Abstract
Background:
Social comparisons (SCs) are common among cancer patients, but their prospective associations are not well understood. This study examined concurrent and prospective relationships of SCs with health-related quality of life (HRQOL) and depressive symptoms during the first year of breast cancer treatment.
Methods:
Non-metastatic breast cancer patients (N=240) enrolled in a larger intervention trial reported on the frequencies of SCs post-surgery (T1) and 6 months later (T2). Health-related quality of life (HRQOL) and depressive symptoms were assessed at T1, T2, and 12 months after T1 (T3). Path analysis via structural equation modeling was used to assess three models relating SCs to HRQOL and depressive symptoms from T1-T2, T1-T3, and T2-T3, controlling for stage of disease, intervention condition, and dispositional optimism.
Results:
Upward contrast SCs were associated with poorer concurrent HRQOL at T1 and T2, and with more concurrent depressive symptoms at T2. However, upward contrast SC at T1 predicted better T2 and T3 HRQOL. Upward identification SC at T1 predicted more T2 depressive symptoms, and at T2 was associated with poorer concurrent HRQOL and more concurrent depressive symptoms. Downward identification SCs at T1 were associated with poorer concurrent HRQOL. Downward identification SCs at T2 predicted poorer T3 HRQOL.
Conclusions:
Upward SCs were related to compromised concurrent psychosocial well-being, but prospective effects varied by the interpretation of the comparison (i.e., contrast vs. identification). Findings have implications for the development and deployment of group-based psychosocial interventions during the early phases of survivorship, during which opportunities for SC are prevalent.
Keywords: breast cancer, health-related quality of life, depressive symptoms, social comparison
Breast cancer is the most common cancer among women in the U.S. and will account for approximately 30% of new female cancers in 2018.1 After diagnosis and throughout treatment, women may experience many challenges to their physical and emotional well-being, including physical side effects of treatments and challenges to one’s self-view.2 Some experiences inherently have objective markers by which a woman can evaluate her status (e.g., objective physical tests). However, in more ambiguous situations, women with breast cancer may use other women as sources of information to judge her own status, a behavior termed social comparison (SC).3 SC can reduce uncertainty or anxiety associated with the threatening experience of having a cancer diagnosis if one’s own status is similar to others.4,5 Studies show that women with breast cancer regularly encounter potential sources of SC (e.g., in doctors’ office waiting rooms, media coverage),6,7 and cancer patients are interested in SCs as a source of information.8
SCs are characterized by their direction (upward vs. downward) and interpretation (identification vs. contrast) which converge to describe four SCs.9,10 Upward identification focuses on how one is similar to better-off others and may provide information about how to improve one’s situation.11,12 Upward contrast focuses on how one is different from better-off others, which may be threatening.13,14 Downward identification focuses on how one is similar to worse-off others and makes salient the possibility of one’s own condition deteriorating.13,15 Downward contrast, focuses on how one is different from worse-off others and suggests that the differences are large or stable.7,13,15 SC is not unique to cancer and has been widely studied across other contexts (e.g., cardiovascular disease,5 depression,13 occupational burnout16). A recent meta-analysis incorporating 60+ years of research concluded that upward and contrasting SCs were most common.17
Psychosocial well-being is an aspect of the cancer experience for which there are few objective indicators of one’s status. Nonetheless, health-related quality of life (HRQOL) and depressive symptoms are two well-studied aspects of the cancer experience. Prior work in cancer shows that perceiving oneself as better off than patients with worse cancer (downward contrast) can buffer against diminished HRQOL in the context of worsening physical well-being.18 Further, SCs related to coping and emotional reactions to cancer can affect HRQOL up to 3 months post-exposure.19–21 Less work in cancer has focused on relationships between SCs and depressive symptoms. In one study, elevated depressive symptoms were associated with a greater perceived need for SC information.8 A separate study of college students found that downward SCs were helpful for individuals experiencing elevated depressive symptoms;13 however, whether this extends to persons with cancer is not currently known.
There are multiple challenges to interpreting past findings of SCs in cancer patients. First, the majority of research has been cross-sectional, and long-term associations of SCs with psychosocial well-being (e.g., HRQOL, depressive symptoms) are not well understood beyond 3 months. Second, much of the existing research focuses on cancer survivors who have completed treatment10,20,21 or has included patients at various timepoints throughout the cancer trajectory.6,7,22–24 More information is needed regarding SCs early in cancer treatment, when contextual threat may be high. Third, research suggests that factors including type of cancer and treatment regimen may influence reactions to SCs.5 Thus, studies that include diverse cancer diagnoses and stages (i.e., early-stage vs. advanced) may not generalize to specific disease groups. Finally, personality differences can predispose individuals to more positive or negative outcomes as a result of SCs.5 For example, greater dispositional optimism is linked to less negative affect after SC.13,25 Thus, personality characteristics such as optimism should be considered when assessing SCs.
