Abstract
Background:
Spinal anesthesia is a safe anesthetic technique commonly practiced. However, it is associated with hypotension (33%), bradycardia (13%), and shivering which are induced by hypovolemia, sympathetic blockade, and Bezold–Jarisch reflex through intracardiac serotonin (5HT3) receptors and vagus nerve.
Aim:
To study the effect of intravenous (i.v.) ondansetron on hypotension and bradycardia induced by spinal anesthesia.
Setting and Design:
This was a randomized controlled double-blinded study done in a tertiary care teaching hospital.
Methods:
Of 140 patients, 70 in Group A received 2 mL of i.v. ondansetron 4 mg and 70 in the Group B received 2 mL of i.v. normal saline. 3 mL of 0.5% hyperbaric bupivacaine was injected intrathecally. Measurements of blood pressure and heart rate (HR) were taken every 3 min for 30 min after spinal anesthesia was performed. Mean arterial pressure (MAP) drop more than 20% was considered as incidence of hypotension and ephedrine 6 mg i.v. was given. HR drop >20% was regarded as bradycardia and atropine 0.5 mg i.v. was given.
Statistical Tests:
Quantitative data were analyzed using ANOVA test and qualitative data were analyzed using Chi-square test.
Results:
Both groups are comparable in demographic data. Four (5.7%) patients in Group B and no patients in Group A had incidence of bradycardia and atropine requirement (P = 0.120). There was no statistically significant difference in systolic blood pressure, diastolic blood pressure, and MAP. 19 (27%) patients in Group A and 33 (47.1%) in Group B required ephedrine with P = 0.029. 12 (17.1%) in Group B and no patients in Group A had shivering with P = 0.0001.
Conclusion:
Our study indicates that prophylactic use of ondansetron before spinal anesthesia significantly reduces the requirement of ephedrine and shivering.
Keywords: Bezold–Jarisch reflex, ondansetron, spinal anesthesia
INTRODUCTION
Spinal anesthesia is a simple, reliable, and most common anesthetic technique practiced worldwide.[1,2] However, spinal anesthesia is associated with side effects such as hypotension, bradycardia, and shivering.[3,4] The mechanism involved in the occurrence of hypotension is decrease in vascular resistance caused by sympathetic blockade which in turn causes vasodilatation and finally leads to drop in arterial pressure.[5,6] Parasympathetic overactivity, activation of Bezold–Jarisch reflex (BJR), and increased baroreceptor activity may lead to hypotension and bradycardia. BJR is triggered by chemoreceptors and mechanoreceptors which are serotonin sensitive.[2] Animal and human studies proved that 5HT3 antagonists prevent serotonin-induced BJR.[3,7] Serotonin is an additive trigger for BJR in hypovolemic patients[8] Ondansetron has 5HT3 antagonizing effects with minor side effects.[9,10] Ondansetron has less chances of causing cognitive side effects such as headache, agitation, and confusion.[11,12,13]
Even though spinal anesthesia is a simple and safe procedure, rare complications such as unresponsive hypotension and bradycardia are real anesthetic challenges. It is preferred to prevent hypotension rather than treating it. Hence, in the recent past, most of the studies are focusing on prophylactic management of hypotension; ondansetron is such a drug gaining popularity in the prevention of hypotension in patients who underwent subarachnoid block.
We hypothesized that blocking type 3 serotonin receptors with intravenous (i.v.) ondansetron reduces the hypotension induced by spinal anesthesia. The purpose of this prospective, randomized, double-blind study was to compare the efficacy of i.v. ondansetron with a placebo in reducing the incidence of hypotension and bradycardia caused by spinal anesthesia as primary outcome and perioperative nausea, vomiting, and shivering as secondary outcomes.
METHODS
A comparative randomized controlled double-blinded study was done on patients who were posted for elective orthopedic, gynecological, and general surgical procedures under spinal anesthesia. After approval from the Institutional Ethics Review Board, 140 American Society of Anesthesiologists (ASA) Physical Status Classes I and II patients were enrolled in the study. The protocol was explained in a detailed manner to all patients in their own understandable language and informed consent was taken.
