Abstract
Technological advances have enabled increasingly sophisticated attempts to remotely monitor heart failure. This should allow earlier identification of decompensation, better adherence to lifestyle changes and medication and interventions (such as diuretic dosage changes) that reduce the need for hospitalisation. This review discusses telemonitoring approaches in heart failure, and the evidence for their impact. It is not difficult to collect data remotely, but converting more data into better decision-making that improves the outcome of care is challenging. Policy-makers and technology companies are enthusiastic about the potential of digital technologies to transform healthcare and bring expertise to the patient, rather than the other way round, but guideline writers are not yet convinced, due to the lack of consistent findings in randomised trials.
Keywords: Remote monitoring, telemonitoring, heart failure, disease management, cardiac implantable electronic devices
Heart failure (HF) is increasing in prevalence globally, and is associated with considerable ill health, healthcare costs and mortality. Prevalence increases steeply with age, and the average age of a person admitted to hospital with decompensation in developed countries such as the UK is in the high 70s.[1] Comorbidity is the rule, with half of hospitalised patients having at least five comorbidities.[2,3]
Frailty is common and, even when HF is diagnosed in the community, almost 10% of patients are admitted as an emergency with worsening symptoms within 1 year.[4] In-hospital mortality is in the range of 5–10% in most series, and emergency readmission within 1 month is as high as 25% in some studies.[5] Length of stay varies between 7 and 11 days in most developed countries, and the overall economic impact on health budgets is therefore substantial. In European and North American countries, approximately 2% of the healthcare budget is spent on HF.[6] In the US, projections suggest that, by 2030, the total cost of HF will increase by almost 130% to US$70 billion annually.[7]
Much attention has focused on identifying decompensation of the HF syndrome before there is a need for emergency hospital admission. International guidelines recommend disease management programmes with education and support for individuals and families who wish to become more skilled in self-monitoring and management.[8–10] The hope is that more intensive monitoring in the community can identify decompensation early, support adherence to lifestyle and medication, and prompt intervention (such as changes to diuretic dosage) in those who are no longer euvolaemic.
Technological advances in the past three decades have allowed increasingly sophisticated attempts to remotely monitor and manage the HF syndrome. Simple, telephone-call based, remote assessment by a HF nurse specialist, standalone home-based systems, implanted devices (such as cardiac resynchronisation therapy and ICDs) and now wearable technologies have opened up a world of possibilities. It is not difficult to collect data remotely, but it has been a challenge to find a way to integrate such potentially continuous data streams into systems of care, and to convert more data into better decision-making that improves the outcome or experience of care.
Policy-makers and technology companies are enthusiastic about the potential of digital technologies to transform the healthcare system into a more personalised, responsive and effective process that brings the expertise to the patient, rather than the other way round.[11]
The field is rapidly changing, as are the technologies that can be used, and regulators, reimbursement authorities and healthcare professionals often struggle to assess the value of the technologies. Medical guideline writers are sceptical and are lukewarm in their current recommendations (Table 1), on the basis that there is a lack of large-scale, randomised trials that show a consistent effect of the introduction of remote monitoring (RM).
Table 1: Guidelines on Remote Monitoring in Heart Failure.
|
European Society of Cardiology (2016):[8] “Telemedicine in HF, which is also termed remote patient management, has variable clinical trial results. Several meta-analyses suggest clinical benefits, but numerous prospectively initiated clinical trials including >3700 patients have not confirmed this.”
|
|
American College of Cardiology Foundation/American Heart Association Guideline (2013):[9] Systems of Care to Promote Care Coordination for Patients With Chronic HF “The quality of evidence is mixed for specific components of HF clinical management interventions, such as home-based care, disease management, and remote telemonitoring programs… Overall, very few specific interventions have been consistently identified and successfully applied in clinical practice.” Evidence Gaps and Future Research Directions “What is critically needed is an evidence base that clearly identifies best processes of care, especially in the transition from hospital to home.” |
|
Heart Failure Society of America White Paper (2018):[10] “Based on available evidence, routine use of external remote patient management devices is not recommended. Implanted devices that monitor pulmonary arterial pressure and/or other parameters may be beneficial in selected patients or when used in structured programs, but the value of these devices in routine care requires further study. Future research is also warranted to better understand the cost-effectiveness of these devices.” |
HF = heart failure; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction, IN-TIME = INfluence of Home Monitoring on The clinical Management of heart failurE patients with impaired left ventricular function.