This study sought to address these limitations by examining SCs among women newly diagnosed with non-metastatic breast cancer across the first year of cancer treatment while controlling for stage of disease, dispositional optimism, and intervention condition (see below) within the framework of social comparison theory. We aimed to assess the cross-sectional associations of SCs with HRQOL and depressive symptoms at two timepoints (post-diagnosis/surgery but before beginning adjuvant therapies, and 6 months later). We hypothesized that upward identification and downward contrast SCs would be associated with better HRQOL and less depressive symptoms at both timepoints, whereas upward contrast and downward identification SCs would be associated with poorer HRQOL and more depressive symptoms. We also aimed to assess the prospective associations of SCs with HRQOL and depressive symptoms from baseline to 6 months, from baseline to 12 months, and from 6 months to 12 months. We hypothesized the same directions of associations of SCs with HRQOL and depressive symptoms as in the first aim.
Method
Participants and Procedures
This study was part of a larger trial testing the effects a Cognitive Behavioral Stress Management (CBSM) intervention for promoting positive adjustment a breast cancer (ClinicalTrial.gov ID NCT01422551).26,27 The institutional review board approved all study procedures. Women with stage 0-III breast cancer were recruited through advertising and physician referrals from a large academic medical cancer center and private practices. All women were 2–10 weeks post-breast cancer surgery and before beginning additional adjuvant treatment (e.g., chemotherapy). Exclusion criteria included a prior cancer diagnosis, prior treatment for a serious psychiatric disorder, and lack of English fluency.
We approached 502 women for this study, of whom 106 did not meet inclusion criteria and 156 declined to participate. In total, 240 women provided written informed consent, enrolled, and completed a baseline assessment (T1). Participants self-reported demographic and socioeconomic information at enrollment. The baseline assessment included self-reported measures of SCs, optimism, and HRQOL, and a structured interview to assess depressive symptoms. Participants were reassessed 6 (T2) and 12 months (T3) later.
Participants were randomized to CBSM or 1-day psychoeducational control group. Women in CBSM participated in a 10-week group-based intervention including elements of cognitive-behavioral therapy and relaxation training (see reports by Antoni and colleagues).26,27 The control condition received a condensed educational version of the intervention material as part of a 1-day psychoeducational seminar but did not benefit from group support or practice of intervention techniques. CBSM was not designed to affect SCs, and SCs were not discussed in the intervention or control conditions.
Measures
Social comparisons.
We used a self-constructed scale to assess SC frequencies, each with one item: How often do you think about… “yourself as being worse off than women who don’t have breast cancer?” (upward contrast), “getting to be as well off as women who’ve completely recovered from their breast cancer?” (upward identification), “women whose breast cancer is worse than yours and think about the possibility of being in their situation?” (downward identification), “women whose breast cancer is worse than yours and think about how much better off you are than they are?” (downward contrast). Responses ranged from 0=not at all to 4=almost constantly.
HRQOL.
The 27-item Functional Assessment of Cancer Therapy-General assesses four domains of well-being in the context of cancer over the past week: physical (“I have nausea” [reverse]), social/family (“I feel close to my friends”), emotional (“I feel sad” [reverse]), and functional (“I am sleeping well”).28 Respondents indicated their agreement with statements on a Likert scale from 0=not at all to 5=very much. Items were summed so higher scores represent better HRQOL. Chronbach’s alphas were good (range 0.89–0.91).
Depressive symptoms.
The 17-item interview-based Hamilton Rating Scale for Depression assessed for the presence and severity of depressive symptoms over the past week.29 A clinical psychologist with extensive training in using the HRSD trained study assessors using the structured interview guide.30 Possible scores range from 0–23 with higher scores reflecting greater symptomatology. Chronbach’s alphas were acceptable (range 0.78–0.80).
Dispositional optimism.
On the six-item (plus three filler items) Life Orientation Test-Revised,31 participants indicated their agreement with statements such as “in uncertain times, I usually expect the best” on a Likert scale from 1=I agree a lot to 4=I disagree. After appropriate reverse scoring, items were summed so higher scores represent greater optimism. Cronbach’s alpha was acceptable (0.77).