Based on the previous study conducted by Marashi et al.,[1] it was found that there was a significant difference in the mean arterial pressure (MAP) values between ondansetron group and control group. In the control group, 14% had bradycardia (heart rate [HR] <50 b.p.m.) and 17% of patients had hypotension (MAP <80 mmHg) requiring vasopressors compared to ondansetron group (MAP >80 mmHg). In the present study, to detect difference of 20% reduction in MAP and HR among the groups. Considering significant level of 5% and assuming a power of 95%, the sample size was estimated to be 68 in each group, and a convenient sample size of 70 was taken in each group.
Subjects with age between 20 and 60 years, ASA Grade I–II were included in the study. Patients in whom spinal anesthesia is contraindicated, patients having known allergy to ondansetron, patients having hypertension (HTN) and coronary artery disease, patients who are taking selective serotonin reuptake inhibitors and parturients, and patients having autonomic neuropathy were excluded from the study.
One hundred and forty patients scheduled for elective infraumbilical surgeries were enrolled in the study. The randomization process of allocating into the two groups was done using computer-generated random numbers. Group A patients received i.v. ondansetron 4 mg (2 mL). Group B patients received normal saline of 2 mL. The drug was labeled by an anesthesiologist who was not involved in the study and the patient was monitored by a different anesthesiologist. Grouping was done in such a way that both patient and the monitoring anesthesiologist were blinded to the study. Informed consent was taken from all the patients who are included in the study. On arrival to operative room, standard monitor was connected to all patients which include, pulse oximeter (SpO2), electrocardiography, and noninvasive arterial blood pressure (NIBP). Supplemental oxygen was given by face mask at rate of 5 lpm. After securing i.v. cannula (18 G/20 G) in the upper limb, i.v. fluids were given at a volume of 5 mL/kg during the study period.
An unlabeled 2 mL syringe was given to the monitoring anesthesiologist and he/she injected that 2 mL of drug 5 min before performing spinal anesthesia. The person performing spinal anesthesia and monitoring was blinded to the study. All patients were given spinal anesthesia in sitting position. 25G Quincke spinal needle was inserted by midline approach into L3-L4 or L4-L5 interspace. After ensuring the correct position of the needle by checking the free flow of cerebrospinal fluid, 3 mL (15 mg) 0.5% hyperbaric bupivacaine was instilled intrathecally and the patient was positioned supine. No surgery-related procedures that include patient positioning, tourniquet placement, and urinary catheterization were performed during the study period. Level of sensory block was evaluated every 5 min for 20 min for cold sensation using alcohol swabs. At the same time, motor blockade was assessed by Bromage scale. Bromage scale: 0 – no paralysis, 1 – inability to lift the thigh, 2 – inability to flex the knee, 3 – inability to move any joint in leg.[2]
All hemodynamic parameters such as HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), and MAP were recorded every 3 min for 30 min. The patients whose HR decreased by 20% of baseline value or less than 45 b.p.m received atropine 0.5 mg i.v. Ephedrine 6 mg i.v. was given in case of hypotension when blood pressure (MAP) decreased by 20% of baseline value. When ephedrine or atropine administration was necessary during the procedure, only values obtained before the medications were considered. Number of doses of ephedrine and atropine needed and timings were recorded. Intraoperative nausea, vomiting, and shivering were recorded. Patients were monitored for rare side effects of ondansetron such as prolonged Qtc, headache, and dizziness.
Statistical methods
Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented on mean ± standard deviation (min–max) and results on categorical measurements are presented in number (%). Significance is assessed at 5% level of significance. The following assumptions on data are made: (1) Dependent variables should be normally distributed, (2) samples drawn from the population should be random, and (3) cases of the samples should be independent.
Student's t-test (two-tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups (intergroup analysis) on metric parameters. Student's t-test (two-tailed, dependent) has been used to find the significance of study parameters on continuous scale within each group.
Chi-square/Fisher's exact test has been used to find the significance of study parameters on categorical scale between two or more groups, nonparametric setting for qualitative data analysis. Fisher's exact test was used when cell samples are very small. P value ≤ 0.05 was considered statistically significant.
Statistical software
The statistical software namely Statistical package for social sciences 18.0 was used for the analysis of the data and Microsoft Word and Excel have been used to generate graphs, tables, etc.
