Abstract
Retinal pigment epithelium (RPE) alterations in age-related macular degeneration occur in patches, potentially involving long-distance communication between damaged and healthy areas. Communication along the epithelium might be mediated by extracellular vesicles (EVs). To test this hypothesis, EVs were collected from supernatants of polarized ARPE-19 and primary porcine RPE monolayers for functional and biochemical assays. EVs from oxidatively stressed donor cells reduced barrier function in recipient RPE monolayers when compared to control EVs. The effect on barrier function was dependent on EV uptake, which occurred rapidly with EVs from oxidatively stressed donor cells. Mass spectrometry-based proteomic analysis of EVs identified HDAC6, which is known to reduce tight junction stability. Activity assays confirmed the presence of HDAC6 in EVs, and EV transfer assays using HDAC6 inhibitors confirmed its effect in monolayers. These findings demonstrate that EVs can communicate stress messages to healthy RPE cells, potentially contributing to RPE dysfunction.
Keywords: retinal pigment epithelium, extracellular vesicles, exosomes, tight junctions, HDAC6, age-related macular degeneration
Graphical Abstract
Introduction
The bystander effect has garnered a lot of attention in biology in particular in the context of the spreading of pathology. Specifically, it refers to the phenomenon of the induction of biological effects in cells that are not directly targeted [1]. The impacts in neighboring cells can be mediated via direct cell-cell communication via gap junctions (GJs), or long-distance via secreted material. The first mechanism typically occurs in cells that are part of a network and information can spread from the treated to the untreated cell via GJs or across membranes. GJs allow small signaling molecules to pass from one cell to another [e.g., [2, 3]], whereas membrane current or reactive oxygen species (ROS) can be transmitted directly across membranes [e.g., [4]]. Long-distance communication on the other hand involves the secretion of biologically active material, with recipient cells being either part of the same network or cell type, or of different origin. The biologically active material might be secreted as individual molecules, or packaged in extracellular vesicles such as exosomes [5–7].
The retinal pigment epithelium (RPE) is a monolayer of cells localized between the light-sensitive layer of the retina and the choroidal blood supply, forming the outer blood-retina barrier. In this network, intercellular communication is mediated by GJs made up of connexin43 and connexin46 [8], whereas it’s barrier function is dependent on tight- and adherence junctions.
In age-related macular degeneration (AMD), the leading cause of visual impairment and blindness in the elderly worldwide, the RPE appears to be a primary site of damage. Persistent oxidative stress is recognized as one of the important underlying risk factors that lead to AMD pathology. Oxidative stress in the RPE is thought to originate from multiple sources and relates to the biological functions of the tissue. Specifically, the RPE’s biological role is to digest photoreceptor outer segments in a diurnal fashion. These outer segments contain high levels of polyunsaturated fatty acids and photosensitizers, which together increase the production of ROS in the presence of oxygen as well as light [9, 10]. Additional risk factors such as smoking, hypercholesterolemia, excessive light exposure and dysregulated para-inflammation, augment the generation of ROS [11, 12].
However, RPE pathology does not get triggered in a single location to spread uniformly from there; but rather RPE damage occurs in multiple locations within the central part of the eye, that finally coalesce to form a region of atrophy (geographic atrophy, GA) [13]. This observation suggests that pathology occurs in susceptible regions, while healthy regions are protected; and that damage may occur randomly, in a stochastic fashion, or once triggered in a susceptible area, might spread via yet to be discovered means to other receptive areas.
This bystander effect in the RPE might be mediated via extracellular vesicles (EVs). These EVs might represent microvesicles, exosomes or apoptotic bodies, which can be distinguished based on size, the direction of blebbing (outward or inward), their content[5] as well as their sedimentation. Apoptotic bodies, due to their large size (1–5 μm in diameter) sediment at 1,200 × g (summarized in [14]), whereas microvesicles and exosomes are smaller (0.1–1 vs 0.04–0.15 μm, respectively [5]) and sediment by high-speed centrifugation (10,000–20,000 × g) or ultracentrifugation (100,000 × g for 70 minutes), respectively. EVs are secreted by almost every cell type in the body, and are involved in several biological and pathological processes [15, 16]. EVs are key facilitators of intercellular communication with broad biological and medical implications [17, 18], including cell growth, migration, differentiation, neuronal signaling, and immune cell modulation. EVs transport functional molecules, such as messenger RNA (mRNA), microRNA (miRNA) and proteins into target tissues or cells [19–21]. These bioactive molecules are considerably stable and can modulate cell behaviors in recipient cells [22]. The biogenesis of exosomes within a donor cell has four major steps, membrane formation (initiation), endocytosis and multivesicular body (MVB) formation, MVB fusion with the plasma membrane, followed by the release of the internal vesicles (i.e., exosomes) into the extracellular milieu [15]. The biogenesis of microvesicles on the other hand involves trafficking of the cargo to be packaged to the plasma membrane, a redistribution of membrane lipids, followed by the pinching off of vesicles. Surface markers of microvesicles hence tend to be reflective of the composition of the membrane of origin.[23] Due to the complications to unequivocally identify the EVs as exosomes or microvesicles, many reports will not aim to distinguish between the two types, but rather refer to them combined as EVs. The same strategy is followed here. In recipient cells, EVs can mediate their effects in multiple ways. Proteins integral to the EV membrane or bound on the extravesicular side might serve directly as ligands for receptor-mediated effects via EV binding to the recipient cell, or these proteins might get cleaved off and serve as soluble ligands. Alternatively, EVs might fuse directly with the recipient cell membrane to release their content into the cytosol, or are taken up by the recipient cells via one of multiple mechanisms, including endocytosis or phagocytosis, with the EV content gaining access to the endosomal/lysosomal compartment of the cell.
Here, we investigated whether oxidatively stressed highly polarized RPE cells can communicate stress messages to healthy neighboring cells (recipient cells) via EVs, and the potential cargo it might involve.
Material and Methods
Cell Culture
Human ARPE-19 cells and primary porcine RPE cells were used for this study. All cell culture products were obtained from Gibco/ThermoFisher Scientific. ARPE-19 cells were grown as monolayers on 6-well Transwell filters as described previously [24]. Primary porcine RPE (pRPE) were cultured according to a published protocol [25], using eyes obtained from a local abattoir. Pooled cells from 10 eyes were suspended in 10% FBS Growth Medium (high glucose DMEM with L-glutamine, sodium pyruvate, 1% nonessential amino acids, 10% FBS and Penicillin and Streptomycin), expanded in T25 cell culture flasks and transferred to 12-well plates (3×105 cells/cm2). For both cell types, upon reaching confluency, tight junction formation was enabled by step-wise FBS reduction to 1%. Monolayer integrity was assessed by Transepithelial resistance (TER) measurements using an EVOM volt-ohmmeter (World Precision Instruments), with monolayers being considered stable when TER was repeatedly measured as ~40–45 Ωcm2 (ARPE-19 cells) or 150 Ωcm2 (pRPE cells).
Prior to each experiment, monolayers were washed with FBS-free medium and maintained with FBS-free medium for 24–48 hours to avoid the contamination with FBS-derived EVs.
Isolation of extracellular vesicles
To trigger the release of EVs, monolayers on transwells were stimulated apically with 0.5 mM H2O2 (Sigma Aldrich, 216763) once a day for 3 days without changing medium. Pulsed exposure of H2O2 leads to rapid depletion of the oxidant rather than build-up over time, while eliciting the desired effect.[26] Supernatants were collected from both apical and basal sides, spun at 3000g and used for EV isolation using Exoquick-TC (Systems Biosciences) according to the manufacturer’s instructions. In short, 2 ml of Exoquick-TC was added to 10 ml of culture media and incubated overnight at 4°C, centrifuged at 1600g for 35 minutes to collect the EV pellet and resuspended in 50 μL of sterile PBS.
Zetaview nanoparticle tracking analysis (NTA)
NTA was performed using the ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany) and its corresponding software (ZetaView 8.02.28), using instrument settings previously described by us [27]. For each sample, 5–10 μL of the resuspended pellet were diluted into 2 ml of 1X PBS and loaded into the NTA cell for analysis to obtain the diameter size (modal) and EV particle concentration of each sample.
