Abstract
IMPORTANCE:
Survival rates following in-hospital cardiac arrest (IHCA) vary significantly among United States centers; whether this variation is due to differences in IHCA care quality is unknown.
OBJECTIVES:
We evaluated hospital-level variation to determine if IHCA care quality hospital process composite measures were associated with patient outcomes.
DESIGN, SETTING, AND PARTICIPANTS:
We analyzed 35,283 IHCA patients treated at 261 Get With The Guidelines-Resuscitation (GWTG-R) United States hospitals (2010–2012). We calculated the hospital process composite performance score for IHCA using five guideline-recommended process measures. Opportunity-based scores were calculated for all patients, aggregated at the hospital level, divided into quartiles, and then associated with risk-standardized survival and neurologic status by a test for trend, evaluated through hierarchical logistic regression, and reported as odds ratios per 10% increment in process composite performance adherence.
INTERVENTIONS:
Acute treatments for in-hospital cardiac arrest.
MAIN OUTCOME MEASURES:
Our primary outcome of interest was survival to discharge measured as risk standard survival rates, and the secondary outcome was favorable neurologic status at hospital discharge.
RESULTS:
The median IHCA hospital process composite performance was 89.7% (IQR 85.4, 93.1) and varied among hospitals quartiles from 82.6% (lowest) to 94.8% (highest). IHCA hospital process composite performance was linearly associated with risk-standardized hospital survival to discharge rates: 21.1%, 21.4%, 22.8%, and 23.4% from lowest to highest performance quartiles (p<0.001). After adjustment, each 10% increase in a hospital’s process composite performance was associated with a 22% higher odds of survival (adjusted OR, 1.22 [95% CI, 1.08–1.38]). Hospital process composite quality performance was also associated with favorable neurologic status at discharge (p=0.004).
CONCLUSIONS AND RELEVANCE:
The quality of guideline-based care for IHCA varies significantly among United States hospitals and is associated with patient survival and neurologic outcomes.
Keywords: survival, cardiac arrest, quality of care
More than 200,000 patients are treated for in-hospital cardiac arrest (IHCA) annually in the United States (U.S.).1,2 IHCA is associated with poor survival, yet survival to discharge rates vary significantly among U.S. hospitals.3 Some process of care measures, such as shorter time to defibrillation, are associated with better survival after IHCA.4–6 The Joint Commission, National Quality Forum, and American Heart Association (AHA) have expressed significant interest in developing performance measures specific to IHCA in hopes of facilitating benchmarking and ultimately improving patient outcomes.7 A recent AHA consensus statement identified several strategies for improving survival from cardiac arrest and pinpointed best practices related to structure, care pathways, and quality improvement care opportunities.1
We conducted this study to examine: 1) the variability in IHCA process quality of care across U.S. hospitals; and 2) whether or not there is an association between a hospital measure of IHCA process quality of care and patient outcomes. To our knowledge, no previous study has examined variation between IHCA quality of care and outcomes using a hospital process composite performance measure. Understanding the relationship between process and survival may clarify the utility of these process measures to inform hospital-level quality for IHCA care.
Methods
Data Source
The AHA’s Get With the Guidelines-Resuscitation (GWTG-R) program is an ongoing prospective hospital-based clinical registry and quality improvement program for patients with IHCA. This registry was created to: 1) collect cardiac resuscitation care and outcomes data from hospitals; and 2) generate evidence-based guidelines.
The design of GWTG-R has been previously described in detail.8 Briefly, all full-code patients with a confirmed IHCA (defined as apnea, unresponsiveness, and lack of a palpable central pulse) who received cardiopulmonary resuscitation (CPR) are identified and enrolled by dedicated staff at participating hospitals. Cases are recognized by centralized collection of cardiac arrest flow sheets, review of hospital paging-system logs, routine checks of code carts (carts stocked with emergency medications and equipment), pharmacy tracer drug records, and hospital billing charges for code-cart charges. GWTG-R uses standardized Utstein Style definitions for clinical variables and outcomes. Utstein Style refers to consensus reporting guidelines for cardiac resuscitation which originated from an international multidisciplinary meeting in 1990 and has been updated several times.9,10 Data completeness and accuracy are ensured by rigorous training, certification of hospital staff, and the use of standardized software with internal data checks.8,11 A prior report shows an error rate in data abstraction of 2.4.8 This study was approved by the Duke Institutional Review Board.
Study Population
From January 1, 2010 through December 31, 2012, we identified 48,189 adults ≥18 years of age with IHCA across 351 U.S. GWTG-R hospitals. In order to avoid inflation in variance due to small numbers, patients were excluded if they were enrolled at sites with fewer than 20 admissions overall, averaged fewer than five cardiac arrests per year, or had participated in GWTG-R for less than a year (eTable 1). Additionally, we excluded hospitals whose hospital characteristics were missing (n=16). Only the index cardiac arrest for each patient was included in this analysis. Within GWTG-R hospitals, we excluded cardiac arrests occurring in operating rooms, procedural suites, and the emergency department, since these arrests are known to be different and have distinct survival from those occurring in wards and intensive care units (eTable 1). After these exclusions, our final study population included 35,283 patients from 261 GWTG-R hospitals.
Statistical Analysis
Five American College of Cardiology/AHA guideline-recommended acute resuscitation process measures were evaluated among individuals who were eligible to receive them. The guideline-recommended process of care measures chosen a priori by consensus of our research team were: 1) device confirmation of correct endotracheal tube placement5; 2) a monitored or witnessed cardiac arrest event6; 3) time to first chest compression less than or equal to one minute; 4) time to first defibrillation delivered less than or equal to two minutes for ventricular tachycardia/ventricular fibrillation (VT/VF)4; and 5) administration of epinephrine or vasopressin for pulseless events (pulseless VT/VF or pulseless electrical activity/asystole) within five minutes.12 These five measures were chosen based on standard guideline recommendations, evidence showing association of individual measure with outcome,4,5,6 and completeness of data.12 Inclusion and exclusion criteria for each process measure were defined according to standard eligibility definitions for inhospital cardiac arrest put forth by the AHA for GWTG-R (eTable 2). Another measure, time to second defibrillation >2 minutes, was considered for inclusion in this study, but was removed at the request of the AHA GWTG-R task force after an observational analysis called into question the practice of withholding shocks for at least 2 minutes to allow for CPR. Additionally, three measures were not selected for inclusion given suspected universal adherence: time to assisted ventilation, chest compressions provided, and defibrillation shock provided.