This article briefly discusses a variety of telemonitoring approaches that have been used in HF management, and the evidence for their impact.
What is Telemonitoring?
Telemonitoring or RM encompasses the use of audio, video and other telecommunication technologies to monitor patient status at a distance.[12] Examples include:
Structured telephone support for patients from the HF team, typically provided by HF specialist nurses as part of a disease management programme or a post-discharge service.
Standalone devices for use at home which can measure, e.g. blood pressure, heart rate, weight and oxygen saturation (often supplemented by automated questions on a variety of symptoms). Trends in these data or movement of any one variable outside preset limits may be used by the HF team to trigger a variety of actions, including a telephone call or clinic review for further assessment, or recommendations on lifestyle and medication changes, or even urgent admission to hospital (Figure 1).
Cardiac implantable electronic devices, which can provide useful physiological data to aid HF management, either as a dedicated implant to monitor haemodynamics, or a part of the wealth of physiological data recorded by devices such as pacemakers and ICDs, implanted primarily for therapeutic purposes.
Most recently, a range of wearable technologies, including patches, watches or textiles that can monitor, e.g. ECG, body temperature, blood glucose concentration and body posture.
Figure 1: Schema for Remote Monitoring.
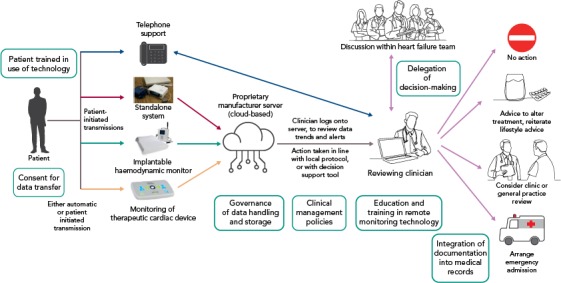
Boxes show key considerations for a remote monitoring clinical service. Arrows indicate the actions taken.
Evidence for the Benefit of Telemonitoring Technologies
Telephone Support
This was one of the earliest methods of RM to be adopted. Patients were called by a member of the HF team to discuss their symptoms and review their compliance with lifestyle measures and drug treatment. Patients could be asked to weigh themselves, which they then verbally reported, or identify when their weight had increased over a set level and contact the HF team for advice. These approaches have become a standard part of disease management programmes, based on the evidence from many relatively small studies showing such programmes reduce all-cause mortality and HF (but not all-cause) hospitalisation rates compared with usual care (Figure 2).[13,14]
Figure 2: Impact of Structured Telephone Support on All-cause Mortality on Meta-analysis.
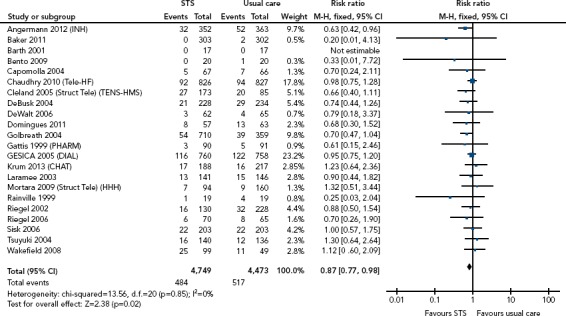
M-H = Mantel Haenszel; STS = structured telephone support. Source: Inglis et al. 2017.[14] Reproduced with permission from BMJ Publishing Group.