Transmission Electron microscopy (TEM)
For negative staining of EVs, freshly isolated EV suspensions were applied to copper mesh Formvar coated carbon stabilized grids, fixed in 4% paraformaldehyde for 1 hour, and stained with 1% aqueous uranyl acetate. For labelling with anti-CD81 (BioRad, 1D6) [28], EV samples were fixed in 4% paraformaldehyde diluted in 0.1M cacodylate buffer (pH 7.4). Grids were floated sequentially onto drops of 1M ammonium chloride, blocking buffer (0.4% BSA in PBS), blocking buffer (negative control) or primary antibody (CD81; 1:100), 1.4 nm anti-rabbit nanogold (Nanoprobes, Inc; 1:1000), HQ Silver (gold enhancement reagent, Nanoprobes, Inc.) and 2% aqueous uranyl acetate. After air drying, TEM examination was performed using a JEM 1230 transmission electron microscope (JEOL USA Inc., Peabody, MA) at 110 kV and imaged with an UltraScan 4000 CCD camera & First Light Digital Camera Controller (Gatan Inc., Pleasanton, CA) [27].
Transfer Assays
Transfer assays were performed to study cell-cell communication using either EV-containing media, or purified EVs. EV-containing media were prepared by sequential centrifugation at 2000g followed by 20,000g [29]; alternatively, EVs were isolated by Exoquick-TC as described above and resuspended in fresh media equal in amount to the starting material. Material (2 mL or 0.5 mL of EV-containing media or resuspended EVs for 6- and 12-well plates, respectively) collected from donor monolayers were transferred to recipient monolayers of the same age and TER as donor cells. TER measurements were performed prior to the transfer (0 hr) and after incubation of 4 hrs.
Endocytosis/Exosome Uptake Assays
EVs were labeled with ExoGlow according to the manufacturer’s instructions (Systems Biosciences, EXOC300A-1) and tracked with live-cell imaging of recipient cells. Annexin A2 is localized on the surface of EVs and is required for EV uptake by recipient cells [30]. Annexin A2 was knocked down in ARPE-19 cells using lentiviral vector-mediated siRNA transfer (Santa Cruz, sc-270151), and monolayers used as EV donors. Annexin 2 knockdown cells released quantities of EVs indistinguishable from those of control cells (data not shown). Endocytosis of EVs was further investigated in the presence of 100 μM dynasore (Abcam, ab120192), a cell-permeable dynamin inhibitor.
Live Cell Imaging
ARPE-19 cells (recipient cells) cultured on glass bottom culture dishes as described [4] were labeled with plasma membrane marker wheat germ agglutinin (WGA) Alexa Fluor 647 (Life Technologies, Eugene, OR, USA) and exposed to ExoGlow-labeled EVs. For time-lapse movies, Z-stack images were acquired using the UltraViewVoX spinning disk confocal microscope equipped for total internal reflection fluorescence (TIRF) microscopy (Eclipse Ti, Nikon, Tokyo, Japan), running Volocity software (Perkin Elmer, Wokingham, UK) on a Windows 64- bit system as described previously [4]. 3D images were reconstructed offline converted into tiff files and processed using ImageJ software (NIH).
HDAC6 activity assays
HDAC activity was measured with a homogenous fluorescence release HDAC deacetylase assay. EVs (supernatant from 1 compartment per transwell plate) or cell lysates (3 μg protein) were incubated with (AMC)-K(Ac)GL-Ac substrate (synthesized as published by us, >95% purity, [31]) to assess class I HDACs (HDACs 1, 2, 3, 6 and 10) enzyme activity in the presence of MS-275 (inhibitor of HDAC 1, 2 and 3 activities [32], Selleckchem). Deacetylated AMC-KGL is sensitive toward lysine peptidase (1 mg/ml trypsin), generating free fluorogenic 4-methylcoumarin-7-amide (excitation 355 nm, emission 460 nm; Fluoroskan Ascent Microplate Fluorometer [Labsystems]). Assay conditions were set with excess substrate concentrations to ensure linear deacetylation kinetics. Data were standardized using the control, and absolute deacetylated substrates were calculated based on the standard curve generated by nonacetylated AMC-KGL substrate under the same conditions [33].
HDAC6 activity analysis in recipient monolayers was confirmed using inhibitors Trichostatin A (TSA; Sigma) and Tubastatin A (Tub A; Sigma) at concentrations indicated in the text.
Immunostaining
Cells cultured on 6-well transwell culture dishes were fixed with pre-chilled 4% paraformaldehyde, blocked with blocking solution (10% Normal Goat Serum, 3% BSA and 0.4% Triton X-100 in 1X PBS), incubated overnight with anti-acetyl α tubulin antibody (Santa Cruz Biotechnology, 6–11B-1; 1:200), followed by secondary staining with Alexa-488 conjugated goat anti-rabbit antibody (Invitrogen; 1:500). Staining was examined by fluorescence microscopy (Zeiss, Thornwood, NY) equipped with a digital black-and-white camera (Spot camera; Diagnostic Instruments, Sterling Heights, MI) [34].
Western Blotting
Western Blotting was performed using previously published protocols [35], using primary antibodies (1:1,000) against annexin A2 (Abcam, ab41803) or acetyl α-tubulin (Santa Cruz Biotechnology, 6–11B-1) and β-actin (Cell Signaling Technologies, #8457S) as control. Proteins were visualized with horseradish peroxidase–conjugated secondary antibodies (Santa Cruz Biotechnology), followed by incubation with Clarity™ Western ECL Blotting Substrate (Bio-Rad Laboratories, Inc.) and chemiluminescent detection. Protein bands were scanned, and densities were quantified using ImageJ software normalized to β-actin.
ELISA
The syntenin ELISA was carried out according to the manufacturer instructions using the human syntenin-1 ELISA (My BioSource, MBS763611). ApoB ELISA assay was performed as previously published,[36] using the Human ApoB ELISA development kit (MabTech, 3715–1H-20). EVs were isolated by ExoQuick-TC and pellet dissolved in 50 μL ice cold PBS by vortexing. Protein was measured by NanoDrop Microvolume Spectrophotometry (ThermoFisher Scientific) and equal amounts (5 μg in 100 μL dilution buffer) were subjected to the assay procedure as directed by the manufacturer. Purified human syntenin-1 (20–0.1625 ng/mL) and ApoB (10–0.48 ng/mL) were used as a standard. Measurements were obtained using the microplate reader set at 450 nm.
Statistics
Data are presented as mean ± SD. Each TER experiment reflects the average of 3 independent experiments with 3 biological replicates each, unless otherwise indicated. Experiments using all other methods were performed at least 3 times. Single comparisons were analyzed using unpaired t-tests, with mean value differences considered significant at P ≤ 0.05. Differences in EV diameter were analyzed by Z-test (P < 0.05). For time course analysis (EV uptake), data was analyzed by one-factor ANOVA.
