Abstract
OBJECTIVE
To estimate the efficacy of common treatments for vulvodynia: topical lidocaine monotherapy, oral desipramine monotherapy, and lidocaine-desipramine combined therapy.
METHODS
A 12-week randomized, double-blinded, placebo-controlled trial was conducted on 133 vulvodynia-afflicted women assigned to four treatment arms: placebo tablets-placebo cream, desipramine tablets–placebo cream, placebo tablets–lidocaine cream, and desipramine tablets–lidocaine cream. The tampon test was selected as primary end point using a modified intention-to-treat analysis. Twelve secondary end points were also examined. At completion of the 12-week randomized phase, women were examined “open label” through 52 weeks postrandomization.
RESULTS
All treatment arms reported substantial tampon-test pain reduction: 33% reduction placebo creamplacebo tablet, 20% reduction lidocaine cream–placebo tablet, 24% reduction placebo cream–desipramine tablet, and 36% reduction lidocaine cream-desipramine tablet. Compared with placebo, we found no significant difference in tampon-test pain reduction with desipramine (t=0.90;P=.37) or lidocaine (t=1.27;P=.21). Of the remaining 12 outcome measures, only the Index of Sexual Satisfaction, improved with desipramine compared with placebo (t=−2.81;P=.006). During the open-label phase, women undergoing vestibulectomy surgery reported significantly improved pain as measured by cotton swab test and the McGill Pain Scale compared with nonsurgical alternatives.
CONCLUSION
Oral desipramine and topical lidocaine, as monotherapy or in combination, failed to reduce vulvodynia pain more than placebo. Placebo or placeboindependent effects are behind the substantial pain improvement seen in all treatment allocations.
CLINICAL TRIAL REGISTRATION
LEVEL OF EVIDENCE
I
Women with localized provoked vulvodynia, formerly known as vulvar vestibular syndrome, are afflicted with chronic burning pain to light touch that is classically limited to the vulvar vestibule. Two classes of medications empirically selected for vulvodynia treatment include oral tricyclic antidepressants or topical anesthetics such as lidocaine.1 Despite widespread empiric use for vulvodynia, neither oral tricyclic antidepressants nor topical lidocaine has been studied in placebo-controlled randomized controlled trials (RCTs). Oral tricyclic antidepressants and lidocaine differ in the location of main pharmacologic action with oral tricyclic antidepressants modulating pain, centrally, at the level of the dorsal horn of the spinal cord and topical lidocaine blocking, peripherally, at mucocutaneous nerve endings.2,3 Recent research suggests that localized provoked vulvodynia may be associated with both central and peripheral neuropathology.4,5. Therefore increased therapeutic efficacy may result from combined therapy with central (oral tricyclic antidepressants) and peripheral (lidocaine) effects. Within the oral tricyclic antidepressant class of drugs, secondary amines like desipramine are recommended over tertiary amines like amitriptyline, because of equal neuropathic pain relief efficacy with other oral tricyclic antidepressants but better side-effect tolerance.6
The Vulvar Vestibulitis Clinical Trial was designed to study the efficacy of two commonly used medical treatments for localized provoked vulvodynia: topical lidocaine monotherapy, oral desipramine monotherapy, and combined topical lidocaine-oral desipramine. We hypothesized that treatment with “peripherally acting” lidocaine or “centrally acting” desipramine would be more efficacious than placebo, and that combined therapy, desipramine plus lidocaine, would be more effective than monotherapy.
MATERIALS AND METHODS
The Vulvar Vestibulitis Clinical Trial was a placebocontrolled, double-blinded RCT to study the efficacy of four medical treatments for localized provoked vulvodynia: 1) topical lidocaine, 2) oral desipramine, 3) combined lidocaine and desipramine, and 4) placebo cream and tablets. The study was conducted at Strong Memorial Hospital of the University of Rochester between August 2002, and July 2007, and the protocol was approved by the University of Rochester Research Subjects Review Board (RSRB #8677). The study consisted of three phases: 1) a 2-week preintervention “baseline” phase, 2) a 12-week randomized, blinded phase, and 3) postintervention “open-label” phase with scheduled visits at 16, 26, and 52 weeks postrandomization. Clinical response from baseline to week 12 (the end of the randomized, double-blinded phase of the trial) was assessed by multiple outcome measures including change in tampon insertion pain (tampon test), change in daily pain intensity, change in intercourse pain intensity, the frequency of intercourse, cotton swab test, Vulvar Algesiometer, and a battery of health-related quality-of-life measures described below.
Women were eligible to participate if they reported greater than 3 continuous months of insertional (entryway) dyspareunia, pain, or both with tampon insertion, and were between 18 and 50 years of age. After informed consent, all study candidates completed a standard 115-question history and physical examination. Participants needed to fulfill Friedrich’s criteria for the diagnosis of localized provoked vulvodynia, which included tenderness localized within the vestibule confirmed by cotton swab test using the modified diagnostic criteria of Bergeron et al.7 In four defined points (1:00, 5:00, 7:00, and 11:00) within the vulvar vestibule, the participants should report a mean score equal to or greater than 4 out of 10 on a numeric rating scale of pain intensity. The localized nature of pain was confirmed by finding all remaining cotton swab test points tested in the lower vagina, labia majora, and labia minora to be nonpainful, defined as a mean score equal to or less than 2 out of 10 in pain on the numeric rating scale. Eligibility required a second clinician-examiner to independently concur with the diagnosis of localized provoked vulvodynia by cotton swab test. Additionally, eligible individuals did not demonstrate any other specific neuropathology, atrophic vaginitis, dermatoses such as lichen sclerosus, or pathogens such as culture- or smear-proven Candida species or herpes simplex. Study candidates who opted not to participate or who did not meet inclusion or exclusion criteria were referred for appropriate clinical care.