RESULTS
In our study, 140 patients were randomly assigned to two groups by computer-generated random number table. Demographically, both Group A and Group B are age and gender matched. There is no statistically significant difference in weight, height, body mass index, and ASA class between the two groups [Table 1]. There was no statistically significant difference in the level of sensory blockade and Bromage scale grading at 5th, 10th, 15th, and 20th min [Table 2]. There was no significant difference between level of sensory blockade and incidence of hypotension. None of the patients in Group A and 4 (5.7%) patients in Group B required atropine which is not statistically significant (P = 0.12) [Table 3]. There was no significant difference in SBP, DBP, and MAP between both Group A and B at any point of 3 min interval in span of 30 min. P > 0.05 was found at all time intervals in the span of 30 min [Table 4 and Graph 1]. In Group A, 17 (24.3%) patients required one dose of ephedrine, 1 (1.4%) patient required two doses, and 1 (1.4%) patient required three doses. In Group B, 23 (32.9%) patients required one dose of ephedrine, 7 (10%) patients required two doses, and 3 (4.3%) patients required three doses. A total of 19 (27.1%) and 33 (47.1%) patients required ephedrine in Group A and Group B, respectively, with a significant P = 0.029 [Table 3]. In Group A, none of the patients and 1 (1.4%) patient in Group B had nausea which is statistically not significant with P = 0.496. None of the patients in both groups have incidence of vomiting. 12 (17%) patients in Group B had shivering and none of the patients in Group A had shivering. It was statistically significant with P = 0.0001 [Table 5].
Table 1.
Demographic distribution of patients
| Group A | Group B | Total | P | |
|---|---|---|---|---|
| Age (years) | 39.39±11.62 | 40.19±11.66 | 39.79±11.60 | |
| Sex (%) | ||||
| Male | 48 (68.6) | 47 (67.1) | 95 (67.9) | 0.856 |
| Female | 22 (31.4) | 23 (32.9) | 45 (32.1) | |
| BMI (kg/m2) | 23.82±4.01 | 23.34±4.36 | 23.58±4.18 | 0.498 |
| ASA grade (%) | ||||
| Grade 1 | 40 (57.1) | 41 (58.6) | 81 (57.9) | 0.864 |
| Grade 2 | 30 (42.9) | 29 (41.4) | 59 (42.1) |
BMI=Body mass index, ASA=American Society of Anesthesiologist
Table 2.
Sensory level distribution and Bromage scale grade in two groups of patients
| Group A (n=70), n (%) | Group B (n=70), n (%) | Total (n=140), n (%) | P | |
|---|---|---|---|---|
| Sensory level | ||||
| 5 min | ||||
| T12 | 26 (37.1) | 37 (52.9) | 63 (45) | 0.134 |
| T10 | 27 (38.6) | 17 (24.3) | 44 (31.4) | |
| T8 | 6 (8.6) | 9 (12.9) | 15 (10.7) | |
| T6 | 3 (4.3) | 1 (1.4) | 4 (2.9) | |
| L2 | 1 (1.4) | 3 (4.3) | 4 (2.9) | |
| L1 | 7 (10) | 3 (4.3) | 10 (7.1) | |
| 10 min | ||||
| T12 | 8 (11.4) | 6 (8.6) | 14 (10) | 0.301 |
| T10 | 15 (21.4) | 23 (32.9) | 38 (27.1) | |
| T8 | 32 (45.7) | 23 (32.9) | 55 (39.3) | |
| T7 | 2 (2.9) | 0 | 2 (1.4) | |
| T6 | 11 (15.7) | 16 (22.9) | 27 (19.3) | |
| T5 | 0 | 1 (1.4) | 1 (0.7) | |
| T4 | 1 (1.4) | 1 (1.4) | 2 (1.4) | |
