervous system. Further development of a sufficient number of AOPs that are relevant to DNT will take time, as more mechanisms of DNT need to be unravelled. This situation should, however, not delay development and implementation of a testing strategy such as IATA. Therefore, it was suggested during the recent OECD/EFSA DNT Workshop (Brussels, October 2017) that besides the KEs defined in the existing DNT AOPs, the fundamental neurodevelopmental processes critical for normal brain development could serve as a base for developing a battery of test methods for DNT testing (Fritsche et al., 2017a). This assumes that nervous system development will be impaired when key biological processes are sufficiently disturbed (Lein et al., 2005; Smirnova et al., 2014). In other words, the assays anchored to AOP KEs and key neurodevelopmental processes will serve to predict adverse DNT outcomes. Based on this assumption, readiness of in vitro assays anchored to these critical DNT processes (Fig. 1) have been evaluated (Table 3) to decide which assays are ready to be included in IATA. The information presented in Table 3 suggests that assays permitting evaluation of cell migration, proliferation, neurite outgrowth, synaptogenesis and neuronal network formation and function are ready to be used for screening purposes. The acceptable level of uncertainty for screening can be higher when compared to other regulatory purposes such as hazard or risk assessment. It is advisable that this battery of in vitro DNT tests is based on in vitro neuronal/glial models, originating from human induced pluripotent stem cells (hiPSCs) in order to be as close as possible to human biology.
The above selected in vitro assays are supported by recently developed DNT AOPs (Bal-Price and Meek, 2017; Bal- Price et al., 2015b) in which impairment of these critical neurodevelopmental processes has been identified as late KEs, leading to adverse outcome e.g. learning and memory deficit in children (AOP 135; AOP 541). Interestingly enough, these AOPs (Table 2A in Bal-Price and Meek, 2017) are triggered by various molecular initiating events (MIEs) and different early KEs, but KEs close to adverse outcome such as neuronal differentiation, synaptogenesis or neuronal network formation and function are shared common KEs (CKEs) in several AOPs. Therefore, the assays that permit in vitro evaluation of these CKEs are relevant candidates for inclusion in IATA battery of DNT tests. The existing DNT AOPs (Bal-Price and Meek, 2017; Bal-Price et al., 2015b) provide a mechanistic understanding of the linked KEs and AOs, thus increasing scientific confidence in the relevance of the selected in vitro test methods, and providing mechanistic/biological context for IATA development (Tollefsen et al., 2014). Further development of AOPs relevant to DNT is strongly encouraged, as AOP-informed IATA will play a pivotal role in shifting emphasis from traditional DNT toxicity testing that is entirely based on animals to more tailored, hypothesis-based and predictive approaches taking into account existing mechanistic information at various levels of biological organization.
Since there are only few identified DNT compounds, the outlined IATA (Fig. 6 and 7) is proposed for screening and prioritisation of chemicals of unknown DNT effects. The first stage in IATA work-flow aims to gather existing information on chemical form and structure, the relevant route of entry and whether it passes e.g. the placenta or blood-brain barrier (Schultz et al., 2015). If there is not enough existing information, then the IATA refers to the scenario where new data must be generated to take a decision. The purpose of this IATA is priority setting, i.e. is the compound of DNT concern or not? This is a problem formulation relevant of course for chemicals regulated under the US Toxic Substance Control Act (TSCA) where there is no data available. However, it is also relevant in very data rich scenarios such as pesticides, since it has been concluded that the triggers for requiring DNT studies in the pesticide regulations are not sensitive enough and do not have adequate biological coverage in terms of toxicity pathways, since very different and even unique pathways are operating during development of the nervous system (Fritsche et al., 2017b). Consequently, DNT data are often not available, and therefore screening and priority setting is also warranted. In Figure 6 an outline for a decision tree is proposed. Obviously, if no effects are detected, then there is no immediate concern in regard to DNT. If DNT effects are detected in in vitro assay(s), then there might be a need to extrapolate the in vitro concentrations to in vivo concentration (QIVIVE) (Yoon et al., 2012), as a default the lowest effect level should be chosen. Depending on the regulatory context, other data might be available and a Health –Based Reference Value (HBRV) may already exist (as for pesticides) or not. In both scenarios, a decision can be made on comparing the effect levels to a risk- management-defined acceptable safety margin and the compound can be deemed of low or high priority. In the latter case, further hazard and risk characterisation or exposure data are required.
Figure 6: An integrated Approach to Testing and Assessment (IATA) designed for DNT screening/prioritization purposes.
The IATA was designed for screening/prioritization purposes, and it was coupled to a decision tree for the DNT regulatory decision making. The IATA integrates multiple sources of existing information (human data, in vivo, in vitro and non-testing data) and guides the targeted generation of new data when required. If further testing is required, then the battery of in vitro DNT tests that permit evaluations of key neurodevelopmental processes and KE identified in the relevant AOPs, combined with non-testing methods (e.g. QSARs and read-across) are proposed to be included in the DNT IATA for chemical screening and prioritization. KE: key event; AOP: adverse outcome pathway; DNT: developmental toxicity; QSAR: quantitative structure activity relationship; QIVIVE: quantivative in vitro in vivo extrapolation; HBRV: health –based reference value; MoE: margin of exposure.
Figure 7: Incorporation of potential endocrine effects into an IATA for DNT hazard identification/characterization.
Before applying the IATA it would be important to determine whether any DNT hazard could potentially be due to an endocrine mediated mode of action. Assays and models are in place (or under development) for regulatory purposes (for estrogen, androgen, steroid and thyroid (EATS) modalities). For the regulatory decision making, any further characterization of DNT effects by the proposed IATA should be integrated with the EATS information. IATA: integrated approach to testing and assessment; DNT: developmental toxicity; QSAR: quantitative structure activity relationship; ADME: absorption, distribution, metabolism and excretion; AOP: adverse outcome pathway.
AOP 12: https://aopwiki.org/aops/12
AOP 13: https://aopwiki.org/aops/13
AOP 42: https://aopwiki.org/aops/42
AOP 54: https://aopwiki.org/aops/54
An IATA for DNT hazard identification and characterisation is also envisaged (Fig. 7). Since DNT effects can also be mediated by endocrine modes of action (e.g. AOP 54) and assays and models are already in place to detect effects at least for oestrogen, androgen and steroidogenesis modalities and partly for the thyroid (McCarthy, 2008; Bernal, 2015), it would be relevant to first establish whether such modes of action are involved. If this is not the case, then the IATA for hazard identification and characterisation of non-ED mediated DNT effects should be applied. In this case, if further information is needed for regulatory decision making, a tiered testing strategy should be applied, where the in vitro DNT battery would be the first tests to be conducted. If further data are needed, then higher tiers would include testing in alternative species (e.g., zebrafish) and if necessary ultimately rodent models. In such a scenario, it is obviously crucial that there is confidence in the adequacy of the biological coverage of the first (lower) tier tests. The advantage of such an approach is that the data collected in the lower tiers could probably inform on the relevant testing in vivo and thus a targeted design only focussing on producing required information, applying certain selected endpoints would be adequate –thus avoiding the full-scale, costly TG426 study. The regulatory decision has to integrate all other relevant data and if DNT effects occur this could result in proposals for classification and labelling and/or establishment of HBRVs.
For regulatory decisions, if the compound has no effect in the lower tier tests, there would most likely not be a concern if the compound is within the applicability domain of the assay/QSAR. If DNT effect(s) are observed, the lowest effect concentration from the most sensitive assay should be extrapolated into in vivo concentrations by QIVIVE (Quantitative in vitro in vivo extrapolation). For this, test methods and algorithms for prediction of toxicokinetic properties (not covered in this report) would be essential (e.g., Wetmore, 2015; Meek and Lipscomb, 2015). The required data do not necessarily need to be derived from animals (Daneshian et al., 2015); there are complex in vitro models available that predict metabolism and distribution of toxicants (e.g. Schildknecht et al., 2015; Gordon et al., 2015). There are also new high-throughput toxicokinetics models available that can be run with simple in vitro derived kinetics parameters (Pearce et al., 2017).
The IATA integrates multiple sources of existing information (human data, in vivo, in vitro and non-testing data) and guides the targeted generation of new data when required. In the tiered testing strategy, it is proposed to first test in the battery of in vitro DNT assays (see Fig. 4 and if relevant, to further test in Zebrafish assays. If further in vivo testing is required (rodent test), the design of these tests could be informed by the in vitro DNT battery/zebrafish assays and in this way a more tailored and cost- effective test than the TG426 or TG443 could be conducted. For any further regulatory decision making including classification and labelling and/or establishment of Health-Based Reference Doses (HBRD), the data derived from the IATA should be integrated with other effect data.