Results
Characterization of extracellular vesicles isolated from ARPE-19 cells
ARPE-19 cells were grown as monolayers for 5–6 weeks to form polarized cells and stimulated with 0.5 mM H2O2 to trigger vesicle release. EVs were isolated from both the apical and basal culture media of control and H2O2-treated monolayers for characterization. TEM, a standard tool to characterize morphology, confirmed that the isolated particles contained vesicles within the expected size range for exosomes and microvesicles (120–150 nm) (Fig. 1A–D) as well as smaller ones, with some of them showing the central depression characteristic for exosomes (Fig. 1C). NTA analysis (Fig. 1G,H) confirmed that the average diameter of EVs was 127.5 ±10.0 nm; with no significant difference in size between apically and basally released EVs, or those secreted under control or stress conditions (Fig. 1G). The average zeta-potential, a measure of nanoparticle stability, was identified as −25.70 ±3.07 mV; again with no difference between apically and basally released exosomes, or those secreted under control or stress conditions. In contrast, the number of EVs differed significantly between apically and basally released vesicles, with more ending up in the supernatant collected from the apical side when compared to the basal side, and under stress conditions, significantly increased numbers were identified in the apical side of H2O2-treated cells. (Fig. 1H). Immunogold labelling EM was performed, confirming the presence of the tetraspanin protein CD81, a marker that is traditionally known to be enriched in exosomes [37, 38] (Fig. 1E,F). Finally, as the ExoQuick-TC method used here for vesicle isolation might also isolate lipoprotein particles [39] and RPE cells release a range of modified lipoprotein particles, [40] ELISA measurements were performed to determine the ratio between syntenin-1 (exosome marker) [41] and ApoB (modified lipoprotein particle and secreted by ARPE-19 cells) [42]. All samples contained syntenin-1, whereas no ApoB was detected (data not shown). These data suggest that the isolated extracellular vesicles are a mixture of microvesicles and exosomes based on size range, morphology, zeta-potential and exosome-specific protein enrichment, and will be referred to as extracellular vesicles henceforth (EVs).
Figure 1. Characterization of Extracellular Vesicles (EVs).
(A) Representative image of particles as by Transmission electron microscopy (TEM). (B-D) TEM of particles isolated from supernatants of control (B, C) and H2O2-treated (D) ARPE-19 cell monolayers. Shapes included both smooth exosomes and those with characteristic central depressions. (E,F) Immunogold labelling reveals surface staining for the tetraspanin CD81. (G) Particle diameter (modal size, reflecting the most abundant peak in the measurement) as assessed by Zetaview is characteristic for exosomes and small microvesicles. (H) Concentration (EVs per ml of conditioned media) of EVs in apical and basal supernatants revealed polarized secretion, which can be augmented by stress. Bar graphs represent mean ±SD (n=5). *p<0.05; determined by Z-test.
Extracellular vesicles mediate bystander effect on barrier function in naïve cells
The RPE forms the outer blood-retinal barrier, a function dependent on the presence of apically-located tight junctions [43]. To determine whether rapid effects of EV cargo on recipient cells might alter barrier function, EVs were added to recipient ARPE-19 cells of the same age (i.e., cultured in parallel) followed by TER measurements at 0 and 4 hrs. Initial transfer assays were performed using either EV-containing media after sequential centrifugation (Fig. 2A) or purified EVs isolated by Exoquick-TC and resuspended in fresh media (Fig. 2D). Both treatments contain EVs corresponding to 2 mL of supernatant. The transfer of EV-containing media from the apical side of donor cells treated with H2O2 (H2O2 apical) to the apical side of recipient cells reduced TER by 25–35% (30 ± 5 %; p < 0.005). Similarly, the transfer of EVs from the basal side H2O2-stressed donor cells to the apical side of recipient cells reduced TER. In contrast, the transfer of EVs from the apical (Fig. 2A) or basal side (data not shown) of control donor cells had no significant effect on TER in recipient cells. Purified EVs from the apical side of H2O2-stressed donor cells resuspended in fresh media had the same effect (Fig. 2D). To confirm that this effect is universal, and not an artifact of an RPE-cell line in culture, it was confirmed that EVs released from pig primary RPE cells (pRPE) grown in culture have the same effect on recipient pRPE monolayers. Apical EVs from H2O2-treated donor pRPE reduced TER significantly in recipient pRPE, whereas control EVs have no effect (Fig. 2C). Finally, elimination of EVs from the apical supernatant either by high-speed centrifugation (100,000 g) or by pre-clearing the supernatant with ExoQuick-TC, completely prevented the effect of supernatants on TER, confirming that the effects on TER were mediated by EVs, and not by other soluble compounds in the media (Fig. 2A). From here on, we will use EV-containing apical media after sequential centrifugation for experiments unless otherwise noted, and refer to the treatment as EV treatment. Apical (donor) to apical (recipient) treatment rather than basal to apical was selected since that reflects the physiologically relevant parameter; basal to basal cannot be performed since EV delivery to the underside of the transwell is not feasible.
Figure 2. Transfer assays to study cell-cell communication.
Transepithelial resistance (TER) assays are used to study bystander effects elicited by vesicle transfer. TER measurements between 0 (baseline) and 4 hour time point (post treatment) are compared. (A) Extracellular vesicle (EV)-containing media was collected from the apical or basal compartment of ARPE-19 cell monolayers (donor cells) of control or stressed (H2O2) and transferred directly to the apical side of naïve monolayers (recipient cells). EVs from both the apical and the basal surface of stressed cells elicited a bystander effect. To ensure physiologically relevant experiments, transfer of apical EVs to apical side of recipients is performed from hereon. (B) Bystander effect elicited by EVs develops rapidly. TER measurements in recipient cells after the transfer of H2O2-induced EVs demonstrated a time dependent decrease of TER, with the first measurement at 15 minutes revealing a significant decrease, and reaching a maximum in around 2.5 hrs. (C) This effect appears universal for RPE cells; EVs from the apical surface of stressed primary porcine RPE cells elicited a bystander effect in naive porcine RPE monolayers. (D) Transfer assay of purified EVs isolated from control or stressed donor ARPE-19 cells supernatants, resuspended in the original amount of media and transferred to the recipient cells elicited a comparable effect on TER. Bar graphs represent mean ±SD. **p<0.005; ***p<0.0005; determined by Student’s t test.
Extracellular vesicle recognition and internalization
The initial transfer assays were performed with a readout of TER after 4 hours. To start examining the potential signaling mechanism of EVs, the time course of EV signaling on TER was performed. TER was reduced as quickly as 15 mins after the transfer of the EV-containing media to around 85% of baseline values, and was further reduced by 2.5 hrs to around 75% (Fig. 2B).
Annexin A2, a Ca2+-dependent phospholipid-binding protein has recently been shown to be present on the outer leaflet of EV membranes [30], and annexin A2 regulates phagocytosis in RPE cells [44]. EVs were generated from ARPE-19 cells depleted of annexin A2 using small interfering RNA, a treatment that did not interfere with EV secretion (data not shown). Apical EVs from control siRNA cells treated with H2O2 produced the expected reduction in TER, whereas those generated from annexin A2 knockdown treated with H2O2 cells had no effect (Fig. 3B).
Figure 3. Bystander effect elicited by extracellular vesicles (EVs) requires EV uptake by recipient cells.
Transepithelial resistance (TER) assays are used to study bystander effects elicited by EV transfer under conditions interfering with EV recognition and uptake. (A) Western blotting confirmed the almost complete loss of annexin A2 in annexin-A2 siRNA transfected ARPE-19 cells. (B) Transfer of apical EV-containing media from control siRNA and annexin-A2 siRNA transfected ARPE-19 donor cells from control or H2O2-stressed to the recipient ARPE-19 cells. Loss of annexin A2 prevented the bystander effect. (C) Dynasore (100 μM), a dynamin inhibitor, significantly reduced the bystander effect elicited by the transfer of apical EV-containing medium from stressed donor ARPE-19 cells. Bar graphs represent mean ±SD. **p<0.005; determined by Student’s t test.
Similarly, dynasore, a cell-permeable GTPase inhibitor of dynamin that blocks endocytosis, prevented the EV-induced effect on TER in recipient cells. Specifically, recipient cells pretreated with 100 μM dynasore prior to the application of H2O2-induced apical EVs showed little change in TER when compared to those pretreated with vehicle only (Fig. 3C).