| L1 | 1 (1.4) | 0 | 1 (0.7) | |
| 15 min | ||||
| T12 | 2 (2.9) | 2 (2.9) | 4 (2.9) | 0.812 |
| T10 | 11 (15.7) | 12 (17.1) | 23 (16.4) | |
| T9 | 0 | 1 (1.4) | 1 (0.7) | |
| T8 | 27 (38.6) | 27 (38.6) | 54 (38.6) | |
| T7 | 1 (1.4) | 0 | 1 (0.7) | |
| T6 | 25 (35.7) | 25 (35.7) | 50 (35.7) | |
| T5 | 0 | 1 (1.4) | 1 (0.7) | |
| T4 | 4 (5.7) | 2 (2.9) | 6 (4.3) | |
| 20 min | ||||
| T12 | 2 (2.9) | 1 (1.4) | 3 (2.1) | 0.298 |
| T11 | 0 | 1 (1.4) | 1 (0.7) | |
| T10 | 6 (8.6) | 10 (14.3) | 16 (11.4) | |
| T9 | 0 | 2 (2.9) | 2 (1.4) | |
| T8 | 29 (41.4) | 20 (28.6) | 49 (35) | |
| T7 | 2 (2.9) | 0 | 2 (1.4) | |
| T6 | 27 (38.6) | 32 (45.7) | 59 (42.1) | |
| T4 | 4 (5.7) | 4 (5.7) | 8 (5.7) | |
| Bromage scale | ||||
| 0 min | ||||
| 0 | 68 (97.1) | 67 (95.7) | 135 (96.4) | 1.000 |
| 1 | 2 (2.9) | 3 (4.3) | 5 (3.6) | |
| 5 min | ||||
| 1 | 21 (30) | 28 (40) | 49 (35) | 0.452 |
| 2 | 28 (40) | 23 (32.9) | 51 (36.4) | |
| 3 | 21 (30) | 19 (27.1) | 40 (28.6) | |
| 10 min | ||||
| 1 | 2 (2.9) | 1 (1.4) | 3 (2.1) | 0.100 |
| 2 | 10 (14.3) | 20 (28.6) | 30 (21.4) | |
| 3 | 58 (82.9) | 49 (70) | 107 (76.4) | |
| 15 min | ||||
| 1 | 0 | 0 | 0 | 1.000 |
| 2 | 5 (7.1) | 4 (5.7) | 9 (6.4) | |
| 3 | 65 (92.9) | 66 (94.3) | 131 (93.6) | |
| 20 min | ||||
| 1 | 0 | 0 | 0 | 1.000 |
| 2 | 1 (1.4) | 0 | 1 (0.7) | |
| 3 | 69 (98.6) | 70 (100) | 139 (99.3) |
Chi-square test/Fisher’s exact test. T=Thoracic vertebrae, L=Lumbar vertebrae
Table 3.
Atropine and ephedrine usage in two groups of patients
| Group A (n=70), n (%) | Group B (n=70), n (%) | Total (n=140), n (%) | P | |
|---|---|---|---|---|
| Doses of atropine (number of bolus) | ||||
| 0 | 70 (100) | 66 (94.3) | 136 (97.1) | 0.120 |
| 1 | 0 | 4 (5.7) | 4 (2.9) | |
| Doses of ephedrine (number of bolus) | ||||
| 0 | 51 (72.9) | 37 (52.9) | 88 (62.9) | 0.029* |
| 1 | 17 (24.3) | 23 (32.9) | 40 (28.6) | |
| 2 | 1 (1.4) | 7 (10) | 8 (5.7) | |
| 3 | 1 (1.4) | 3 (4.3) | 4 (2.9) | |
| Total patients requiring ephedrine | 19 (27.1) | 33 (47.1) |
Chi-square test/Fisher’s exact test. *P value <0.05 is significant
Table 4.
Comparison of mean arterial pressure (mmHg) distribution in two groups of patients
| MAP (mmHg) | Group A | Group B | Total | P |
|---|---|---|---|---|
| Baseline | 96.29±9.68 | 98.89±8.71 | 97.59±9.27 | 0.097 |
| 0 min | 94.54±9.76 | 97.26±8.29 | 95.90±9.12 | 0.078 |
| 3 min | 90.30±11.21 | 92.39±12.52 | 91.34±11.89 | 0.301 |
| 6 min | 86.34±14.55 | 89.50±12.44 | 87.90±13.59 | 0.173 |
| 9 min | 83.73±12.63 | 86.54±11.75 | 85.14±12.23 | 0.174 |
| 12 min | 83.36±11.27 | 85.75±11.09 | 84.53±11.21 | 0.223 |
| 15 min | 83.08±11.64 | 84.53±11.06 | 83.77±11.34 | 0.480 |
| 18 min | 82.55±11.66 | 84.18±11.13 | 83.30±11.40 | 0.438 |
| 21 min | 83.95±11.39 | 84.48±11.14 | 84.19±11.23 | 0.804 |
| 24 min | 83.81±10.41 | 85.60±10.31 | 84.57±10.36 | 0.392 |
| 27 min | 84.86±10.05 | 84.86±10.07 | 84.86±10.01 | 0.999 |
| 30 min | 85.70±9.67 | 84.12±9.25 | 85.03±9.48 | 0.422 |
Student t-test. MAP=Mean arterial pressure
Graph 1.