Supplementary Material
References
- Adamo AM, Aloise PA, Soto EF et al. (1990) Neonatal Hyperthyroidism in the Rat Produces an Increase in the Activity of Microperoxisomal Marker Enzymes Coincident with Biochemical Signs of Accelerated Myelination. J Neurosci Res 25, 353–359. [DOI] [PubMed] [Google Scholar]
- Addae C, Yi X, Gernapudi R et al. (2012) All-trans-retinoid acid induces the differentiation of encapsulated mouse embryonic stem cells into GABAergic neurons. Differentiation 83, 233–241, doi: 10.1016/j.diff.2012.03.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeleye Y, Andersen M, Clewell R et al. (2015) Implementing Toxicity Testing in the 21st Century (TT21C): Making safety decisions using toxicity pathways, and progress in a prototype risk assessment. Toxicology 332, 102–11. [DOI] [PubMed] [Google Scholar]
- Alépée N, Bahinski A, Daneshian M et al. (2014) State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology. ALTEX 31, 441–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Champagne DL and Richardson MK (2012). Behavioral profiling of zebrafish embryos exposed to a panel of 60 water-soluble compounds. Behav Brain Res 228, 272–283. doi: 10.1016/j.bbr.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ et al. (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29(3):730–741. [DOI] [PubMed] [Google Scholar]
- Aschner M, Ceccatelli S, Daneshian M et al. (2017) Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use. ALTEX 34, 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Yan Y, Canfield S et al. (2013) Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg 116, 869–880, doi: 10.1213/ANE.0b013e3182860fc9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal R, Erdogan S,, Theophilidis G et al. (2010) Assessing the effects of the neonicotinoid insecticide imidacloprid in the cholinergic synapses of the stellate cells of the mouse cochlear nucleus using whole-cell patch-clamp recording. Neurotoxicology 31, 113–120. [DOI] [PubMed] [Google Scholar]
- Balmer NV, Klima S, Rempel E et al. (2014a) From transient transcriptome responses to disturbed neurodevelopment: role of histone acetylation and methylation as epigenetic switch between reversible and irreversible drug effects. Arch Toxicol 88, 1451–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer NV and Leist M (2014b) Epigenetics and transcriptomics to detect adverse drug effects in model systems of human development. Basic Clin Pharmacol Toxicol 115, 59–68. [DOI] [PubMed] [Google Scholar]
- Balmer NV, Weng M, Zimmer B et al. (2012). Epigenetic changes and disturbed neural development in a human embryonic stem cell-based model relating to the fetal valproate syndrome. Hum Mol Genet 21, 4104–4114 [DOI] [PubMed] [Google Scholar]
- Bal-Price A and Meek MEB (2017a) Adverse outcome pathways: Application to enhance mechanistic understanding of neurotoxicity. Pharmacol Ther pii: S0163–7258(17)30125–0. doi: 10.1016/j.pharmthera.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price A, Lein PJ, Keil KP et al. (2017b) Developing and applying the adverse outcome pathway concept for understanding and predicting neurotoxicity. Neurotoxicology. 59, 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price A, Crofton KM, Leist M et al. (2015a) International STakeholder NETwork (ISTNET): creating a developmental neurotoxicity (DNT) testing road map for regulatory purposes. Arch Toxicol 89, 269–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price A, Crofton KM, Sachana M et al. (2015b) Putative adverse outcome pathways relevant to neurotoxicity. Crit Rev Toxicol 45, 83–91. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price AK, Coecke S, Costa L et al. (2012) Advancing the science of developmental neurotoxicity (DNT): testing for better safety evaluation. ALTEX 29, 202–15. [DOI] [PubMed] [Google Scholar]
- Bal-Price A,K, Hogberg HT,, Buzanska L (2010) In vitro developmental neurotoxicity (DNT) testing: relevant models and endpoints. Neurotoxicology 31, 545–54. [DOI] [PubMed] [Google Scholar]
- Barenys M, Gassmann K, Baksmeier C et al. (2017) Epigallocatechin gallate (EGCG) inhibits adhesion and migration of neural progenitor cells in vitro. Arch Toxicol 91, 827–837. [DOI] [PubMed] [Google Scholar]
- Baumann J ., Barenys M, Gassmann K et al. (2014) Comparative human and rat “neurosphere assay” for developmental neurotoxicity testing. Curr Protoc Toxicol 59, 12.21.1–24. [DOI] [PubMed] [Google Scholar]
- Baumann J, Dach K, Barenys M et al. (2015) Application of the Neurosphere Assay for DNT Hazard Assessment: Challenges and Limitations In: . Methods in Pharmacology and Toxicology. Humana Press [Google Scholar]
- Baumann J, Gassmann K Masjosthusmann S et al. (2016) Comparative human and rat neurospheres reveal species differences in chemical effects on neurodevelopmental key events. Arch Toxicol 90, 1415–1427, doi: 10.1007/s00204-015-1568-8 (2016). [DOI] [PubMed] [Google Scholar]
- Baumann J, Dach K, Barenys M, et al. , (2015) Application of the Neurosphere Assay for DNT Hazard Assessment: Challenges and Limitations Methods in Pharmacology and Toxicology, Springer Protocols, Humana Press, DOI 10.1007/7653_2015_49. [DOI] [Google Scholar]
- Bayer SA and Altman J (2007). Atlas of Human Central Nervous System Development, Volumes 1–5 (CRC Press; ). [Google Scholar]
- Begum AN, Guoynes C, Cho J et al. (2015) Rapid generation of sub-type, region specific neurons and neural networks from human pluripotent stem cell-derived neurospheres. Stem Cell Res 15, 731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M, Hsieh JH, Shafer TJ et al. (2015) Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol Teratol 52, 181–93. [DOI] [PubMed] [Google Scholar]
- Behl M, Rice JR, Smith MV et al. (2016) Editor’s Highlight: Comparative Toxicity of Organophosphate Flame Retardants and Polybrominated Diphenyl Ethers to Caenorhabditis elegans. Toxicol Sci 154, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R, Graus-Porta D, Goebbels S et al. (2007) Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci 27, 13854–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff J, Grunze H, Schindler C et al. (2013) Ziprasidone--not haloperidol--induces more de-novo neurogenesis of adult neural stem cells derived from murine hippocampus. Pharmacopsychiatry 46, 10–15, doi: 10.1055/s-0032-1311607 (2013). [DOI] [PubMed] [Google Scholar]
- Berbel P, Auso E, Garcia-Velasco JV et al. (2001) Role of thyroid hormones in the maturation and organisation of rat barrel cortex. Neuroscience 107, 383–394. [DOI] [PubMed] [Google Scholar]
- Bernal J, (2015). Thyroid Hormones in Brain Development and Function Endotext. https://www.ncbi.nlm.nih.gov/books/NBK285549/
- Bond AM, Bhalala OG and Kessler JA (2012) The dynamic role of bone morphogenetic proteins in neural stem cell fate and matura- tion. Dev Neurobiol 72, 1068–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V and Götz M (2014) Role of radial glial cells in cerebral cortex folding. Curr Opin Neurobiol 27, 39–46. [DOI] [PubMed] [Google Scholar]
- Brad M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramanti V, Tomassoni D, Avitabile M et al. (2010) Biomarkers of glial cell proliferation and differentiation in culture. Front Biosci (Schol Ed) 2, 558–570 (2010). [DOI] [PubMed] [Google Scholar]
- Breier JM, Radio NM, Mundy WR et al. (2008) Development of a high-throughput screening assay for chemical effects on proliferation and viability of immortalized human neural progenitor cells. Toxicol Sci 105, 119–133, doi: 10.1093/toxsci/kfn115 (2008). [DOI] [PubMed] [Google Scholar]
- Brinkley JF, Borromeo C, Clarkson M et al. (2013) The ontology of craniofacial development and malformation for translational craniofacial research. Am J Med Genet C Semin Med Genet 163C, 232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Hall D, Frank CL et al. (2016) Evaluation of a Microelectrode Array-Based Assay for Neural Network Ontogeny Using Training Set Chemicals. Toxicol Sci 154, 126–139. [DOI] [PubMed] [Google Scholar]
- Brown JP, Lynch BS, Curry-Chisolm IM et al. (2017) Assaying Spontaneous Network Activity and Cellular Viability Using Multi-well Microelectrode Arrays. Methods Mol Biol 1601, 153–170. [DOI] [PubMed] [Google Scholar]
- Browne P, Judson RS, Casey W et al. (2017) Correction to Screening Chemicals for Estrogen Receptor Bioactivity Using a Computational Model. Environ Sci Technol 51, 9415–9415. [DOI] [PubMed] [Google Scholar]
- Brunelli L, Llansola M, Felipo V et al. (2012) Insight into the neuroproteomics effects of the food-contaminant non-dioxin like polychlorinated biphenyls. J Proteomics 75, 2417–2430. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen Y, Liu W et al. (2012a) Developmental lead acetate exposure induces embryonic toxicity and memory deficit in adult zebrafish. Neurotoxicol Teratol 34, 581–586, doi: 10.1016/j.ntt.2012.09.001 (2012). [DOI] [PubMed] [Google Scholar]
- Chen X, Huang C, Wang X et al. (2012b) BDE-47 disrupts axonal growth and motor behavior in developing zebrafish. Aquat Toxicol 120–121, 35–44. https://www.ncbi.nlm.nih.gov/pubmed/22609740 [DOI] [PubMed] [Google Scholar]
- Choi SH, Kim YH, Hebisch M et al. (2014) A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 515, 274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim YH, Quinti L et al. (2016) 3D culture models of Alzheimer’s disease: a road map to a “cure-in-a-dish”. Mol Neurodegener 11, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH (1989) The effects of methylmercury on the developing brain. Prog Neurobiol 32, 447–470 [DOI] [PubMed] [Google Scholar]
- Chow ES, Hui MN, Lin CC et al. (2008) Cadmium inhibits neurogenesis in zebrafish embryonic brain development. Aquat Toxicol 87, 157–169, doi: 10.1016/j.aquatox.2008.01.019 (2008). [DOI] [PubMed] [Google Scholar]
- Claudio L, Kwa WC, Russell AL et al. (2000) Testing methods for developmental neurotoxicity of environmental chemicals. Toxicol Appl Pharmacol 164, 1–14. [DOI] [PubMed] [Google Scholar]
- Coecke S, Goldberg AM, Allen S et al. (2007) Workgroup report: incorporating in vitro alternative methods for developmental neurotoxicity into international hazard and risk assessment strategies. Environ Health Perspect 115, 924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM Jr. and Kreiborg S (1990) The central nervous system in the Apert syndrome. Am J Med Genet 35, 36–45 [DOI] [PubMed] [Google Scholar]
- Cohen S and Greenberg ME (2008) Communication between the synapse and the nucleus in neuronal development, plasticity and disease. Annu Rev Cell Dev Biol 24, 183–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleoni S, Galli C, Gaspar JA et al. (2011) Development of a neural teratogenicity test based on human embryonic stem cells: response to retinoic acid exposure. Toxicol Sci 124, 370–377, doi: 10.1093/toxsci/kfr245 (2011). [DOI] [PubMed] [Google Scholar]
