Abstract
Objective:
Atrial fibrillation (AF) has a demonstrable effect on quality of life (QOL). Recurrent stroke occurs in 10% of patients with AF. The objective of this study was to demonstrate proof of concept that thoracoscopic pulmonary vein isolation and atrial appendage ligation (TPVIAL) could prevent recurrent stroke and could potentially improve QOL in patients with AF with a previous stroke.
Methods:
The study was a National Institutes of HealthYfunded single-center proof-of-concept design that randomized 23 patients with AF-related stroke to TPVIAL (n = 12) or to medical management (n = 11). Quality of life was the primary outcome variable; secondary end points included restoration of rhythm, recurrent stroke, and surgical morbidity.
Results:
Quality-of-life subscores at 3 and 6 months revealed improvements in energy and decreases in fatigue in the TPVIAL arm [baseline, 33 (19.8); 3 months, 49.5 (20.6), P = 0.01; 6 months, 55.5 (14.4), P = 0.03]. At 12-month follow-up, there were no recurrent strokes in the TPVIAL group. In the medically treated arm, two patients at 6 months (P = 0.22) and three total patients at 12 months (P = 0.09) had recurrent ischemic stroke. There was one death in the medical management arm. In the TPVIAL arm, no AF recurrence occurred in patients with paroxysmal AF, and one patient had recurrence of persistent and long-standing AF. Seven patients in the TPVIAL arm discontinued warfarin therapy for secondary stroke prevention.
Conclusions:
This small proof-of-concept study showed that TPVIAL improved QOL on two subscores and restored normal sinus rhythm in all but one patient, and it showed the potential to prevent secondary stroke. A larger study will be needed.
Keywords: Atrial fibrillation, Surgical ablation, Minimally invasive surgery, Stroke, Left atrial appendage ligation
Atrial fibrillation (AF) is an important cause of disabling stroke as patients with AF are six times more likely to have a stroke compared with patients in normal sinus rhythm (NSR).1 Overall, AF is responsible for 15% of all strokes, with the atrial appendage implicated as the predominant source of emboli in most patients with AF.2 As the population ages, stroke incidence in AF is expected to increase.3 The major risk factor for thromboembolic complications in patients with AF is a history of stroke or transient ischemic attack (TIA). This highlights the importance of secondary stroke prevention in these patients, which was a driver for this proof-of-concept pilot study.4
Patients with AF receiving medical therapy including anticoagulation and rate control have a stroke risk of 1% per year.5 More importantly, patients with AF with previous stroke have a 10% risk of recurrence even with optimal anticoagulation strategies.6
Minimally invasive thoracoscopic pulmonary vein isolation and appendage ligation (TPVIAL) has been shown to have great procedural success (990%) in the elimination of AF and prevention of thromboembolic events.7–9 This minimally invasive surgical approach includes ligation of the atrial appendage, the predominant site of thrombus formation and the site thought to be most important in the genesis of AF-related stroke.8
To our knowledge, there have not been any previous trials comparing TPVIAL with standard medical therapy. Therefore, we designed a small randomized controlled clinical trial to examine the efficacy of TPVIAL on quality of life (QOL) improvement, NSR maintenance, and secondary stroke prevention in patients with AF with previous stroke.
MATERIALS AND METHODS
Study Design and Patient Selection
This was a National Institutes of HealthYfunded, prospective, single-center, randomized, and controlled pilot proof-of-concept study conducted from April 2011 to April 2013, which received institutional review board approval. Data were collected in accordance with the Declaration of Helsinki. The primary end point chosen was QOL improvement in patients undergoing TPVIAL versus medical management (MED) because it was unclear whether there would be a reduction in recurrent stroke. The trial was designed to be a clinical trial planning grant, which would provide the necessary information for a future multicenter study including selection of primary and secondary end points for such a study. Secondary end points included recurrent stroke on surveillance pretreatment and posttreatment brain magnetic resonance imaging (MRI), repeat interventions, bleeding, and surgical morbidity.