Statistical Analysis
Power calculations were based on our prior study in breast cancer. We projected that with 210 participants retained at T3 and a 0.05 alpha level, we would have a power of 0.95 to detect medium intervention effects on the primary outcomes. We screened all variables for outliers (>3 standard deviations from the mean) and inspected distributions for skewness, kurtosis, and multivariate assumptions of normality.32 We used means, standard deviations, frequencies, and general linear modeling to characterize the sample and assess changes in study variables over time.
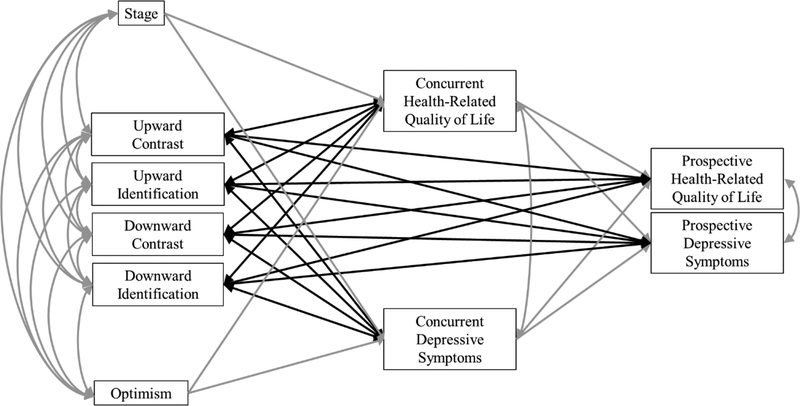
We conducted path analysis using structural equation modeling in Mplus Version 7.33 The main model (Figure 1) was specified representing concurrent and prospective associations of SCs with HRQOL and depressive symptoms across three timespans (T1-T2, T1-T3, and T2-T3) controlling for disease stage and optimism.5 Models 1 and 2 tested the associations of T1 SCs with T1 HRQOL and depressive symptoms, and predicted T2 (Model 1) and T3 (Model 2) HRQOL and depressive symptoms from T1 SCs. T1 HRQOL and depressive symptoms were control variables in the prediction of T2 and T3 outcomes. All exogenous variables were specified to covary (disease stage, optimism, T1 SCs), and HRQOL and depressive symptoms were specified to correlate at each time point. Model 3 tested the same pattern of associations but correlating T2 SCs with T2 HRQOL and depressive symptoms and predicting T3 HRQOL and depressive symptoms from T2 SCs. T2 HRQOL and depressive symptoms were control variables in the prediction of T3 outcomes. All exogenous variables were specified to covary (disease stage, optimism, T2 SCs), and HRQOL and depressive symptoms were specified to correlate at T2 and T3. Although CBSM was not designed to affect SCs, it is possible that cognitive-behavioral techniques (e.g., cognitive reframing) could affect the types of SCs women make over time. Thus, after establishing the main models, study condition (CBSM vs. control) was added as a covariate.
Figure 1.
Conceptual model relating social comparisons with health-related quality of life and depressive symptoms controlling for stage of disease and optimism.
Missing data were estimated using full information maximum likelihood, which yields population estimates using all observed data to ensure that each participant is represented in the analyses. We interpreted four indices for model fit: chi-square test (χ2) p>0.05, confirmatory fit index (CFI) >0.95, root mean squared error of approximation (RMSEA) <0.06, and standardized root mean square residual (SRMR) <0.08.32 Standardized coefficients were interpreted at a two-tailed significance level of 0.05 as measures of effect sizes at the following levels: 0.1=small; 0.3=medium; 0.5=large.34
Results
Sample Characteristics
See Table 1 for descriptive information. Women were on average 50.34 years old (SD=9.03) and the majority identified as non-Hispanic white (63.3%). Most participants had stage I (34.6%) or II (37.9%) disease. Roughly half of participants underwent adjuvant chemotherapy (52.9%) and radiation therapy (55.8%), and two thirds were prescribed hormone therapy (67.1%).
Table 1.