Drug assignments were determined by the Department of Biostatistics using a permuted block randomization scheme by means of a computer-based random numbers generator. Identical-appearing pills and creams were packaged and distributed by the Investigational Drug Service, following the randomized sequence and identified by nonconsecutive numbers. During the blinded phase, two oral regimens were distributed: desipramine 25-mg tablets and an identical-appearing oral placebo tablet containing 25 mg lactose. Dosing began with one daily tablet for week 1, two daily tablets for week 2, three daily tablets for week 3, four daily tablets for week 4, five daily tablets for week 5, and six daily tablets for weeks 6 through 12. Participants were asked to take the oral medication at one time, preferably at bedtime. Participants were instructed to advance to a total dose of six tablets daily, regardless of point of response (pain relief). In the event of side effects, without significant medical implications, the participant was advised to decrease tablet dose by one and to remain at that dose for the remainder of the clinical trial. In the event of further side effects the reduction by one tablet was repeated on an every-7th-day basis until a tolerable dose was found. Those not able to tolerate the oral drug regimen at any dose were advised to stop the oral drug but continue the topical regimen; these participants were analyzed on an intention-to-treat basis. Two topical regimens were distributed: lidocaine 5% (buffered) in Moisturel (active agents petrolatum+dimethicone, compounded by Strong Memorial Hospital Pharmacy) and an identical-appearing and identically packaged placebo cream, pure Moisturel. Participants were instructed with aid of a mirror and given written instructions to apply the cream lightly over the painful region four times daily, every day. They were asked to refrain from cream application on the days of follow-up study visits. For the small proportion of patients not able to tolerate topical application of lidocaine, the participant was asked to continue oral therapy and was analyzed on an intention-to-treat basis. An unblinding officer and unblinding protocol were available at all times through the trial. During the blinded phase of the trial, pain “rescue medication” was provided through oral acetaminophen, 650 mg every 6 hours. The use of other analgesics, such as opioid analgesics, nonsteroidal antiinflammatory drugs, and topical “caines” were documented as protocol violations.
The primary trial end point was the tampon test, performed once weekly. Detailed methods, reliability, and convergent and discriminant validity of this measure have been reported in detail elsewhere.8 Briefly, the tampon test required the participant to insert and immediately remove a tampon (Tampax Original Regular) and record the degree of pain during the entire insertion-removal experience on a 0–10 pain numeric rating scale–0 indicating no pain and 10 indicating the worst possible pain–in her Vulvar Vestibulitis Clinical Trial logbook. Instructions concerning the performance and documentation of the weekly tampon test, the daily 24-hour pain diary, and intercourse pain log were given to each participant on the first prerandomization visit by the research nurse or coordinator. All information was reviewed and recorded during weekly telephone calls by the research nurse or coordinator and later confirmed by review of the study logbook on scheduled study visits. During the prerandomization phase of the trial, eligible individuals were required to demonstrate an adequate baseline level of pain (average 4 out of 10 or greater) on the tampon test to proceed to randomization. On a daily basis during the trial, participants also recorded whether they experienced sexual intercourse in the past 24 hours. The possible responses were: 1–No, too painful; 2 –No, not interested; 3–No, no opportunity; and 4–Yes. If intercourse was confirmed, then the participant recorded her level of pain on a 0–10 numeric rating scale in the study logbook. Participants were also asked to record intensity of general pain experienced over the past 24 hours on a 0–10 numeric rating scale and to record any side-effects experienced while taking study medication. Side effects were listed individually and included a severity estimate (mild, moderate, or severe).
During scheduled study visits, participants were evaluated with physical examination, cotton swab test, Vulvar Algesiometer, a battery of health-related quality-of-life measures, and laboratory testing. All components of the examination were routinely performed by the same examiner (D.C.F.) in identical fashion to the first prerandomization visit. Cotton swab test was performed on defined points of the labia majora, minora, and lower vagina, as previously described. During pelvic examination, participants underwent a selective digital palpation of pelvic floor muscles including levator ani, obturator internus, and piriformis muscle groups. The participant received explicit instructions to focus on palpation of the muscle groups by the examiner’s fingertip while attempting to overlook coexisting entryway pain. Notation was made for each muscle group, anatomic side, and pain level on a 0–3 scale corresponding to none, mild, moderate, and severe pain, respectively. The Vulvar Algesiometer, supplied by Curnow and Morrison (Plymouth, UK), consisted of a mechanical pulse generator that drove a probe against the mucocutaneous surface of the vulva for a calibrated distance and force ranging from 176 mN to 1868 mN in eight increments.9 Using a previously published technique,10 four anatomic sites of the vestibule were tested and end point was defined by the method of limits with the first of two consecutively positive pain responses to probe stimulus designated as pain threshold.11 Algesiometer score was computed by the summation of the pain thresholds from the four designated vestibular sites (0–28 score range with higher score corresponding with less vestibular pain). A short test battery was administered during each study visit that included the Brief Pain Inventory,12 Short Form-McGill Pain Questionnaire,13 and the Neuropathic Pain Scale.14 In addition, a more comprehensive battery was added during weeks 0, 12, 26, and 52 that included the Profile of Mood States,15 Beck Depression Inventory,15,16 and Index of Sexual Satisfaction.17 During every study visit, participants underwent laboratory testing that included microscopic wet mount smears, Rakoff stain for vaginal maturation index, and phenazine test tape for vaginal pH. At the baseline visit, participants underwent a pregnancy test, an electrocardiogram to evaluate specifically the QT interval, and colorimetry of the least sun-exposed skin using the Minolta CR 200. At week 12, each participant provided a blood sample for desipramine and lidocaine serum levels.