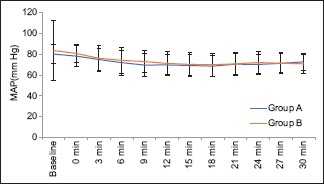
Distribution of mean arterial pressure
Table 5.
Frequency of nausea, vomiting, and shivering distribution in two groups of patients
| Group A (n=70), n (%) | Group B (n=70), n (%) | Total (n=140), n (%) | P | |
|---|---|---|---|---|
| Nausea | 0 | 1 (1.4) | 1 (0.7) | 0.496 |
| Vomiting | 0 | 0 | 0 | - |
| Shivering | 0 | 12 (17.1) | 12 (8.6) | 0.0001* |
*P value <0.05 is significant
DISCUSSION
Spinal anesthesia is a safe anesthetic technique practiced commonly worldwide. Hemodynamic changes such as hypotension and bradycardia occur after spinal anesthesia are usually benign and respond by the fluid therapy and vasopressors. However, rarely, it may cause severe bradycardia and cardiac arrest.[3,4,5,14] Sympathetic blockade causes decrease in systemic vascular resistance, leads to blood redistribution, and finally leads to decrease preload which in turn causes hypotension.[15,16] The decrease in preload stimulates chemoreceptors and mechanoreceptors in the ventricular wall, which are also serotonin sensitive stimulate BJR.[2] Bradycardia is due to parasympathetic overactivity, increased baroreceptor activity, and BJR. Serotonin is an additive trigger to activate BJR in a hypovolemic patient.[1] Hence, measures to prevent or treat the hemodynamic changes caused by spinal anesthesia are required.
Various methods of preventing cardiovascular consequences of subarachnoid block include preloading and coloading with i.v. infusion, administration of sympathomimetic, administration of atropine, and patient positioning facilitating venous return.[2,17,18] Volume preload may cause fluid overload and cardiovascular collapse in labile patients.[19] Prophylactic use of vasopressors has no role in preventing hypotension in turn which may cause HTN and increase cardiac workload.[20,21]
BJR demonstrated by hypotension, bradycardia, and vasodilatation is known to be caused by chemoreceptors and mechanoreceptors but also result by stimulation of 5HT3 receptors in vagal nerve ending. Various studies have proved that chemoreceptors on ventricular wall are serotonin sensitive.[8,21,22]
Tsikouris et al. found that granisetron 5HT3 blocker prevents the incidence of HR fluctuations during head-up tilt table test which might be related to BJR.[23]
In our study, we did not find statistically significant difference between ondansetron and control groups in SBP, DBP, and MAP. However, we found that 19 (27.1%) patients in Group A and 33 (47%) patients in saline group required ephedrine with statistically significant P = 0.029. As we have considered a decrease in >20% basal MAP as hypotension for the use of ephedrine at any of the 3 min time interval in the span of 30 min, possibly our results showed significant difference in vasopressor usage.
Four (5.7%) patients in Group B and no patients in Group A had episode of bradycardia (HR <45 b.p.m or fall >20% of basal HR) and required atropine which is not statistically significant with P = 0.12.
In the study conducted by Marashi et al., they compared two different doses of ondansetron 6 mg, 12 mg with placebo group (210 patients). 12% of patients in control group had hypotension and required vasopressors. 45% had shivering, 14% had bradycardia.[1] Our study has shown similar results in vasopressor consumption but no difference in results with regard to bradycardia.