Characteristics of the sample
| Variable | T1 | T2 | T3 |
|---|---|---|---|
| Age (years); M (SD) | 50.34 (9.03) | ||
| Race/ethnicity; n (%) | |||
| White non-Hispanic | 152 (63.3) | ||
| Hispanic | 61 (25.4) | ||
| Black | 21 (8.8) | ||
| Asian | 5 (2.1) | ||
| Not reported | 1 (0.4) | ||
| Education; n (%) | |||
| Less than high school | 3 (1.2) | ||
| High school | 27 (11.2) | ||
| Some college | 73 (26.3) | ||
| College and above | 147 (61.3) | ||
| Annual household income (thousands of dollars); M (SD) | 79.62 (67.08) | ||
| Stage of disease; n (%) | |||
| 0 | 42 (17.5) | ||
| I | 83 (34.6) | ||
| II | 91 (37.9) | ||
| III | 23 (9.6) | ||
| Missing | 1 (0.4) | ||
| Surgical procedure; n (%) | |||
| Lumpectomy | 122 (50.8) | ||
| Mastectomy | 118 (49.2) | ||
| Received chemotherapy; n (%) | 127 (52.9) | ||
| Received radiation; n (%) | 134 (55.8) | ||
| Received hormone therapy; n (%) | 161 (67.1) | ||
| Received more than one treatment (e.g., chemotherapy and radiation); n (%) | 153 (63.7) | ||
| Social comparisons; M (SD) | |||
| Upward contrast | 0.91 (0.84) | 0.66 (0.65) | 0.61 (0.65) |
| Upward identification | 2.14 (1.18) | 1.63 (1.27) | 1.49 (1.29) |
| Downward contrast | 1.76 (1.13) | 1.68 (1.14) | 1.70 (1.10) |
| Downward identification | 1.13 (0.88) | 1.05 (0.85) | 1.01 (0.90) |
| Health-related quality of life; M (SD) | 79.47 (14.59) | 84.28 (14.77) | 86.98 (14.07) |
| Depressive symptoms; M (SD) | 7.52 (5.46) | 5.97 (4.91) | 5.92 (4.88) |
| Optimism; M (SD) | 20.02 (3.39) |
Note. M=mean; SD=standard deviation; n=frequency; T1=baseline; T2=6 months; T3=12 months; T1 n=240; T2 n=194; T3 n=192.
At T1, upward identification was the most frequently endorsed SC. Upward identification (F[2, 174]=20.09, p<0.001) and upward contrast both decreased from T1-T3 (F[2, 175]=9.46, p<0.001), but there were no changes in downward contrast or downward identification (ps>0.05). HRQOL improved over time (F[2, 173]=36.67, p<0.001) with the lowest mean HRQOL at T1. Depressive symptoms decreased over time (F[2, 164]=9.94, p<0.001) with the highest mean depressive symptoms at T1.
Model 1: T1-T2
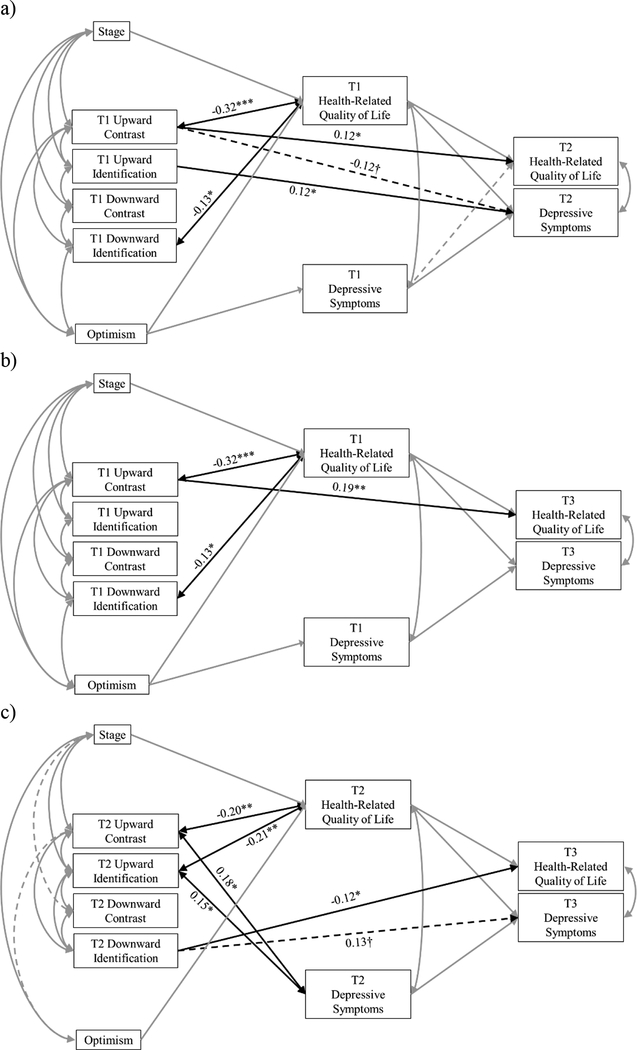
Model 1 (Figure 2a) fit the data well (χ2[4]=5.70, p=0.223; CFI=1.00; RMSEA=0.04; SRMR=0.01). See Table 2 for all standardized coefficients.