Patients with ischemic stroke or TIA with documented paroxysmal or persistent AF were eligible for the study if they demonstrated an ischemic stroke on neuroimaging studies or had transient symptoms consisting of hemiparesis, aphasia, or hemineglect. Exclusion criteria included age of less than 18 or greater than 80 years, left atrial appendage thrombus on computed tomography or echocardiography, stroke within 30 days of screening, ejection fraction of less than 25%, previous cardiac or thoracic surgery or empyema, left atrial diameter of greater than 55 mm, contraindication to anticoagulation with warfarin, or mitral valve insufficiency (>2+).
Randomization
Patients were randomized to TPVIAL or MED after being initially evaluated in a stroke clinic or hospital after an acute stroke. Simple randomization was applied using a computer-generated number sequence. At baseline, all patients underwent remote 10-day telemetry monitoring (Medicomp Inc, Melbourne, FL USA), echocardiogram, and brain MRI. A 64-slice three-dimensional chest computed tomographic scan was performed in patients randomized to surgery to determine the anatomy and baseline dimensions of the pulmonary veins.
Surgery
Thoracoscopic pulmonary vein isolation was performed as described by Wolf with modifications including atrial appendage ligation with the AtriClip device (AtriCure, Westchester, OH USA)9,10 (Fig. 1). Transesophageal echocardiography was performed to rule out atrial thrombus upon induction of anesthesia. Thoracoscopic port access was obtained, and the pericardium was opened overlying the pulmonary veins on the right, with careful attention to preserve the phrenic nerve. The AtriCure bipolar radiofrequency energy ablation device (AtriCure, Westchester OH USA) was placed around the hilum of the right pulmonary veins for ablation, and the entire procedure was then repeated on the left side, along with ligation of the atrial appendage at its base under transesophageal echocardiography guidance with confirmation of appendage ligation at the base11 (Fig. 1).
FIGURE 1.
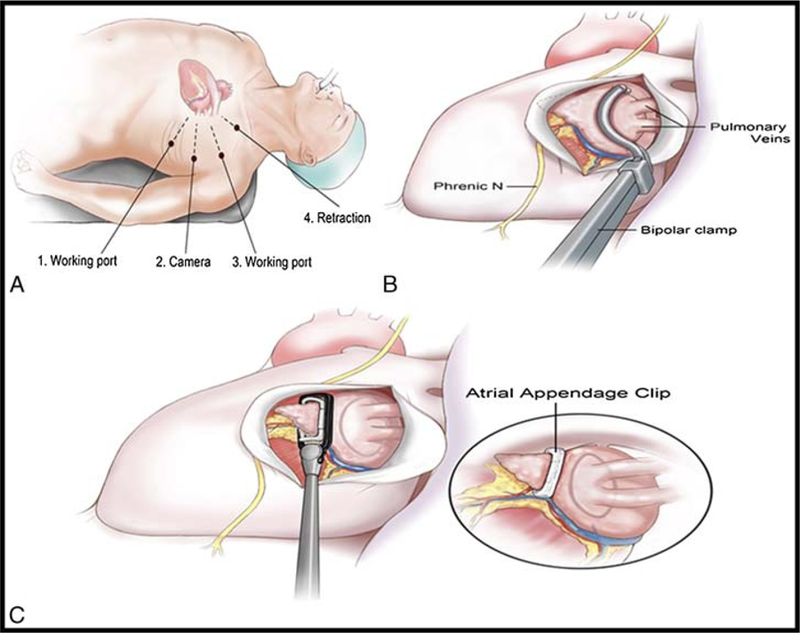
A, Thoracoscopic pulmonary vein isolation and atrial appendage ligation procedure. B, Patient position and ports on left side. C, Bipolar ablation clamp being placed around pulmonary vein hilum. Clip being placed at the base of the left atrial appendage.
Medical Therapy
Optimal medical therapy in patients with AF with TIA or stroke included anticoagulation with warfarin (international normalized ratio goal of 2.0‒3.0) or a novel anticoagulant, dabigatran (Boehringer Ingelheim, Ridgefield, CT USA). All patients in the medical arm were followed by a single cardiologist with either rate control or rhythm control according to patient preference as one large trial found no difference in either strategy.5
Quality of Life
Quality of life was assessed with the RAND 36-Item Health Survey questionnaire,12 which consists of eight components as follows: physical functioning, role limitations caused by physical health, role limitations caused by emotional problems, energy/fatigue, emotional well-being, social functioning, pain, and general health. Each component was scored on a 0-to-100 range, with higher scores showing a more favorable QOL in every component. Participants independently completed the QOL assessment at baseline and repeated at 3 and 6 months.