However, one of the largest randomised trials of telephone-based HF monitoring (Tele-HF) in the US does not support such an approach.[15] In this study, 1,653 patients were randomised to usual care or a telephone-based interactive voice-response system (Tel-Assurance™, Pharos Innovations), which patients dialled into and were then asked to respond to questions about their symptoms and weight, with the results reviewed by their clinician. There was no significant difference in the primary endpoint of death or hospitalisation within 180 days of enrolment, which occurred in 51.5% and 52.3% of patients respectively (p=0.75). It was also noted that 14% of patients randomised to the intervention never used it and, by the final week of the study, only half of them were still using the system three times per week as instructed. As is often the case with telemonitoring technologies, the initial results from a single highly engaged centre – the initial pilot study showed a 44% decrease in hospitalisation – could not be reproduced when the system was expanded into a much larger, multicentre programme.
Nevertheless, telephone support for patients in a HF programme remains central to many services, but is generally targeted at more unstable patients, those who have recently returned home after an admission for HF or who live at some considerable distance from the HF service.
Standalone Telemonitoring Systems
Standalone systems allow patients, usually in their own homes, to send noninvasively measured data to their healthcare team, by either telephony-based systems or the internet. In many countries, internet access is wireless, and may be via mobile telecommunication networks. The HF team review the data on a regular basis (usually looking for trends over several days) or can be sent an alert if any variable falls outside a preset limit. Action based on the data can be taken at the healthcare professional’s discretion, or there may be a local guideline or protocol that has to be followed.[16]
One of the earliest randomised studies was the Trans-European Network – Home-Care Management System study (TEN-HMS).[17] This study recruited 426 patients with HF with reduced ejection fraction (HFrEF) from across Europe, and randomised them in a 2:2:1 ratio to home telemonitoring with a standalone system, nurse telephone support or usual care. The primary outcome of days lost to death or hospitalisation was not different between the groups, but there was a reduction in the length of hospital stay for the home telemonitoring group and lower mortality in the telephone support and telemonitoring patients compared to usual care. Meta-analysis with other (small) studies suggested mortality benefit and a reduction in HF (but not all-cause) hospitalisation (Figure 3).[14]
Figure 3: Impact of Noninvasive Telemonitoring on All-cause Mortality on Meta-analysis.
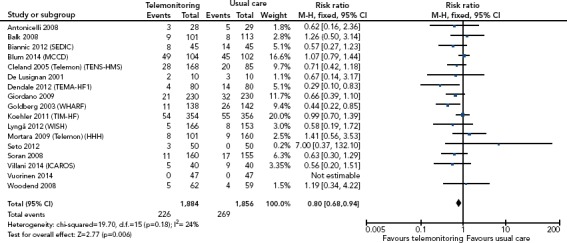
M-H = Mantel Haenszel. Source: Inglis et al. 2017.[14] Reproduced with permission from BMJ Publishing Group.
The first Telemedical Interventional Monitoring in HF (TIM-HF) study with centralised RM run from a telemonitoring centre in Berlin failed to demonstrate any improvement in outcomes in 710 patients randomised and followed up for a minimum of 12 months.[18] However, the larger, follow-on randomised study in 1,571 patients (which required patients to have had a HF hospitalisation in the 12 months preceding enrolment and no evidence of major depression), using a wireless system with a digital tablet to send daily transmissions of weight, blood pressure, heart rate, ECG, oxygen saturation and health status questionnaire, reported a borderline statistically significant reduction of just under 7 days in the number of days lost due to unplanned cardiovascular hospital admissions or death compared to the control group (17.8 versus 24.2 days per year, p=0·046).[19] There was also a significant decrease in the secondary endpoint of all-cause mortality, but not cardiovascular mortality.