- Colleoni S, Galli C, Gaspar JA et al. (2012) Characterisation of a neural teratogenicity assay based on human ESCs differentiation following exposure to valproic acid. Curr Med Chem 19, 6065–6071 (2012). [PubMed] [Google Scholar]
- Coronas V, Durand M, Chabot JG et al. (2000). Acetylcholine induces neuritic outgrowth in rat primary olfactory bulb cultures. Neuroscience 98, 213–219 (2000). [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M and Monteggia LM (2013) mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Neurosci 16, 1537–1543. [DOI] [PubMed] [Google Scholar]
- Cotterill E, Hall D, Wallace K et al. (2016) Characterization of Early Cortical Neural Network Development in Multiwell Microelectrode Array Plates. J Biomol Screen 21, 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowden J, Padnos B, Hunter D et al. (2012) Developmental exposure to valproate and ethanol alters locomotor activity and retino-tectal projection area in zebrafish embryos. Reprod Toxicol 33, 165–173. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Makris SL, Sette WF, et al. (2004) A qualitative retrospective analysis of positive control data in developmental neurotoxicity studies. Neurotoxicol Teratol 26, 345–52. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Mundy WR, Lein PJ et al. (2011) Developmental neurotoxicity testing: recommendations for developing alternative methods for the screening and prioritization of chemicals. ALTEX 28, 9–15. [PubMed] [Google Scholar]
- Crofton KM, Mundy WR and Shafer TJ (2012) Developmental neurotoxicity testing: a path forward. Congenit Anom (Kyoto) 52, 140–146. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Mundy WR, Lein PJ et al. (2011) Developmental neurotoxicity testing: recommendations for developing alternative methods for the screening and prioritization of chemicals. ALTEX 28, 9–15. [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ and Slotkin TA (2000) Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factors involved in cell replication and differentiation. Brain Res 857, 87–98 (2000). [DOI] [PubMed] [Google Scholar]
- Culbreth ME, Harrill JA, Freudenrich TM et al. (2012) Comparison of chemical-induced changes in proliferation and apoptosis in human and mouse neuroprogenitor cells. Neurotoxicology 33, 1499–1510. [DOI] [PubMed] [Google Scholar]
- D’Ercole AJ and Ye P (2008) Minireview: Expanding the mind: insulin-like growth factor I and brain development. Endocrinology 149, 5958–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Grueter BA, Malenka RC et al. (2012) Dopaminergic neurons from midbrain-specified human embryonic stem ceel-derived neural stem cells engrafted in a monkey model of Parkinson’s Disease. PLOS One 7, e41120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dach K, Bendt F, Huebenthal U et al. (2017) BDE-99 impairs differentiation of human and mouse NPCs into the oligodendroglial lineage by species-specific modes of action. Sci Rep 7:44861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshian M, Busquet F, Hartung T et al. (2015) Animal use for science in Europe. ALTEX 32, 261–274 [DOI] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G et al. (2016) Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 19, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Escobar GM, Obregon MJ and del Rey FE (2004). Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18, 225–248. [DOI] [PubMed] [Google Scholar]
- Delrue N, Sachana M, Sakuratani Y et al. (2016) The adverse outcome pathway concept: A basis for developing regulatory decision-making tools. Altern Lab Anim 44, 417–429. [DOI] [PubMed] [Google Scholar]
- Devakumar D, Bamford A, Ferreira MU et al. , (2017) Infectious causes of microcephaly: epidemiology, pathogenesis, diagnosis, and management. Lancet Infect Dis pii: S1473–3099(17)30398–5. doi: 10.1016/S1473-3099(17)30398-5. [DOI] [PubMed] [Google Scholar]
- Dingemans MM, Schütte MG, Wiersma DM et al. (2016) Chronic 14-day exposure to insecticides or methylmercury modulates neuronal activity in primary rat cortical cultures. Neurotoxicology 57, 194–202. [DOI] [PubMed] [Google Scholar]
- Dou C and Zhang J (2011) Effects of lead on neurogenesis during zebrafish embryonic brain development. J Hazard Mater 194, 277–282, doi: 10.1016/j.jhazmat.2011.07.106 (2011). [DOI] [PubMed] [Google Scholar]
- Dreser N, Zimmer B, Dietz C. et al. (2015) Grouping of histone deacetylase inhibitors and other toxicants disturbing neural crest migration by transcriptional profiling. Neurotoxicology 50, 56–70, doi: 10.1016/j.neuro.2015.07.008 (2015). [DOI] [PubMed] [Google Scholar]
- Druwe I, Freudenrich TM, Wallace K et al. (2016) Comparison of Human Induced Pluripotent Stem Cell-Derived Neurons and Rat Primary Cortical Neurons as In vitro Models of Neurite Outgrowth. Appl In vitro Toxicol 2, 26–36. [Google Scholar]
- Druwe I, Freudenrich TM, Wallace K et al. (2015) Sensitivity of neuroprogenitor cells to chemical-induced apoptosis using a multiplexed assay suitable for high-throughput screening. Toxicology 333, 14–24. [DOI] [PubMed] [Google Scholar]
- Dupin E and Sommer L (2012) Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol 366, 83–95. [DOI] [PubMed] [Google Scholar]
- Easton RM, Cho H, Roovers K et al. (2005) Role for Akt3/Protein Kinase Bγ in Attainment of Normal Brain Size. Mol Cell Biol 25, 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edoff K, Raciti M, Moors M et al. (2017) Gestational Age and Sex Influence the Susceptibility of Human Neural Progenitor Cells to Low Levels of MeHg. Neurotox Res. doi: 10.1007/s12640-017-9786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremova L, Chovancova P, Adam M et al. (2017) Switching from astrocytic neuroprotection to neurodegeneration by cytokine stimulation. Arch Toxicol, 91, 231–246 [DOI] [PubMed] [Google Scholar]
- Efremova L, Schildknecht S, Adam M et al. (2015) Prevention of human dopaminergic neurodegeneration in an astrocytes co-culture system allowing endogenous drug metabolism. Br J Pharmacol,172, 4119–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Plant Protection Products and their Residues (2013) Scientific Opinion on the developmental neurotoxicity potential of acetamiprid and imidacloprid. EFSA Journal 11, 3471. [Google Scholar]
- Ehrlich DE and Josselyn SA (2016) Plasticity-related genes in brain development and amygdala-dependent learning. Genes Brain Behav 15, 125–143. [DOI] [PubMed] [Google Scholar]
- EPA 2016. Process for Evaluating & Implementing Alternative Approaches to Traditional in vivo Acute Toxicity Studies for FIFRA Regulatory Use. US EPA Office of Pesticide Programs, 4 February 2016. https://www.epa.gov/sites/production/files/2016-03/documents/final_alternative_test_method_guidance_2-4-16.pdf [Google Scholar]
- Evans RM, Martin OV, Faust M et al. (2016) Should the scope of human mixture risk assessment span legislative/regulatory silos for chemicals? Sci Total Environ 543(Pt A), 757–764. [DOI] [PubMed] [Google Scholar]
- Faivre L, Gosset P, Cormier-Daire V et al. (2002) Overgrowth and trisomy 15q26.1-qter including the IGF1 receptor gene: report of two families and review of the literature. Eur J Hum Genet 10, 699–706. [DOI] [PubMed] [Google Scholar]
- Falsig J, Porzgen P, Lund S et al. (2006). The inflammatory transcriptome of reactive murine astrocytes and implications for their innate immune functions. J Neurochem 96, 893–907. [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y et al. (2007) Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 282, 11221–11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick JM and Patlewicz G (2017) Application of IATA - A case study in evaluating the global and local performance of a Bayesian network model for skin sensitization. SAR QSAR Environ Res 2017 (4), 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Diekmann H and Goldsmith P (2013). Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS One 8, e77548. doi: 10.1371/journal.pone.0077548. https://www.ncbi.nlm.nih.gov/pubmed/24147021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Sarnat L, Sarnat HB, Dávila-Gutiérrez G et al. (2003) Hemimegalencephaly: part 2. Neuropathology suggests a disorder of cellular lineage. J Child Neurol 18, 776–785. [DOI] [PubMed] [Google Scholar]
- Foti SB, Chou A, Moll AD et al. (2013) HDAC inhibitors dysregulate neural stem cell activity in the postnatal mouse brain. Int J Dev Neurosci 31, 434–47. [DOI] [PubMed] [Google Scholar]
- Frank CL, Brown JP, Wallace K et al. (2017) Developmental neurotoxicants disrupt formation of cortical networks on microelectrode arrays: Screening 86 compounds in the neural network formation assay. Toxicol Sci 160, 121–35 [DOI] [PubMed] [Google Scholar]
- Fraser MM, Zhu X, Kwon CH et al. (2004) Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res 64, 7773–7779 [DOI] [PubMed] [Google Scholar]
- Fritsche E, Crofton KM, Hernandez AF et al. (2017a) OECD/EFSA workshop on developmental neurotoxicity (DNT): The use of non-animal test methods for regulatory purposes. ALTEX 34, 311–315. [DOI] [PubMed] [Google Scholar]
- Fritsche et al. (2017b) Report on Integrated Testing Strategies for the identification and evaluation of chemical hazards associated with the developmental neurotoxicity (DNT), to facilitate discussions at the Joint EFSA/OECD Workshop on DNT OECD ENV/JM/MONO(2017)4/AN1
- Fritsche E, Alm H, Baumann J et al. (2015). Literature review on in vitro and alternative developmental neurotoxicity (DNT) testing methods. EFSA Support Publications 12, 4 10.2903/sp.efsa.2015.EN-778 [DOI] [Google Scholar]
- Fuller LC, Cornelius SK, Murphy CW et al. (2002) Neural crest cell motility in valproic acid. Reprod Toxicol 16, 825–839. [DOI] [PubMed] [Google Scholar]
- Garcia G,R, Noyes P,D, Tanguay RL (2016) Advancements in zebrafish applications for 21st century toxicology. Pharmacol Ther 161:11–21. doi: 10.1016/j.pharmthera.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann K, Abel J, Bothe H et al. (2010) Species-specific differential AhR expression protects human neural progenitor cells against developmental neurotoxicity of PAHs. Environ Health Perspect 118, 1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann K, Baumann J, Giersiefer S et al. (2012) Automated neurosphere sorting and plating by the COPAS large particle sorter is a suitable method for high-throughput 3D in vitro applications. Toxicol In vitro 26, 993–1000. [DOI] [PubMed] [Google Scholar]
- Gerhardt E, Kügler S, Leist M et al. (2001). Cascade of caspase activation in potassium-deprived cerebellar granule neurons: Targets for treatment with peptide and protein inhibitors of apoptosis. Mol Cell Neurosci, 17, 717–731. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Ahlbom E, Dare E et al. (2001). Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: age and gender-dependent effects. Environ Toxicol Pharmacol 9, 61–70. [DOI] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Schulz C and Geissmann F (2013) Development and homeostasis of “resident” myeloid cells: the case of the microglia. Glia 61, 112–120. [DOI] [PubMed] [Google Scholar]