Brain MRI
All patients underwent a clinical brain MRI study with diffusion sequence (repetition time, 7800 milliseconds; echo rime, 89 milliseconds; 220-mm field of view; 4-mm slice thickness; and 1.5 × 1.5-mm pixel size) at baseline and at 6 month follow-up. A board-certified neuroradiologist blinded to patient treatment arm examined the MRI scans for onset of new strokes from pretreatment to posttreatment time points.
Follow-up
Patients were seen by both a neurologist and a cardiologist at 3, 6, and 12 months to assess for adverse events including new focal neurologic findings and cardiac dysrhythmia. As per protocol, amiodarone was discontinued at 3 months, and anticoagulation was discontinued at 6 months in patients who had ligation of their atrial appendage if there was no evidence of AF on extended telemetry monitoring.
Statistical Analysis
Continuous variables are presented as the mean (SD), and categorical data are expressed as frequency and percentage. Baseline difference in QOL metrics between the two groups was assessed through Mann-Whitney U-test. Statistical comparison of the eight QOL items before and after therapy within groups was performed by Wilcoxon matched-paired test. Spearman correlation was used to find possible correlation between QOL improvement and clinical factors. Intention-to-treat analysis was performed using Fisher exact and χ2 tests for the recurrence rate of stroke at 6 and 12 months. All analyses were performed using SPSS Version 22 (IBM Co., Armonk, NY USA). Two-tailed P < 0.05 was considered significant.
RESULTS
Baseline Demographics and Risk Factors
Five hundred fifty patients were screened in the hospital, stroke and cardiology clinics. Cerebrovascular events were confirmed to be related to AF by a board-certified vascular neurologist based on history, clinical examination, and imaging characteristics according to TOAST criteria.13 Five hundred twenty-seven patients (95.81%) did not meet the inclusion criteria or refused to participate (Fig. 2). Overall, 23 patients, including 14 (60.86%) male and 9 (39.13%) female patients, met qualification criteria and were enrolled. There was no significant difference in baseline characteristics between groups (Table 1). CHADS2 risk factors14 for stroke including left ventricular systolic dysfunction with/without heart failure, age of 75 years or older, hypertension, and diabetes mellitus were similar between the two groups (all P values > 0.05).
FIGURE 2.
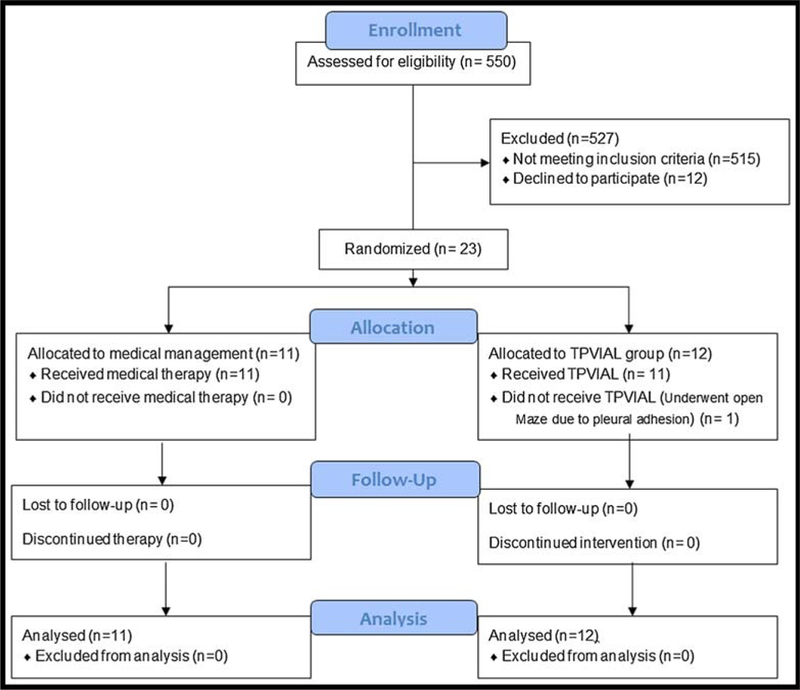
Flow diagram—Venrollment data.