Hospital process composite performance scores were calculated using opportunity-based scoring, which is defined as the sum of correct care divided by total care opportunities.13 Each patient at a GWTG-R hospital contributed care opportunities to the relevant hospital’s overall composite adherence score.14 Each patient, depending on the initial rhythm, could contribute either a maximum of four (non-shockable) or five (shockable) opportunities to the model. For example, if a patient arrested and was found to have a shockable rhythm during rhythm analysis, then he/she would potentially be “eligible” to contribute all five care opportunities to the hospital’s performance score. If correct care only occurred for the monitored/witnessed event and time to defibrillation was <2 minutes, then only two of the five opportunities were count as “received.” In contrast, a patient with pulseless electrical activity/asystole as an initial rhythm would only contribute a maximum of four opportunities to the model. Opportunity scoring implicitly weights each measure in proportion to the percentage of eligible patients at each hospital.13 Hospitals were divided into equal quartile after rank-ordering of process of care composite adherence score.
Demographic, cardiac arrest event, and hospital characteristics were compared by hospital adherence quartiles. Individual process measures (eligibility and received) were also compared across performance quartiles. Pearson X2 tests compared categorical variables across hospital quartile performance; Kruskal-Wallis test compared continuous variables across hospital performance quartiles. Categorical variables were presented as percentages, and continuous variables were presented as medians and interquartile ranges. A Cochran-Armitage trend test was used to compare differences across quartiles for individual performance measures. Our primary outcome of interest was survival to discharge measured as risk-standardized survival rates (RSSR), and the secondary outcome was favorable neurologic status at hospital discharge.
RSSR was calculated based on a previously validated model, which was developed to facilitate comparisons across hospitals.15 Validation and derivation characteristics for this model have been previously described.15 According to this method, RSSR was calculated for each hospital by dividing a hospital’s predicted survival by the expected survival, multiplied by the cohort’s unadjusted survival. Survival to discharge was modeled by hierarchical logistic regression, including patient risk factors as fixed effects and a random intercept for hospitals. Hospital-level predicted survival was calculated as the average of model-based predictions across patients at a given hospital, including an empirical Bayes prediction of the hospital effect.16,17 Hospital-level expected survival was calculated in the same way, using only the fixed effects portion of the model, with the hospital effect set equal to 0 (representing a typical hospital). This same process was implemented to obtain RSSR with patients grouped by hospital quartile. The full model included the following 18 risk factors: age, sex, event location, initial cardiac arrest rhythm, myocardial infarction present on admission, prior heart failure, renal insufficiency, hepatic insufficiency, hypotension, septicemia, acute stroke, diabetes, metabolic/electrolyte abnormality, metastatic/hematologic malignancy, major trauma, mechanical ventilation, dialysis, and intravenous vasopressor use. Race was not included in the published RSSR model because it has been found to be associated with the quality of the treating hospital.15 We performed a sensitivity analysis, adding race to the RSSR model to determine if its addition attenuated the relationship between quality of care and outcomes (eTable 3). Patient race was self-identified and was abstracted from the medical records by GWTG-R staff. Differences in hospital RSSR and risk-adjusted favorable neurologic status across hospital process composite performance quartiles were assessed using the linear regression weighted by the number of patients within a hospital to address non-constant variance.
Differences between unadjusted outcomes across hospital process composite performance quartiles was assessed by linear regression weighted by the number of patients at each site. The association between hospital process composite performance and risk-adjusted survival was also evaluated directly through hierarchical logistic regression. Specifically, the process composite performance score was added as a continuous covariate to the hierarchical model described above. Variable inclusion for this model was age, race, sex, event location, initial cardiac arrest rhythm, whether or not myocardial infarction was present on admission, prior heart failure, renal insufficiency, hepatic insufficiency, hypotension, septicemia, acute stroke, diabetes, metabolic/electrolyte abnormality, metastatic/hematologic malignancy, major trauma, mechanical ventilation, dialysis, and intravenous vasopressor use. The linear association with survival was tested by a Chi-square test, and odds ratios were reported per 10% increment in hospital process composite performance score. This differs from the previous assessment of RSSR in that the linear association is evaluated on the log-odds scale, rather than the absolute scale.
To determine the number of lives that could be saved if all hospitals operated at the level of the best performing hospital, we first identified the best performing hospital as the one with the highest risk-adjusted survival. From the full covariate adjusted hierarchical model we estimated the “effect” of being treated at this hospital. For every patient in the dataset, we used the hierarchical model to predict their survival probability, given their fixed covariates and the “best hospital effect.” The predictions were summed over all patients in the sample to estimate the overall predicted survival if all patients were treated at the best hospital.
Favorable neurologic status at discharge was assessed as a secondary outcome for each patient with cardiac arrest. Cerebral performance categories (CPC) are defined as follows: 1, good cerebral performance; 2, moderate disability; 3, severe disability; 4, coma or vegetative state; 5, brain death. Favorable neurologic status was defined as a having a CPC score of 1 or 2 at hospital discharge and is defined according to Utstein Style criteria.10 We also report clinically significant favorable neurologic status with a CPC score of 1 (eTable 4).
In our analysis, the missing CPC score rate was 17.7%, which aligns with previously published estimates of missing CPC data in GWTG-R. After a histogram review, we discovered that several hospitals were missing more than 50% of CPC data (eFigure 1). As a result, we excluded hospitals with <75% of CPC scores on its patients (n=88) for this secondary analysis. The subsequent missing discharge CPC score rate among 173 hospitals was 2.3%.
In order to avoid survivor bias and to facilitate adequate hospital-level comparisons for neurologic status, all patients were included in this analysis. Patients who died during the hospitalization were assigned a CPC score of 5 (brain death).
C-indices were calculated to determine model diagnostics. The c-index for calculation of predicted survival for the RSSR rates is 0.694 and 0.704 for risk-standardized favorable neurologic status. After race is added to the aforementioned models for the sensitivity analysis, c-indices were 0.697 for predicted survival in RSSR and 0.708 for favorable neurologic status. For our continuous risk-adjusted models, the c-indices are 0.716 and 0.733 for survival and favorable neurologic status, respectively. All p-values were two-sided and significant at p<0.05.