- Gordon S, Daneshian M, Bouwstra J et al. (2015) Non-animal models of epithelial barriers (skin, intestine and lung) in research, industrial applications and regulatory toxicology. ALTEX 32, 327–378 [DOI] [PubMed] [Google Scholar]
- Grandjean P and Landrigan PJ (2006). Developmental neurotoxicity of industrial chemicals. Lancet 368, 2167–2178. doi: 10.1016/S0140-6736(06)69665-7 [DOI] [PubMed] [Google Scholar]
- Guadagnoli T, Caltana L, Vacotto M et al. (2016) Direct effects of ethanol on neuronal differentiation: An in vitro analysis of viability and morphology. Brain Res Bull 127, 177–186, doi: 10.1016/j.brainresbull.2016.09.013 (2016). [DOI] [PubMed] [Google Scholar]
- Gulisano M, Pacini S Punzi T et al. (2009) Cadmium modulates proliferation and differentiation of human neuroblasts. J Neurosci Res 87, 228–237, doi: 10.1002/jnr.21830 (2009). [DOI] [PubMed] [Google Scholar]
- Gut IM, Beske PH, Hubbard KS et al. (2013) Novel application of stem cell-derived neurons to evaluate the time- and dose-dependent progression of excitotoxic injury. PLOS One 8, e64423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC et al. (1999) Maternal Thyroid Deficiency during Pregnancy and Subsequent Neuropsychological Development of the Child. N Engl J Med 341, 549–555 [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui J, Parker P et al. (2010) Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561. [DOI] [PubMed] [Google Scholar]
- Hansson O, Castilho RF, Kaminski Schierle GS et al. (2000). Combination treatment with caspase inhibitor and lazaroid additively improves the survival of transplanted rat and human embryonic dopamine neurons. Exp. Neurol, 164, 102–111. [DOI] [PubMed] [Google Scholar]
- Harrill JA, Chen H, Streifel KM et al. (2015a) Ontogeny of biochemical, morphological and functional parameters of synaptogenesis in primary cultures of rat hippocampal and cortical neurons. Mol Brain 8:10. doi: 10.1186/s13041-015-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill JA, Robinette BL, Freudenrich TM et al. (2015b) Media formulation influences chemical effects on neuronal growth and morphology. In vitro Cell Dev Biol Anim 51, 612–629 [DOI] [PubMed] [Google Scholar]
- Harrill JA, Freudenrich TM, Robinette BL et al. (2011a) Comparative sensitivity of human and rat neural cultures to chemical-induced inhibition of neurite outgrowth. Toxicol Appl Pharmacol 256, 268–280, doi: 10.1016/j.taap.2011.02.013 (2011). [DOI] [PubMed] [Google Scholar]
- Harrill JA, Robinette BL and Mundy WR (2011b) Use of high content image analysis to detect chemical-induced changes in synaptogenesis in vitro. Toxicol In vitro 25, 368–387. [DOI] [PubMed] [Google Scholar]
- Harrill JA, Freudenrich TM, Machacek DW et al. (2010) Quantitative assessment of neurite outgrowth in human embryonic stem cell-derived hN2 cells using automated high-content image analysis. Neurotoxicology 31, 277–290. [DOI] [PubMed] [Google Scholar]
- Hartung T, FitzGerald RE, Jennings P et al. (2017b) Systems Toxicology: Real World Applications and Opportunities. Chem Res Toxicol 30, 870–882. doi: 10.1021/acs.chemrestox.7b00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T, Kavlock R and Sturla SJ (2017a) Systems Toxicology II: A Special Issue. Chem Res Toxicol 30, 869. doi: 10.1021/acs.chemrestox.7b00038. [DOI] [PubMed] [Google Scholar]
- Hartung T and Leist M (2008). Food for thought … on the evolution of toxicology and phasing out of animal testing. ALTEX 25, 91–96. [DOI] [PubMed] [Google Scholar]
- Hartung T, Bremer S, Casati S et al. (2004) A modular approach to the ECVAM principles on test validity. Altern Lab Anim 32, 467–472. [DOI] [PubMed] [Google Scholar]
- He X, Gao J, Dong T et al. (2016) Developmental Neurotoxicity of Methamidophos in the Embryo-Larval Stages of Zebrafish. Int J Environ Res Public Health. 14,(1). pii: E23. doi: 10.3390/ijerph14010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Imanishi S, Sone H et al. (2012) Effects of methylmercury exposure on neuronal differentiation of mouse and human embryonic stem cells. Toxicol Lett 212, 1–10, doi: 10.1016/j.toxlet.2012.04.011 (2012). [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Smith DL, Ram PT et al. (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4, 988–1004 [DOI] [PubMed] [Google Scholar]
- Henrichs J, Bongers-Schokking JJ, Schenk JJ et al. (2010) Maternal Thyroid Function during Early Pregnancy and Cognitive Functioning in Early Childhood: The Generation R Study. J Clin Endocrinol Metab 95, 4227–4234, [DOI] [PubMed] [Google Scholar]
- Hevner RF (2005) The cerebral cortex malformation in thanatophoric dysplasia: neuropathology and pathogenesis. Acta Neuropathol 110, 208–221. [DOI] [PubMed] [Google Scholar]
- Hevner RF (2015) Brain overgrowth in disorders of RTK-PI3K-AKT signaling: a mosaic of malformations. Semin Perinatol 39, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer DB and Meredith RM (2017). Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders. Neurotoxicology 58, 23–41. [DOI] [PubMed] [Google Scholar]
- Hirsch C, Striegl B, Mathes S et al. (2017) Multiparameter toxicity assessment of novel DOPO-derived organophosphorus flame retardants. Arch Toxicol 91, 407–425, doi: 10.1007/s00204-016-1680-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt U, Gantner F and Leist M (2000). Phagocytosis of non-apoptotic cells dying by caspase-independent mechanisms. J. Immunol 164, 6520–6529. [DOI] [PubMed] [Google Scholar]
- Gantner F, Leist M, Küsters S et al. (1996). T-cell stimulus-induced crosstalk between lymphocytes and liver cells results in augmented cytokine-release. Exp. Cell Res. 229, 137–146 [DOI] [PubMed] [Google Scholar]
- Hoareau C, Hazane F, Le Pen G et al. (2006) Postnatal effect of embryonic neurogenesis disturbance on reelin level in organotypic cultures of rat hippocampus. Brain Res 1097, 43–51, doi: 10.1016/j.brainres.2006.04.075. [DOI] [PubMed] [Google Scholar]
- Hoelting L, Klima S, Karreman C et al. (2016). Stem Cell-Derived Immature Human Dorsal Root Ganglia Neurons to Identify Peripheral Neurotoxicants. Stem Cells Transl Med 5, 476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelting L, Scheinhardt B, Bondarenko O et al. (2013) A 3-dimensional human embryonic stem cell (hESC)-derived model to detect developmental neurotoxicity of nanoparticles. Arch Toxicol 87, 721–733, doi: 10.1007/s00204-012-0984-2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg HT, Sobanski T, Novellino A et al. (2011a) Application of micro-electrode arrays (MEAs) as an emerging technology for developmental neurotoxicity: evaluation of domoic acid-induced effects in primary cultures of rat cortical neurons. Neurotoxicology 32, 158–168. [DOI] [PubMed] [Google Scholar]
- Hogberg HT and Bal-Price AK (2011b) Domoic Acid-Induced Neurotoxicity Is Mainly Mediated by the AMPA/KA Receptor: Comparison between Immature and Mature Primary Cultures of Neurons and Glial Cells from Rat Cerebellum. J Toxicol. 2011:543512. doi: 10.1155/2011/543512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg HT, Bressler J, Christian KM et al. (2013) Toward a 3D model of human brain development for studying gene/environment interactions. Stem Cell Res Ther 4 Suppl 1, S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg HT, Sobanski T, Novellino A et al. (2011) Application of micro-electrode arrays (MEAs) as an emerging technology for developmental neurotoxicity: evaluation of domoic acid-induced effects in primary cultures of rat cortical neurons. Neurotoxicology 32, 158–168. [DOI] [PubMed] [Google Scholar]
- Hondebrink L, Kasteel EEJ, Tukker AM et al. (2017) Neuropharmacological characterization of the new psychoactive substance methoxetamine. Neuropharmacology 123, 1–9, doi: 10.1016/j.neuropharm.2017.04.035. [DOI] [PubMed] [Google Scholar]
- Hondebrink L, Verboven AHA, Drega WS et al. (2016) Neurotoxicity screening of (illicit) drugs using novel methods for analysis of microelectrode array (MEA) recordings. Neurotoxicology 55, 1–9, doi: 10.1016/j.neuro.2016.04.020. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF et al. (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Yuan Y, Wang D et al. (2016) Heterogeneous astrocytes: Active players in CNS. Brain Res Bull 125, 1–18. [DOI] [PubMed] [Google Scholar]
- Huber KM, Klann E, Costa-Mattioli M et al. (2015) Dysregulation of mammalian target of rapamycin signaling in mouse models of autism. The Journal of neuroscience: the official journal of the Society for Neuroscience 35, 13836–13842. doi: 10.1523/JNEUROSCI.2656-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ and Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72, 609–642. [DOI] [PubMed] [Google Scholar]
- Huang X and Saint-Jeannet JP (2004) Induction of the neural crest and the opportunities of life on the edge. Dev Biol 275, 1–11. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD et al. (2003) Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci 26, 509–563. [DOI] [PubMed] [Google Scholar]
- Hutson MS, Leung MCK, Baker NC et al. (2017) Computational Model of Secondary Palate Fusion and Disruption. Chem Res Toxicol 30, 965–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaconelli J, Lalonde J, Watmuff B et al. (2017) Lysine Deacetylation by HDAC6 Regulates the Kinase Activity of AKT in Human Neural Progenitor Cells. ACS Chem Biol doi: 10.1021/acschembio.6b01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarema KA, Hunter DL, Shaffer RM et al. (2015) Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol Teratol 52(Pt B), 194–209. doi: 10.1016/j.ntt.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Zhu Z, Wang Y et al. (2016) The fungicide imazalil induces developmental abnormalities and alters locomotor activity during early developmental stages in zebrafish. Chemosphere 153, 455–161. doi: 10.1016/j.chemosphere.2016.03.085. [DOI] [PubMed] [Google Scholar]
- Kadereit S, Zimmer B, van Thriel C et al. (2012). Compound selection for in vitro modeling of developmental neurotoxicity. Front Biosci (Landmark Ed) 17, 2442–2460. [DOI] [PubMed] [Google Scholar]
- Kapucu FE, Tanskanen JM, Mikkonen JE et al. (2012) Burst analysis tool for developing neuronal networks exhibiting highly varying action potential dynamics. Front Comput Neurosci 6, 38. doi: 10.3389/fncom.2012.00038. eCollection 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KM, Collier AD, Meshalkina DA (2017) Zebrafish models in neuropsychopharmacology and CNS drug discovery. Br J Pharmacol 174, 1925–1944. doi: 10.1111/bph.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor YM, Soga T and Parhar IS (2013) Caffeine neuroprotects against dexamethasone-induced anxiety-like behaviour in the Zebrafish (Danio rerio). Gen Comp Endocrinol 181, 310–315, doi: 10.1016/j.ygcen.2012.09.021 (2013). [DOI] [PubMed] [Google Scholar]