TABLE 1.
Patients’ Characteristics and Study Outcomes
| Variables | TPVIAL (n = 12) | Medically Managed (n = 11) | P |
|---|---|---|---|
| Age, mean (SD), y | 67 (9.4) | 68 (8.4) | 0.70 |
| Montreal cognitive assessment, mean (SD) | 25 (3.7) | 22.9 (6.2) | 0.36 |
| Education level, mean (SD) | 14.6 (4.3) | 12.5 (1.7) | 0.13 |
| Male, n (%) | 5 (42) | 9 (82) | 0.09 |
| Hypertension, n (%) | 10 (83) | 7 (64) | 0.37 |
| Smoker, n (%) | 5 (42) | 6 (55) | 0.68 |
| Current smoker, n (%) | 2 (17) | 1 (9) | 1 |
| Diabetes, n (%) | 2 (17) | 4 (36) | 0.37 |
| Congestive heart failure, n (%) | 3 (25) | 1 (9.1) | 0.59 |
| Peripheral vascular disease, n (%) | 0 | 3 (27.3) | 0.09 |
| Type of atrial fibrillation | |||
| Paroxysmal, n (%) | 5 (42) | 6 (55) | 0.68 |
| Persistent/long-standing persistent, n (%) | 7 (58) | 5 (45) | |
| Previous cardioversion, n (%) | 3 (25) | 2 (18.2) | 1 |
| Previous catheter ablation, n (%) | 2 (17) | 0 | 0.20 |
| AF recurrence after TPVIAL | |||
| ▅ Paroxysmal (n = 5) | 0 | ||
| ▅ Persistent/long standing persistent AF (n = 7) | 1 | ||
| Previous stroke/transient ischemic attack, n (%) | 12 (100) | 11 (100) | 1 |
| CHADS2 score, mean (SD) | 3.25 (0.87) | 3.28 (1.1) | 0.96 |
| CHADS2-VASc score, mean (SD) | 4.5 (1.2) | 4.6 (1.3) | 0.80 |
| HAS-BLED score, mean (SD) | 3.41 (0.90) | 4.0 (1.09) | 0.18 |
| Type of medical management, n (%) | ‒ | Rhythm control, 1 (9.1) | |
| Rate control, 10 (90.9) | |||
| Successful warfarin withdrawal, n (%) | 7 (58.3) | 0 | 0.005 |
| Stroke recurrence, n (%) | 0 | 2 (18.2) (6 mo) | 0.22 |
| 3 (27.3) (12 mo) | 0.09 |
AF, atrial fibrillation; TPVIAL, thoracoscopic pulmonary vein isolation and appendage ligation.
Recurrence of Stroke, Procedural Complications, and Mortality
The American Heart Association expert panel definition of stroke includes any pathological, imaging, or other objective evidence of focal ischemic cerebral injury.15 All patients in the study had baseline and 6-month brain MRI studies and were successfully clinically followed up for 12 months. For TPVIAL, no secondary stroke or TIA was detected at 6 or 12 months. For the MED group, 2 (18%) of 11 patients had recurrent ischemic stroke at 6 months (P = 0.22), and a total of 3 of (27%) 11 patients at 12 months (P = 0.09).
The TPVIAL surgical procedure including left atrial appendage ligation was performed successfully without complications in all patients in the minimally invasive surgery group except for one, who had pleural adhesions preventing thoracoscopy. This patient subsequently underwent an open Maze surgery via sternotomy and was included as per intention-to-treat analysis. One patient died in the MED group because of secondary stroke, and no death occurred among TPVIAL patients.
Maintenance of NSR
For the 12 TPVIAL patients, all 5 patients with paroxysmal AF remained in NSR after the procedure, whereas 6 of the patients with persistent/long-standing persistent AF were in NSR with 1 having a reoccurrence of AF at 12 months. In the MED arm, all patients remained in NSR on medical therapy through 12-month follow-up using rhythm control (n = 1) and rate control (n = 10) strategies. The difference in AF recurrence was not significant between the two groups.