Outside this centralised 24/7 telemonitoring service in Germany, other large randomised trials have failed to show benefit. In a study in academic centres in California, an RM approach combined with intensive coaching of patients did not show any improvement in mortality or hospitalisation over a 6-month period.[20]
In the UK, the Whole System Demonstrator project involved the remote exchange of data between 3,230 patients with diabetes, chronic obstructive pulmonary disease or HF in 179 general practices over 1 year in three areas in England. After adjustment for baseline differences, there was a statistically significant reduction in mortality and length of stay for those hospitalised, but no difference in emergency admission rates for those remotely monitored.[21] Overall savings to the healthcare system were small (geometric mean £242 per patient) and cost-effectiveness was poor.[22]
Remote Monitoring Through Therapeutic Cardiac Implantable Electronic Devices
The past decade has seen a revolution in the use of remote monitoring of therapeutic devices, such as pacemakers and defibrillators. RM is now standard in many centres for device function and safety reasons.[23,24] For patients with HF, remote monitoring offers the additional possibility of detecting decompensation earlier.
One of the first intrathoracic technologies developed was Optivol™ (Medtronic), a measure of intrathoracic impedance that is undertaken by direct measurement between the RV lead and pulse generator of the device. The Medtronic Impedance Diagnostics in Heart Failure Trial (Mid-HEFT) was a prospective, observational study investigating the use of intrathoracic impedance as a marker of HF deterioration. Of the 33 patients who had the device implanted, 10 patients had 25 hospitalisations over the course of 21 months’ follow-up.[25] Retrospective review of the impedance data showed a decrease in the 2 weeks preceding HF hospitalisation, well in advance of the symptoms. There was also an increase in intrathoracic impedance as patients underwent diuresis.
This early promise was not confirmed with subsequent larger trials of the technology. Using an alert system to identify when the Optivol score had increased above a predefined threshold that suggested HF deterioration, the Diagnostic Outcome Trial in Heart Failure (DOT-HF) study randomised 335 patients to management with physician and patient access to alerts, or not. The alert arm saw a 79% increase (p=0.02) in the HF hospitalisation rate.[26] Overall, the specificity of the alert system was not acceptable, particularly in the early period after implantation, leading to a high false positive rate and increased hospital admission by the physicians caring for the patients.[27]
Further improvements in the positive predictive value of monitoring have been achieved by adding further parameters into algorithms that incorporate intrathoracic impedance. The Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure (PARTNERS HF) and Integrated Diagnostic for Heart Failure (TRIAGE-HF) trials have shown promise in identifying which patients are at risk of hospitalisation.[28,29]
To date, the Remote Management of Heart Failure Using Implantable Electronic Devices study (REM-HF) is the largest prospective randomised clinical trial conducted on RM through implanted devices.[30] In this trial, 1,650 patients with HF who had an implanted cardiac device were randomised to active weekly review of remote monitoring data or usual care across nine UK hospitals, with an average follow-up of 2.8 years. The primary outcome of death or hospitalisation from cardiovascular causes was the same in the RM group (42.4%) and the control group (40.8%) of patients (p=0.87), despite considerable extra activity being triggered by the remotely collected data.
Thus far, only the INfluence of Home Monitoring on The clinIcal Management of heart failurE patients with impaired left ventricular function (IN-TIME) study has provided prospective randomised data of benefit in clinical outcomes for remote monitoring of implanted devices.[31] For this study, 664 patients were randomly assigned to multiparameter RM in addition to standard care or standard care alone. The composite clinical score, which incorporated all-cause death, HF hospitalisation, change in New York Heart Association (NYHA) class, and change in patient global self-assessment, was better in the RM population, largely driven by a lower death rate in the RM group (estimated 1 year mortality 2.7% versus 6.8% (HR 0.37; 95% CI [0.16–0.83], p=0.012).