Results
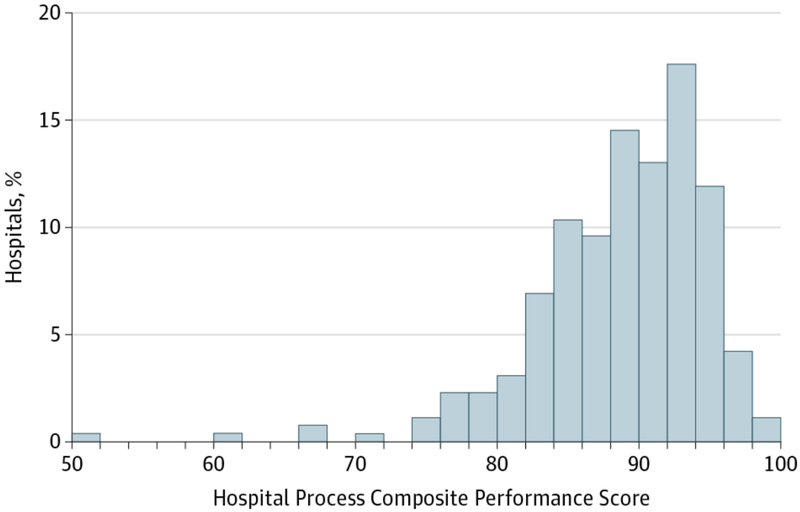
Our analysis included 35,283 IHCA patients from 261 GWTG-R hospitals between January 1, 2010 and December 31, 2012. The overall eligibility population for each measure ranged from 14.5% for time to defibrillation, to 97.0% for time to first compressions ≤1 minute (Table 1). The median hospital process composite performance score was 89.7% (interquartile range [IQR] 85.4, 93.1). The hospital process composite performance varied significantly among the GWTG-R hospitals and ranged from 47.6% to 94.2% (Figure 1). Hospitals in quartiles 1, 2, 3, and 4 had a median composite performance score of 82.6% (IQR: 78.9, 84.3), 88.0% (86.7, 88.9), 91.5% (90.4, 92.3), and 94.8% (93.9, 95.9%), respectively (eFigure 2).
Table 1.
Variation in Adherence to Individual Guideline Recommended Process Measures by Hospital Quartile Composite Performance
| Overall | 1st Quartilea (Lowest) | 2nd Quartilea | 3rd Quartilea | 4th Quartilea (Highest) | p-value | |
|---|---|---|---|---|---|---|
| (n=35,283) | (n=4,576) | (n=7,883) | (n=10,900) | (n=11,924) | ||
| Device confirmation of correct endotracheal tube placement | ||||||
| Eligible patients | 16,572 (47.0) | 2,380 (52.0) | 3,996 (50.7) | 4,797 (44.0) | 5,399 (45.3) | 0.17 |
| Received measure | 14,217 (85.8) | 1,684 (70.8) | 3,126 (78.2) | 4,317 (90.0) | 5,090 (94.3) | |
| Percent pulseless cardiac events monitored or witnessed | ||||||
| Eligible patients | 32,014 (90.7) | 4,057 (88.7) | 7,121 (90.3) | 10,009 (91.8) | 10,827 (90.8) | 0.36 |
| Received measure | 30,093 (94.0) | 3,627 (89.4) | 6,574 (92.3) | 9,413 (94.0) | 10,479 (96.8) | |
| Time to first chest compressions ≤1 min | ||||||
| Eligible patients | 34,215 (97.0) | 4,359 (95.3) | 7,624 (96.7) | 10,471 (96.1) | 11,761 (98.6) | 0.18 |
| Received measure | 32,607 (95.3) | 3,915 (89.8) | 7,105 (93.2) | 10,038 (95.9) | 11,549 (98.2) | |
| Time to first shock ≤2 mins for VF/VT | ||||||
| Eligible patients | 5,113 (14.5) | 684 (14.9) | 1,156 (14.7) | 1,563 (14.3) | 1,710 (14.3) | 0.08 |
| Received measure | 3,051 (59.7) | 338 (49.4) | 649 (56.1) | 926 (59.2) | 1,138 (66.5) | |
| Time to IV/IO epinephrine/vasopressin bolus administered to pulseless adults ≤5 mins | ||||||
| Eligible patients | 22,003 (62.4) | 2,599 (56.8) | 4,675 (59.3) | 7,088 (65.0) | 7,641 (64.1) | 0.14 |
| Received measure | 19,731 (89.7) | 2,148 (82.6) | 4,107 (87.9) | 6,353 (89.6) | 7,123 (93.2) |
Abbreviations: ET, endotracheal tube; IQR, interquartile range; IV/IO, intravenous/intraosseous; min, minutes; VF/VT, ventricular fibrillation/ventricular tachycardia
Quartiles are divided into equal hospital quartiles based on sequentially ordered composite quality performance scores.
Figure 1. Hospital Process Composite Performance Scores.
Frequency distribution of hospital process composite performance scores in GWTG-R cohort Abbreviations: GWTG-R, Get With The Guidelines-Resuscitation
Table 1 shows the variation in use of individual guideline-recommended IHCA process quality of care measures among patients in our cohort. Hospitals in the highest quartile for the process composite performance had significantly higher adherence to all individual guideline measures for IHCA compared with hospitals in other quartiles. There was significant variability in adherence to guideline-recommended IHCA process quality of care measures. The greatest increase across quartiles for hospital performance of individual measures was seen for confirmation of endotracheal tube placement (70.8% in quartile [Q]1 to 94.3% in Q4, p=0.014) and first defibrillation shock ≤2 minutes for VT/VF (49.4% in Q1 to 66.5% in Q4, p=0.01). The measures with both the greatest overall adherence and the lowest degree of variance (although significant) were: 1) monitored or witnessed cardiac events (p-value for increase across quartiles=0.004) and 2) time to first compressions ≤1 minute (p-value for increase across quartiles=0.014; Table 1).
Table 2 shows baseline characteristics of patients and hospitals within each quartile of hospital process composite performance. Compared with patients treated at hospitals with the lowest quartile of process composite performance, patients at the hospitals with the highest quartile process composite performance were younger, slightly less likely to be male, more likely to be black, and were less likely to be in VT/VF at the time of cardiac arrest. Patients at the hospitals with the highest quartile of process composite performance were more likely to be in the intensive care unit at the time of arrest, less likely to have cardiac arrests at night, and were more likely to have interventions such as mechanical ventilation, hemodialysis, vasopressors, arterial catheters, and vascular access.
Table 2.