Maintenance of Anticoagulation
There was a significant association with the TPVIAL procedure versus MED therapy with successful warfarin withdrawal (P = 0.005), which was considered for patients with no AF on telemetry monitoring. Overall, seven patients (58.3%) were successfully taken off warfarin in the TPVIAL group and remained on aspirin for secondary stroke prevention. Despite the CHADS2 and the CHADS2-VASc scores being similar in both groups, no patients in the MED group were discontinued from anticoagulation.
Quality-of-Life Measures
At baseline, the QOL metrics were not different between the two groups (all P values > 0.05). Within pretreatment to posttreatment, pairwise analyses showed significant improvement in energy and decrease in fatigue in the TPVIAL group at both 3 (z = ‒2.49, P = 0.017) and 6 months (z = ‒2.09, P = 0.036). Moreover, physical functioning, role limitations caused by physical health, social function, and general health metrics all improved in the surgical group, without reaching statistical significance (Table 2, Fig. 3). No correlation between improvement of QOL metrics and age, education, reading ability, or other clinical factors was found.
TABLE 2.
Improvement (Increased Mean From Baseline to 6 Months) of Quality-of-Life Metrics in the Minimally Invasive Surgical Group
| QOL Items | Medically Managed |
TPVIAL* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline, Mean (SD) | 3 mo, Mean (SD) | P | 6 mo, Mean (SD) | P* | Baseline, Mean (SD) | 3 mo, Mean (SD) | P | 6 mo, Mean (SD) | P | |
| Energy/fatigue | 46.25 (25.4) | 49.16 (23.1) | 0.49 | 54.37 (22.1) | 0.06 | 33 (19.8) | 49.5 (20.6) | 0.01 | 55.5 (14.4) | 0.03 |
| Physical function | 65 (33.9) | 60.13 (27.3) | 0.73 | 65.55 (20.8) | 0.88 | 50.16 (23.5) | 49.22 (20.7) | 0.95 | 54.88 (26.5) | 0.44 |
| Role limitation caused by physical health | 46.87 (47.1) | 53.12 (47.1) | 0.85 | 40.62 (42.1) | 0.85 | 22.5 (41.5) | 32.5 (40.2) | 0.41 | 50 (42.4) | 0.17 |
| Social function | 73.43 (24.4) | 71.87 (19.76) | 1.0 | 73.43 (18.2) | 0.72 | 66.25 (21.2) | 75 (26.3) | 0.55 | 80 (24.4) | 0.28 |
| General health | 61.87 (25.9) | 60.62 (23.9) | 1.0 | 61.25 (25.8) | 0.86 | 62.87 (18.9) | 69.5 (15.3) | 0.34 | 72.5 (20.9) | 0.29 |
| Pain | 72.5 (23.9) | 69.37 (23) | 0.68 | 79.37 (18.5) | 0.12 | 73.75 (27.7) | 79.75 (19.5) | 0.28 | 76 (23.1) | 0.55 |
| Limitations caused by emotional problems | 66.66 (39.8) | 66.66 (39.8) | 0.50 | 70.83 (33) | 0.50 | 63.33 (42) | 60 (40.9) | 0.68 | 70 (42.8) | 0.79 |
| Emotional well-being | 72 (19.4) | 69.83 (15.5) | 0.52 | 71.5 (16.4) | 0.88 | 74.1 (9.7) | 71.2 (20.2) | 0.52 | 67.3 (19.7) | 0.28 |
Baseline versus 6 months.
QOL, quality of life; TPVIAL, Thoracoscopic Pulmonary Vein Isolation and Appendage Ligation.
FIGURE 3.