The primary end point was defined as the percent change of mean tampon-test pain of weeks (10, 11, and 12) from the mean of weeks (−2, −1, and 0), labeled as baseline. The primary analysis of this 2×2 factorial design involved fitting an analysis of covariance (ANCOVA) model to the percent change of mean tampon-test pain with the two treatment variables as the predictors while adjusting for the covariate age. Interaction of the two treatments was first tested in the ANCOVA model at the .05 level of significance. If the interaction effect was not significant, it would be dropped from the model and the conclusion would be drawn from the model with main effects only. If significant, the model with interactions would be adopted. SAS Proc GLM was used in the analysis.
If interaction between treatments was significant, a hierarchical testing strategy18 was adopted as follows: the first stage would compare desipramine or lidocaine individually with placebo with multiplicity-adjusted P values. If a significant difference (one or both null hypotheses rejected) was found for either or both individual agents, the analysis would proceed to the second stage of hypothesis, which would compare the effects of the active desipramine-active lidocaine treatment with those of the double placebo. If a significant difference (null hypothesis rejected) was found for combined therapy over placebo based on the multiplicity-adjusted P value, then the final (tertiary) stage of comparison would be performed comparing combined therapy to individual therapy. In this strategy if at least one hypothesis has been rejected, then the next stage of hypotheses would be tested, and the family-wise error rate would be controlled at the .05 level.
In the case of nonsignificant interaction, the primary analysis would be based on the ANCOVA model with main effects of treatments and adjusting for age with a Bonferroni corrected alpha level of 0.025 (two-sided). The significance of the main effect of each treatment was assessed by t tests in the ANCOVA model. The aim of the primary analysis was to estimate whether each treatment was superior to placebo, and if both hypotheses held, the double treatment therapy would be most effective under the additive effect assumption of the ANCOVA model.
Twelve secondary end points were analyzed as the absolute change of mean of weeks (10, 11, and 12) from the mean of weeks (−2, −1, and 0), labeled as baseline. Statistical analysis conformed to the tampon-test approach described above. Because secondary end points were considered exploratory, no corrections for multiplicity were performed. Outcome variables and drug safety and side effect data were analyzed according to a modified intention to treat with last observations carried forward for missing data and included all participants who took at least one dose of study drug.
Power analysis was based on pilot data (Foster DC, Duguid KM. Open-label study of oral desipramine and topical lidocaine for the treatment of vulvar vestibulitis [abstract]. International Conference on Mechanism and Treatment of Neuropathic Pain. Rochester, NY, 1998). We estimated that the response would be a 20% decrease in pain from baseline for the double placebo group, a 50% decrease from baseline for each treatment used alone, and an 80% decrease when the two treatments were used together. Thus each treatment would increase the response rate by 30% irrespective of whether the other treatment was used. Power analysis for the main effects (desipramine compared with placebo and lidocaine compared with placebo) used a Bonferroni corrected 80% power level with alpha=0.025 (two-sided test), and estimated that a total of 104 participants would be needed to complete the trial. Assuming a 20% dropout rate, we therefore estimated that 130 participants would be needed.
RESULTS
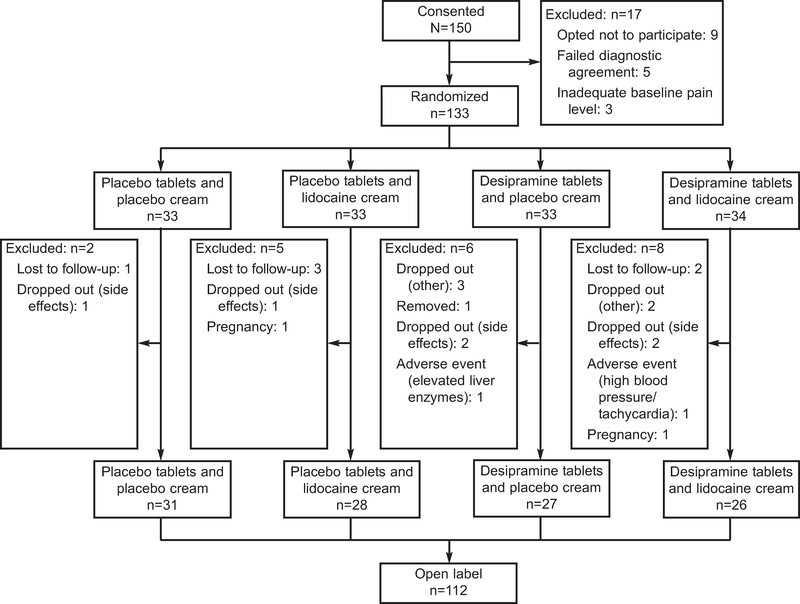
Figure 1 summarizes the disposition of participants from baseline visit to week 12. From September 2003 through March 2007, a total of 173 women were screened to identify 150 who met entry criteria. Of the 150 women who consented to participate in the Vulvar Vestibulitis Clinical Trial, 133 were selected and 112 completed the randomized, blinded phase of the trial (week 12). Of the 17 participants who were excluded or who withdrew before drug randomization, nine decided not to participate in the trial, five did not receive diagnostic agreement by examiners, and three did not demonstrate adequate levels of pain (4 out of 10 or greater) on initial tampon test. Of the 21 individuals randomly assigned to study drug who did not complete the trial, there were two pregnancies, two individuals removed by research staff because of adverse events (one hypertension and tachycardia and one elevated liver enzymes), one person removed by research staff because of data falsified by the participant, five who withdrew from the study owing to side-effects, five who withdrew from the study for other reasons, and six who were lost to follow-up. Analyses were based on 132 participants selected, with one individual removed based on falsified data from the initial 133.
Fig. 1.
Vulvar Vestibulitis Clinical Trial flow diagram.
Foster. Desipramine and Lidocaine for Vulvodynia. Obstet Gynecol 2010.