Baseline Patient Characteristics by Hospital Performance Quartilea
| Quartile | ||||||
|---|---|---|---|---|---|---|
| Overall (n=261)b | 1 (lowest) (n=65)c | 2 (n=65)d | 3 (n=66)e | 4 (Highest) (n=65)f | P Value | |
| Patient characteristics | ||||||
| Age, median (IQR), y | 67 (56–78) | 69 (57–79) | 68 (57–79) | 66 (55–77) | 66 (55–77) | <0.001 |
| Male | 20,428 | 2,695 (58.9) | 4,729 (60.0) | 6,224 (57.1) | 6,780 (56.9) | <0.001 |
| Race | ||||||
| White | 23,612 (67.3) | 3,257 (71.6) | 5,621 (71.6) | 6,901 (64.0) | 7,833 (66.0) | <0.001 |
| Black | 7,615 (21.7) | 739 (16.2) | 1,412 (18.0) | 2,615 (24.2) | 2,849 (24.0) | |
| Asian/pacific Islander | 439 (1.3) | 61 (1.3) | 62 (0.8) | 141 (1.3) | 175 (1.5) | |
| American Indian/Eskimo | 132 (0.4) | 27 (0.6) | 27 (0.3) | 48 (0.4) | 30 (0.3) | |
| Hispanic | 1,672 (4.8) | 169 (3.7) | 244 (3.1) | 631 (5.8) | 628 (5.3) | |
| Other | 1,589 (4.5) | 299 (6.6) | 483 (6.2) | 453 (4.2) | 354 (3.0) | |
| Initial rhythm | ||||||
| VF or pulseless VT | 5,547 (17.1) | 742 (18.4) | 1,257 (17.4) | 1,690 (16.8) | 1,858 (16.7) | <0.001 |
| Asystole or PEA | 26,845 (82.9) | 3,290 (81.6) | 5,949 (82.6) | 8,342 (83.2) | 9,264 (83.3) | |
| Location of arrest | ||||||
| ICU | 21,698 (61.5) | 2,514 (54.9) | 4,449 (56.4) | 6,889 (63.2) | 7,846 (65.8) | <0.001 |
| Monitored unit | 6,788 (19.2) | 732 (16.0) | 1,577 (20.0) | 2,120 (19.4) | 2,359 (19.8) | |
| Nonmonitored unit | 6,797 (19.3)) | 1,330 (29.1) | 1,857 (23.6) | 1,891 (17.3) | 1,719 (14.4) | |
| Time of arrest | ||||||
| Arrest at night (11 pm to 7am) | 13,293 (38.0) | 1,781 (39.8) | 3,078 (39.4) | 4,053 (37.5) | 4,381 (36.9) | <0.001 |
| Arrest on weekend | 9,796 (27.8) | 1,213 (26.6) | 2,204 (28.0) | 3,014 (27.7) | 3,365 (28.2) | 0.10 |
| Recurrent IHCA after index IHCA | 5,343 (15.1) | 573 (12.5) | 1,114 (14.1) | 1,706 (15.7) | 1,950 (16.4) | <0.001 |
| Preexisting conditions | ||||||
| MI during admission | 3,556 (13.7) | 414 (11.3) | 815 (13.2) | 1,164 (14.5) | 1,163 (14.4) | <0.001 |
| Prior MI | 3,525 (13.6) | 440 (12.0) | 760 (12.3) | 1,272 (15.9) | 1,053 (13.1) | 0.02 |
| Arrhythmia | 7,614 (29.4) | 895 (24.5) | 1,915 (31.0) | 2,702 (33.7) | 2,102 (26.1) | 0.20 |
| CHF during admission | 4,466 (17.2) | 548 (15.0) | 1,010 (16.4) | 1,405 (17.5) | 1,503 (18.7) | <0.001 |
| Prior CHF | 5,284 (20.4) | 758 (20.7) | 1, 215 (19.7) | 1,729 (21.6) | 1,582 (19.6) | 0.49 |
| Diabetes mellitus | 8,125 (31.4) | 1,089 (29.8) | 1,929 (31.3) | 2,510 (31.3) | 2,597 (32.2) | 0.01 |
| Malignant tumor | 3,176 (12.3) | 410 (11.2) | 660 (10.7) | 1,059 (13.2) | 1,047 (13.0) | <0.001 |
| HIV positive | 241 (0.9) | 39 (1.1) | 43 (0.7) | 69 (0.9) | 90 (1.1) | 0.14 |
| AIDS | 159 (0.6) | 23 (0.6) | 28 (0.5) | 43 (0.5) | 65 (0.8) | 0.04 |
| Stroke | 981 (3.8) | 133 (3.6) | 215 (3.5) | 289 (3.6) | 344 (4.3) | 0.02 |
| Respiratory insufficiency | 11,180 (43.2) | 1,161 (31.7) | 2,438 (39.5) | 3,652 (45.6) | 3,929 (48.8) | <0.001 |
| Pneumonia | 3,984 (15.4) | 532 (14.5) | 997 (16.2) | 1,134 (14.1) | 1,321 (16.4) | 0.10 |
| Hepatic insufficiency | 1, 984 (7.7) | 290 (7.9) | 425 (6.9) | 647 (8.1) | 622 (7.7) | 0.39 |
| Renal insufficiency | 9,485 (36.6) | 1,158 (31.6) | 2,122 (34.4) | 3,058 (38.1) | 3,147 (39.1) | <0.001 |
| Septicemia | 4,665 (18.0) | 516 (14.1) | 1,020 (16.5) | 1,537 (19.2) | 1,592 (19.8) | <0.001 |
| Metabolic or electrolyte abnormality | 3,794 (14.6) | 432 (11.8) | 774 (12.5) | 1,306 (16.3) | 1,282 (15.9) | <0.001 |
| Illness category | ||||||
| Medical | 27,442 (77.8) | 3,591 (78.5) | 6,289 (79.8) | 8,571 (78.6) | 8,991 (75.4) | <0.001 |
| Surgical | 7,646 (21.7) | 821 (17.9) | 1,577 (20.0) | 2,320 (21.3) | 2,928 (24.6) | |
| Interventions in place at the time of event | ||||||
| Vascular access | 33,197 (94.1) | 4,171 (91.1) | 7,169 (90.9) | 10,363 (95.1) | 11,494 (96.4) | <0.001 |
| Arterial catheter | 4,442 (36.1) | 349 (21.4) | 695 (27.1) | 1,596 (42.2) | 1,802 (41.6) | <0.001 |
| Vasopressors | 2,598 (28.5) | 226 (1839) | 466 (23.0) | 871 (30.8) | 1,035 (33.8) | <0.001 |
| Hemodialysis | 1,084(13.6) | 124 (11.0) | 212 (11.5) | 280 (11.7) | 468 (17.9) | 0.001 |
| IABP | 472 (6.3) | 41 (3.9) | 108 (6.1) | 154 (6.7) | 169 (7.1) | <0.001 |
| Mechanical ventilation | 12,028 (67.1) | 1,287 (56.1) | 2,326 (62.4) | 4,174 (72.2) | 4,241 (69.3) | <0.001 |
| Length of stay, meian (IQR), d | 6 (2–15) | 5 (2–12) | 5 (2–14) | 6 (2–15) | 7 (2–17) | <0.001 |
| CPC score at hospital discharge, mean (SD) |
4.3 (1.5) | 4.3 (1.5) | 4.3 (1.4) | 4.3 (1.4) | 4.2 (1.5 | <0.001 |
Abbreviations: See Table 3.
Data are presented as numbers (percentage) of patients unless otherwise indicated
Overall population, 35,283 patients; quartile 1, 4,576 patients; quartile 2, 7,883 patients; quartile 3, 10,900 patients; quartile 4, 11,924 patients.
Compared with hospitals having the lowest quartile process composite performance, hospitals with the highest quartile process composite performance were more likely to have cardiac surgery capabilities and more likely to be teaching hospitals. Best performing hospitals were also more likely to have a mean medium and large number of hospital beds (Table 2).