Figure 2.
a) Significant effects of baseline (T1) social comparisons (SCs) on concurrent and 6-month (T2) health-related quality of life (HRQOL) and depressive symptoms; b) Significant effects of T1 SCs on concurrent and 12-month (T3) HRQOL and depressive symptoms; c) Significant effects of 6-month (T2) SCs on concurrent and T3 HRQOL and depressive symptoms. Solid lines represent significant paths (p<0.05). Dotted lines represent marginally significant paths (0.05<p<0.10). Grey lines represent covariate paths. Significant standardized coefficients only shown for associations between SCs, HRQOL, and depressive symptoms. †p<0.10; *p<0.05; **p<0.10; ***p<0.001.
Table 2.
Standardized coefficients of effects of T1 and T2 social comparisons on T1, T2, and T3 health-related quality of life and depressive symptoms.
| T1 UC | T1 UI | T1 DC | T1 DI | T1 HRQOL | T1 Dep | T2 UC | T2 UI | T2 DC | T2 DI | T2 HRQOL | T2 Dep | T3 HRQOL | T3 Dep | Stage | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 UC | |||||||||||||||
| T1 UI | 0.18** | ||||||||||||||
| T1 DC | 0.09 | 0.19** | |||||||||||||
| T1 DI | 0.29*** | −0.05 | 0.43*** | ||||||||||||
| T1 HRQOL | −0.32*** | −0.08 | −0.06 | −0.13* | |||||||||||
| T1 Dep | 0.06 | 0.07 | 0.01 | 0.04 | −0.47*** | ||||||||||
| T2 UC | |||||||||||||||
| T2 UI | 0.21** | ||||||||||||||
| T2 DC | 0.10 | 0.34*** | |||||||||||||
| T2 DI | 0.19** | 0.19** | 0.48*** | ||||||||||||
| T2 HRQOL | 0.12* | −0.07 | 0.01 | 0.03 | 0.68*** | −0.11† | −0.20** | −0.21** | 0.06 | −0.12 | |||||
| T2 Dep | −0.12† | 0.12* | 0.08 | −0.09 | −0.40*** | 0.31*** | 0.18* | 0.15* | −0.03 | 0.01 | −0.41*** | ||||
| T3 HRQOL | 0.19** | −0.01 | −0.01 | −0.07 | 0.72*** | −0.08 | 0.02 | 0.00 | 0.04 | −0.12* | 0.66*** | −0.09 | |||
| T3 Dep | 0.12 | 0.02 | −0.02 | −0.01 | −0.33*** | 0.30*** | −0.03 | −0.10 | −0.08 | 0.13† | −0.31*** | 0.32*** | −0.38*** | ||
| Stage | 0.14* | 0.19** | −0.23*** | −0.07 | −0.18** | 0.09 | 0.18* | 0.20** | −0.14† | −0.02 | −0.16* | 0.10 | |||
| Optimism | −0.22*** | −0.06 | 0.02 | 0.16* | 0.33*** | −0.27*** | −0.14† | −0.03 | 0.07 | −0.05 | 0.25*** | −0.07 | −0.17** |
Note.
p<0.10;
p<0.05;
p<0.10;
p<0.001;
UC=upward contrast; UI=upward identification; DC=downward contrast; DI=downward identification; HRQOL=health-related quality of life; Dep=depressive symptoms; T1=baseline; T2=6 months; T3=12 months.
Cross-sectional associations.
T1 upward contrast and downward identification were negatively associated with concurrent HRQOL. That is, in the time after breast cancer diagnosis/surgery and before beginning other adjuvant therapies (baseline), more frequently thinking that you are worse off than women who don’t have breast cancer (upward contrast) and that you are similar to women with worse breast cancer (downward identification) were associated with poorer HRQOL.
Prospective associations.
T1 upward contrast predicted better T2 HRQOL and less T2 depressive symptoms (marginal association), and T1 upward identification predicted more T2 depressive symptoms. In other words, at baseline, more frequently thinking that you are worse off than women who don’t have breast cancer (upward contrast) predicted better HRQOL and less depressive symptoms (marginally) 6 months later. More frequently thinking that you are similar to women who have recovered from breast cancer (upward identification) predicted more depressive symptoms 6 months later.
Intervention effects.