As noted in Table 1, the mean age of participants ranged from 27 to 31 years, the duration of pain ranged from 4.4 to 6.5 years, and the mean total years of education was reported to range from 15.6 to 16.4 years. Participants were predominantly white, nonHispanic (82–100%), were married or partnered (69–82%), and most commonly reported a chief complaint of pain with intercourse (66–78%). When asked “what preceded the onset of vulvodynia pain,” the most common response was “did not know” (31–50%); the second most common response was “it began after intercourse” (16–29%). The number of participants therapeutically naive to tricyclic antidepressants (69–88%) and topical anesthetics (56–70%) did not significantly differ between treatment arms. All participants(100%) were therapeutically naive to oral desipramine. All participants (100%) were therapeutically naive to daily, repeated applications of topical 5% lidocaine. Before study entry, topical anesthetic-experienced individuals reported only sporadic use shortly before attempted intercourse. Common comorbidities in study participants included endometriosis (13 [10.6%]), irritable bowel syndrome (30 [23.8%]), fibromyalgia (8 [6.4%]), interstitial cystitis (3 [2.4%]), and rape or sexual abuse (17 [13.6%]). No common comorbidity differed across treatment allocations (data not shown). Comparison of the 21 individuals who failed to complete the randomized phase found no difference in age, race, years of education, marital status, or duration of disease compared with participants completing the randomized phase.
Table 1.
Characteristics of Participants
| Placebo-Placebo | Lidocaine-Placebo | Desipramine-Placebo | Desipramine-Lidocaine | P | |
|---|---|---|---|---|---|
| Age (y) | 33 (27.7±6.3) | 32 (31.6±8.4) | 32 (31.0±7.7) | 34 (31.3 ±6.8) | .11* |
| Duration of pain (y) | 30 (5.9±6.3) | 30 (5.8±5.7) | 31 (4.4±3.7) | 33 (6.5±7.0) | .53* |
| Years of education | 32 (16.3±3.0) | 31 (16±3.0) | 32 (16.4±2.4) | 33 (15.6±3.4) | .61* |
| Race | |||||
| White (non-Hispanic) | 29 (0.88) | 32 (1.00) | 30 (0.94) | 28 (0.82) | .21† |
| Other | 4 (0.12) | — | 2 (0.06) | 6 (0.18) | |
| Currently married/partnered | 22 (0.67) | 23 (0.72) | 26 (0.81) | 28 (0.82) | .53† |
| Nullipara | 26 (0.79) | 24 (0.75) | 23 (0.72) | 28 (0.82) | .76† |
| Main problem | |||||
| Intercourse pain | 22 (0.67) | 21 (0.66) | 25 (0.78) | 23 (0.68) | .92† |
| Spontaneous pain | 7 (0.21) | 8 (0.25) | 5 (0.16) | 10 (0.29) | .44‡ |
| Pain start | |||||
| After intercourse | 7 (0.21) | 6 (0.19) | 5 (0.16) | 10 (0.29) | .40‡ |
| Yeast infection | 2 (0.06) | 7 (0.22) | 3 (0.09) | 4 (0.12) | .37‡ |
| Surgical procedure | 1 (0.03) | 4 (0.13) | 2 (0.06) | 1 (0.03) | .56‡ |
| Other | 11 (0.33) | 5 (0.16) | 6 (0.19) | 6 (0.18) | .22‡ |
| Do not know | 12 (0.36) | 10 (0.31) | 16 (0.50) | 13 (0.38) | .35‡ |
| Prior vulvar pain therapy | |||||
| Antidepressant | 4 (0.12) | 10 (0.31) | 4 (0.12) | 4 (0.12) | .10† |
| Lidocaine | 10 (0.30) | 14 (0.44) | 13 (0.41) | 11 (0.32) | .62† |
Data are n (mean±σ) or n (%) unless otherwise specified: “—” indicates 0.
Analysis of variance.
χ2.
Fisher’s exact test.
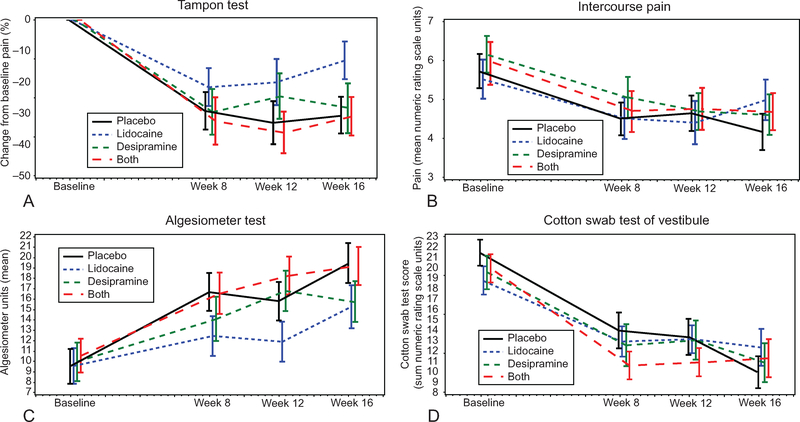
Figure 2 displays the baseline phase, 12-week randomized phase, and the first 4 weeks of open label through week 16. The primary outcome variable, the tampon test, is displayed as the percent change from baseline (mean±SEM) by intention to treat. For comparative purposes, Figure 2 includes absolute changes from baseline (mean± SEM) of three other outcome measures: intercourse pain, Vulvar Algesiometer, and cotton swab test of the vestibule. By week 12, a substantial improvement in tampon-test pain had occurred in all treatment arms: 33% pain reduction for placebo cream–placebo tablet, 20% pain reduction for lidocaine cream-placebo tablet, 24% pain reduction for placebo cream–desipramine tablet, and 36% pain reduction for lidocaine cream–desipramine tablet. The tampon-test percent change from baseline to week 12, analyzed by ANCOVA, showed a significant desipramine tablet-lidocaine cream interaction (t=−2.13; P=.04). As defined above, subsequent ANCOVA model using the heirarchial strategy18 produced no significant difference beyond placebo for desipramine alone (t=0.90; P=.37) or lidocaine alone (t=1.27; P=.21). Post hoc nonparametric analysis found no significant effect beyond placebo for desipramine alone (Wilcoxon rank-sum; P=.88) or lidocaine alone (Wilcoxon rank-sum; P=.91). Similar to the tampon test although in mirror image (Fig. 2), the Algesiometer (mean absolute change±SEM) found topical lidocaine–placebo tablet tended toward less improvement compared with other treatment arms including placebo cream–placebo tablet. Intercourse pain and cotton swab test (Fig. 2) graphically illustrate very similar improvement of all treatment allocations, including placebo cream-placebo tablet, over the 12-week phase with no distinguishable trends by allocation.