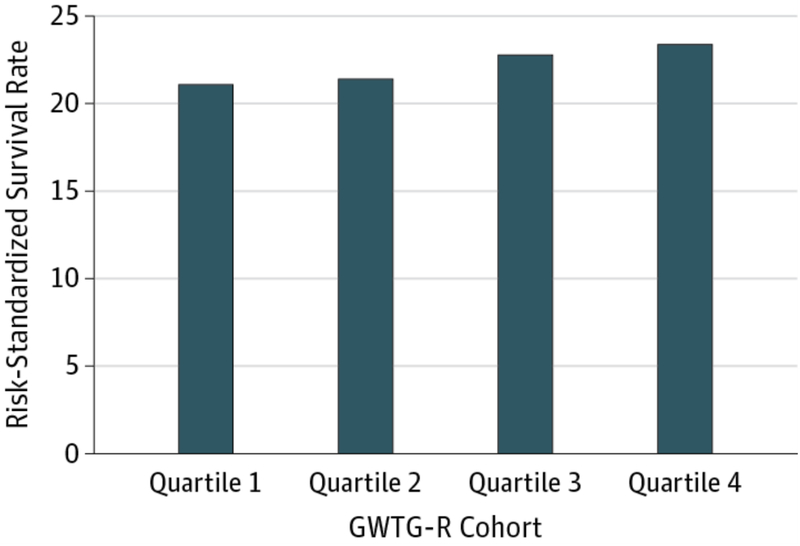
Unadjusted survival to discharge was 22.4% overall, ranging from 20.7% in the lowest quartile to 23.6% in the highest quartile (p<0.001; Table 3). After adjusting for patient and event characteristics, RSSR was 21.1%, 21.4%, 22.8%, and 23.4% from lowest to highest quartile, respectively (p<0.001 for trend; Figure 2). Each 10% increase in a hospital’s composite performance was associated with a 22% higher adjusted odds of survival (adjusted odds ratio [OR], 1.22 [95% CI, 1.09–1.37]; Table 3).
Table 3.
Baseline Hospital and Patient Characteristics by Hospital Adherence Quartilea
| Overallb | 1st Quartilec (Lowest) | 2nd Quartiled | 3rd Quartilee | 4th Quartilef (Highest) | p-value | |
|---|---|---|---|---|---|---|
| (n=261) | (n=65) | (n=65) | (n=65) | (n=65) | ||
| Hospital characteristicsg | ||||||
| Hospital type | ||||||
| Teaching (major or minor) | 144 (55.2) | 27 (41.5) | 33 (50.8) | 42 (63.6) | 42 (64.6) | 0.003 |
| Trauma services (level 1,2) | 107 (45.7) | 27 (49.1) | 22 (37.9) | 34 (56.7) | 24 (39.3) | 0.72 |
| Cardiac surgery capabilities | 172 (73.5) | 35 (63.6) | 44 (75.9) | 48 (80.0) | 45 (73.8) | 0.21 |
| Hospital bed size | 0.01 | |||||
| Small (<250 beds) | 91 (34.9) | 31 (47.7) | 25 (38.5) | 15 (22.7) | 20 (30.8) | |
| Medium (250–499 beds) | 109 (41.8) | 22 (33.8) | 27 (41.5) | 31 (47.0) | 29 (44.6) | |
| Large (≥500 beds) | 61 (23.4) | 12 (18.5) | 13 (20.0) | 20 (30.3) | 16 (24.6) | |
| Nurse-to-bed ratio | 0.02 | |||||
| <1 | 16 (6.1) | 8 (12.3) | 2 (3.1) | 4 (6.1) | 2 (3.1) | |
| ≥1, <1.5 | 76 (29.1) | 25 (38.5) | 20 (30.8) | 14 (21.2) | 17 (26.2) | |
| ≥1.5, <2 | 89 (34.1) | 17 (26.2) | 20 (30.8) | 30 (45.5) | 22 (33.8) | |
| ≥2, <2.5 | 56 (21.5) | 8 (12.3) | 18 (27.7) | 13 (19.7) | 17 (26.2) | |
| ≥2.5, <3 | 16 (6.1) | 5 (7.7) | 3 (4.6) | 4 (6.1) | 4 (6.2) | |
| ≥3 | 8 (3.1) | 2 (3.1) | 2 (3.1) | 1 (1.5) | 3 (4.6) | |
| Percentage of ICU beds | 0.87 | |||||
| Low (<5%) | 49 (18.8) | 17 (26.2) | 7 (10.8) | 8 (12.1) | 17 (26.2) | |
| Medium (5–10%) | 155 (59.4) | 33 (50.8) | 42 (64.6) | 46 (69.7) | 34 (52.3) | |
| High (>10%) | 26 (10.0) | 5 (7.7) | 6 (9.2) | 6 (9.1) | 9 (13.8) | |
| Geographic region | 0.56 | |||||
| Northeast | 40 (15.3) | 10 (15.4) | 12 (18.5) | 11 (16.7) | 7 (10.8) | |
| South | 116 (44.4) | 27 (41.5) | 29 (44.6) | 28 (42.4) | 32 (49.2) | |
| Midwest | 52 (19.9) | 15 (23.1) | 11 (16.9) | 14 (21.2) | 12 (18.5) | |
| West | 53 (20.3) | 13 (20.0) | 13 (20.0) | 13 (19.7) | 14 (21.5) | |
| Location | 0.08 | |||||
| Urban | 240 (92.0) | 55 (84.6) | 62 (95.4) | 63 (95.5) | 60 (92.3) | |
| Rural | 21 (8.0) | 10 (15.4) | 3 (4.6) | 3 (4.5) | 5 (7.7) | |
| Ownership | 0.42 | |||||
| Private | 34 (13.0) | 11 (16.9) | 5 (7.7) | 9 (13.6) | 9 (13.8) | |
| Government | 42 (16.1) | 12 (18.5) | 10 (15.4) | 8 (12.1) | 12 (18.5) | |
| Non-profit | 185 (70.9) | 42 (64.6) | 50 (76.9) | 49 (74.2) | 44 (67.7) | |
| Patient characteristics | ||||||
| Demographics | ||||||
| Age, yr, median (IQR) | 67 (56,78) | 69 (57,79) | 68 (57,79) | 66 (55,77) | 66 (55,77) | <0.001 |
| Male | 20,428 (57.9) | 2,695 (58.9) | 4,729 (60.0) | 6,224 (57.1) | 6,780 (56.9) | <0.001 |
| Race | <0.001 | |||||
| White | 23,612 (67.3) | 3,257 (71.6) | 5,621 (71.6) | 6,901 (64.0) | 7,833 (66.0) | |
| Black | 7,615 (21.7) | 739 (16.2) | 1,412 (18.0) | 2,615 (24.2) | 2,849 (24.0) | |
| Asian/Pacific Islander | 439 (1.3) | 61 (1.3) | 62 (0.8) | 141 (1.3) | 175 (1.5) | |
| American Indian/Eskimo | 132 (0.4) | 27 (0.6) | 27 (0.3) | 48 (0.4) | 30 (0.3) | |
| Hispanic | 1,672 (4.8) | 169 (3.7) | 244 (3.1) | 631 (5.8) | 628 (5.3) | |
| Other | 1,589 (4.5) | 299 (6.6) | 483 (6.2) | 453 (4.2) | 354 (3.0) | |
| Cardiac arrest characteristics | ||||||
| Initial rhythm | <0.001 | |||||
| VF/pulseless VT | 5,547 (17.1) | 742 (18.4) | 1,257 (17.4) | 1,690 (16.8) | 1,858 (16.7) | |