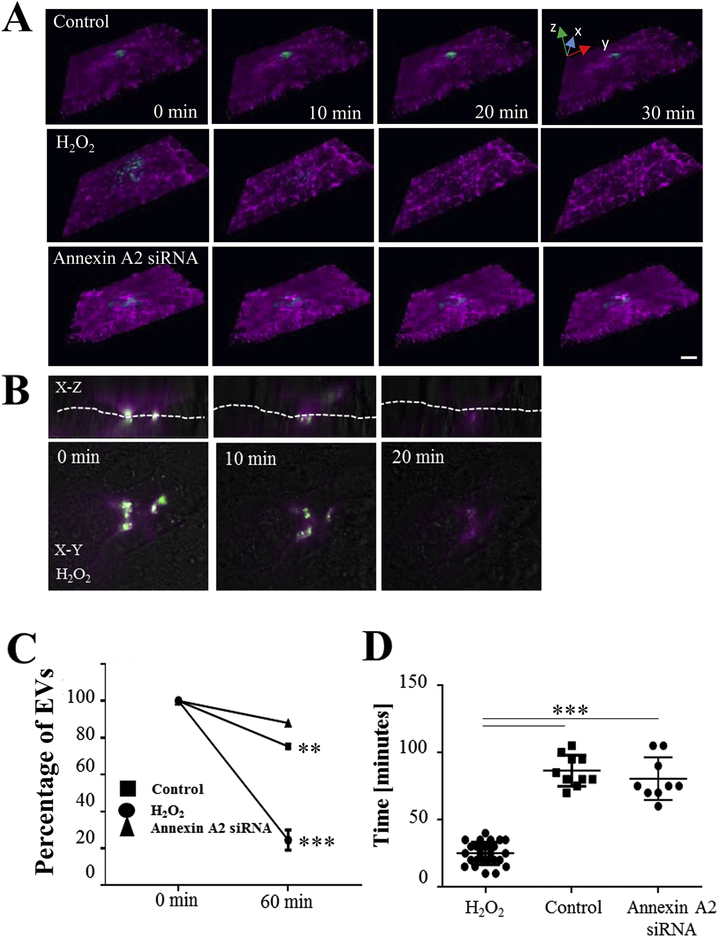
Internalization of EVs by RPE monolayers was confirmed by live cell imaging, using EVs isolated by ExoQuick-TC and labelled with the protein dye ExoGlow (green). To aid in the 3D reconstruction of the recipient ARPE-19 cells, membranes were labelled with WGA (magenta; Fig. 4A). Z-stack analyses demonstrated that H2O2-induced apical EVs were internalized readily by recipient cells in as quickly as ~10–20 mins, such that by 60 minutes, only ~20% of the EVs were still present on the plasma membrane. In comparison, control EVs and annexin A2 knockdown EVs were internalized slowly, taking ~100 mins, with ~80% of the exosomes remaining on the plasma membrane (Fig. 4A, C, D). EV internalization was confirmed by TIRF imaging (Fig. 4B). These data thus far suggests that the effects of EVs on recipient cells are fast, they potentially require recognition by the recipient cells involving EV annexin A2 signaling or some other stress-mediated ligand, and they require for the EVs to be endocytosed.
Figure 4. Live cell imaging investigating internalization of extracellular vesicles (EVs).
(A) 3D images (reconstructed from multiple Z-stacks) of live ARPE-19 cell monolayers exposed to EVs delivered via picospritzer. EVs were labelled with ExoGlow (green), the recipient cell membranes were labelled with wheat germ agglutinin (WGA; magenta). Internalization of EVs isolated from control, H2O2-stressed wildtype or H2O2-stressed annexin-A2 siRNA transfected donor cells was investigated. (B) 3D-reconstructed cross sections and surface focal image of H2O2 EVs captured by TIRF image confirmed the uptake of EVs. Dashed line indicates cell surface border. (C) Percentage of EVs remaining on the cell surface and that are not being internalized after 1 hr; and (D) internalization times confirm the differences between control EVs, and EVs isolated from H2O2-treated wildtype and annexin A2 knockdown cells. Graphs represent mean ±SD (n=3, examining 55.2 ±6.9 cells per experiment). **p<0.005, ***p<0.0005; data in C was analyzed by 1-factor ANOVA, date in D determined by Student’s t test.
Extracellular vesicle-mediated intracellular signaling requires HDAC6 activity
To identify the potential signaling molecule present in H2O2-induced apical EVs, preliminary mass spectrometry-based proteomic analyses were performed on material isolated by ExoQuick-TC, comparing control and H2O2-induced apical and basal EVs, and identifying proteins based on gene ontology involved in tight junction stability. Levels for HDAC6, an enzyme previously shown to be involved in endothelial [45, 46] and epithelial (RPE) barrier function [47], were found to be increased in H2O2 apical EVs as compared to controls.
To ascertain that the effect on TER in recipient cells is mediated by HDAC6 activity contained within the EVs, two approaches of HDAC6 inhibition were included. Recipient cells were pretreated with HDAC6 inhibitors TSA (pan class I and II HDAC inhibitor; 100 nM) and Tubastatin A (HDAC6-specific inhibitor; 1 μM) for 1 hour prior to the addition of media containing EVs. Pre-treatment with HDAC6 inhibitors prevented the reduction of TER upon treatment with H2O2 apical EVs in ARPE-19 cell monolayers (Fig. 5A) as well as pRPE (Fig. 5B). In addition, pre-incubation of the EVs with the respective HDAC6 inhibitors in a total of 10 μL prior to their transfer (diluted in 2 mL) prevented the reduction in TER when compared to EVs pretreated with vehicle (Fig. 5C). HDAC inhibitor alone at that concentration had no effect (Fig. 5C).
Figure 5. Bystander effect elicited by extracellular vesicles (EVs) is dependent on HDAC6 signaling.

Transepithelial resistance (TER) assays are used to study bystander effects elicited by EV transfer under conditions interfering with HDAC6 signaling. Loss of TER in recipient cells in response to the transfer of EV medium from RPE cells stressed with H2O2 is prevented by pre-treatment of recipient cells with HDAC6 inhibitors, TSA and Tubastatin A in both ARPE-19 cells (A) and primary porcine RPE cells (B). (C) Loss of TER in recipient cells in response to the transfer of EVs from ARPE-19 cells stressed with H2O2 is prevented by pre-treatment of EVs with HDAC6 inhibitors, TSA and Tubastatin A. Bar graphs represent mean ±SD. **p<0.005; determined by Student’s t test.
In HDAC6 activity assays, levels were elevated in EVs isolated from H2O2-treated cells when compared to control cells, specifically and uniquely in those released apically. This observation was confirmed for EVs derived from both ARPE-19 cells and pRPE cells (Fig. 6A, B). HDAC6 activity within recipient cells was only elevated above baseline upon transfer of EVs released apically from H2O2-treated when compared to control cells (Fig. 6C).
Figure 6. Extracellular vesicles (EVs) contain active HDAC6.

HDAC activity was measured with a homogenous fluorescence release HDAC deacetylase assay in the presence of MS-275. HDAC6 activity was significantly elevated in EVs isolated from the apical compartment of H2O2-stressed ARPE-19 cells (active EVs) (A) or primary porcine RPE cells (B), when compared to EVs isolated from the basal compartment or EVs released under control conditions (inactive EVs). (C) HDAC6 activity was significantly elevated in recipient cells treated with active EVs, when compared to inactive EVs or control conditions. Bar graphs represent mean ±SD (n=3 for EVs from ARPE-19 cells and EV-treated recipient cells, n=2 for EVs from primary pig RPE cells). **p<0.005; ***p<0.0005; determined by Student’s t test.
Finally, HDAC6 activity was further confirmed by analyzing deacetylation of one of its substrates, α-tubulin [48]. Immunohistochemistry of treated recipient cells demonstrated basal level of acetylated tubulin in control cells, reduced levels after transfer of H2O2 apical EVs, and elevated levels above baseline in the presence of EVs plus HDAC6 inhibitors (Fig. 7A). Similar results were obtained by Western blotting, showing quantitative differences (Fig. 7B,C). These data clearly demonstrate that the effects of EVs on barrier function in recipient cells are mediated by HDAC6 activity transferred within the EVs, resulting in deacetylation of α-tubulin required for tight junction stability [47]. They also provide indirect evidence that the EVs did not enter the degradation pathway, but rather release their content in the recipient cells.
Figure 7. HDAC6 activity in recipient cells treated with extracellular vesicles (EVs).