Fig. 2.
Response change for selected outcome measures (mean±standard error of the mean [SEM]) from baseline through week 16 for the four treatment allocations: tampon test (A), intercourse pain (B), algesiometer test (C), cotton swab test-vestibule (D). Week 16 represents open label after completion of randomized phase (baseline to week 12).
Foster. Desipramine and Lidocaine for Vulvodynia. Obstet Gynecol 2010.
Table 2 lists the 12 secondary outcome measures, baseline values, and absolute changes from week 0 to week 12. Two significant desipramine tablets-lidocaine cream interactions were found, for cotton swab test (t=−2.16; P=.03) and for the McGill-Short Form total score (t=−2.17; P = .03). Subsequent ANCOVA analysis using the heirarchial strategy18 of individual and combined effects of desipramine tablets and lidocaine cream did not significantly differ from placebo. Of all 12 secondary outcome measures following the three active treatments (Table 2), only the Index of Sexual Satisfaction improved with desipramine tablets compared with placebo (t=−2.81.; P=.006). All other outcome measures demonstrated a trend toward improvement over time but no superiority to placebo, similar to the results for the tampon test. Secondary outcome measures displayed 30 –50% improvement from week 0 to week 12 and continued improvement during open label through week 16, similar to the tampon test, in the placebo tablet–placebo cream group.
Table 2.
Vulvar Vestibulitis Clinical Trial Secondary Outcomes by Treatment Allocation
| Placebo-Placebo |
Lidocaine-Placebo |
|||||
|---|---|---|---|---|---|---|
| n | Mean Baseline | Mean Change | n | Mean Baseline | Mean Change | |
| Daily pain log | 32 | 1.97 | −0.47±0.77 | 32 | 2.01 | − 0.53±1.63 |
| Intercourse pain log | 26 | 5.72 | −1.97±2.47 | 21 | 5.52 | −1.92±1.82 |
| Intercourse frequency (per wk) | 32 | 0.13 | −0.02±0.12 | 33 | 0.15 | −0.02±0.10 |
| Cotton swab test–vestibule | 33 | 21.48 | −8.65±6.59 | 32 | 19.03 | −6.42±7.90 |
| Algesiometer | 32 | 9.53 | 6.97±8.18 | 32 | 9.88 | 3.10±9.64 |
| Pelvic muscle pain (maximum pain) | 33 | 1.15 | −0.12±0.86 | 32 | 1.16 | −0.23±0.92 |
| McGill-Short Form total score | 29 | 13.74 | − 4.57±5.86 | 27 | 12.32 | − 3.10±6.77 |
| Neuropathic Pain Scale | 31 | 41.90 | −13.39±14.42 | 30 | 44.78 | −12.17±17.02 |
| Profile of Mood State Summary | 24 | 85.58 | − 4.68±17.37 | 29 | 88.17 | − 2.15±20.75 |
| Becks Depression Inventory | 29 | 8.62 | −1.92±5.44 | 30 | 11.17 | −0.86±5.90 |
| Brief Pain Inventory Interference | 31 | 20.9 | − 6.55±13.81 | 30 | 21.37 | −3.80±20.25 |
| Index of Sexual Satisfaction | 28 | 59.82 | 0.69±9.28 | 24 | 64.92 | 0.43±11.71 |
Mean change is week 0 to 12±standard deviation.
Only significant desipramine–lidocaine interactions are displayed.
During the 12-week randomized phase, daily tablet intake increased to a mean of 4.2±1.9 active desipramine tablets daily (1 tablet= 25 mg desipramine) and 3.9± 1.2 applications of active 5% lidocaine topically. In the two active desipramine treatment arms, the mean desipramine serum level at week 12 was 106 ± 121 ng/mL, with a wide range less than 20 to 532 ng/mL. Comparing the lower 50th percentile and upper 50th percentile desipramine serum levels to tampon-test end points, no significant dose-response was found (t= 1.55; P=.13). In the two active lidocaine treatment arms, the mean serum level was below assayable (less than 0.1 micrograms/mL; range, less than 0.1–0.2 micrograms/mL). Conforming to study instructions, no participant in either group applied lidocaine cream within 1 hour of examination on week 12. Of the individuals who continued through week 12, one participant reported a drug-protocol violation (initiation of gabapentin). This participant had been randomly assigned to receive desipramine tablet-placebo cream, and outcome data were carried forward from the time point preceding the protocol violation.
The two desipramine treatment arms demonstrated the greatest, albeit modest, dropout proportion (8%). Most significant side effects were found with desipramine use, including dry mouth (24% desipramine compared with 8% no desipramine; P=.017), hot flushes (21% desipramine compared with 5% no desipramine; P=.009), dizzy or lightheaded (21% desipramine compared with 8% no desipramine; P=.047). Soreness and tenderness were more common in participants not taking desipramine (9% desipramine compared with 23% no desipramine). Desipramine intake resulted in a significant weight loss (−1.6 kg over 12 weeks) compared with a slight weight gain with placebo tablets (0.04 kg over 12 weeks); P=.03. Of greatest clinical and statistical significance was tachycardia (more than 100 beats per minute) at rest (16% desipramine compared with 2% no desipramine; P<.001). Two adverse events occurred: one case of mild elevation in liver enzymes (desipramine tablets-placebo cream) and one case with hypertension and tachycardia (desipramine tablets-lidocaine cream). No U.S. Food and Drug Administration-defined serious adverse events occurred during the trial. Before randomization, electrocardiograms showed no prolonged QT intervals, (absolute QT more than 500 milliseconds).19 After completion of week 12, 67% of the participants correctly guessed desipramine randomization status and 56% of the participants correctly guessed lidocaine randomization status.