- Kiecker C and Lumsden A (2005) Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci 6, 553–564. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP et al. (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29, 13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiiski H, Aänismaa R, Tenhunen J et al. (2013) Healthy human CSF promotes glial differentiation of hESC-derived neural cells while retaining spontaneous activity in existing neuronal networks. Biol Open 2, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG and Lee YI (2012) Differential Expressions of Synaptogenic Markers between Primary Cultured Cortical and Hippocampal Neurons. Exp Neurobiol 21, 61–67. doi: 10.5607/en.2012.21.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindlundh-Hogberg AM, Pickering C, Wicher G et al. (2010) MDMA (Ecstasy) decreases the number of neurons and stem cells in embryonic cortical cultures. Cell Mol Neurobiol 30, 13–21, doi: 10.1007/s10571-009-9426-y (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiderman S, Gutbier S, Tufekci KU, Ortega F, Sá JV, Teixeira AP, Brito C, Glaab E, Berninger B, Alves PM, Leist M. (2016a) Conversion of non-proliferating astrocytes into neurogenic neural stem cells: control by FGF2 and IFN-gamma. Stem Cells 34, 2861–2874 [DOI] [PubMed] [Google Scholar]
- Kleiderman S, Sá JV, Teixeira AP, Brito C, Gutbier S, Evje LG, Hadera MG, Glaab E, Henry M, Agapios S, Alves PM, Sonnewald U, Leist M (2016b) Functional and phenotypic differences of pure populations of stem cell-derived astrocytes and neuronal precursor cells Glia 64, 695–715 [DOI] [PubMed] [Google Scholar]
- Kleinstreuer N, Dix D, Rountree M, et al. (2013) A Computational Model Predicting Disruption of Blood Vessel Development. PLoS Comput Biol 9, e1002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B and Gibb R (2011) Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry 20, 265–276. [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S and Martinez-Cerdeno V (2006) Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nature Rev. Neurosci 7, 883–890. [DOI] [PubMed] [Google Scholar]
- Krstic D and Knuesel I (2013) Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol 9, 25–34. [DOI] [PubMed] [Google Scholar]
- Krstic D, Madhusudan A, Doehner J et al. (2012) Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation 9, 151. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug AK, Balmer NV Matt F et al. (2013a) Evaluation of a human neurite growth assay as specific screen for developmental neurotoxicants. Arch Toxicol 87, 2215–2231, doi: 10.1007/s00204-013-1072-y (2013). [DOI] [PubMed] [Google Scholar]
- Krug AK, Balmer NV, Matt F et al. (2013b). Evaluation of a human neurite growth assay as specific screen for developmental neurotoxicants. Arch Toxicol 87, 2215–2231. doi: 10.1007/s00204-013-1072-y [DOI] [PubMed] [Google Scholar]
- Kuegler PB, Baumann BA, Zimmer B et al. (2012) GFAP-independent inflammatory competence and trophic functions of astrocytes generated from murine embryonic stem cells.. Glia, 60, 218–228 [DOI] [PubMed] [Google Scholar]
- Kuegler PB, Zimmer B, Waldmann T et al. (2010). Markers of murine embryonic and neural stem cells, neurons and astrocytes: reference points for developmental neurotoxicitiy testing – a review by the Translantic Think Tank for Toxicology (t4). altex 27, 17–42 [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA et al. (2013) Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenza I, Pallocca G, Mennecozzi M et al. (2013) A human pluripotent carcinoma stem cell-based model for in vitro developmental neurotoxicity testing: effects of methylmercury, lead and aluminum evaluated by gene expression studies. Int J Dev Neurosci 31, 679–691 [DOI] [PubMed] [Google Scholar]
- Lee G, Papapetrou EP, Kim H et al. (2009) Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi J, el Abdellaoui N, van Pomeren M et al. (2015) Comparability of behavioural assays using zebrafish larvae to assess neurotoxicity. Environ Sci Pollut Res Int 22, 16277–16289. doi: 10.1007/s11356-014-3805-8. [DOI] [PubMed] [Google Scholar]
- Lein P, Silbergeld E, Locke P et al. (2005) In vitro and other alternative approaches to developmental neurotoxicity testing (DNT). Environ Toxicol Pharmacol 19, 735–744. [DOI] [PubMed] [Google Scholar]
- Leist M, Hartung T, Nicotera P (2008a). The dawning of a new age of toxicology. ALTEX 25, 103–114 [PubMed] [Google Scholar]
- Leist M, Bremer S, Brundin P et al. (2008b). The biological and ethical basis of the use of embryonic stem cells for in vitro tests and cell therapy. ALTEX 25, 163–190 [PubMed] [Google Scholar]
- Leist M, Efremova L, Karreman C (2010) Food for thought on considerations and guidelines for basic test method descriptions in toxicology. ALTEX 27, 309–317 [DOI] [PubMed] [Google Scholar]
- Leist M, Hasiwa N, Daneshian M et al. (2012) Validation and quality control of replacement alternatives – current status and future challenges. Toxicol Res 1, 8–22 (doi: 10.1039/C2TX20011B) [DOI] [Google Scholar]
- Leist M, Hasiwa N, Rovida C et al. (2014) Consensus report on the future of animal-free systemic toxicity testing. ALTEX, 31, 341–356 [DOI] [PubMed] [Google Scholar]
- Lesiak A, Zhu M, Chen H et al. (2014) The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J Neurosci 34, 717–725. doi: 10.1523/JNEUROSCI.2884-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MCK, Hutson MS, Seifert AW et al. (2016) Computational modeling and simulation of genital tubercle development. Reprod Toxicol 64, 151–161. [DOI] [PubMed] [Google Scholar]
- Lewitus E, Kelave I and Huttner WB 2013. Conical expansion of the outer subventricular zone and the role of ceocortical folding in evolution and development. Front Hum Neurosci 7, 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, He X, Sun Y et al. (2016) Developmental exposure to paraquat and maneb can impair cognition, learning and memory in Sprague-Dawley rats. Mol. BioSyst 12, 3088–3097. [DOI] [PubMed] [Google Scholar]
- Li L, Liu F, Salmonsen RA et al. (2002) PTEN in neural precursor cells: regulation of migration, apoptosis, and proliferation. Mol Cell Neurosci 20, 21–29 [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JO and Sahin M (2014) The neurology of mTOR. Neuron 84, 275–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE and Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623. [DOI] [PubMed] [Google Scholar]
- López-Espíndola D, Morales-Bastos C, Grijota-Martínez C et al. (2014) Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J Clin Endocrinol Metab 99, E2799–2804. doi: 10.1210/jc.2014-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotharius J, Barg S, Wiekop P et al. (2002) Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem 277, 38884–38894. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K and Lu B (2008) BDNF: a key regulator for protein synthesis dependent LTP and long-term memory? Neurobiol Learn Mem 89, 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Porzgen P, Mortensen AL et al. (2006). The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol 180, 71–87 [DOI] [PubMed] [Google Scholar]
- Madill M, Fitzgerald D, O’Connell KE et al. (2016) In vitro and ex vivo models of multiple sclerosis. Drug Discov Today 21, 1504–1511. [DOI] [PubMed] [Google Scholar]
- Manning BD, and Toker A (2017) AKT/PKB Signaling: Navigating the Network. Cell 169, 381–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus T, Hansson SR, Cronberg T et al. (2010) beta-Adrenoceptor activation depresses brain inflammation and is neuroprotective in lipopolysaccharide-induced sensitization to oxygen-glucose deprivation in organotypic hippocampal slices. J Neuroinflammation 7, 94, doi: 10.1186/1742-2094-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx U, Andersson TB, Bahinski A et al. (2016) Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing using animals. ALTEX 33, 272–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx-Stoelting P, Adriaens E, Ahr HJ et al. (2009) A review of the implementation of the embryonic stem cell test (EST). The report and recommendations of an ECVAM/ReProTect Workshop. Altern Lab Anim 37, 313–328. [DOI] [PubMed] [Google Scholar]
- Mattson MP (2008) Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci 1144, 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Zhang X, Darnell SB et al. (2011) Cocaine alters BDNF expression and neuronal migration in the embryonic mouse forebrain. J Neurosci 31, 13400–13411, doi: 10.1523/JNEUROSCI.2944-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the devloping brain. Physiology Reviews. 88(1): 91–124 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek ME, Lipscomb JC (2015) Gaining acceptance for the use of in vitro toxicity assays and QIVIVE in regulatory risk assessment. Toxicology. 5, 332:112–23. doi: 10.1016/j.tox.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Menegola E, Broccia ML, Di Renzo F et al. (2005) Craniofacial and axial skeletal defects induced by the fungicide triadimefon in the mouse. Birth Defects Res B Dev Reprod Toxicol 74, 185–195. [DOI] [PubMed] [Google Scholar]
- Miller JA, Ding SL, Sunkin SM, et al. , (2014) Transcriptional landscape of the prenatal human brain. Nature, 508: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV and Deneend B (2015) Astrocyte development: A Guide for the Perplexed. Glia 63, 1320–1329. [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG and Honegger P (1993) Evaluation of the toxicity of different metal compounds in the developing brain using aggregating cell cultures as a model. Toxicol In vitro 7, 335–339. [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG and Honegger P (1996) Comparison of the developmental effects of two mercury compounds on glial cells and neurons in aggregate cultures of rat telencephalon. Brain Res 741, 52–59. [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG, Schilter B et al. (2000) Maturation-dependent effects of chlorpyrifos and parathion and their oxygen analogs on acetylcholinesterase and neuronal and glial markers in aggregating brain cell cultures. Toxicol Appl Pharmacol 165, 175–183 (2000). [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG, Sorg O et al. (1999) The naturally occurring food mycotoxin fumonisin B1 impairs myelin formation in aggregating brain cell culture. Neurotoxicology 20, 41–48. [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM et al. (2004) Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA 101, 10827–10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors M, Bose R Johansson-Hague K et al. (2012) Dickkopf 1 mediates glucocorticoid-induced changes in human neural progenitor cell proliferation and differentiation. Toxicol Sci 125, 488–495, doi: 10.1093/toxsci/kfr304 (2012). [DOI] [PubMed] [Google Scholar]
- Moors M, Vudattu NK, Abel J et al. (2010) Interleukin-7 (IL-7) and IL-7 splice variants affect differentiation of human neural progenitor cells. Genes Immun 11, 11–20. [DOI] [PubMed] [Google Scholar]