The difference in results between REM-HF (UK) and IN-TIME (Europe, Israel and Australia) are as yet unexplained, but may be down to different healthcare settings, lower symptom severity, devolved rather than centralised monitoring of the data and weekly remote monitoring, rather than daily review and intervention in REM-HF compared with IN-TIME. It is likely that the impact of RM is highly context dependent, with the processes that support decision-making on remote data being as important as the data and the monitoring tools themselves.
One solution that combines multiparametric RM with complex data processing is the HeartLogic™ algorithm (Boston Scientific). Developed in 900 patients as part of the Multisensor Chronic Evaluations in Ambulatory Heart Failure Patients (MultiSENSE) study, this algorithm incorporates heart sounds, respiratory rate, heart rate, activity levels and intrathoracic impedance to generate a ‘HeartLogic’ score.[32] The algorithm uses the patient as their own control, calculating changes from baseline, which removes the need for the clinician to assess the different data streams for all patients. Using a preset threshold provides a 70% sensitivity for identifying a HF event (e.g. one that requires HF hospitalisation or IV therapy) with an unexplained alert rate of 1.5 per patient-year. The alert gives a median lead time before a HF event of 34 days, with 90% of patients being alerted 2 weeks before an event.
The Multiple Cardiac Sensors for the Management of Heart Failure (MANAGE-HF) trial, a large, multicentre outcome study recruiting 2,700 patients, is under way to test whether HeartLogic alert-based management can improve mortality and morbidity from HF when used in more routine care (NCT03237858).
Remote Monitoring Through Implantable Haemodynamic Monitors
Implantable devices offer an opportunity to assess disturbances in haemodynamic parameters promptly, rather than relying on the measurement of less direct measures of HF decompensation which may take longer to become abnormal. Left ventricular filling pressure may be the best measure of the control of the HF syndrome, and several technologies have been developed to measure this directly or indirectly. The aim is to then optimise therapy to maintain filling pressure within an optimal range. Meta-analysis of published studies suggests benefit in preventing hospitalisation (Figure 4).[33]
Figure 4: Meta-analysis of Effect of Implantable Haemodynamic Monitoring on Heart Failure Hospitalisation.
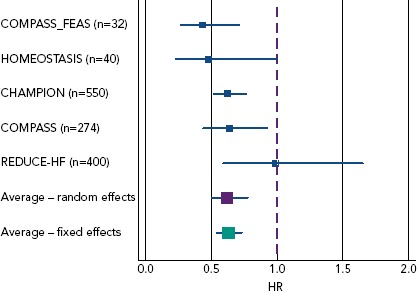
Using a random effect model, the reduction is 38% (HR 0.62, 95% CI [0.50–0.78], p<0.001) and 37% (HR 0.63, 95% CI [0.54–0.73] p<0.001) using a fixed-effects model. Source: Adamson et al. 2016.[33] Reproduced with permission from John Wiley and Sons.
The Chronicle™ implantable haemodynamic monitor (Medtronic) was designed as a subcutaneous device with a transvenous sensor, much like a pacing lead, that could be deployed in the right ventricular (RV) outflow track, measuring RV pressures to estimate pulmonary artery diastolic pressures, alongside recording heart rate, temperature and physical activity. The subcutaneous device transmitted information intermittently to a home monitor, which would upload the information to a remote server for clinicians to review. The Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) study randomised 274 patients who had the device implanted to receive optimal medical care guided by the device (n=134) or a control group with optimal care alone (n=140).[34] While the device met the safety endpoints at 6 months, the reduction in the primary composite outcome of HF-related hospitalisations, emergency-department visits or urgent clinic visits was non-significant at 21% (p=0.33). On the basis on these results, the Food and Drug Administration’s Circulatory System Devices Panel voted against approving the Chronicle device.
The CardioMEMS™ HF System (Abbott Vascular) has Food and Drug Administration approval and a CE mark, and has been implanted in more than 10,000 patients with HF worldwide. The CardioMEMS device is a pulmonary artery wireless microelectromechanical sensor that is implanted using transcatheter techniques and fluoroscopic guidance. The device is permanent and becomes covered with endothelium in the weeks after implantation. A patient electronics system (a pillow-like device) is used to collect daily pulmonary artery haemodynamic measurements, and the patient’s physician or nurse can access the data via a secure internet connection.