All significant associations held after controlling for study condition. Condition was not associated with T1 SCs, stage, or optimism, or with T2 HRQOL or depressive symptoms (ps>0.05).
Model 2: T1-T3
Model 2 (Figure 2b) fit the data well (χ2[4]=5.61, p=0.230; CFI=1.00; RMSEA=0.04; SRMR=0.01). See Table 2 for all standardized coefficients.
Cross-sectional associations.
Cross-sectional associations between T1 SCs, HRQOL, and depressive symptoms in this model were identical to those reported in Model 1.
Prospective associations.
Consistent with Model 1, T1 upward contrast predicted better T3 HRQOL.
Intervention effects.
All significant associations held after controlling for study condition. CBSM was associated with better T3 HRQOL (β=0.11, p=0.028) and fewer T3 depressive symptoms (β=−0.14, p=0.027).
Model 3: T2-T3
Model 3 (Figure 2c) fit the data acceptably (χ2[4]=9.91, p=0.042; CFI=0.98; RMSEA=0.08; SRMR=0.02). See Table 2 for all standardized coefficients.
Cross-sectional associations.
T2 upward contrast and upward identification were both negatively associated with HRQOL and positively associated with depressive symptoms. That is, approximately 6 months after initiating adjuvant therapies, more frequently thinking that you are worse off than women who don’t have breast cancer (upward contrast) and similar to women who have recovered from breast cancer (upward identification) was associated with poorer HRQOL and more depressive symptoms.
Prospective associations.
T2 downward identification predicted poorer T3 HRQOL and more T3 depressive symptoms (marginal association). In other words, approximately 6 months after initiating adjuvant therapies, more frequently thinking that you are similar to women with worse breast cancer (downward identification) predicted poorer HRQOL and more depressive symptoms (marginally) 6 months later (12 months post-diagnosis/surgery).
Intervention effects.
All significant associations held after controlling for study condition. CBSM was marginally associated with upward contrast at T2 (β=0.12, p=0.080). All other associations with study condition were non-significant (ps>0.050).
Discussion
This study examined the associations of SCs with HRQOL and depressive symptoms among women with newly diagnosed non-metastatic breast cancer at three timepoints – post-diagnosis/surgery and before beginning adjuvant therapies (T1), and 6 (T2) and 12 months later (T3). Consistent with our hypotheses, upward contrast was associated with poorer concurrent HRQOL at T1 and T2 (medium and small effects, respectively) and with more concurrent depressive symptoms at T2 (small effect) but not at T1. Contrary to our hypotheses, T1 upward contrast subsequently predicted better HRQOL at T2 and T3 (small effects) and less depressive symptoms at T3 (marginal association). Further, T1 upward identification predicted more T2 depressive symptoms (small effect), and T2 upward identification was associated with poorer concurrent HRQOL and depressive symptoms (small effects). As expected, T1 downward identification was associated with poorer concurrent HRQOL (small effect), and T2 downward identification predicted poorer T3 HRQOL (small effect) and more T3 depressive symptoms (marginal association).
Results suggest that in the time after diagnosis/surgery, recognizing the differences between oneself and women without breast cancer (upward contrast) and identifying with other breast cancer patients (downward identification), essentially facing the reality of a breast cancer diagnosis, are detrimental to immediate HRQOL. Indeed, almost one third of newly diagnosed breast cancer patients experience clinically elevated distress.35 However, we found that facing this new reality was protective for HRQOL up to 12 months later. This is consistent with studies showing that coping through denial post-breast cancer diagnosis is detrimental over time, whereas post-diagnosis acceptance predicts more positive adjustment.36,37 It is possible that our observed pattern of SCs reflect how denial may be initially protective and acceptance threatening, yet have the opposite effects over time.
Our findings suggest that upward comparisons (both contrast and identification) approximately 6 months post-diagnosis/surgery were associated with poorer concurrent psychosocial well-being. This is consistent with work suggesting that upward comparisons are threatening, inasmuch as they place the self in an unfavorable position.13,14 There may be something unique about this particular timepoint, at which many women were completing primary treatment, which makes upward comparisons particularly threatening.