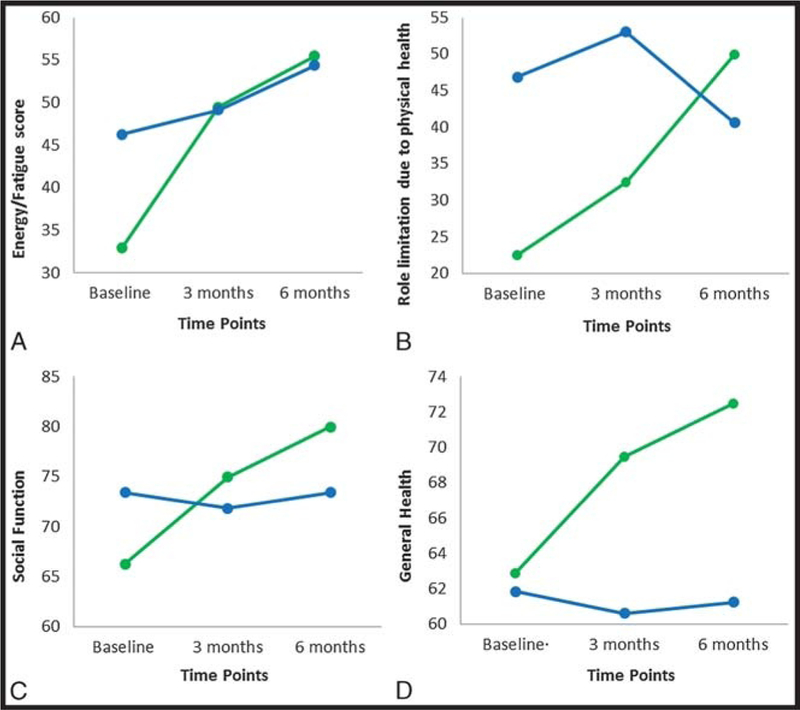
Quality-of-life analysis. A, Energy domain showed significant improvement in the minimally invasive surgical group from baseline. BYD, Physical health, social function, and general health did not change from baseline in the medical group, although some improvement was noted in the surgical group (medical, blue; surgery, green).
DISCUSSION
Atrial fibrillation is the most common cardiac arrhythmia, affecting more than 2.5 million Americans.16 Atrial fibrillation is responsible for 15% to 30% of strokes and costs Medicare more than $7 billion annually.17 Although stroke prevention with AF includes effective anticoagulant therapy, anticoagulant management with warfarin can be challenging (even in monitored clinical trials, appropriate international normalized ratio ranges between 44% and 83%).18 Even with optimal anticoagulation, patients with AF with previous stroke have an 8% to 10% risk for recurrent stroke as reported in several major studies.6,19,20 Furthermore, some patients with AF may elect a single interventional procedure to avoid the risks of lifelong anticoagulation therapy.21
The left atrial appendage is the major source of emboli in AF, and in this study, the AtriClip (AtriCure Inc, Westchester, OH USA), a dacron-covered nitinol clip, was placed epicardially to close the appendage at the same time pulmonary vein isolation was performed to restore NSR. Although the Watchman device (Boston Scientific Inc, Marlborough, MA USA) has shown promise for appendage closure via catheter delivery, it should be noted in the latest randomized PREVAIL trial that the Watchman device did not meet the end point for reduction in ischemic stroke or systemic embolism as compared with warfarin.22 There also remains concern about residual leak around the Watchman, which by design is circular and is not a perfect fit for the oblong appendage.23–25 Furthermore, a recent meta-analysis of 2406 patients in the Watchman Prevail and Prevent trials and associated registries found that intracranial hemorrhage was lower with Watchman versus warfarin (hazard ratio, 0.22; P = 0.004), but ischemic stroke was higher (hazard ratio, 1.95; P = 0.05).26 Moreover, cardiologists have been reluctant to perform a catheter-based endocardial intervention in a patient after a recent acute stroke, and new approaches are therefore needed.
Appendage closure alone does not eliminate the risk for ischemic recurrent stroke, as observed earlier in the PREVAIL trial.22 Actually, restoration of NSR with a surgical MAZE procedure was found more effective than atrial appendage ligation for the prevention of embolic stroke by Bando et al27 during mitral valve replacement.27 Minimally invasive thoracoscopic pulmonary vein isolation has the potential to eliminate AF with up to 90% success rate9,10,28,29; however, controlled trials such as the one performed in this proof-of-concept study are needed for confirmation.