The pivotal CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial demonstrated the effectiveness of patient management guided by such daily pulmonary artery pressure readings.[35] In this trial, 550 patients with NYHA class III symptoms who had the sensor implanted were randomised to management guided by readings from the device (n=270) or a control group of standard care (n=280). There was a 33% (95% CI [20–45%]) decrease in HF hospitalisations over an average of 18 months of follow-up in the randomised phase of the study (p<0.0001).[36]
A number of studies assessing the utility of pulmonary artery haemodynamic measurements in a broader cohort of patients and in different healthcare settings are under way. The Hemodynamic-GUIDEd Management of Heart Failure (GUIDE-HF study) (NCT03387813) will enrol 3,600 patients with NYHA class II–IV symptoms in the US, and the CardioMEMS HF System OUS Post Market Study (NCT02954341) and the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF) trial (NCT02693691) will recruit patients in Europe and Australia to investigate effectiveness in these populations outside the US.
A further development in RM through implantable devices was a strategy that focused on empowering the patient in self-management, rather than relying on direct input from their HF clinical team after reviewing remotely collected data. The HeartPOD™ implantable sensor lead (St Jude Medical) allowed measurement of left atrial pressure (LAP) which was then visible to the patient, who could then alter their own treatments based on education delivered to them by the HF team.
The Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy (LAPTOP-HF) study planned to enrol 730 patients (with NYHA class III symptoms and HF hospitalisation in the past 12 months or elevated B-type natriuretic peptide levels) and randomise them to physician-directed patient self-management based on LAP readings taken twice daily or to usual care.[37] The trial was stopped prematurely after 486 patients had been recruited because of an excess of procedure-related complications related to the atrial transseptal puncture. At that point in time, there was a 41% decrease in annualised HF hospitalisations among the patients enrolled (p=0.005).[38]
Wearable Technologies
The concept of multiple sensors contributing to an HF alert system is also the focus of the Wearable Congestive HF Management System (WCHFS, also known as SimpleSENSE), which incorporates various sensors in a wearable undergarment. The observational Nanowear Heart Failure Management Multi-sensor Algorithm (Nanosense) cohort study (NCT03719079) is recruiting patients with the aim of demonstrating which sensors are of diagnostic use in predicting HF deterioration.
This may be the first of many wearables that are developed for remote monitoring of HF, alleviating the need for implants in patients who do not have defibrillators or pacemakers.[39] There are many other mobile health (also called m-health) technologies in development for HF, but evidence on their benefits awaits robust assessment.[40]
Current Challenges and Future Technologies
There is no difficulty in identifying technologies that can accurately measure a physiological variable, or record a patient report of symptoms or quality of life, and accurately transmit this back to the healthcare team. The problem has been in identifying which data point or points provide signal rather than just noise, and identifying when a healthcare team members (or patient themselves) should act. Artificial intelligence may assist human intelligence in this process in the near future.[41,42]
The key question is how these technologies can be used to ensure better, timely decision-making, rather than just to generate a higher workload with more decisions and action to be taken. The huge range of technologies available and the lack of a consistent evidence base is a challenge to the healthcare system, including to those responsible for approving funding. The design of clinical studies to robustly assess impact on clinically important outcomes, patient experience, workflow and cost is evolving, as is the framework of regulators and reimbursement authorities. Challenges remain around what evidence is considered useful by the many stakeholders involved in the process of implementation of RM and other digital technologies into traditional healthcare settings.