- Moors M, Rockel TD, Abel J et al. (2009) Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect 117, 1131–1138, doi: 10.1289/ehp.0800207 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors M, Cline JE, Abel J et al. (2007) ERK-dependent and -independent pathways trigger human neural progenitor cell migration. Toxicol Appl Pharmacol 221, 57–67. [DOI] [PubMed] [Google Scholar]
- Morte B, Díez D, Ausó E et al. (2010). Thyroid hormone regulation of gene expression in the developing rat fetal cerebral cortex: prominent role of the Ca2+/calmodulin-dependent protein kinase IV pathway. Endocrinology 151, 810–820. [DOI] [PubMed] [Google Scholar]
- Mueller T and Wullimann M (2016). Atlas of Early Zebrafish Brain Development (Second Edition). A Tool for Molecular Neurogenetics. Academic Press, ISBN 978-0-12-418669-9. 10.1016/B978-0-12-418669-9.09987-6 [DOI] [Google Scholar]
- Mundy WR, Radio NM and Freudenrich TM (2010) Neuronal models for evaluation of proliferation in vitro using high content screening. Toxicology 270, 121–130. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Padilla S, Breier JM et al. (2015) Expanding the test set: Chemicals with potential to disrupt mammalian brain development. Neurotoxicol Teratol 52, 25–35. [DOI] [PubMed] [Google Scholar]
- Nagappan G and Lu B (2005) Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci 28, 464–471. [DOI] [PubMed] [Google Scholar]
- Nash R, Krishnamoorthy M, Jenkins A et al. (2012) Human embryonic stem cell model of ethanol-mediated early developmental toxicity. Exp Neurol 234, 127–135, doi: 10.1016/j.expneurol.2011.12.022 (2012). [DOI] [PubMed] [Google Scholar]
- Neal AP and Guillarte RT (2010) Molecular neurobiology of lead (Pb2+): effect on synaptic function. Mol Neurobiol 42, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TJ and Alkon DL (2015) Molecular regulation of synaptogenesis during associative learning and memory. Brain Res 1621, 239–251 [DOI] [PubMed] [Google Scholar]
- Ninomiya E, Hattori T, Toyoda M et al. (2014) Glucocorticoids promote neural progenitor cell proliferation derived from human induced pluripotent stem cells. SpringerPlus 3, 527, doi: 10.1186/2193-1801-3-527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Murakami S, Ashikawa Y (2015) Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit Anom (Kyoto) 55, 1–16. doi: 10.1111/cga.12079. [DOI] [PubMed] [Google Scholar]
- Noyes PD, Haggard DE, Gonnerman GD et al. (2015) Advanced morphological - behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol Sci 145, 177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyffeler J, Karreman C, Leisner H et al. (2017a) Design of a high-throughput human neural crest cell migration assay to indicate potential developmental toxicants. ALTEX 34, 75–94. doi: 10.14573/altex.1605031. [DOI] [PubMed] [Google Scholar]
- Nyffeler J, Dolde X, Krebs A et al. (2017b) Combination of multiple neural crest migration assay to identify environmental toxicants from a proof-of-concept chemical library. Arch Toxicol, in press doi: 10.1007/s00204-017-1977-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard M, Wahlstrom GM, Gustafsson MV et al. (2003) Hormone-Dependent Repression of the E2F-1 Gene by Thyroid Hormone Receptors. Mol Endocrinol 17, 79–92. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R and Muller F (2006). The Embryonic Human Brain: An Atlas of Developmental Stages, Third Edition (Wiley-Liss; ). [Google Scholar]
- OECD 2014a. OECD n 207, ENV/JM/MONO(2014)23, OECD Thyroid Hormone scoping document: ,(http://www.oecd-ilibrary.org/environment/new-scoping-document-on-in-vitro-and-ex-vivo-assays-for-the-identification-of-modulators-of-thyroid-hormone-signalling_9789264274716-en)
- OECD 2014b. Guidance Document for describing non-guideline in vitro test methods (Series on Testing and Assessment No. 211). ENV/JM/MONO(2014)35.
- OECD 2016a. Guidance Document for the use of Adverse Outcome Pathways in developing Integrated Approaches to Testing and Assessment (IATA). ENV/JM/MONO(2016)67.
- OECD. Guidance Document on the reporting of Defined Approaches and Individual Information Sources to be used within Integrated Approaches to Testing and Assessment for Skin Sensitisation. 2016b. ENV/JM/MONO(2016)29.
- OECD. Guidance Document on the Reporting of Defined Approaches to be used within Integrated Approaches to Testing and Assessment. 2016c. ENV/JM/MONO(2016)28)0.
- OECD 2017. Draft guidance document on Good In vitro Method Practices (GIVIMP) for the development and implementation of in vitro methods for regulatory use in human safety assessment. OECD Paris. Deadline for comments 13 September 2017. http://www.oecd.org/chemicalsafety/testing/draft-guidance-review-documents-monographs.htm; http://www.oecd.org/env/ehs/testing/OECD%20Draft%20GIVIMP_v05%20-%20clean.pdf
- Orsolits B, Borsy A, Madarasz E et al. (2013) Retinoid machinery in distinct neural stem cell populations with different retinoid responsiveness. Stem Cells Dev 22, 2777–2793, doi: 10.1089/scd.2012.0422 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla S, Hunter DL, Padnos B et al. (2011) Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicol Teratol 33, 624–630. doi: 10.1016/j.ntt.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Pal R, Mamidi MK, Das AK et al. (2011) Human embryonic stem cell proliferation and differentiation as parameters to evaluate developmental toxicity. J Cell Physiol 226, 1583–1595. doi: 10.1002/jcp.22484. [DOI] [PubMed] [Google Scholar]
- Pallocca G, Fabbri M, Sacco MG et al. (2013) miRNA expression profiling in a human stem cell-based model as a tool for developmental neurotoxicity testing. Cell Biol Toxicol 29, 239–257, doi: 10.1007/s10565-013-9250-5 (2013). [DOI] [PubMed] [Google Scholar]
- Pallocca G, Grinberg M, Henry M et al. (2016) Identification of transcriptome signatures and biomarkers specific for potential developmental toxicants inhibiting human neural crest cell migration. Arch Toxicol 90, 159–180, doi: 10.1007/s00204-015-1658-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JA, Poenitzsch AM, Smith SM et al. (2012) Metabolic biomarkers of prenatal alcohol exposure in human embryonic stem cell-derived neural lineages. Alcohol Clin Exp Res 36, 1314–1324, doi: 10.1111/j.1530-0277.2011.01732.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamies D, Barreras P, Block K et al. (2017a) A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. ALTEX 34, 362–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamies D, Bal-Price A, Simeonov A et al. (2017b) Good Cell Culture Practice for stem cells and stem-cell-derived models. ALTEX 34, 95–132. doi: 10.14573/altex.1607121. [DOI] [PubMed] [Google Scholar]
- Pamies D and Hartung T (2017c) 21st Century Cell Culture for 21st Century Toxicology. Chem Res Toxicol 30, 43–52. doi: 10.1021/acs.chemrestox.6b00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Chen YC, Priyadarshini M et al. (2010) The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis 40, 46–57. [DOI] [PubMed] [Google Scholar]
- Parker MO, Annan LV, Kanellopoulos AH et al. (2014) The utility of zebrafish to study the mechanisms by which ethanol affects social behavior and anxiety during early brain development. Prog Neuropsychopharmacol Biol Psychiatry 55, 94–100, doi: 10.1016/j.pnpbp.2014.03.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MO, Brock AJ, Walton RT et al. (2013) The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front Neural Circuits 7, 63. doi: 10.3389/fncir.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlewicz G, Kuseva C, Kesova A et al. (2014) Towards AOP application--implementation of an integrated approach to testing and assessment (IATA) into a pipeline tool for skin sensitization. Regul Toxicol Pharmacol 69, 529–545. [DOI] [PubMed] [Google Scholar]
- Pearce R,G, Setzer RW, Davis JL (2017) Evaluation and calibration of high-throughput predictions of chemical distribution to tissues. J Pharmacokinet Pharmacodyn doi: 10.1007/s10928-017-9548-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gomez A and Tasker RA (2012) Enhanced neurogenesis in organotypic cultures of rat hippocampus after transient subfield-selective excitotoxic insult induced by domoic acid. Neuroscience 208, 97–108, doi: 10.1016/j.neuroscience.2012.02.003 (2012). [DOI] [PubMed] [Google Scholar]
- Piersma AH, Bosgra S, van Duursen MB et al. (2013) Evaluation of an alternative in vitro test battery for detecting reproductive toxicants. Reprod Toxicol 38, 53–64. [DOI] [PubMed] [Google Scholar]
- Piersma AH, Ezendam J, Luijten M et al. (2014) A critical appraisal of the process of regulatory implementation of novel in vivo and in vitro methods for chemical hazard and risk assessment. Crit Rev Toxicol 44, 876–894. [DOI] [PubMed] [Google Scholar]
- Pistollato F, Louisse J, Scelfo B et al. (2014) Development of a pluripotent stem cell derived neuronal model to identify chemically induced pathway perturbations in relation to neurotoxicity: effects of CREB pathway inhibition. Toxicol Appl Pharmacol 280, 378–388. doi: 10.1016/j.taap.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Pistollato F, Canovas-Jorda D, Zagoura D et al. (2017) Protocol for the Differentiation of Human Induced Pluripotent Stem Cells into Mixed Cultures of Neurons and Glia for Neurotoxicity Testing. J Vis Exp. 2017 June 9;(124). doi: 10.3791/55702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova D and Jacobsson SO (2014) A fluorescence microplate screen assay for the detection of neurite outgrowth and neurotoxicity using an antibody against betaIII-tubulin. Toxicol In vitro 28, 411–418, doi: 10.1016/j.tiv.2013.12.009 (2014). [DOI] [PubMed] [Google Scholar]
- Puehringer D, Orel N, Lüningschrör P et al. (2013) EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat Neurosci 16, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quasthoff S and Hartung HP (2002) Chemotherapy-induced peripheral neuropathy. J Neurol 249, 9–17. [DOI] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM et al. (2016) Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radio NM, Breier JM, Reif DM, et al. , (2015) Use of Neural Models of Proliferation and Neurite Outgrowth to Screen Environmental Chemicals in the ToxCast Phase I Library. Applied In vitro Toxicology 1, 131–139. [Google Scholar]
- Radonjic M, Cappaert NL, de Vries EF et al. (2013) Delay and impairment in brain development and function in rat offspring after maternal exposure to methylmercury. Toxicol Sci 133, 112–124. doi: 10.1093/toxsci/kft024. [DOI] [PubMed] [Google Scholar]
- Raffaele KC, Rowland J, May B et al. (2010) The use of developmental neurotoxicity data in pesticide risk assessments. Neurotoxicol Teratol 32, 563–572. [DOI] [PubMed] [Google Scholar]
- Ryan KR, Sirenko O, Parham F et al. (2016) Neurite outgrowth in human induced pluripotent stem cell-derived neurons as a high-throughput screen for developmental neurotoxicity or neurotoxicity. Neurotoxicology 53, 271–281. [DOI] [PubMed] [Google Scholar]