Table 3 displays the treatment choices during the 40-week open-label phase (week 12–52). The largest percentage of participants (27%) opted for topical lidocaine and oral desipramine through 52-week trial completion and reported continued improvement, albeit not statistically significant. Participants (8%) taking combination of oral gabapentin and topical lidocaine demonstrated significantly greater pain (0.8±3.7; t=−2.39; P=.02) with the cotton swab test. Individuals (9%) who elected to undergo surgery (perineoplasty with vaginal advancement) reported significantly improved pain with the cotton swab test (−9.2±10.9; t=2.27; P=.03) and the McGill-short form total score (−5.4±6.5, t=2.25; P=.03) and approached significant improvement with the Vulvar Algesiometer (9.2± 10.9, t= 1.87; P=.06) and the Neuropathic Pain Scale (−14.8±20.3, t= 1.74; P=.08).
Table 3.
Outcomes After Open-Label Treatment Selection by Vulvar Vestibular Clinical Trial Participants During Weeks 13 to 52 Postrandomization
| Open-Label Treatment Choices | n (%) | Tampon Test | Sex Pain | Cotton Swab Test–Vestibule | Algesiometer | McGill Short Form Total | Neuropathic Pain Scale Total |
|---|---|---|---|---|---|---|---|
| Desipramine+lidocaine | 35 (27) | − 0.9 (1.2) | − 0.1 (2.4) | − 3.8 (8.1) | 4.4 (10.6) | − 0.9 (4.2) | − 6.3 (13.4) |
| Desipramine only | 9(7) | − 0.4 (0.7) | − 0.9 (1.8) | −4.9 (9.1) | 3.0 (10.3) | −2.0 (3.7) | − 7.7 (17.0) |
| Lidocaine only | 24 (18) | − 0.5 (0.9) | − 0.2 (2.3) | −4.5 (7.1) | 1.7 (9.5) | − 1.5 (7.8) | − 4.4 (19.6) |
| Gabapentin+lidocaine | 10 (8) | 0.8 (3.7) | 1.0 (4.3) | 3.3 (12.1)* | 3.9 (8.7) | 0.3 (7.1) | − 2.3 (17.9) |
| Gabapentin only | 5 (4) | − 1.2 (1.1) | − 1.9 (3.5) | − 6.6 (4.4) | 9.8 (11.7) | − 3.6 (5.3) | − 2.8 (23.5) |
| Surgery during trial | 12 (9) | − 1.3 (2.6) | − 0.4 (1.5) | − 9.2 (10.9)† | 9.2 (10.9) | − 5.4 (6.5)‡ | − 14.8 (20.3) |
| None | 16 (12) | 0.2 (2.2) | 1.3 (2.5) | −0.2 (9.3) | 1.1 (11.1) | − 1.4 (5.6) | −4.1 (20.6) |
| Other | 1 (1) | ||||||
| Unknown | 20 (15) | ||||||
| Total participants | 132 |
Data are mean (standard deviation) unless otherwise specified.
t= −2.39; P=.02.
t= −2.27; P=.03.
t=− 2.25; P=.03.
DISCUSSION
The Vulvar Vestibulitis Clinical Trial stands out from previously published vulvodynia clinical trials by investigating two of the most commonly prescribed medications, using a placebo-controlled, double-blinded design, and measuring a large number of validated, multidimensional end points. Our major findings can be summarized in four points. First, oral desipramine and topical lidocaine, as monotherapy or in combination, failed to reduce vulvodynia pain better than placebo. This conclusion is based on a lack of significant treatment effect found for the primary end point tampon test and further supported by the lack of treatment effect found for multiple secondary end points including daily pain diary, intercourse pain log, intercourse frequency log, cotton swab test, Vulvar Algesiometer, the McGill-SF Pain Questionnaire, Profile of Mood States, Beck Depression Inventory, Brief Pain Inventory, and the Neuropathic Pain Scale. Second, a substantial reduction in vulvodynia pain occurred over the 12-week doubleblind phase for all groups including those randomly assigned to placebo tablets and cream, ranging from 24% to 43%. The basis for this substantial improvement may be placebo effect or placebo-independent effects (see below). Third, participants who selected the surgical option (vestibulectomy and vaginal advancement) during the open-label phase (weeks 12–52), reported the greatest improvement in end points of any intervention. Fourth, lidocaine cream monotherapy tended to produce the least pain improvement, even less than the identical cream base without lidocaine. This finding suggests that repeated topical applications of lidocaine may counteract ongoing improvement in vulvodynia pain and disagrees with conclusions of earlier research without placebo control.20
The modest overall dropout rate of 15.7% reflected the excellent adherence to the drug regimen by study participants. Serious adverse events did not occur for any treatments. Oral desipramine and topical lidocaine were both well tolerated by a majority of participants with significant, albeit limited, adverse events associated with desipramine use. A higher dropout rate (19.6% compared with 10.6%) was found in the desipramine treatment arms and was likely based on tachycardia and anticholinerginc effects associated with tricyclic antidepressants and displayed in Table 3. Recognizable side effects of desipramine also may have resulted in a higher unblinding effect for participants so assigned. We found that 67% of individuals correctly guessed randomization to desipramine, which is modestly higher than chance (50%). The effect of this unblinding, however, is probably insignificant given the absence of a demonstrated beneficial (or harmful) treatment effect found for either medication. We found a wide range of desipramine serum levels during the final (maximum dosage) phase of the RCT. The wide range in serum levels reflected both variation in the maximum number of desipramine tablets taken and individual variation in drug metabolism.