Supporting healthcare team members to deal with remotely collected data is essential: who is responsible for looking at the data? How often? What happens out of hours or at the weekend? How is data security maintained? Which patients should be offered which technology (if any), and at what stage in their disease pathway? Clinical guidelines are silent on these issues, and most studies provide scant detail on how the flow of data was integrated in the usual care pathway. In any case, without reimbursement, there is little incentive for a healthcare service to introduce RM, as it may just increase the non-contact workload while reducing income from face-to-face clinical reviews. Figure 1 shows a schema that illustrates some of the key issues that require discussion before a remote monitoring service is established. Very recently, key national and international organisations, as well as health policy-makers, have recognised the challenges around bringing digital technologies into the healthcare system.[43] It is likely that only co-ordinated efforts from all the key stakeholders, including patients themselves, will allow the value of particular technologies to be established.
However, the potential is enormous. If we look at how diabetes is managed, we can see a model where people living with the condition rarely have to seek professional assistance, and how their blood glucose concentrations can easily be incorporated into data platforms that can be accessed by patients, their carers and their healthcare professionals at any point in time and from anywhere there is internet access to the cloud-based server.[44] For those requiring insulin, there are now patches that monitor blood glucose every few minutes, and wirelessly communicate with an insulin pump to help ensure stable blood glucose control.[45] We are far from this situation for HF, which may intrinsically be a more complex syndrome but, undoubtedly, RM will find an important place for those living with HF and the professionals advising them.
References
- 1.National Institute of Cardiovascular Outcomes Research; London: www.nicor.org.uk/wp-content/uploads/2019/02/crm-devices-national-audit-report-2015-16_v2.pdf National Audit of Cardiac Rhythm Management Devices. April 2015–March 2016. NICOR, 2017. Available at: (accessed 6 May 2019) [Google Scholar]
- 2.Centers for Medicare & Medicaid Services; Baltimore, MD: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/2012ChartBook.html Chronic Conditions Among Medicare Beneficiaries. Chartbook: 2012 edition. Centers for Medicare & Medicaid Services, 2012. Available at: (accessed 6 May 2019) [Google Scholar]
- 3.Conrad N, Judge A, Tran J et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–80. doi: 10.1016/S0140-6736(17)32520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottle A, Kim D, Hayhoe B Frailty and comorbidity predict first hospitalisation after heart failure diagnosis in primary care: population-based observational study in England. Age Ageing. 2019. epub ahead of press. [DOI] [PubMed]
- 5.Cowie MR, Anker SD, Cleland JG Improving care for patients with acute heart failure: Before, during and after hospitalisation. Oxford: Oxford PharmaGenesis, 2014. Available at: www.oxfordhealthpolicyforum.org/AHFreport (accessed 6 May 2019) [DOI] [PubMed]
- 6.Soundarraj D, Singh V, Satija V, Thakur RK. Containing the costs of heart failure management: a focus on reducing readmissions. Heart Fail Clin. 2017;13:21–8. doi: 10.1016/j.hfc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Virani SS, Callaway CW et al. Heart disease and stroke statistics – 2018 update. A report from the American Heart Association. Circulation. 2018;137:e67–492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 8.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association of the ESC. Eur Heart J. 2016;14(37):2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson MG, Allen LA, Albert NA et al. Remote monitoring of patients with heart failure: a white paper from the Heart Failure Society of America scientific statements committee. J Card Fail. 2018;24:682–94. doi: 10.1016/j.cardfail.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 11.https://www.gov.uk/government/publications/the-future-of-healthcare-our-vision-for-digital-data-and-technology-in-health-and-care/the-future-of-healthcare-our-vision-for-digital-data-and-technology-in-health-and-care Department of Health and Social Care. Policy paper. The future of healthcare: our vision for digital, data and technology in health and care. London: DHSC, 2018. Available at: (accessed 6 May 2019)