In the study conducted by Trabelsi et al., in 80 parturients posted for elective lower segment caesarean section, they found that vasopressor consumption is significantly more in saline group as compared with the ondansetron group (P < 0.0001).[6]
In a study conducted by Rashad and Farmawy in 60 parturient females undergoing elective cesarean section, they concluded that patients who received i.v. ondansetron 4 mg before subarachnoid block significantly decreased both the hypotension and the doses of vasopressors consumption (P = 0.005).[14]
In the study conducted by Owczuk et al., they premedicated 71 patients who were undergoing surgeries under subarachnoid block with i.v. ondansetron 8 mg and found that there was a significant difference between MAP and SBP between study and control group, but there was no significant difference in HR.[2]
A meta-analysis conducted by Gao et al. included 10 randomized controlled trials with 863 patients who underwent surgical procedures under spinal anesthesia. This database review suggested that prophylactic administration of i.v. ondansetron reduces the incidence of spinal anesthesia-induced hypotension and vasopressor consumption in both obstetric and nonobstetric patients.[24]
In the study conducted by Fassoulaki et al., they concluded that ondansetron antagonizes the sensory block produced by lignocaine which may be the potential reason to explain about attenuation of hemodynamic response by ondansetron. However, in our study, we have not observed any significant difference in sensory level attained between ondansetron group and control group. However, we have not monitored the regression of spinal anesthesia. There was no difference seen in Bromage scale grade in both the groups.[15]
In a study conducted by Terkawi et al. in 86 parturients for cesarean section who were premedicated with i.v. ondansetron 8 mg, they showed no significant difference in SBP, DBP, MAP, and HR between the ondansetron and placebo groups.[25] Our results are similar to the above study whereas the consumption of vasopressors is significantly less in ondansetron group.
Shivering is a response to hypothermia caused by spinal anesthesia. Shivering may cause increased metabolic activity and oxygen consumption consequently may cause lactic acidosis and arterial hypoxemia.[1,26] Spinal anesthesia causes internal redistribution of blood from core to peripheral compartment. Loss of vasoconstriction capacity postsubarachnoid block causes increased heat loss from patient body surface mainly from lower extremities. Serotonin is a thermoregulatory neurotransmitter which decreases the core temperature and triggers shivering. Ondansetron is inhibitor of 5HT3 system which attenuates the serotonin-induced effects on thermoregulation. Ondansetron inhibits the serotogenic actions at the level of hypothalamus where bulk of thermoregulatory control occurs.[1] Shivering is more deleterious in older patients with low cardiopulmonary reserve, so preventing perioperative shivering plays a significant role in patient outcome.
In our study, we found that 12 (17%) patients had shivering in control group whereas none in ondansetron group (Group A) had shivering (P = 0.0001). In a study conducted by Marashi et al. in 210 patients divided into three groups, they compared 6 mg and 12 mg of ondansetron with a control group (Group B). They have not found any significant difference in incidence of shivering between two ondansetron groups, but 32 (45%) patients had incidence of shivering in control group.[1]
In a study by Kalkasha et al., in 75 patients undergoing spinal anesthesia, they compared incidence of shivering in patients who received i.v. ondansetron 8 mg and meperidine 0.4 mg/kg immediately before spinal anesthesia. They concluded that ondansetron has similar effects such as meperidine in reducing incidence of shivering.[27]
Nausea and vomiting commonly occur during spinal anesthesia with incidence of 18% and 7%, respectively. Unopposed vagal activity occurs by sympathetic blockade and cerebral hypoxia caused by spinal anesthesia-induced hypotension are some of the mechanisms involved in spinal anesthesia-induced nausea and vomiting.[4,10] 5-HT type serotonin receptors are present peripherally on vagal nerve terminals and centrally on the chemoreceptor trigger zone of the area postrema, which is known to be associated with nausea and vomiting.[28]
In a study conducted by Rashad and Farmawy in 60 parturients, they concluded that i.v. ondansetron prevents spinal anesthesia induced nausea as compared with saline group having P = 0.008.[14]
However, in our study, no patients in ondansetron group (Group A) and only one patient (1.4%) in control group (Group B) had nausea with a P = 0.452. None of the patients in either group had incidence of vomiting. There is no significant difference in incidence of nausea and vomiting between ondansetron group and saline group. Our results are similar to the results of Terkawi et al., who conducted study on 68 parturients and concluded that premedicating patients with ondansetron 8 mg before spinal anesthesia has no role in preventing nausea and vomiting.[25]
The main aim of our study is to bring forward how a very routinely used anti-emetic drug ondansetron can attenuate hemodynamic disturbances caused by spinal anesthesia and there are more chances of tachyphylaxis with routinely used drugs such as mephentermine. This study focused on anti-shivering effect of ondansetron because shivering has very deleterious effect especially in geriatric patients which will interfere with postoperative monitoring of electrocardiogram and pulse oximetry.
This study and the finding will be very useful in pregnant patients where the use of vasopressors and sympathomimetic effects the uterine contractions and uterine blood flow and elderly patients who cannot tolerate excessive fluid administration as therapeutic measure of hypotension due to ongoing cardiovascular decomposition.