- Reif DM, Martin MT, Tan SW et al. (2010) Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ Health Perspect 118, 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif DM, Sypa M, Lock EF et al. (2013) ToxPi GUI: an interactive visualization tool for transparent integration of data from diverse sources of evidence. Bioinformatics 29, 402–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif D, Truong L,, Mandrell D, et al. (2016) High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch Toxicol 90, 1459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel E, Hölting L, Waldmann T et al. (2015) A transcriptome-based classifier to identify developmental toxicants by stem cell testing: design, validation and optimization for histone deacetylase inhibitors. Arch Toxicol 89, 1599–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AM, Judson RS, Houck KA, et al. , (2016) ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem Res Toxicol. 2016 August 15;29(8):1225–51. [DOI] [PubMed] [Google Scholar]
- Rigamonti A, Repetti G, Sun C et al. (2016) Large-Scale Production of Mature Neurons from Human Pluripotent Stem Cells in a Three-Dimensional Suspension Culture System. Stem Cell Reports 6, 93–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette BL, Harrill JA, Mundy WR et al. (2011) In vitro assessment of developmental neurotoxicity: use of microelectrode arrays to measure functional changes in neuronal network ontogeny. Front Neuroeng. 4:1. doi: 10.3389/fneng.2011.00001. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovida C, Asakura S, Daneshian M et al. (2015) Toxicity Testing in the 21st Century Beyond Environmental Chemicals. ALTEX 32, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH (2004) Glial specification in the vertebrate neural tube. Nat Rev Neurosci 5, 409–419. [DOI] [PubMed] [Google Scholar]
- Salamon N, Andres M, Chute DJ et al. (2006) Contralateral hemimicrencephaly and clinical-pathological correlations in children with hemimegalencephaly. Brain 129, 352–365. [DOI] [PubMed] [Google Scholar]
- Sànchez-Màrtin FJ, Fan X, Lindquist DM et al. (2013) Lead induces similar gene expression changes in brains of gestationally exposed adult mice and in neurons differentiated from mouse embryonic stem cells. PLOS one 8, e80558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom von Tobel J, Zoia D, Althaus J et al. (2014) Immediate and delayed effects of subchronic Paraquat exposure during an early differentiation stage in 3D-rat brain cell cultures. Toxicol Lett 230, 188–197. [DOI] [PubMed] [Google Scholar]
- Sandstrom J, Broyer A, Zoia D et al. (2017a) Potential mechanisms of development-dependent adverse effects of the herbicide paraquat in 3D rat brain cell cultures. Neurotoxicology 60, 116–124. [DOI] [PubMed] [Google Scholar]
- Sandstrom J, Eggermann E, Charvet I et al. (2017b). Development and characterization of a human embryonic stem cell-derived 3D neural tissue model for neurotoxicity testing. Toxicol In vitro 38, 124–135. [DOI] [PubMed] [Google Scholar]
- Santos-Fandila A, Vasquez E, Barranco A et al. (2015) Analysis of 17 neurotransmitters, metabolites and precursors in zebrafish through the life cycle using ultrahigh performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 1001, 191–201. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Di Monte DA, Pape R et al. (2017) Tipping points and endogenous determinants of nigrostriatal degeneration by MPTP Trends Pharmacol Sci 38, 541–555. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Pape R, Meiser J et al. (2015) Preferential extracellular generation of the active parkinsonian toxicant MPP+ by transporter-independent export of the intermediate MPDP+. Antioxid Redox Signal 23, 1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BZ, Lehmann M, Gutbier S et al. (2017) In vitro acute and developmental neurotoxicity screening: an overview of cellular platforms and high-throughput technical possibilities. Arch Toxicol 91, 1–33. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Strähle U and Scholpp S (2013) Neurogenesis in zebrafish - from embryo to adult. Neural Dev 8:3. doi: 10.1186/1749-8104-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuck MR, Temme T, Dach K et al. (2017) Omnisphero: a high-content image analysis (HCA) approach for phenotypic developmental neurotoxicity (DNT) screenings of organoid neurosphere cultures in vitro. Arch Toxicol 91, 2017–2028. [DOI] [PubMed] [Google Scholar]
- Scholz D, Pöltl D, Genewsky A et al. (2011). Rapid, complete and large-scale generation of post-mitotic neurons from the human LUHMES cell line. J Neurochem 119, 957–971. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Gassmann K, Götz C et al. (2010) Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Perspect 118, 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh RA, Lein PJ, Beckles RA et al. (2002) Noncholinesterase mechanisms ofchlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol Appl. Pharmacol 182, 176–185. [DOI] [PubMed] [Google Scholar]
- Schultz L, Zurich MG, Culot M et al. (2015) Evaluation of drug-induced neurotoxicity based on metabolomics, proteomics and electrical activity measurements in complementary CNS in vitro models. Toxicol In vitro 30, 138–65. [DOI] [PubMed] [Google Scholar]
- Schwartz MP, Hou Z, Propson NE et al. (2015). Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci U S A 112, 12516–12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selderslaghs IW, Hooyberghs J, De Coen W et al. (2010) Locomotor activity in zebrafish embryos: A new method to assess developmental neurotoxicity. Neurotoxicology and Teratology 32, 460–471. [DOI] [PubMed] [Google Scholar]
- Selderslaghs IW, Hooyberghs J, Blust R et al. (2013) Assessment of the developmental neurotoxicity of compounds by measuring locomotor activity in zebrafish embryos and larvae. Neurotoxicol Teratol 37, 44–56. [DOI] [PubMed] [Google Scholar]
- Senut MC, Sen A, Cingolani P et al. (2014) Lead exposure disrupts global DNA methylation in human embryonic stem cells and alters their neuronal differentiation. Toxicol Sci 139, 142–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang EH and Zhdanova IV (2007) The circadian system is a target and modulator of prenatal cocaine effects. PLoS One 2, e587, doi: 10.1371/journal.pone.0000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Scott MA, Ghosh B et al. (2011) Synapse microarray identification of small molecules that enhance synaptogenesis. Nat Commun 2, 510–519, doi: 10.1038/ncomms1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde V, Hoelting L, Perumal SS et al. (2016) Definition of transcriptome-based indices for quantitative characterization of chemically disturbed stem cell development - Introduction of the STOP-Toxukn and STOP-Toxukk. Arch Toxicol 91, 839–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde V, Klima S, Sureshkumar PS et al. (2015). Human pluripotent stem cell based developmental toxicity assays for chemical safety screening and systems biology data generation. J Vis Exp e52333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki Y, Shibata K, Yoshida K et al. (2017). Transformation of Astrocytes to a Neuroprotective Phenotype by Microglia via P2Y1 Receptor Downregulation. Cell Rep 19, 1151–1164. [DOI] [PubMed] [Google Scholar]
- Simão D, Terrasso AP, Teixeira AP et al. (2016) Functional metabolic interactions of human neuron-astrocyte 3D in vitro networks. Sci Rep 6, 33285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis JC,, Pochareddy S, Zhu Y et al. (2016) The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron, 89, 248–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W Jr., Liu F, Rainosek SW et al. (2015) Ketamine-Induced Toxicity in Neurons Differentiated from Neural Stem Cells. Mol Neurobiol 52, 959–969. [DOI] [PubMed] [Google Scholar]
- Slotkin TA and Seidler FJ (2012) Developmental neurotoxicity of organophosphates targets cell cycle and apoptosis, revealed by transcriptional profiles in vivo and in vitro. Neurotoxicol Teratol 34, 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P et al. (2002) Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex 12, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova L, Harris G, Delp J et al. (2016) A LUHMES 3D dopaminergic neuronal model for neurotoxicity testing allowing long-term exposure and cellular resilience analysis. Arch Toxicol 90, 2725–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova L, Hogberg HT, Leist M et al. (2014) Developmental neurotoxicity - challenges in the 21st century and in vitro opportunities. ALTEX 31, 129–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulé J, Messaoudi E and Bramham CR (2006) Brain-derived neurotrophic factor and control of synaptic consolidation in the adult brain. Biochem Soc Trans 34, 600–604. [DOI] [PubMed] [Google Scholar]
- Souza BS, Sampaio GL, Pereira CS, et al. (2016) Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci Rep 6, 39775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler NV, Krug AK, Matt F et al. (2011) Assessment of chemical-induced impairment of human neurite outgrowth by multiparametric live cell imaging in high-density cultures. Toxicol Sci 121, 73–87. [DOI] [PubMed] [Google Scholar]
- Strickland JD, Martin M, Houck T et al. (2017) Screening the ToxCast Phase II Libraries for Neuroactivity using Cortical Neurons Grown on Multi-well Microelectrode Array (mwMEA) Plates. Archives of Toxicology, doi: 10.1007/s00204-017-2035-5. (Available online Aug 2, 2017.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummann TC, Hareng L and Bremer S (2009) Hazard assessment of methylmercury toxicity to neuronal induction in embryogenesis using human embryonic stem cells. Toxicology 257, 117–126. [DOI] [PubMed] [Google Scholar]
- Suarez-Isla BA, Pelto DJ, Thompson JM et al. (1984) Blockers of calcium permeability inhibit neurite extension and formation of neuromuscular synapses in cell culture. Brain Res 316, 263–270. [DOI] [PubMed] [Google Scholar]
- Talens-Visconti R, Sanchez-Vera I, Kostic J et al. (2011) Neural differentiation from human embryonic stem cells as a tool to study early brain development and the neuroteratogenic effects of ethanol. Stem Cells Dev 20, 327–339. [DOI] [PubMed] [Google Scholar]
- Tamm C and Ceccatelli S (2017) Mechanistic insight into neurotoxicity induced by developmental insults. Biochem Biophys Res Commun 482, 408–418. [DOI] [PubMed] [Google Scholar]
- Tang H, Hammack Ch., Ogden SC et al. (2016) Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell, 18, 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasneem S, Farrell K, Lee MY and Kothapalli CR (2016) Sensitivity of neural stem cell survival, differentiation and neurite outgrowth within 3D hydrogels to environmental heavy metals. Toxicol Lett 242, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller Thomas and Wullimann Mario (2016). Atlas of Early Zebrafish Brain Development (Second Edition). A Tool for Molecular Neurogenetics. Academic Press, ISBN 978-0-12-418669-9. 10.1016/B978-0-12-418669-9.09987-6 [DOI] [Google Scholar]