| Asystole/PEA | 26,845 (82.9) | 3,290 (81.6) | 5,949 (82.6) | 8,342 (83.2) | 9,264 (83.3) | |
| Location of arrest | <0.001 | |||||
| ICU | 21,698 (61.5) | 2,514 (54.9) | 4,449 (56.4) | 6,889 (63.2) | 7,846 (65.8) | |
| Monitored unit | 6,788 (19.2) | 732 (16.0) | 1,577 (20.0) | 2,120 (19.4) | 2,359 (19.8) | |
| Non-monitored unit | 6,797 (19.3) | 1,330 (29.1) | 1,857 (23.6) | 1,891 (17.3) | 1,719 (14.4) | |
| Time of arrest | ||||||
| Arrest at night (11pm–7am) | 13,293 (38.0) | 1,781 (39.8) | 3,078 (39.4) | 4,053 (37.5) | 4,381 (36.9) | <0.001 |
| Arrest on weekend | 9,796 (27.8) | 1,213 (26.6) | 2,204 (28.0) | 3,014 (27.7) | 3,365 (28.2) | 0.10 |
| Recurrent IHCA after index IHCA | 5343 (15.1) | 573 (12.5) | 1114 (14.1) | 1706 (15.7) | 1950 (16.4) | <0.001 |
| Preexisting conditions | ||||||
| MI during admission | 3,556 (13.7) | 414 (11.3) | 815 (13.2) | 1,164 (14.5) | 1,163 (14.4) | <0.001 |
| Prior MI | 3,525 (13.6) | 440 (12.0) | 760 (12.3) | 1,272 (15.9) | 1,053 (13.1) | 0.02 |
| Arrhythmia | 7,614 (29.4) | 895 (24.5) | 1,915 (31.0) | 2,702 (33.7) | 2,102 (26.1) | 0.20 |
| CHF during admission | 4,466 (17.2) | 548 (15.0) | 1,010 (16.4) | 1,405 (17.5) | 1,503 (18.7) | <0.001 |
| Prior CHF | 5,284 (20.4) | 758 (20.7) | 1,215 (19.7) | 1,729 (21.6) | 1,582 (19.6) | 0.49 |
| Diabetes mellitus | 8,125 (31.4) | 1,089 (29.8) | 1,929 (31.3) | 2,510 (31.3) | 2,597 (32.2) | 0.01 |
| Malignancy | 3,176 (12.3) | 410 (11.2) | 660 (10.7) | 1,059 (13.2) | 1,047 (13.0) | <0.001 |
| HIV positive | 241 (0.9) | 39 (1.1) | 43 (0.7) | 69 (0.9) | 90 (1.1) | 0.14 |
| AIDS | 159 (0.6) | 23 (0.6) | 28 (0.5) | 43 (0.5) | 65 (0.8) | 0.04 |
| Stroke | 981 (3.8) | 133 (3.6) | 215 (3.5) | 289 (3.6) | 344 (4.3) | 0.02 |
| Respiratory insufficiency | 11,180 (43.2) | 1,161 (31.7) | 2,438 (39.5) | 3,652 (45.6) | 3,929 (48.8) | <0.001 |
| Pneumonia | 3,984 (15.4) | 532 (14.5) | 997 (16.2) | 1,134 (14.1) | 1,321 (16.4) | 0.10 |
| Hepatic insufficiency | 1,984 (7.7) | 290 (7.9) | 425 (6.9) | 647 (8.1) | 622 (7.7) | 0.39 |
| Renal insufficiency | 9,485 (36.6) | 1,158 (31.6) | 2,122 (34.4) | 3,058 (38.1) | 3,147 (39.1) | <0.001 |
| Septicemia | 4,665 (18.0) | 516 (14.1) | 1,020 (16.5) | 1,537 (19.2) | 1,592 (19.8) | <0.001 |
| Metabolic/electrolyte abnormality | 3,794 (14.6) | 432 (11.8) | 774 (12.5) | 1,306 (16.3) | 1,282 (15.9) | <0.001 |
| Illness category | <0.001 | |||||
| Medical | 27,442 (77.8) | 3,591 (78.5) | 6,289 (79.8) | 8,571 (78.6) | 8,991 (75.4) | |
| Surgical | 7,646 (21.7) | 821 (17.9) | 1,577 (20.0) | 2,320 (21.3) | 2,928 (24.6) | |
| Interventions in place at the time of event | ||||||
| Vascular access | 33,197 (94.1) | 4,171 (91.1) | 7,169 (90.9) | 10,363 (95.1) | 11,494 (96.4) | <0.001 |
| Arterial catheter | 4,442 (36.1) | 349 (21.4) | 695 (27.1) | 1,596 (42.2) | 1,802 (41.6) | <0.001 |
| Vasopressors | 2,598 (28.5) | 226 (18.9) | 466 (23.0) | 871 (30.8) | 1,035 (33.8) | <0.001 |
| Hemodialysis | 1,084 (13.6) | 124 (11.0) | 212 (11.5) | 280 (11.7) | 468 (17.9) | <0.001 |
| IABP | 472 (6.3) | 41 (3.9) | 108 (6.1) | 154 (6.7) | 169 (7.1) | 0.001 |
| Mechanical ventilation | 12,028 (67.1) | 1,287 (56.1) | 2,326 (62.4) | 4,174 (72.2) | 4,241 (69.3) | <0.001 |
| Length of stay, days, median (IQR) | 6 (2, 15) | 5 (2, 12) | 5 (2, 14) | 6 (2, 15) | 7 (2, 17) | <0.001 |
| CPC score at hospital discharge, mean (SD) | 4.3 (1.5) | 4.3(1.5) | 4.3 (1.4) | 4.3 (1.4) | 4.2 (1.5) | <0.001 |
Abbreviations: AIDS, acquired immune deficiency syndrome; CHF, congestive heart failure; CPC, cerebral performance category; IABP, intra-aortic balloon pump; ICU, intensive care unit; IQR, interquartile range; HIV, human immunodeficiency virus; MI, myocardial infarction; PA, pulmonary artery; PEA, pulseless electrical activity; SD, standard deviation; All other abbreviations can be found in Table 1.