The results of our pilot proof-of-concept study suggest that TPVIAL may provide improved outcome relative to medical management alone for patients with AF with a history of stroke. In this study, three patients in the medical arm patients had a stroke at 12 months, including one patient who died of a secondary stroke. Thoracoscopic pulmonary vein isolation and atrial appendage ligation restored the NSR and had zero recurrent strokes, allowed discontinuation of anticoagulation in select patients, and improved energy and fatigue on QOL subscores, with an acceptable safety profile. Thoracoscopic pulmonary vein isolation and atrial appendage ligation has the potential to reduce adverse events from anticoagulation, as 14% to 40% of patients with AF cannot take anticoagulant drugs.30,31
Although the findings in this proof-of-concept study did not reach statistical significance, the data will help us to appropriately design a larger multicenter clinical trial that could use recurrent stroke as the primary end point along with QOL end points.
Study Limitations
The limitations of the study included the small number of patients with interventions only performed by a single surgeon at a single institution. Extended remote telemetry monitoring was not performed in the medical arm; therefore, patients may have had an undetected recurrence of AF. This study is too small to draw any major conclusions, and a future clinical trial could overcome these issues by powering the study appropriately and by randomizing patients to three arms as follows: (1) TPVIAL, (2) appendage ligation alone, and (3) MED therapy. Each arm would use extended telemetry monitoring during follow-up, and the primary outcome variable would be recurrent stroke.
CONCLUSIONS
This small proof-of-concept pilot clinical trial suggests that a minimally invasive TPVIAL procedure could be performed in patients with AF with recent stroke. Surgical patients had improved QOL scores for energy and fatigue and had no recurrent stroke but this did not reach significance. A redesigned larger multicenter clinical trial will be required to answer the question as to the utility of this procedure in clinical practice.
CLINICAL PERSPECTIVE.
This study was an National Institutes of Health-funded single-center proof-of-concept trial that randomized 23 patients with atrial fibrillation-related strokes to thoracoscopic pulmonary vein isolation and atrial appendage ligation (TPVIAL) or to medical management. At 12-month follow-up, there were no recurrent strokes in the TPVIAL group. In the medically treated arm, two patients at 6 months and three patients at 12 months had recurrent ischemic strokes. Quality-of-life subscores at 3 and 6 months revealed improvements in energy and decreases in fatigue in the TPVIAL arm. This very small study showed that TPVIAL may have a potential for improving quality of life and preventing further strokes in patients who had had an atrial fibrillation-related stroke.
These preliminary data are encouraging but require verification in a larger multicenter randomized clinical trial. At the moment, the ability of TPVIAL to prevent recurrent strokes remains unproven.
ACKNOWLEDGMENTS
The authors thank Debra Robertson, RN, for her help in coordinating the study and collecting patient data, and David Peace, artist for the figures. The authors (T.M.B., M.F.W.) acknowledge The National Institutes of Health, National Heart, Lung and Blood Institute, for funding support for this project (RC1HL100195–01). The authors also thank Michael S. Okun for his helpful comments.
Supported by NIH (RC1HL100195–01).
Footnotes
Presented at the Annual Scientific Meeting of the International Society for Minimally Invasive Cardiothoracic Surgery, June 3Y6, 2015, in Berlin, Germany.
Disclosures: Thomas M. Beaver, MD, is a member of the educational steering committee and a study site principal investigator for AtriCure, Inc, West Chester, OH USA; however, there is no reported conflict of interest. William M. Miles, MD, is a consultant to Medtronic, Inc, Minneapolis, MN USA. Vishnumurthy Shushrutha Hedna, MD, Anna Y. Khanna, MD, Catherine C. Price, PhD, Ilona M. Schmalfuss, MD, Seyed Hossein Aalaei-Andabili, MD, and Michael F. Waters, MD, PhD, declare no conflicts of interest.