One limitation of our study was we used fixed dose of ondansetron 4 mg in all patients irrespective of weight of patients. Another limitation was we monitored NIBP, probably invasive blood pressure monitoring would have been more reliable. The use sympathomimetics such as ephedrine for treatment of hypotension which has a tendency to increase both blood pressure and HR would have masked the incidence of bradycardia. Hence, this could possibly one of the reasons for not getting significant values in the incidence of bradycardia.
Further studies with larger samples and randomized controlled studies are required to understand the significance of ondansetron effect hypotension and bradycardia in patients undergoing surgeries under subarachnoid block
CONCLUSION
Our study indicates that prophylactic use of i.v. ondansetron 4 mg reduces the requirement of ephedrine in patients undergoing surgeries under subarachnoid block. Ondansetron showed no significant effect on bradycardia in our study. Our study revealed that ondansetron significantly decreases the incidence of shivering after spinal anesthesia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Marashi SM, Soltani-Omid S, Soltani Mohammadi S, Aghajani Y, Movafegh A. Comparing two different doses of intravenous ondansetron with placebo on attenuation of spinal-induced hypotension and shivering. Anesth Pain Med. 2014;4:e12055. doi: 10.5812/aapm.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owczuk R, Wenski W, Polak-Krzeminska A, Twardowski P, Arszułowicz R, Dylczyk-Sommer A, et al. Ondansetron given intravenously attenuates arterial blood pressure drop due to spinal anesthesia: A double-blind, placebo-controlled study. Reg Anesth Pain Med. 2008;33:332–9. doi: 10.1016/j.rapm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Yamano M, Ito H, Kamato T, Miyata K. Characteristics of inhibitory effects of serotonin (5-HT) 3-receptor antagonists, YM060 and YM114 (KAE-393), on the von Bezold-Jarisch reflex induced by 2-methyl-5-HT, veratridine and electrical stimulation of vagus nerves in anesthetized rats. Jpn J Pharmacol. 1995;69:351–6. doi: 10.1254/jjp.69.351. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter RL, Caplan RA, Brown DL, Stephenson C, Wu R. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992;76:906–16. doi: 10.1097/00000542-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kinsella SM, Tuckey JP. Perioperative bradycardia and asystole: Relationship to vasovagal syncope and the Bezold-Jarisch reflex. Br J Anaesth. 2001;86:859–68. doi: 10.1093/bja/86.6.859. [DOI] [PubMed] [Google Scholar]
- 6.Trabelsi W, Romdhani C, Elaskri H, Sammoud W, Bensalah M, Labbene I, et al. Effect of ondansetron on the occurrence of hypotension and on neonatal parameters during spinal anesthesia for elective caesarean section: A Prospective, randomized, controlled, double-blind study. Anesthesiol Res Pract. 2015;2015:158061. doi: 10.1155/2015/158061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nallam SR, Dara S. Effect of intravenous ondansetron on reducing the incidence of hypotension and bradycardia events during shoulder arthroscopy in sitting position under interscalene brachial plexus block: A prospective randomized trial. Indian J Anaesth. 2015;59:353–8. doi: 10.4103/0019-5049.158739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campagna JA, Carter C. Clinical relevance of the Bezold-Jarisch reflex. Anesthesiology. 2003;98:1250–60. doi: 10.1097/00000542-200305000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Zhuo L, Wang Q, Shen MK, Yu YY, Yu JJ, et al. Efficacy of prophylactic intravenous ondansetron on the prevention of hypotension during cesarean delivery: A dose-dependent study. Int J Clin Exp Med. 2014;7:5210–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Shen Z, You J, Zhu X, Tang QF. Effect of ondansetron in preventing postoperative nausea and vomiting under different conditions of general anesthesia: A preliminary, randomized, controlled study. Ups J Med Sci. 2013;118:87–90. doi: 10.3109/03009734.2013.768315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McQuaid KR. Drugs used in the treatment of gastrointestinal diseases. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and Clinical Pharmacology. 12th ed. New York: McGraw Hill; 2012. pp. 1097–9. [Google Scholar]