- Tiedeken JA, Ramsdell JS and Ramsdell AF (2005) Developmental toxicity of domoic acid in zebrafish (Danio rerio). Neurotoxicol Teratol 27, 711–717. [DOI] [PubMed] [Google Scholar]
- Tofighi R, Wan Ibrahim WN, Rebellato P et al. (2011) Non-dioxin-like polychlorinated biphenyls interfere with neuronal differentiation of embryonic neural stem cells. Toxicol Sci 124, 192–201. [DOI] [PubMed] [Google Scholar]
- Tokuda S, Mahaffey CL, Monks B, Faulkner CR (2011) A novel Akt3 mutation associated with enhanced kinase activity and seizure susceptibility in mice. Hum Mol Genet 20, 988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen KE,, Scholz S, Cronin MT et al. (2014) Applying Adverse Outcome Pathways (AOPs) to support Integrated Approaches to Testing and Assessment (IATA). Regul Toxicol Pharmacol 70, 629–640. [DOI] [PubMed] [Google Scholar]
- Tonduti D, Vanderver A, Berardinelli A et al. (2013) MCT8 deficiency: extrapyramidal symptoms and delayed myelination as prominent features. J Child Neurol 28, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonk ECM, Pennings JLA, and Piersma AH (2015) An adverse outcome pathway framework for neural tube and axial defects mediated by modulation of retinoic acid homeostasis. Reprod Toxicol 55, 104–113. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Hashemzadeh-Gargari H, McGlothan JL et al. (2002). Developmental Pb exposure alters NMDAR subtypes and reduces CREB phosphorylation in the rat brain. Develop Brain Res 139, 217–226. [DOI] [PubMed] [Google Scholar]
- Tschopp O, Yang ZZ, Brodbeck D (2005) Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132, 2943–54. [DOI] [PubMed] [Google Scholar]
- Tsuji R, Crofton KM (2012) Developmental neurotoxicity guideline study: issues with methodology, evaluation and regulation. Congenit Anom (Kyoto) 52(3):122–8. doi: 10.1111/j.1741-4520.2012.00374.x. Review [DOI] [PubMed] [Google Scholar]
- Tukker AM, de Groot MW, Wijnolts FM et al. (2016) Is the Time Right for In vitro Neurotoxicity Testing Using Human iPSC-Derived Neurons? ALTEX 33, 261–271. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR et al. (2002) From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem 9, 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbisch D, Mehling A, Guth K et al. (2015) Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul Toxicol Pharmacol. 71, 337–51. [DOI] [PubMed] [Google Scholar]
- van Thriel C, Westerink RH, Beste C et al. (2012) Translating neurobehavioural endpoints of developmental neurotoxicity tests into in vitro assays and readouts. Neurotoxicology 33, 911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo A, Chiappalone M, De Camargos L,R et al. (2017) A multi-laboratory evaluation of microelectrode array-based measurements of neural network activity for acute neurotoxicity testing. Neurotoxicology 60, 280–292. [DOI] [PubMed] [Google Scholar]
- Viberg H (2009) Neonatal ontogeny and neurotoxic effect of decabrominated diphenyl ether (PBDE 209) on levels of synaptophysin and tau. Int J Dev Neurosci. 27, 423–429. [DOI] [PubMed] [Google Scholar]
- Vinken M (2013) The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology 312, 158–165. [DOI] [PubMed] [Google Scholar]
- Visan A, Hayess K Sittner D et al. (2000) Neural differentiation of mouse embryonic stem cells as a tool to assess developmental neurotoxicity in vitro. Neurotoxicology 33, 1135–1146. [DOI] [PubMed] [Google Scholar]
- Volbracht C, Leist M and Nicotera P (1999). ATP controls neuronal apoptosis triggered by microtubule breakdown or potassium deprivation. Mol. Med 5, 477–489. [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Makris SL (2015) Assessment of learning, memory, and attention in developmental neurotoxicity regulatory studies: synthesis, commentary, and recommendations. Neurotoxicol Teratol. 52:109–15. [DOI] [PubMed] [Google Scholar]
- Wagenaar DA, Pine J and Potter SM (2006). An extremely rich repertoire of bursting patterns during the development of cortical cultures. BMC Neurosci 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T, Rempel E, Balmer NV et al. (2014). Design principles of concentration-dependent transcriptome deviations in drug-exposed differentiating stem cells. Chem Res Toxicol 27, 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T, Grinberg M, König A et al. (2017) Stem cell transcriptome responses and corresponding biomarkers that indicate the transition from adaptive responses to cytotoxicity. Chem Res Toxicol 30, 905–922. [DOI] [PubMed] [Google Scholar]
- Wallace K, Strickland JD, Valdivia P et al. (2015). A multiplexed assay for determination of neurotoxicant effects on spontaneous network activity and viability from microelectrode arrays. Neurotoxicology 49, 79–85. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu Y, Li S et al. (2015) Wnt signaling pathway participates in valproic acid-induced neuronal differentiation of neural stem cells. Int J Clin Exp Pathol 8, 578–585 (2015). [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhou K, Fu Z et al. (2017) Brain Development and AKT Signaling: the Crossroads of Signaling Pathway and Neurodevelopmental Diseases. J Mol Neurosci 61, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M, Zimmer B, Pöltl D et al. (2012). Extensive transcriptional regulation of chromatin modifiers during human neurodevelopment. PlosOne, 7, e36708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng MK, Nataraj K, Scholz D et al. (2014) Lineage-specific regulation of epigenetic modifier genes in human liver and brain.PlosOne 9, e102035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore BA (2015) Quantitative in vitro-to-in vivo extrapolation in a high-throughput environment. Toxicology 332, 94–101. doi: 10.1016/j.tox.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Graham JR and Ball AJ (2014) Multiparametric High Content Analysis for assessment of neurotoxicity in differentiated neuronal cell lines and human embryonic stem cell-derived neurons. Neurotoxicology 42, 33–48. [DOI] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B et al. (2013) Modeling Transformations of Neurodevelopmental Sequences across Mammalian Species. J. Neurosci, 33, 7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth AP and Patlewicz G (2016) Integrated Approaches to Testing and Assessment. Adv Exp Med Biol 856, 317–342. [DOI] [PubMed] [Google Scholar]
- Wu C, Orozco C, Boyer J et al. (2009) BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10(11), R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Ibhazehiebo K, Iwasaki T et al. (2012) An in vitro method to study the effects of thyroid hormone-disrupting chemicals on neuronal development. Neurotoxicology 33, 753–757. [DOI] [PubMed] [Google Scholar]
- Yang D, Kania-Korwel I, Ghogha A et al. (2014) PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol Sci 138, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Peng SX, Xu YD et al. (2017) Unexpected Neuroprotective Effects of Loganin on 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Neurotoxicity and Cell Death in Zebrafish. J Cell Biochem 118, 615–628. [DOI] [PubMed] [Google Scholar]
- Yoon M, Campbell J,L,, Andersen M,E,, Clewell H,J Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit Rev Toxicol. 2012. September;42(8):633–52. [DOI] [PubMed] [Google Scholar]
- York R,G, Barnett J, Girard M,F, (2005) Refining the effects observed in a developmental neurobehavioral study of ammonium perchlorate administered orally in drinking water to rats. II. Behavioral and neurodevelopment effects. Int J Toxicol 24(6):451–67. [DOI] [PubMed] [Google Scholar]
- Yu JS and Cui W (2016) Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determi- nation. Development 143, 3050–3060. [DOI] [PubMed] [Google Scholar]
- Zagoura D, Canovas-Jorda D, Pistollato F et al. (2017) Evaluation of the rotenone-induced activation of the Nrf2 pathway in a neuronal model derived from human induced pluripotent stem cells. Neurochem Int 106, 62–73. [DOI] [PubMed] [Google Scholar]
- Zheng J, Feng X, Hou L et al. (2011) Latanoprost promotes neurite outgrowth in differentiated RGC-5 cells via the PI3K-Akt-mTOR signaling pathway. Cell Mol Neurobiol 31, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Liu Y, Tan XJ et al. (2015) Inhibitory effect of arsenic trioxide on neuronal migration in vitro and its potential molecular mechanism. Environ Toxicol Pharmacol 40, 671–677. [DOI] [PubMed] [Google Scholar]
- Zhou J, Parada LF (2012) PTEN signaling in autism spectrum disorders. Curr Opin Neurobiol 22, 873–9. [DOI] [PubMed] [Google Scholar]
- Zhu YP, Xi SH, Li. MY et al. (2017) Fluoride and arsenic exposure affects spatial memory and activates the ERK/CREB signaling pathway in offspring rats. Neurotoxicology 59, 56–64. [DOI] [PubMed] [Google Scholar]
- Zimmer B, Kuegler PB, Baudis B et al. (2011a). Coordinated waves of gene expression during neuronal differentiation of embryonic stem cells as basis for novel approaches to developmental neurotoxicity testing. Cell Death Differ 18, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B, Schildknecht S, Kuegler PB et al. (2011b) Sensitivity of dopaminergic neuron differentiation from stem cells to chronic low-dose methylmercury exposure. Toxicol Sci 12, 357–367. [DOI] [PubMed] [Google Scholar]
- Zimmer B, Lee G, Balmer NV et al. (2012) Evaluation of developmental toxicants and signaling pathways in a functional test based on the migration of human neural crest cells. Environ Health Perspect 120, 1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B, Pallocca G, Dreser N et al. (2014) Profiling of drugs and environmental chemicals for functional impairment of neural crest migration in a novel stem cell-based test battery. Arch Toxicol 88, 1109–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann FF, Gaspary KV, Leite CE et al. (2015) Embryological exposure to valproic acid induces social interaction deficits in zebrafish (Danio rerio): A developmental behavior analysis. Neurotoxicol Teratol 52, 36–41. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Cai J,Wang X et al. (2009) Acute administration of tributyltin and trimethyltin modulate glutamate and N-methyl-D-aspartate receptor signalling pathway in Sebastiscus marmoratus. Aquat Toxicol 92, 44–49. [DOI] [PubMed] [Google Scholar]
- Zurich MG, Eskes C, Honegger P et al. (2002) Maturation-dependent neurotoxicity of lead aceate in vitro: Implication of glial reactions. J Neurosc Res 70, 108–116. [DOI] [PubMed] [Google Scholar]
- Zurich MG, Honegger P, Schilter B et al. (2000) Use of aggregating brain cell cultures to study developmental effects of organophosphorus insecticides. Neurotoxicology 21, 599–605. [PubMed] [Google Scholar]
- Zurich MG, Stanzel S, Kopp-Schneuder A et al. (2012). Evaluation of aggregating brain cell cultures for the detection of acute organ-specific toxicity. Toxic in vitro 27, 1416–1424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.