HDAC6 activity was analyzed by immunofluorescence and western blotting of acetyl-α-tubulin after EV transfer. (A) ARPE-19 cell recipient monolayers were stained for acetyl-α-tubulin after transfer of EVs. EVs from H2O2-treated donor cells reduced the amount of acetylated α-tubulin in recipient cells, an effect that was reversed by pre-treatment with HDAC6 inhibitors, TSA and Tubastatin A. (B) Western blotting for acetyl-α-tubulin confirmed the effects seen by imaging, and are expressed as (C) relative acetyl-α-tubulin levels from three independent experiments. Bar graph represents mean ±SD. **p<0.05; determined by Student’s t test.
Extracellular vesicle-mediated TER reduction is not a universal effect of stressed RPE.
We have reported previously that RPE cells respond differently to specific cellular stressors, and that while other AMD-associated compounds might elicit oxidative stress in ARPE-19 cell monolayers, their downstream effects on barrier function differ [49]. Here, other stressors known to result in oxidative stress were investigated. Rotenone is a widely used inhibitor of mitochondrial complex I, and rotenone has been shown to lead to ROS formation [50], RPE dysfunction [51] and exosome release [52]. Treatment of RPE donor cells with 25 μM rotenone resulted in the release of exosomes that were able to reduce TER in recipient cells similar to H2O2 (Fig. 8A). On the other hand, exosomes released in response to 5% smoke-extract exposure or treatment with 100 μM ferric ammonium citrate (FAC) [53], both inducers of oxidative stress in ARPE-19 cells as shown by us previously [49], did not reduce TER significantly as compared to control EVs (Fig. 8B). The effects of EVs produced in response to rotenone could be inhibited by HDAC6 inhibition (Fig. 8A); whereas no significant change in HDAC6 activity could be detected in EVs released from smoke or FAC-stressed cells (Fig. 8C). Taken together, EV-mediated TER reduction is not a universal effect of stressed RPE, and the triggers resulting in HDAC6 packaging in EVs generated upon H2O2 or rotenone stress remains to be determined.
Figure 8. HDAC6 activity in extracellular vesicles (EVs) is not a universal effect of stressed RPE cells.
Transepithelial resistance (TER) assays are used to study bystander effects elicited by EVs collected from cells under different stress conditions. (A) Apical EVs collected from donor cells treated with 25 μM rotenone were found to significantly reduce TER in naïve recipient cells, an effect that could be prevented by HDAC6 inhibition. (B) Apical EVs collected from donor cells treated exposed to 5% smoke extract or 100 μM ferrous ammonium citrate (FAC) had no significant effect on TER in naïve recipient cells. (C) EVs isolated from cells exposed to smoke extract or FAC did not contain elevated HDAC6 activity when compared to controls. Bar graphs represent mean ±SD. ***p<0.0005; N.S - Non Significant; determined by Student’s t test.
Discussion
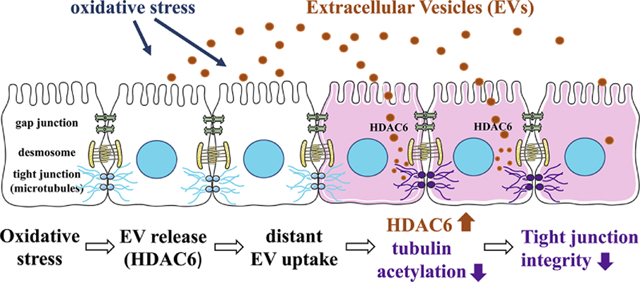
Microvesicles and exosomes are extracellular vesicles between 30–1000 nm size that are secreted by almost every cell type in the body [23, 54]. Exosomes were initially considered waste disposal material and have been investigated in RPE dysfunction in that context [50, 55]. However, more recently, there is accumulating evidence that EVs play a key role in intercellular communication both within, and between organs. And while the discovery of microvesicles dates back to 1967 [14] and exosomes were discovered in the 1980s [16, 56], interest only started growing once it was described that in addition to peptides and proteins, exosomes and microvesicles can carry mRNA and miRNA [19–21, 57]. Here, we aimed at elucidating a potential role for EVs derived from oxidatively stressed RPE in long-distance communication within the RPE monolayer. The main results of this study were as follows: (a) RPE cells release EVs, some of which have characteristics of exosomes, in a polarized fashion; (b) increased levels of EVs were found to be released from the apical side of the RPE monolayer in response to oxidative stress induced by H2O2; (c) in transfer assays, apical EVs released from H2O2-treated donor cells were found to rapidly induce changes in barrier function in naïve recipient cells; (d) this bystander effect was rapid, and required endocytosis of the vesicles; (e) uptake appeared to be ligand-dependent, as removal of annexin A2 from H2O2-generated EVs or control EVs from unstressed cells resulted in poor uptake of exosomes; (f) mass spectrometric analysis combined with pathway analysis of the protein content of active EVs (apically released, H2O2-generated) suggested a role for HDAC6 in mediating the bystander effect; (g) HDAC6 activity assays and inhibitor studies confirmed elevated levels of HDAC6 activity in active EVs and upon EV transfer in recipient RPE cells; (h) α-tubulin was identified as a potential target for HDAC6 activity; (i) and finally, HDAC6 packaging into EVs released from RPE cells in response is not universal; but rather is dependent on the type of stressor used. Taken together, our data suggest that EVs from oxidatively-stressed donor cells can be taken up rapidly by recipient cells to communicate stress messages. Here we have focused on rapid, protein-mediated effects on naïve recipient cells, but in future studies we expect to examine long-term consequences of the bystander effect mediated by RNA species.
Here we focused on triggers of EV release, internalization and cellular response rather than EV biogenesis per se. Vesicle release was monitored using multiple methodologies. TEM and NTA analyses (Fig. 1) demonstrated that vesicles ranging from ~30 to ~200 nm were present in the supernatants isolated by differential centrifugation (20,000 g) or ExoQuick-TC, with an average diameter of 127.5 ±10.0 nm. While some of the vesicles exhibit exosome characteristics (characteristic dimple, immunopositive for CD81) and the preparation contains syntenin-1 positive vesicles (specific for exosomes), the vesicle preparation is most likely not pure. The presence of significant amounts of lipoprotein particles was excluded based on the lack of ApoB in the samples, however small microvesicles are presumed to be present based on size, binding of microvesicles to polyethylene glycol (ExoQuick-TC) [58] and the presence of cargo typically associated with microvesicles (integrins, MMPs identified in preliminary mass spec analysis) [14].
EV targeting and adhesion to recipient cells might be mediated by surface proteins present on the vesicles, interacting with corresponding receptors on recipient cells. Information exchange between EVs and recipient cells be mediated via four general mechanisms: (1) ligands present as vesicle surface proteins might be cleaved off to directly interact with corresponding receptors to trigger intracellular signaling pathways (soluble signaling); (2) EVs via their surface proteins might bind directly to cell surface receptors leading to juxtracrine signaling; (3) EVs could fuse with the recipient cell membrane to deliver functional proteins, lipids or genetic particles to recipient cells; and (4) EVs could be taken up via phagocytosis or receptor-/raft-mediated endocytosis to transfer cargo [16, 59, 60]. Once EVs either bind to or are taken up by the recipient cell, the cargo can then affect the function and/or the cellular phenotype of the recipient cell. Responses in the recipient cell however will depend on the cell type, the stimulus in the donor cell and the metabolic state of the recipient cell. Our data on RPE monolayers, analyzing EV transfer via the apical membrane in recipient cells using live-cell imaging (Fig. 5) suggest that EVs are taken up via phagocytosis and/or receptor-/raft-mediated endocytosis. EV fusion with the recipient cell membrane would have been visible by live-cell imaging, generating EV membrane patches (green) within the recipient extracellular membrane (purple), an effect that was not observed. Soluble and juxtracrine signaling were excluded for the observed effect of EVs on TER as the blocker of endocytosis (dynosore) prevented activity (Fig. 3C). Likewise, our data suggests that annexin A2 present on EVs might be involved in exosome uptake (Fig. 3B). Annexin A2 has been shown to mediate cell-cell interaction [61], mediate phagocytosis in RPE cells [44] and is present on the surface of extracellular vesicles [30], making it an attractive candidate to mediate binding of EVs to recipient cells. Vesicle uptake is likely to involve a ligand/receptor mediated mechanism, since uptake is ~5–10 times faster in EVs from stressed cells when compared to those derived from control cells. However, it is unclear to date what epitopes are exposed on the outside of EVs from stressed RPE cells. Proteolytic surface protein shaving assays in which EVs are treated with proteinase K to remove proteins accessible on the vesicle surface, coupled with mass spectral analysis might help with their identification.