- 12.Sanders-Bush E, Mayer SE. 5HT (Serotoinin) receptor agonists and antagonists. In: Bruton LL, Lazo JS, editors. The Pharmacological Basis of Therapeutics, Goodman and Gillman's. 11th ed. New York: McGraw Hill; 2006. pp. 297–315. [Google Scholar]
- 13.Villalón CM, Centurión D. Cardiovascular responses produced by 5-hydroxytriptamine: A pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:45–63. doi: 10.1007/s00210-007-0179-1. [DOI] [PubMed] [Google Scholar]
- 14.Rashad MM, Farmawy MS. Effects of intravenous ondansetron and granisetron on hemodynamic changes and motar and sensory blockade induces by spinal anesthesia in parturients undergoing caesarean section. Egypt J Anaesth. 2013;29:369–74. [Google Scholar]
- 15.Fassoulaki A, Melemeni A, Zotou M, Sarantopoulos C. Systemic ondansetron antagonizes the sensory block produced by intrathecal lidocaine. Anesth Analg. 2005;100:1817–21. doi: 10.1213/01.ANE.0000152616.57107.F6. [DOI] [PubMed] [Google Scholar]
- 16.Lim HH, Ho KM, Choi WY, Teoh GS, Chiu KY. The use of intravenous atropine after a saline infusion in the prevention of spinal anesthesia-induced hypotension in elderly patients. Anesth Analg. 2000;91:1203–6. doi: 10.1097/00000539-200011000-00029. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Zhuo L, Shen MK, Yu YY, Yu JJ, Wang M, et al. Ondansetron preloading with crystalloid infusion reduces maternal hypotension during cesarean delivery. Am J Perinatol. 2014;31:913–22. doi: 10.1055/s-0033-1364189. [DOI] [PubMed] [Google Scholar]
- 18.Somboonviboon W, Kyokong O, Charuluxananan S, Narasethakamol A. Incidence and risk factors of hypotension and bradycardia after spinal anesthesia for cesarean section. J Med Assoc Thai. 2008;91:181–7. [PubMed] [Google Scholar]
- 19.Morgan P. The role of vasopressors in the management of hypotension induced by spinal and epidural anaesthesia. Can J Anaesth. 1994;41:404–13. doi: 10.1007/BF03009863. [DOI] [PubMed] [Google Scholar]
- 20.Liu SS, McDonald SB. Current issues in spinal anesthesia. Anesthesiology. 2001;94:888–906. doi: 10.1097/00000542-200105000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Watts SW, Davis RP. 5-hydroxtryptamine receptors in systemic hypertension: An arterial focus. Cardiovasc Ther. 2011;29:54–67. doi: 10.1111/j.1755-5922.2010.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinsella SM. Reflex bradycardia and asystole during anaesthesia. Saudi J Anaesth. 2009;3:35–8. [Google Scholar]
- 23.Tsikouris JP, Kluger J, Chow MS, White CM. Usefulness of intravenous granisetron for prevention of neurally mediated hypotension upon head upright tilt testing. Am J Cardiol. 2000;85:1262–4. doi: 10.1016/s0002-9149(00)00743-8. [DOI] [PubMed] [Google Scholar]
- 24.Gao L, Zheng G, Han J, Wang Y, Zheng J. Effects of prophylactic ondansetron on spinal anesthesia-induced hypotension: A meta-analysis. Int J Obstet Anesth. 2015;24:335–43. doi: 10.1016/j.ijoa.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Terkawi AS, Tiouririne M, Mehta SH, Hackworth JM, Tsang S, Durieux ME, et al. Ondansetron does not attenuate hemodynamic changes in patients undergoing elective cesarean delivery using subarachnoid anesthesia: A double-blind, placebo-controlled, randomized trial. Reg Anesth Pain Med. 2015;40:344–8. doi: 10.1097/AAP.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 26.Safavi M, Honarmand A, Mohammadsadeqie S. Prophylactic use of intravenous ondansetron versus ketamine – Midazolam combination for prevention of shivering during spinal anesthesia: A randomized double-blind placebo-controlled trial. Adv Biomed Res. 2015;4:207. doi: 10.4103/2277-9175.166143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelsaka E, Baris S, Karakaya D, Sarihasan B. Comparison of ondansetron and meperidine for prevention of shivering in patients undergoing spinal anesthesia. Reg Anesth Pain Med. 2006;31:40–5. doi: 10.1016/j.rapm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Eidi M, Kolahdouzan K, Hosseinzadeh H, Tabaqi R. A comparison of preoperative ondansetron and dexamethasone in the prevention of post-tympanoplasty nausea and vomiting. Iran J Med Sci. 2012;37:166–72. [PMC free article] [PubMed] [Google Scholar]