| Desipramine-Placebo |
Desipramine-Lidocaine |
||||||
|---|---|---|---|---|---|---|---|
| n | Mean Baseline | Mean Change | n | Mean Baseline | Mean Change | Regression | P |
| 32 | 2.03 | −0.65±1.73 | 33 | 1.6 | −0.52±0.71 | Desipramine | .38 |
| Lidocaine | .66 | ||||||
| 21 | 6.15 | −2.07±2.31 | 21 | 5.92 | − 1.72±1.99 | Desipramine | .86 |
| Lidocaine | .76 | ||||||
| 32 | 0.11 | 0.03±0.14 | 33 | 0.11 | 0.02±0.14 | Desipramine | .12 |
| Lidocaine | .81 | ||||||
| 32 | 19.59 | − 8.07±10.23 | 34 | 19.97 | −11.37±8.00 | Desipramine | .47 |
| Lidocaine | .15 | ||||||
| Desip.–Lido. | .03* | ||||||
| 31 | 9.97 | 7.14±11.69 | 33 | 10.55 | 9.33±9.67 | Desipramine | .09 |
| Lidocaine | .51 | ||||||
| 32 | 1.19 | − 0.34±0.75 | 34 | 0.97 | −0.29±0.91 | Desipramine | .15 |
| Lidocaine | .44 | ||||||
| 32 | 13.21 | − 5.48±7.8 | 30 | 14.78 | −6.44±8.54 | Desipramine | .41 |
| Lidocaine | .42 | ||||||
| Desip.–Lido. | .03* | ||||||
| 32 | 45.68 | −14.87±19.67 | 33 | 42.77 | −20.35±18.15 | Desipramine | .30 |
| Lidocaine | .48 | ||||||
| 28 | 87.54 | −10.42±28.14 | 33 | 88.79 | −5.48±26.17 | Desipramine | .45 |
| Lidocaine | .59 | ||||||
| 27 | 9.15 | −3.33±5.26 | 32 | 9.76 | −1.77±7.58 | Desipramine | .54 |
| Lidocaine | .41 | ||||||
| 32 | 21.59 | − 8.08±15.24 | 34 | 17.82 | − 7.62±12.01 | Desipramine | .13 |
| Lidocaine | .10 | ||||||
| 28 | 56.07 | − 6.86±10.30 | 30 | 60.87 | − 6.32±10.43 | Desipramine | .006 |
| Lidocaine | .97 | ||||||
The substantial improvement with placebo treatment may not necessarily indicate a true placebo effect. Instead, changes may be based on several factors including the Hawthorne effect, a therapeutic benefit of cream massage, or the natural history of vulvodynia.21 Despite nurse-to-participant contact limited to medication instructions and response-data collection, a therapeutic benefit may have accrued from the weekly telephone call based partially on attention to the participant’s pain and distress (Hawthorne effect) and partially on inadvertent recommendations with beneficial effects. Cream massage to the vulvar vestibule, done by all participants in the trial, may have been beneficial by duplicating the soft-tissue manipulation performed by physical therapists, or the active ingredients in Moisturel (petrolatum and dimethicone) may have exerted a direct beneficial effect. Finally, the Vulvar Vestibulitis Clinical Trial experience may reflect an ongoing spontaneous improvement (natural history) of localized provoked vulvodynia as has been observed in an observational study of the Michigan Health Registry.22
The open-label phase displayed in Table 3 demonstrates outcomes following participants’ treatment choices based mostly from the Vulvodynia Guidelines.23 More than 50% of participants remained on combined desipramine-lidocaine or desipramine or lidocaine alone based on the perceived improvement on study drug during the RCT. Of the eight options selected by study participants, surgery (vestibulectomy and vaginal advancement) resulted in the greatest number and degree of improved outcome measures of any therapeutic choice during the trial. No firm conclusion can be made because localized provoked vulvodynia surgery was undertaken outside of the RCT phase. However, our findings do support the results of a previously published RCT demonstrating surgical benefits.24
Several comments should be made concerning study limitations and study method. Although results were based on modified intention-to-treat analyses with last observation carried forward for missing data, some results did not include all participants because of unavailability of baseline values. This is particularly evident in the logbook report of intercourse pain, where a substantial proportion of participants (21–38%), across treatment arms, were sexually inactive during weeks 0–12. Desipramine therapy significantly improved the Index of Sexual Satisfaction over placebo and was the only outcome measure during the double-blind phase of the trial that reflected benefit of active drug. The Index of Sexual Satisfaction provides a measure of interpersonal relationship strength and does not focus on entryway dyspareunia per se, and therefore cannot be considered a specific measure of improvement in sexual function. A newer measure, the Female Sexual Function Index more specifically measures intercourse pain and may be preferred in future vulvodynia RCTs.25 Our research setting used a single institutionsingle clinical examiner, thereby maintaining consistency and reliability of outcome measures over time. However, the lack of a multi-institutional-multiple investigator design may also reduce the ability to generalize our findings to a broader, more heterogeneous patient population. After a negative trial, the question of adequacy of sample size will be inevitably raised. The multiple outcomes of the Vulvar Vestibulitis Clinical Trial, however, show little evidence of trends in data that might achieve statistical significance with a larger sample size, other than a possible exacerbation of pain after repeated use of topical lidocaine. Finally, spontaneous improvement of localized provoked vulvodynia may have been distinguished from placebo effect by inclusion of a notreatment arm in the RCT.26
In conclusion, given the marked improvement seen with active and placebo treatments alike in this trial, the assumption of therapeutic benefits reported by the majority of past localized provoked vulvodynia trials lacking placebo controls must be questioned. Although substantial response across all treatment arms in the Vulvar Vestibulitis Clinical Trial remains unexplained at present, we can offer two recommendations for future vulvodynia research. First, future localized provoked vulvodynia trials should include both a placebo arm and, ideally, a no-treatment arm to better define the true placebo effect.26 Second, vulvodynia treatments under consideration for future trials should show beneficial effects in pilot studies substantially greater than 50%, before embarking on time-consuming and resource-intensive trials. Although limited in scope, the study findings also help to support a broader, two-step, clinical approach. First, choose an initial treatment option with minimal risk and an acceptable side effect profile. The patient afflicted with localized provoked vulvodynia should be provided support, ample time under observation and treatment, and careful measurement of clinical response. Second, in the case of an initial failed clinical response, other options including alternative medications, physical therapy, and surgery should be reviewed with the patient including known risks and success rates.