Exosomes and microvesicles have been known to contain predominantly proteins and lipids, and only recently, since the finding of their nucleic acid contents (mRNAs, miRNAs and mitochondrial DNA), have they garnered new attention as a vehicle to transport this information to other cells [16, 62, 63]. Since then, research into exosomes and microvesicles has become of interest as regulators in cell–cell communications during diverse biological processes [64–69]. Protein transfer, and in particular, functional changes based on protein transfer are difficult to show since only small amounts of proteins can be packaged within EVs. Hence the focus should be on key enzymes, toxic protein, transporters or transcription factors that can make a difference at smaller quantities. In the heart, cardiomyocytes-derived exosomes can transfer functional glucose transporters and glycolytic enzymes to modulate glucose transport and metabolism in recipient endothelial cells [70]. Microglia can spread protein via exosome secretion and depletion of microglia or inhibition of exosome synthesis was shown to significantly reduce tau propagation in vitro and in vivo [71, 72]. In RPE-endothelial cell interactions, it was demonstrated that ethanol-stressed RPE cells release more exosomes than control cells, and those exosomes contain higher levels of VEGF receptors in their membranes, as well as increased levels of VEGFR-1 and −2 mRNA within the exosome and thus promote vasculogenesis/angiogenesis when they interact with endothelial cells [73]. It has been hypothesized that this horizontal transfer of material via exosomes or microvesicles might be particularly important for cells that are not connected via gap junctions [74]. In comparison to many of the published studies discussed here that have examined effects in heterologous cultures, here, the effect of EVs were examined on cells from the same origin (homologous cultures). In addition, EVs were used for activity assays at concentrations released into the media (i.e, EVs were not concentrated prior to transfer). Specifically, EV-derived HDAC6 was identified as a mediator of the stress signal released from the apical side only, leading to TER loss. While EVs from normal control RPE cells contained little active HDAC6 and when transferred to healthy RPE monolayer had no measurable effect on TER (Fig. 2A) or HDAC6 activity (Fig. 6C), apical EVs from stressed (H2O2 or rotenone) RPE cells contained high levels of HDAC6 activity, and when transferred to heathy recipient cells impaired the integrity of tight junctions leading to reduced TER (Figs. 2A, 8A). Importantly, inhibition of HDAC6 activity in either the recipient cells or in EVs prevented the bystander effect induced by EVs (Fig. 5); an effect likely to be mediated by α-tubulin interaction with tight junctions (Fig. 7) [75]. This bystander effect could be elicited in both ARPE-19 as well as pRPE monolayer, making this a general effect in RPE cell-cell communication (Figs. 2B,C). HDAC6 is known to be involved in endothelial cell barrier function [45] and HDAC6 inhibitor, TSA has been reported to improve tight junction stability in various endothelial [76, 77] and epithelial tissues [47, 78]. In support of these findings, histone deacetylase inhibitors have been reported to up-regulate the expression of tight junction proteins in fibroblasts [79]. A-tubulin is a endogenous substrate of HDAC6 [46,80], acetylation of the α-tubulin at Lys40 stabilizes microtubule structure [46, 81] and overexpression of HDAC6 specifically reduces the acetylation levels of α-tubulin [48]. Finally, we have shown in a previous publication that RPE cells respond differently to specific cellular stressors, irrespective of their ability to induce oxidative stress [49]. Here we demonstrated that while both H2O2 and rotenone treatment resulted in the release of apical EVs that contained high levels of HDAC6, resulting in TER reduction upon transfer (Figs. 2A, 8A), continued smoke exposure and FAC-induced EVs did neither contain HDAC6 nor reduce TER significantly when compared to control EVs (Fig. 8B,C). Basal EVs released from H2O2-stressed were effective in reducing TER when added to the apical side of recipient cells (Fig. 2A) but were found not to contain HDAC6 activity. We aim to identify the active molecule in future studies.
How might EV communication contribute to pathology seen in age-related macular degeneration? As indicated above, the RPE is a monolayer located between the light sensitive tissue of the retina and the blood supply of the choroid. Its many functions [reviewed by [43]] include transport of molecules between the subretinal space and the choroidal blood supply; spatial ion buffering; and secretion of molecules that control the stability of photoreceptors, Bruch’s membrane and the choroid. In early AMD, the RPE is thought to contribute to the formation of drusen (extracellular material that builds up between the RPE and Bruch’s membrane). Wang et al., had proposed that exosomes released from aged RPE might contribute to the formation of drusen [50], and polarized release of exosomes from RPE cells has been described in cultured RPE cells [39, 82, 83]. In addition to damaging effects, exosomes released from the RPE are also thought to contribute to neuroptrotection in the retina [84]. In geographic atrophy, the most prevalent form of late AMD, the RPE atrophies start with several small patches, which grow and subsequently coalesce. It is unclear what leads to patchiness, however, it suggests that damage occurs in susceptible areas, and is delayed in more resilient areas. Our data indicates that EVs might further contribute to disease by spreading damage to susceptible areas in the RPE. In addition, EVs released from the RPE might influence other tissues in the eye. In support of this hypothesis that there is cross-talk between the tissues of the eye, Hajrasouliha and coworkers have reported that exosomes released from retinal astroglial cells suppress retinal vessel leakage and inhibit choroidal neovascularization (angiogenesis of choroidal blood vessels) but not RPE exosomes [85].
In conclusion, as demonstrated by the results presented here, we have, for the first time, revealed that EVs released from stressed RPE cells can have an effect on naïve cells of the same origin. HDAC6 was identified as playing a critical role in transferring the information from donor to recipient cells. This enzyme is known to regulate barrier function in epithelial and endothelial cells. These findings increase our basic understanding of cell-cell communication in RPE cells but also contribute to that of the development of pathology in diseases of the RPE such as age-related macular degeneration.
SUMMARY.
Retinal pigment epithelium damage in age-related macular degeneration is triggered in many different locations, suggesting that damage occurs in susceptible areas.
We have identified transfer of a signal to recipient cells by extracellular vesicles (EVs) as a potential mechanism that could mediate the bystander effect.
Active EVs transfer HDAC6 to recipient cells, mediating reduction in barrier function.
Acknowledgements
Funding for this project was provided in part by the National Institutes of Health (NIH) (R01EY019320) (BR), the Department of Veterans Affairs (RX000444, BX003050) (BR), the South Carolina SmartState Endowment (BR), the BrightFocus Foundation (YL), The Glaucoma Foundation (YL), the Glaucoma Research Foundation (YL), and the Startup Fund from the Medical College of Georgia (YL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors have no financial or non-financial competing interests to disclose.