Quartiles are divided into equal hospital quartiles based on sequentially ordered composite quality performance scores
Overall population: 35,283 patients
Quartile 1 population: 4,576 patients
Quartile 2 population: 7,883 patients
Quartile 3 population: 10,900 patients
Quartile 4 population: 11,923 patients
All percentages are based on non-missing values.
Figure 2. Risk-standardized Survival.
Risk-standardized survival by adherence quartile for patients treated at GWTG-R hospitals. p-value for trend <0.001 Abbreviations: GWTG-R, Get With The Guidelines-Resuscitation
There were also significant differences found in favorable neurologic status at discharge based on hospital process quality of care with hospitals in the highest composite performance quartile having the highest percentage of patients with favorable neurologic status After adjustment, favorable neurologic status was 17.7%, 17.0%, 17.5%, and 19.9%, from lowest to highest quartile, respectively (p<0.001 for trend; Table 3, eTable 4). A sensitivity analysis of patients with a CPC score of 1 (clinically significant favorable neurologic status) also revealed improved neurologic status by composite performance quartiles (eTable 4).
Discussion
Successful treatment of IHCA requires rapid implementation of several processes of care within a short and defined time period.1,12 We found significant variation in process quality of care achievement for patients with IHCA treated at U.S. hospitals. Furthermore, we found that patients treated at hospitals with greater adherence to IHCA guideline-recommended therapies had higher survival rates. The relationship between process quality of care measures and outcomes was evident after adjusting for both patient and hospital characteristics. We estimate that an additional 22,990–24,200 lives could be saved if all hospitals had similar IHCA quality to the best performing hospitals.
Time to defibrillation has been established as an important measure for IHCA care. VT/VF patients with timely defibrillation (i.e., within two minutes), were 50% more likely to survive compared with patients who had delays in defibrillation.4 Previous work has also demonstrated a link between variation in hospital performance with time to defibrillation and survival. Hospitals with the best performance for timely defibrillation had 41% higher adjusted survival compared with the worst-performing hospitals (quartile comparisons).3 We found significant variation, not only in time to defibrillation, but also in other processes of guideline-recommended acute care for IHCA.
Our cross sectional analysis supports a relationship between greater adherence to process measures for IHCA and higher survival rates. Every 10% increase in composite adherence for process of care measures among hospitals in our analysis was associated with a 1.22 higher adjusted odds of survival. An association between composite process performance and outcomes has been demonstrated for other cardiovascular conditions such as acute stroke, heart failure, and myocardial infarction care.14,18–20 However, a recent analysis showed that hospitals with better outcomes for heart failure, acute myocardial infarction, and pneumonia did not have the best survival for IHCA.21 In light of this, authors concluded public reporting of IHCA measures could provide new information on hospital quality. We demonstrate that process measures for IHCA care vary appreciably and are significantly associated with survival and neurologic outcome. The Joint Commission and National Quality Forum have proposed performance measures for IHCA care, including survival to discharge, time to defibrillation, and endotracheal tube confirmation. Our work supports the importance of addressing the process quality of IHCA care. We show that several of these process measures contribute important information related to clinical outcomes. As a result, a composite score used with other medical conditions (e.g., congestive heart failure, coronary artery disease)14,22,23 may be a more appropriate measure of quality of care for IHCA patients.
In our analysis, we estimated that survival to discharge of the best performing hospital was predicted to be 34.5% (compared with an observed survival of 22.4%). Based on an estimated 190,000–200,000 in-hospital cardiac arrests per year in the U.S., we estimate an additional 22,990–24,200 lives would be saved per year if all hospitals operated at the level of the highest-performing hospital. While this is an estimate only, it helps to shed light on the impact of ensuring timely and high-quality care for in-hospital cardiac arrest.
Our study has several limitations. First, our data are observational; therefore, we cannot prove causation between process of care measures and outcomes. Additionally, we cannot be sure that unmeasured confounders may have contributed to the association between composite quality of care and outcomes. Second, hospitals participating in GWTG-R may be more interested in improving quality of care compared with non-participating hospitals. While our sample may not be generalizable to all U.S. hospitals, the degree of variability in actual process quality may be even greater among all U.S. hospitals because the GWTG-R hospitals are self-selected with a presumed greater interest in improving outcomes from IHCA. Third, our secondary outcome of neurologic function was only analyzed among a subset of hospitals that did not have large amounts of missing data (173 of 261 hospitals); this may further limit generalizability and interpretation to hospitals that routinely collecting CPC data on most of their cardiac arrest patients. Fourth, we included five guideline-recommended process measures to create our composite score. While we carefully chose measures based on the 2010 AHA guideline recommendations and the availability and completeness of data, we acknowledge that other process or structural measures may be more or less associated with survival and neurologic outcomes. Fifth, we restricted our analysis to data collected between 2010 and 2012 to account for secular trends in survival to discharge (survival known to be lower in earlier years). Sixth, each measure was weighted equally in our analysis, yet some measures may be more associated with outcomes than others. Nonetheless, composite measures developed for other measures have treated individual measures similarly. Seventh, hospital variability in obtaining do-not-attempt-resuscitation (DNAR) orders prior to cardiac arrest likely influences a hospital’s survival, such that hospitals with the best survival may be more aggressive in obtaining DNAR orders. GWTG-R does not include patients in its registry if they were DNAR prior to their IHCA. However, we did not find that better performing hospitals were more likely than their counterparts to establish DNAR orders during the hospitalization (eTable 5). Eight, we used standard definitions provided by the American Heart Association’s GWTG-R to determine the population inclusion and exclusion criteria for each guideline measure. We acknowledge that the population inclusion for these measures may change over time as a result of expert consensus or new evidence; however, our analysis closely mimics feedback provided to each GWTG-R hospital for each measure and reflects consensus of the measure at the time of our analysis. Finally, our data represent a cross-sectional association between adherence to process quality and outcome. A longitudinal study accounting for change in hospital process performance is needed to establish a relationship between quality and outcomes.
Conclusions
Significant opportunities remain for bettering adherence to guideline-recommended care overall, and with individual process of care measures. Importantly, enhancing process quality of care may improve outcomes for the many patients who suffer from IHCA.
Supplementary Material
Table 4.
Outcomes of IHCA for GTWG-R Cohort
| Unadjusted Outcomes by Hospital Adherence Quartilea | ORs | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Overallb (n=261) | 1st Quartilec (Lowest) (n=65) | 2nd Quartiled (n=65) | 3rd Quartilee (n=65) | 4th Quartilef (Highest) (n=65) | p-value | Unadjusted ORg (95% CI) | Adjusted ORg (95% CI) |
| Survival to discharge | 22.4% | 20.7% | 21.7% | 22.5% | 23.6% | 0.011 | 1.09 (0.98, 1.22) | 1.22 (1.08, 1.37) |
| Favorable neurologic statush (CPC 1 or 2) | 18.3% | 17.7% | 17.0% | 17.5% | 19.9% | 0.041 | 1.11 (0.94, 1.31) | 1.32 (1.11, 1.58) |
Abbreviations: CI, confidence interval; GWTG-R, Get With The Guidelines-Resuscitation; IHCA, in-hospital cardiac arrest; OR, odds ratio; All other abbreviations can be found in Table 2.