This study has important strengths. The sample was homogeneous, as all participants were women with newly diagnosed non-metastatic breast cancer. Focusing exclusively on breast cancer patients and recruiting them at a distinct time eliminated many between-subject differences that may influence the associations of interest.5 We included theoretically relevant covariates, ensuring that the observed associations were significant above the effects of potential confounders. Finally, we used a longitudinal design and robust statistical methodology, allowing for relationships to emerge over a longer period of time than has been previously reported.5,11
Study Limitations
Participants reported a high average household income and were well-educated. Data were self-reported and thus vulnerable to issues of self-representation (with the exception of objectively-assessed depressive symptoms). Women self-selected into the larger intervention trial, which could select for women who are more comfortable in group settings in which SCs are likely. Further, we used a self-constructed measure of four SCs, resembling past research.7 A more complete picture could be observed using open-ended assessments to capture nuanced comparisons23 or by using other validated SC measures.9
Clinical Implications
Findings could have implications for long-term health outcomes. For example, greater depressive symptoms in the first year of breast cancer treatment are linked to higher all-cause mortality.38–40 Assessing SCs early in the course of breast cancer treatment could be a unique way to identify those at risk of poorer long-term psychosocial adjustment and who will potentially benefit from supportive care. Further, knowledge of how breast cancer patients make SCs during treatment and how those comparisons can influence HRQOL and depression may be useful in the development and deployment of group-based psychosocial interventions. We did not find associations between the CBSM intervention and SCs in this sample; however, CBSM was not designed to affect SCs. Other work has found that individually-administered interventions designed to induce and manipulate SCs can affect HRQOL over 3 months.19–21 Notably, these studies have been conducted with cancer patients at various points in the cancer trajectory. Our findings suggest that the associations of SCs with psychosocial well-being persist across the first year of breast cancer treatment. Perhaps this timeframe, during which contextual threat is high, lends itself to SC intervention. Future research should investigate this possibility.
Conclusions
This study provides evidence that SCs during the first 6 months after breast cancer diagnosis can affect concurrent HRQOL and depressive symptoms as well as prospective outcomes up to 6 and 12 months later. Upward SCs were related to compromised concurrent psychosocial well-being, but prospective effects varied by the interpretation of the comparison (i.e., contrast vs. identification). Findings have implications for the development and deployment of group-based psychosocial interventions during the early phases of survivorship, during which opportunities for SC are prevalent.
Acknowledgements
This study was funded by NCI grant R01-CA-064710. LCB was funded by NCI training grant T32-CA-193193.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Society AC. Cance Facts & Figures 2018. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 2.Denieffe S, Gooney M. A meta-synthesis of women’s symptoms experience and breast cancer. Eur J Cancer Care (Engl). 2011;20(4):424–35. [DOI] [PubMed] [Google Scholar]
- 3.Festinger L A theory of social comparison processes. Hum Relat. 1954;7:23. [Google Scholar]
- 4.Bellizzi KM, Blank TO, Oakes CE. Social comparison processes in autobiographies of adult cancer survivors. J Health Psychol. 2006;11(5):777–86. [DOI] [PubMed] [Google Scholar]
- 5.Arigo D, Suls JM, Smyth JM Social comparisons and chronic illness: research synthesis and clinical implications. Health Psychol Rev. 2014;8:60. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SE, Aspinwall LG, Guiliano TA, Dakoff KK, Reardon KK Storytelling and coping with stressful events. J Appl Soc Psychol. 1993;23:30. [Google Scholar]
- 7.Wood JV, Taylor SE, Lichtman RR. Social comparison in adjustment to breast cancer. J Pers Soc Psychol. 1985;49(5):1169–83. [DOI] [PubMed] [Google Scholar]
- 8.Bennenbroek FT, Buunk BP, van der Zee KI, Grol B. Social comparison and patient information: what do cancer patients want? Patient Educ Couns. 2002;47(1):5–12. [DOI] [PubMed] [Google Scholar]
- 9.VanderZee K BB, Sanderman R, Botke G, VandenBergh F. Social comparison and coping with cancer treatment. Pers Indiv Differ. 2000;28:17. [Google Scholar]