References
- [1].Hall EJ, The bystander effect, Health Phys, 85 (2003) 31–35. [DOI] [PubMed] [Google Scholar]
- [2].Davidson JO, Green CR, Bennet L, Nicholson LF, Danesh-Meyer H, O’Carroll SJ, Gunn AJ, A key role for connexin hemichannels in spreading ischemic brain injury, Curr Drug Targets, 14 (2013) 36–46. [DOI] [PubMed] [Google Scholar]
- [3].Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR, Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions, J Physiol, 568 (2005) 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ishii M, Rohrer B, Bystander effects elicited by single-cell photo-oxidative blue-light stimulation in retinal pigment epithelium cell networks, Cell Death Discov, 3 (2017) 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bebawy M, Roseblade A, Luk F, Rawling T, Ung A, Grau GE, Cell-derived microparticles: new targets in the therapeutic management of disease, J Pharm Pharm Sci, 16 (2013) 238–253. [DOI] [PubMed] [Google Scholar]
- [6].Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O, Biological properties of extracellular vesicles and their physiological functions, J Extracell Vesicles, 4 (2015) 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ibrahim A, Marban E, Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology, Annu Rev Physiol, 78 (2016) 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hoang Quan V., Qian Haohua, H. Ripps, Functional analysis of hemichannels and gapjunctional channels formed by connexins 43 and 46, Molecular Vision, 16 (2010) 1343–1352. [PMC free article] [PubMed] [Google Scholar]
- [9].Wang S, Koster KM, He Y, Zhou Q, miRNAs as potential therapeutic targets for age-related macular degeneration, Future Med Chem, 4 (2012) 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ding X, Patel M, Chan CC, Molecular pathology of age-related macular degeneration, Prog Retin Eye Res, 28 (2009) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ambati J, Atkinson JP, Gelfand BD, Immunology of age-related macular degeneration, Nat Rev Immunol, 13 (2013) 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abd AJ, Kanwar RK, Kanwar JR, Aged macular degeneration: current therapeutics for management and promising new drug candidates, Drug Discov Today, (2017). [DOI] [PubMed] [Google Scholar]
- [13].Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, Henry EC, Brittain C, The Pathophysiology of Geographic Atrophy Secondary to Age-Related Macular Degeneration and the Complement Pathway as a Therapeutic Target, Retina, 37 (2017) 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meckes DG Jr., Raab-Traub N, Microvesicles and viral infection, J Virol, 85 (2011) 12844–12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thery C, Zitvogel L, Amigorena S, Exosomes: composition, biogenesis and function, Nat Rev Immunol, 2 (2002) 569–579. [DOI] [PubMed] [Google Scholar]
- [16].Raposo G, Stoorvogel W, Extracellular vesicles: exosomes, microvesicles, and friends, J Cell Biol, 200 (2013) 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Meckes DG Jr., Exosomal communication goes viral, J Virol, 89 (2015) 5200–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yuana Y, Sturk A, Nieuwland R, Extracellular vesicles in physiological and pathological conditions, Blood Rev, 27 (2013) 31–39. [DOI] [PubMed] [Google Scholar]
- [19].Ha D, Yang N, Nadithe V, Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges, Acta Pharm Sin B, 6 (2016) 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moldovan L, Batte K, Wang Y, Wisler J, Piper M, Analyzing the circulating microRNAs in exosomes/extracellular vesicles from serum or plasma by qRT-PCR, Methods Mol Biol, 1024 (2013) 129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G, Extracellular vesicles as an emerging mechanism of cell-to-cell communication, Endocrine, 44 (2013) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang J, Sun X, Zhao J, Yang Y, Cai X, Xu J, Cao P, Exosomes: A Novel Strategy for Treatment and Prevention of Diseases, Front Pharmacol, 8 (2017) 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Akers JC, Gonda D, Kim R, Carter BS, Chen CC, Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies, J Neurooncol, 113 (2013) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thurman JM, Renner B, Kunchithapautham K, Ferreira VP, Pangburn MK, Ablonczy Z, Tomlinson S, Holers VM, Rohrer B, Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury, J Biol Chem, 284 (2009) 16939–16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Toops KA, Tan LX, Lakkaraju A, A detailed three-step protocol for live imaging of intracellular traffic in polarized primary porcine RPE monolayers, Exp Eye Res, 124 (2014) 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaczara P, Sarna T, Burke JM, Dynamics of H2O2 availability to ARPE-19 cultures in models of oxidative stress, Free Radic Biol Med, 48 (2010) 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y, A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents, PLoS One, 12 (2017) e0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rohlena J, Volger OL, van Buul JD, Hekking LH, van Gils JM, Bonta PI, Fontijn RD, Post JA, Hordijk PL, Horrevoets AJ, Endothelial CD81 is a marker of early human atherosclerotic plaques and facilitates monocyte adhesion, Cardiovasc Res, 81 (2009) 187–196. [DOI] [PubMed] [Google Scholar]
- [29].Taylor DD, Zacharias W, Gercel-Taylor C, Exosome isolation for proteomic analyses and RNA profiling, Methods Mol Biol, 728 (2011) 235–246. [DOI] [PubMed] [Google Scholar]
- [30].Stewart A, Gessler F, Pluchino S, Moreau K, Inside-out: unpredicted Annexin A2 localisation on the surface of extracellular vesicles, Matters (Zürich), (2016). [Google Scholar]
- [31].Inks ES, Josey BJ, Jesinkey SR, Chou CJ, A novel class of small molecule inhibitors of HDAC6, ACS Chem Biol, 7 (2012) 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chou DH, Holson EB, Wagner FF, Tang AJ, Maglathlin RL, Lewis TA, Schreiber SL, Wagner BK, Inhibition of histone deacetylase 3 protects beta cells from cytokine-induced apoptosis, Chem Biol, 19 (2012) 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Soragni E, Chou CJ, Rusche JR, Gottesfeld JM, Mechanism of Action of 2-Aminobenzamide HDAC Inhibitors in Reversing Gene Silencing in Friedreich’s Ataxia, Front Neurol, 6 (2015) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Obert E, Strauss R, Brandon C, Grek C, Ghatnekar G, Gourdie R, Rohrer B, Targeting the tight junction protein, zonula occludens-1, with the connexin43 mimetic peptide, alphaCT1, reduces VEGF-dependent RPE pathophysiology, Journal of molecular medicine, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Busch C, Annamalai B, Abdusalamova K, Reichhart N, Huber C, Lin Y, Jo EAH, Zipfel PF, Skerka C, Wildner G, Diedrichs-Mohring M, Rohrer B, Strauss O, Anaphylatoxins Activate Ca2+, Akt/PI3-Kinase, and FOXO1/FoxP3 in the Retinal Pigment Epithelium, Front Immunol, 8 (2017) 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cayo MA, Mallanna SK, Di Furio F, Jing R, Tolliver LB, Bures M, Urick A, Noto FK, Pashos EE, Greseth MD, Czarnecki M, Traktman P, Yang W, Morrisey EE, Grompe M, Rader DJ, Duncan SA, A Drug Screen using Human iPSC-Derived Hepatocyte-like Cells Reveals Cardiac Glycosides as a Potential Treatment for Hypercholesterolemia, Cell Stem Cell, 20 (2017) 478–489 e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Berditchevski F, Odintsova E, Tetraspanins as regulators of protein trafficking, Traffic, 8 (2007) 89–96. [DOI] [PubMed] [Google Scholar]
- [38].Andreu Z, Yanez-Mo M, Tetraspanins in extracellular vesicle formation and function, Front Immunol, 5 (2014) 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Klingeborn M, Dismuke WM, Rickman CB, Stamer WD, Roles of exosomes in the normal and diseased eye, Prog Retin Eye Res, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang L, Li CM, Rudolf M, Belyaeva OV, Chung BH, Messinger JD, Kedishvili NY, Curcio CA, Lipoprotein particles of intraocular origin in human Bruch membrane: an unusual lipid profile, Invest Ophthalmol Vis Sci, 50 (2009) 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Imjeti NS, Menck K, Egea-Jimenez AL, Lecointre C, Lembo F, Bouguenina H, Badache A, Ghossoub R, David G, Roche S, Zimmermann P, Syntenin mediates SRC function in exosomal cell-to-cell communication, Proc Natl Acad Sci U S A, 114 (2017) 12495–12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rajapakse D, Peterson K, Mishra S, Wistow G, Serum starvation of ARPE-19 changes the cellular distribution of cholesterol and Fibulin3 in patterns reminiscent of age-related macular degeneration, Exp Cell Res, 361 (2017) 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Strauss O, The retinal pigment epithelium in visual function, Physiol Rev, 85 (2005) 845–881. [DOI] [PubMed] [Google Scholar]