Quartiles are divided into equal hospital quartiles based on sequentially ordered composite quality performance scores.
Overall population: 35,283 patients
Quartile 1 population: 4,576 patients
Quartile 2 population: 7,883 patients
Quartile 3 population: 10,900 patients
Quartile 4 population: 11,923 patients
For every 10% increase in composite adherence, the odds of the outcomes changes by the specified amount.
Outcome included hospitals with less than or equal to 25% of the CPC scores missing. Number of hospitals for CPC variable is 173.
Acknowledgments
The authors would like to thank Barbara Lytle, MS for her project leadership, and Erin Hanley, MS for her editorial contributions to this manuscript. Neither Ms. Lytle nor Ms. Hanley received compensation for their contributions, apart from their employment at the institution where this study was conducted. Both Ms. Lytle and Ms. Hanley are employees of the Duke Clinical Research Institute, Duke University Medical Center, Durham, NC. Dr. Anderson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
The Duke Clinical Research Institute (Durham, NC) is responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Monique Anderson, MD is personally funded by the following career development award: National Institutes of Health Common Fund research supplements to promote diversity in health-related research under award number 3U54AT007748–02S1.
Footnotes
Access to Data
Monique Anderson, MD had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Transparency
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Conflict of Interest Disclosures
ML Anderson: Dr. Anderson TBD
G Nichol: Dr. Nichol TBD
D Dai: Dr. Dai TBD
PS Chan: Dr. Chan reports NHLBI funding (significant), grant #: 1R01HL123980.
L Thomas: Dr. Thomas TBD
SM Al-Khatib: Dr. Al-Khatib TBD
RA Berg: Dr. Berg has no relevant disclosures to report.
SM Bradley: Dr. Bradley has no relevant disclosures to report.
ED Peterson: Dr. Peterson reports industry funding from Janssen; consulting from Boehringer Ingelheim, Janssen, Sanofi, Bayer, Merck, and Astra Zeneca.
References
- 1.Morrison LJ, Neumar RW, Zimmerman JL, et al. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: a consensus statement from the American Heart Association. Circulation. 2013;127(14):1538–1563. [DOI] [PubMed] [Google Scholar]
- 2.Merchant RM, Yang L, Becker LB, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39(11):2401–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265–1273. [DOI] [PubMed] [Google Scholar]
- 4.Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9–17. [DOI] [PubMed] [Google Scholar]
- 5.Phelan MP, Ornato JP, Peberdy MA, Hustey FM. Appropriate documentation of confirmation of endotracheal tube position and relationship to patient outcome from in-hospital cardiac arrest. Resuscitation. 2013;84(1):31–36. [DOI] [PubMed] [Google Scholar]
- 6.Brady WJ, Gurka KK, Mehring B, Peberdy MA, O’Connor RE. In-hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82(7):845–852. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khatib SM, Fonarow GC, Hayes DL, et al. Performance measures to promote quality improvement in sudden cardiac arrest prevention and treatment. Am Heart J. 2013;165(6):862–868. [DOI] [PubMed] [Google Scholar]
- 8.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. [DOI] [PubMed] [Google Scholar]
- 9.Cummins RO, Chamberlain DA, Abramson NS, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. A statement for health proessionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Ustralian Rsuscitation Council. Circulation. 1991;84(2):960–975. [DOI] [PubMed] [Google Scholar]
- 10.Perkins GD, Jacobs IG, Nadkarni VM, et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: A Statement for Healthcare Professionals From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation. 2014. doi: 10.1016/j.resuscitation.2014.11.002. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299(7):785–792. [DOI] [PubMed] [Google Scholar]
- 12.Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S729–767. [DOI] [PubMed] [Google Scholar]
- 13.Peterson ED, DeLong ER, Masoudi FA, et al. ACCF/AHA 2010 Position Statement on Composite Measures for Healthcare Performance Assessment: a report of American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop a Position Statement on Composite Measures). J Am Coll Cardiol. 2010;55(16):1755–1766. [DOI] [PubMed] [Google Scholar]
- 14.Peterson ED, Roe MT, Mulgund J, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295(16):1912–1920. [DOI] [PubMed] [Google Scholar]
- 15.Chan PS, Berg RA, Spertus JA, et al. Risk-standardizing survival for in-hospital cardiac arrest to facilitate hospital comparisons. J Am Coll Cardiol. 2013;62(7):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien SM, Delong ER, Peterson ED. Impact of case volume on hospital performance assessment. Arch Intern Med. 2008;168(12):1277–1284. [DOI] [PubMed] [Google Scholar]
- 17.Normand ST, Shahian DM. Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226. [Google Scholar]
- 18.Hernandez AF, Fonarow GC, Liang L, Heidenreich PA, Yancy C, Peterson ED. The need for multiple measures of hospital quality: results from the Get with the Guidelines-Heart Failure Registry of the American Heart Association. Circulation. 2011;124(6):712–719. [DOI] [PubMed] [Google Scholar]
- 19.Shahian DM, O’Brien SM, Normand SL, Peterson ED, Edwards FH. Association of hospital coronary artery bypass volume with processes of care, mortality, morbidity, and the Society of Thoracic Surgeons composite quality score. J Thorac Cardiovasc Surg. 2010;139(2):273–282. [DOI] [PubMed] [Google Scholar]
- 20.Wang TY, Dai D, Hernandez AF, et al. The importance of consistent, high-quality acute myocardial infarction and heart failure care results from the American Heart Association’s Get with the Guidelines Program. J Am Coll Cardiol. 2011;58(6):637–644. [DOI] [PubMed] [Google Scholar]
- 21.Chen LM, Nallamothu BK, Krumholz HM, Spertus JA, Tang F, Chan PS. Association between a hospital’s quality performance for in-hospital cardiac arrest and common medical conditions. Circ Cardiovasc Qual Outcomes. 2013;6(6):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eapen ZJ, Fonarow GC, Dai D, et al. Comparison of composite measure methodologies for rewarding quality of care: an analysis from the American Heart Association’s Get With The Guidelines program. Circ Cardiovasc Qual Outcomes. 2011;4(6):610–618. [DOI] [PubMed] [Google Scholar]
- 23.Ambardekar AV, Fonarow GC, Dai D, et al. Quality of care and in-hospital outcomes in patients with coronary heart disease in rural and urban hospitals (from Get With the Guidelines-Coronary Artery Disease Program). Am J Cardiol. 2010;105(2):139–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.