Acknowledgments
Supported by grant RO-1 HD040123-05 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Presented as an abstract at the XX World Congress of the International Society for the Study of Vulvovaginal Disease, September 13–17, 2009, Edinburgh, Scotland.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Updike GM, Wiesenfeld HC. Insight into the treatment of vulvar pain: a survey of clinicians. Am J Obstet Gynecol 2005;193:1404–9. [DOI] [PubMed] [Google Scholar]
- 2.Yalcin I, Tessier LH, Petit-Demoulière N, Doridot S, Hein L, Freund-Mercier MJ, et al. Beta2-adrenoceptors are essential for desipramine, venlafaxine or reboxetine action in neuropathic pain. Neurobiol Dis 2009;33:386–94. [DOI] [PubMed] [Google Scholar]
- 3.Amir R, Argoff CE, Bennett GJ, Cummins TR, Durieux ME, Gerner P, et al. The role of sodium channels in chronic inflammatory and neuropathic pain [review]. J Pain 2006;7(5 suppl 3):S1–29. [DOI] [PubMed] [Google Scholar]
- 4.Giesecke J, Reed BD, Haefner HK, Giesecke T, Clauw DJ, Gracely RH. Quantitative sensory testing in vulvodynia patients and increased peripheral pressure pain sensitivity. Obstet Gynecol 2004;104:126–33. [DOI] [PubMed] [Google Scholar]
- 5.Tympanidis P, Casula MA, Yiangou Y, Terenghi G, Dowd P, Anand P. Increased vanilloid receptor VR1 innervation in vulvodynia. Eur J Pain 2004;8:129–33. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007;132: 237–51. [DOI] [PubMed] [Google Scholar]
- 7.Bergeron S, Binik YM, Khalife S, Pagidas K, Glazer HI. Vulvar vestibulitis syndrome: reliability of diagnosis and evaluation of current diagnostic criteria. Obstet Gynecol 2001;98:45–51. [DOI] [PubMed] [Google Scholar]
- 8.Foster DC, Kotok MB, Huang LS, Watts A, Oakes D, Howard FM, et al. The tampon test for vulvodynia treatment outcome research: reliability, construct validity, and responsiveness. Obstet Gynecol 2009;113:825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curnow JS, Barron L, Morrison G, Sergeant P. Vulval algesiometer. Med Biol Eng Comput 1996;34:266–9. [DOI] [PubMed] [Google Scholar]
- 10.Eva LJ, Reid WM, MacLean AB, Morrison GD. Assessment of response to treatment in vulvar vestibulitis syndrome by means of the vulvar algesiometer. Am J Obstet Gynecol 1999;181: 99–102. [DOI] [PubMed] [Google Scholar]
- 11.Dotson RM. Clinical neurophysiology laboratory tests to assess the nociceptive system in humans. J Clin Neurophysiol 1997; 14:32–45. [DOI] [PubMed] [Google Scholar]
- 12.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23: 129–38. [PubMed] [Google Scholar]
- 13.Melzack R The short-form McGill Pain Questionnaire. Pain 1987;30:191–7. [DOI] [PubMed] [Google Scholar]
- 14.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology 1997;48:332–8. [DOI] [PubMed] [Google Scholar]
- 15.McNair DM, Lorr M. An analysis if mood in neurotics. J Abnorm Psychol 1964;69:620–7. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71. [DOI] [PubMed] [Google Scholar]
- 17.Hudson W, Harrison D, Crosscup P. A short-form scale to measure sexual discord in dyadic relationships. J Sex Res 1981;17:157–74. [Google Scholar]
- 18.Dmitrienko A, Wiens BL, Tamhane AC, Wang X. Tree-structured gatekeeping tests in clinical trials with hierarchically ordered multiple objectives [published erratum appears in Stat Med 2008;27:3452]. Stat Med 2007;26:2465–78. [DOI] [PubMed] [Google Scholar]
- 19.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 2004;350:1013–22. [DOI] [PubMed] [Google Scholar]
- 20.Zolnoun DA, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol 2003;102:84–7. [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB, Price DD. What is controlled for in placebocontrolled trials? Mayo Clin Proc 2005;80:1119–21. [DOI] [PubMed] [Google Scholar]
- 22.Reed BD, Haefner HK, Sen A, Gorenflo DW. Vulvodynia incidence and remission rates among adult women: a 2-year follow-up study. Obstet Gynecol 2008;112:231–7. [DOI] [PubMed] [Google Scholar]
- 23.Haefner HK, Collins ME, Davis GD, Edwards L, Foster DC, Hartmann ED, et al. The vulvodynia guideline. J Low Genit Tract Dis 2005;9:40–51. [DOI] [PubMed] [Google Scholar]
- 24.Bergeron S, Binik YM, Khalife S, Pagidas K, Glazer HI, Meana M, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain 2001;91: 297–306. [DOI] [PubMed] [Google Scholar]
- 25.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208. [DOI] [PubMed] [Google Scholar]
- 26.Krogsboll LT, Hrobjartsson A, Gotzsche PC. Spontaneous improvement in randomised clinical trials: meta-analysis of three-armed trials comparing no treatment, placebo and active intervention. BMC Med Res Methodol 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]