- [44].Law AL, Ling Q, Hajjar KA, Futter CE, Greenwood J, Adamson P, Wavre-Shapton ST, Moss SE, Hayes MJ, Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina, Mol Biol Cell, 20 (2009) 3896–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chistiakov DA, Orekhov AN, Bobryshev YV, Endothelial Barrier and Its Abnormalities in Cardiovascular Disease, Front Physiol, 6 (2015) 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yu J, Ma Z, Shetty S, Ma M, Fu J, Selective HDAC6 inhibition prevents TNF-alpha-induced lung endothelial cell barrier disruption and endotoxin-induced pulmonary edema, Am J Physiol Lung Cell Mol Physiol, 311 (2016) L39–47. [DOI] [PubMed] [Google Scholar]
- [47].Desjardins D, Liu Y, Crosson CE, Ablonczy Z, Histone Deacetylase Inhibition Restores Retinal Pigment Epithelium Function in Hyperglycemia, PLoS One, 11 (2016) e0162596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP, HDAC6 is a microtubule-associated deacetylase, Nature, 417 (2002) 455–458. [DOI] [PubMed] [Google Scholar]
- [49].Kunchithapautham K, Bandyopadhyay M, Dahrouj M, Thurman JM, Rohrer B, Sublytic membrane-attack-complex activation and VEGF secretion in retinal pigment epithelial cells, Adv Exp Med Biol, 723 (2011) 23–30. [DOI] [PubMed] [Google Scholar]
- [50].Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH, Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration, PLoS ONE, 4 (2009) e4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W Jr., Ding J, Bowes Rickman C, Boulton M, Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD, Autophagy, 10 (2014) 1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Biasutto L, Chiechi A, Couch R, Liotta LA, Espina V, Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress, Exp Cell Res, 319 (2013) 2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Voloboueva LA, Killilea DW, Atamna H, Ames BN, N-tert-butyl hydroxylamine, a mitochondrial antioxidant, protects human retinal pigment epithelial cells from iron overload: relevance to macular degeneration, Faseb J, 21 (2007) 4077–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tong Y, Zhou YL, Wang YX, Zhao PQ, Wang ZY, Retinal pigment epithelium cell-derived exosomes: Possible relevance to CNV in wet-age related macular degeneration, Med Hypotheses, 97 (2016) 98–101. [DOI] [PubMed] [Google Scholar]
- [55].Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH, Autophagy, exosomes and drusen formation in age-related macular degeneration, Autophagy, 5 (2009) 563–564. [DOI] [PubMed] [Google Scholar]
- [56].Harding CV, Heuser JE, Stahl PD, Exosomes: looking back three decades and into the future, J Cell Biol, 200 (2013) 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Soria FN, Pampliega O, Bourdenx M, Meissner WG, Bezard E, Dehay B, Exosomes, an Unmasked Culprit in Neurodegenerative Diseases, Front Neurosci, 11 (2017) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rider MA, Hurwitz SN, Meckes DG Jr., ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles, Sci Rep, 6 (2016) 23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA, Exosomes: Mechanisms of Uptake, J Circ Biomark, 4 (2015) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C, Exosome/microvesicle-mediated epigenetic reprogramming of cells, Am J Cancer Res, 1 (2010) 98–110. [PMC free article] [PubMed] [Google Scholar]
- [61].Hedhli N, Falcone DJ, Huang B, Cesarman-Maus G, Kraemer R, Zhai H, Tsirka SE, Santambrogio L, Hajjar KA, The annexin A2/S100A10 system in health and disease: emerging paradigms, J Biomed Biotechnol, 2012 (2012) 406273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells, Nat Cell Biol, 9 (2007) 654–659. [DOI] [PubMed] [Google Scholar]
- [63].Guescini M, Genedani S, Stocchi V, Agnati LF, Astrocytes and Glioblastoma cells release exosomes carrying mtDNA, J Neural Transm (Vienna), 117 (2010) 1–4. [DOI] [PubMed] [Google Scholar]
- [64].Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S, Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes, Nat Immunol, 3 (2002) 1156–1162. [DOI] [PubMed] [Google Scholar]
- [65].Simons M, Raposo G, Exosomes--vesicular carriers for intercellular communication, Curr Opin Cell Biol, 21 (2009) 575–581. [DOI] [PubMed] [Google Scholar]
- [66].Gupta SK, Bang C, Thum T, Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease, Circ Cardiovasc Genet, 3 (2010) 484–488. [DOI] [PubMed] [Google Scholar]
- [67].Cocucci E, Racchetti G, Meldolesi J, Shedding microvesicles: artefacts no more, Trends Cell Biol, 19 (2009) 43–51. [DOI] [PubMed] [Google Scholar]
- [68].Simpson RJ, Jensen SS, Lim JW, Proteomic profiling of exosomes: current perspectives, Proteomics, 8 (2008) 4083–4099. [DOI] [PubMed] [Google Scholar]
- [69].Bang C, Thum T, Exosomes: new players in cell-cell communication, Int J Biochem Cell Biol, 44 (2012) 2060–2064. [DOI] [PubMed] [Google Scholar]
- [70].Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A, Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes, Cardiovasc Res, 109 (2016) 397–408. [DOI] [PubMed] [Google Scholar]
- [71].Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M, Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C, J Cell Biol, 189 (2010) 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Xiao T, Zhang W, Jiao B, Pan CZ, Liu X, Shen L, The role of exosomes in the pathogenesis of Alzheimer’ disease, Transl Neurodegener, 6 (2017) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Atienzar-Aroca S, Flores-Bellver M, Serrano-Heras G, Martinez-Gil N, Barcia JM, Aparicio S, Perez-Cremades D, Garcia-Verdugo JM, Diaz-Llopis M, Romero FJ, Sancho-Pelluz J, Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells, J Cell Mol Med, 20 (2016) 1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ratajczak MZ, Ratajczak J, Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later, Clin Transl Med, 5 (2016) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yano T, Matsui T, Tamura A, Uji M, Tsukita S, The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK, J Cell Biol, 203 (2013) 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Edelman JL, Lutz D, Castro MR, Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown, Exp Eye Res, 80 (2005) 249–258. [DOI] [PubMed] [Google Scholar]
- [77].Stewart MW, Anti-VEGF therapy for diabetic macular edema, Curr Diab Rep, 14 (2014) 510. [DOI] [PubMed] [Google Scholar]
- [78].Ablonczy Z, Dahrouj M, Tang PH, Liu Y, Sambamurti K, Marmorstein AD, Crosson CE, Human Retinal Pigment Epithelium Cells as Functional Models for the RPE In Vivo, Invest Ophthalmol Vis Sci, 52 (2011) 8614–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bordin M, D’Atri F, Guillemot L, Citi S, Histone deacetylase inhibitors up-regulate the expression of tight junction proteins, Mol Cancer Res, 2 (2004) 692–701. [PubMed] [Google Scholar]
- [80].Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P, HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo, EMBO J, 22 (2003) 1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Asthana J, Kapoor S, Mohan R, Panda D, Inhibition of HDAC6 deacetylase activity increases its binding with microtubules and suppresses microtubule dynamic instability in MCF-7 cells, J Biol Chem, 288 (2013) 22516–22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gangalum RK, Bhat AM, Kohan SA, Bhat SP, Inhibition of the Expression of the Small Heat Shock Protein alphaB-Crystallin Inhibits Exosome Secretion in Human Retinal Pigment Epithelial Cells in Culture, J Biol Chem, 291 (2016) 12930–12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Klingeborn M, Dismuke WM, Skiba NP, Kelly U, Stamer WD, Bowes Rickman C, Directional Exosome Proteomes Reflect Polarity-Specific Functions in Retinal Pigmented Epithelium Monolayers, Sci Rep, 7 (2017) 4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR, alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells, PLoS ONE, 5 (2010) e12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hajrasouliha AR, Jiang G, Lu Q, Lu H, Kaplan HJ, Zhang HG, Shao H, Exosomes from Retinal Astrocytes Contain Antiangiogenic Components That Inhibit Laser-induced Choroidal Neovascularization, J Biol Chem, 288 (2013) 28058–28067. [DOI] [PMC free article] [PubMed] [Google Scholar]