- 10.Buunk BP, Collins RL, Taylor SE, VanYperen NW, Dakof GA. The affective consequences of social comparison: either direction has its ups and downs. J Pers Soc Psychol. 1990;59(6):1238–49. [DOI] [PubMed] [Google Scholar]
- 11.Wood JV. Theory and research concerning social comparison of personal attributes. Psychol Bull. 1989;106:17. [Google Scholar]
- 12.Taylor SE, Lobel M. Social comparison activity under threat: downward evaluation and upward contacts. Psychol Rev. 1989;96(4):569–75. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons FX. Social comparison and depression: company’s effect on misery. J Pers Soc Psychol. 1986;51(1):140–8. [DOI] [PubMed] [Google Scholar]
- 14.Morse S, Gergen KJ. Social comparison, self-consistency, and the concept of self. J Pers Soc Psychol. 1970;16(1):148–56. [DOI] [PubMed] [Google Scholar]
- 15.Wills TA. Downward comparison principles in social psychology. Psychol Bull. 1981;90:26. [Google Scholar]
- 16.Buunk BP, Schaufeli WB Burnout: A Prospective from Social Comparison Theory In: Schaufeli WB, Maslach C, Marek T, editor. Professional Burnout: Recent Developments in Theory and Research. London: Routledge; 2017. p. 18. [Google Scholar]
- 17.Gerber JP, Wheeler L, Suls J. A social comparison theory meta-analysis 60+ years on. Psychol Bull. 2018;144(2):177–97. [DOI] [PubMed] [Google Scholar]
- 18.Hagedoorn M, Sneeuw KC, Aaronson NK. Changes in physical functioning and quality of life in patients with cancer: response shift and relative evaluation of one’s condition. J Clin Epidemiol. 2002;55(2):176–83. [DOI] [PubMed] [Google Scholar]
- 19.Buunk AP, Bennenbroek FT, Stiegelis HE, van den Bergh AC, Sanderman R, Hagedoorn M Follow-up effects of social comparison information on the quality of life of cancer patients: the moderating role of social comparison orientation. Psychol Health. 2012;27(6):641–54. [DOI] [PubMed] [Google Scholar]
- 20.Brakel TM, Dijkstra A, Buunk AP, Siero FW. Impact of social comparison on cancer survivors’ quality of life: an experimental field study. Health Psychol. 2012;31(5):660–70. [DOI] [PubMed] [Google Scholar]
- 21.Brakel TM, Dijkstra A, Buunk AP. Targeting cancer patients’ quality of life through social comparison: a randomised trial. Psychol Health. 2014;29(8):950–66. [DOI] [PubMed] [Google Scholar]
- 22.Stanton AL, Danoff-Burg S, Cameron CL, Snider PR, Kirk SB. Social comparison and adjustment to breast cancer: an experimental examination of upward affiliation and downward evaluation. Health Psychol. 1999;18(2):151–8. [DOI] [PubMed] [Google Scholar]
- 23.Bogart LM, Helgeson VS Social comparisons among women with breast cancer: a longitudinal investigation. J Appl Soc Psychol. 2000;30:28. [Google Scholar]
- 24.Umstead KL, Kalia SS, Madeo AC, Erby LH, Blank TO, Visvanathan K, et al. Social comparisons and quality of life following a prostate cancer diagnosis. J Psychosoc Oncol. 2018;36(3):350–63. [DOI] [PubMed] [Google Scholar]
- 25.Hemphill KJ, Lehman DR Social comparisons and their affective consequences: the importance of comparison dimension and individual differences. J Soc Clin Psychol. 1991;10:22. [Google Scholar]
- 26.Antoni MH, Lechner SC, Kazi A, Wimberly SR, Sifre T, Urcuyo KR, et al. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006;74(6):1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am J Psychiatry. 2006;163(10):1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams J Structured interview guide for the Hamilton depression rating scale (SIGH-D) 1989. [DOI] [PubMed]
- 31.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67(6):1063–78. [DOI] [PubMed] [Google Scholar]
- 32.Kline RB. Principles and practice of structural equation modeling. 2 ed. New York, NY: Guildford Press; 2005. [Google Scholar]
- 33.Muthen L, Muthen B MPlus user’s guide. 6 ed. Los Angeles, CA: Muthen & Muthen; 2012. [Google Scholar]
- 34.Cohen J Statistical power analysis for the behavioral sciences. 2 ed. New York, NY: Academic Press; 1988. [Google Scholar]
- 35.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28. [DOI] [PubMed] [Google Scholar]
- 36.Carver CS, Pozo C, Harris SD, Noriega V, Scheier MF, Robinson DS, et al. How coping mediates the effect of optimism on distress: a study of women with early stage breast cancer. J Pers Soc Psychol. 1993;65(2):375–90. [DOI] [PubMed] [Google Scholar]
- 37.Stanton AL, Danoff-Burg S, Huggins ME The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment. Psycho-Oncol. 1993;11:9. [DOI] [PubMed] [Google Scholar]
- 38.Antoni MH, Jacobs JM, Bouchard LC, Lechner SC, Jutagir DR, Gudenkauf LM, et al. Post-surgical depressive symptoms and long-term survival in non-metastatic breast cancer patients at 11-year follow-up. Gen Hosp Psychiatry. 2017;44:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40(11):1797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–61. [DOI] [PubMed] [Google Scholar]