REFERENCES
- 1.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449–1457. [PubMed] [Google Scholar]
- 2.Syed TM, Halperin JL. Left atrial appendage closure for stroke prevention in atrial fibrillation: state of the art and current challenges. Nat Clin Pract Cardiovasc Med 2007;4:428–435. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 4.Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(suppl 6):546S–592S. [DOI] [PubMed] [Google Scholar]
- 5.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347: 1825–1833. [DOI] [PubMed] [Google Scholar]
- 6.Kee YY, Brooks W, Bhalla A. Do older patients receive adequate stroke care? An experience of a neurovascular clinic. Postgrad Med J 2009;85: 115–118. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsuka T, Ninomiya M, Nonaka T, et al. Thoracoscopic stand-alone left atrial appendectomy for thromboembolism prevention in nonvalvular atrial fibrillation. J Am Coll Cardiol 2013;62:103–107. [DOI] [PubMed] [Google Scholar]
- 8.McClelland JH, Duke D, Reddy R. Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:1289–1295. [DOI] [PubMed] [Google Scholar]
- 9.Sirak J, Jones D, Sun B, et al. Toward a definitive, totally thoracoscopic procedure for atrial fibrillation. Ann Thorac Surg 2008;86:1960–1964. [DOI] [PubMed] [Google Scholar]
- 10.Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130:797–802. [DOI] [PubMed] [Google Scholar]
- 11.Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 2011;142:1002–1009. [DOI] [PubMed] [Google Scholar]
- 12.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med 2001;33:350–357. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24: 35–41. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Joung B, Son NH, et al. The electroanatomical remodelling of the left atrium is related to CHADS2/CHA2DS2VASc score and events of stroke in patients with atrial fibrillation. Europace 2011;13:1541–1549. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44: 2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 17.Caro JJ. An economic model of stroke in atrial fibrillation: the cost of suboptimal oral anticoagulation. Am J Manag Care 2004;10(suppl 14): S451–S461. [PubMed] [Google Scholar]
- 18.Ansell J, Hirsh J, Dalen J, et al. Managing oral anticoagulant therapy. Chest 2001;119(suppl 1):22S–38S. [DOI] [PubMed] [Google Scholar]
- 19.Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet 1993;342:1255–1262. [PubMed] [Google Scholar]
- 20.Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med 1992;327:1406–1412. [DOI] [PubMed] [Google Scholar]
- 21.Moss JD. Left atrial appendage exclusion for prevention of stroke in atrial fibrillation: review of minimally invasive approaches. Curr Cardiol Rep 2014;16:448. [DOI] [PubMed] [Google Scholar]
- 22.Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 23.Mohrs OK, Wunderlich N, Petersen SE, Pottmeyer A, Kauczor HU. Contrast-enhanced CMR in patients after percutaneous closure of the left atrial appendage: a pilot study. J Cardiovasc Magn Reson 2011;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viles-Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol 2012;59: 923–929. [DOI] [PubMed] [Google Scholar]
- 25.Lam SC, Bertog S, Sievert H. Incomplete left atrial appendage occlusion and thrombus formation after Watchman implantation treated with anticoagulation followed by further transcatheter closure with a second-generation Amplatzer Cardiac Plug (Amulet device). Catheter Cardiovasc Interv 2015;85:321–327. [DOI] [PubMed] [Google Scholar]
- 26.Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol 2015;65:2614–2623. [DOI] [PubMed] [Google Scholar]
- 27.Bando K, Kobayashi J, Hirata M, et al. Early and late stroke after mitral valve replacement with a mechanical prosthesis: risk factor analysis of a 24-year experience. J Thorac Cardiovasc Surg 2003;126:358–364. [DOI] [PubMed] [Google Scholar]
- 28.Beyer E, Lee R, Lam BK. Point: minimally invasive bipolar radiofrequency ablation of lone atrial fibrillation: early multicenter results. J Thorac Cardiovasc Surg 2009;137:521–526. [DOI] [PubMed] [Google Scholar]
- 29.Crijns HJ, Van Gelder IC, Van Gilst WH, Hillege H, Gosselink AM, Lie KI. Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter. Am J Cardiol 1991;68:335–341. [DOI] [PubMed] [Google Scholar]
- 30.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 31.Onalan O, Lashevsky I, Hamad A, et al. Nonpharmacologic stroke prevention in atrial fibrillation. Expert Rev Cardiovasc Ther 2005;3:619–633. [DOI] [PubMed] [Google Scholar]