- 12.www.ncbi.nlm.nih.gov/books/NBK45447 Institute of Medicine. Glossary and abbreviations. In: Field MJ (ed). Telemedicine: A Guide to Assessing Telecommunications in Health Care. Washington, DC: National Academy Press, 1996;239–52. Available at: (accessed 6 May 2019) [PubMed]
- 13.McDonagh TA, Blue L, Clark AL et al. European Society of Cardiology Heart Failure Association standards for delivering heart failure care. Eur J Heart Fail. 2011;13:235–41. doi: 10.1093/eurjhf/hfq221. [DOI] [PubMed] [Google Scholar]
- 14.Inglis SC, Clark RA, Dierckx R et al. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Heart. 2017;103:255–7. doi: 10.1136/heartjnl-2015-309191. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhry SI, Mattera JA, Curtis JP et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–9. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley JP, Cowie MR. Telemonitoring in heart failure. Heart. 2009;95:1964–8. doi: 10.1136/hrt.2007.139378. [DOI] [PubMed] [Google Scholar]
- 17.Cleland JG, Louis AA, Rigby AS et al. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admissions and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45:1654–64. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 18.Koehler F, Winkler S, Schieber M et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123:1873–80. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 19.Koehler F, Koehler K, Deckwart O et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group unmasked trial. Lancet. 2018;392:1047–57. doi: 10.1016/S0140-6736(18)31880-4. [DOI] [PubMed] [Google Scholar]
- 20.Ong MK, Romano PS, Edgington S et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition – Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176:310–8. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steventon A, Bardsley M, Billings J et al. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ. 2012;344:e3874. doi: 10.1136/bmj.e3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson C, Knapp M, Fernández JL et al. Cost effectiveness of telehealth for patients with long term conditions (Whole System Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ. 2013;346:f1035. doi: 10.1136/bmj.f1035. [DOI] [PubMed] [Google Scholar]
- 23.Hernández-Madrid A, Lewalter T, Proclemer A et al. Remote monitoring of cardiac implantable electronic devices in Europe: results of the European Heart Rhythm Association survey. Europace. 2014;16:129–32. doi: 10.1093/europace/eut414. [DOI] [PubMed] [Google Scholar]
- 24.Varma N, Epstein AE, Irimpen A et al. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325–32. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 25.Yu CM, Wang L, Chau E et al. Intrathoracic impedance monitoring in patients with heart failure. Circulation. 2005;112:841–8. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 26.Van Veldhuisen DJ, Braunschweig F, Conraads V et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–26. doi: 10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 27.Conraads VM, Tavazzi L, Santini M et al. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J. 2011;32:2266–73. doi: 10.1093/eurheartj/ehr050. [DOI] [PubMed] [Google Scholar]
- 28.Whellan DJ, Ousdigian KT, Al-Khatib SM et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010;55:1803–10. doi: 10.1016/j.jacc.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 29.Virani SA, Sharma V, McCann M et al. Prospective evaluation of integrated device diagnostics for heart failure management: results of the TRIAGE-HF study. ESC Heart Fail. 2018;5:809–7. doi: 10.1002/ehf2.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan JM, Kitt S, Gill J et al. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38:2352–60. doi: 10.1093/eurheartj/ehx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hindricks G, Taborsky M, Glikson M et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384:583–90. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 32.Boehmer JP, Hariharan R, Devecchi FG et al. A multisensory algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail. 2017;5:216–25. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Adamson PB, Ginn G, Anker SD et al. Remote haemodynamic-guided care for patients with chronic heart failure: a meta-analysis of completed trials. Eur J Heart Fail. 2017;19:426–33. doi: 10.1002/ejhf.638. [DOI] [PubMed] [Google Scholar]
- 34.Bourge RC, Abraham WT, Adamson PB et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073–9. doi: 10.1016/j.jacc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 35.Adamson PB, Abraham WT, Aaron M et al. CHAMPION trial rationale and design: the long-term safety and clinical efficacy of a wireless pulmonary artery pressure monitoring system. J Card Fail. 2011;17:3–10. doi: 10.1016/j.cardfail.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Abraham WT, Stevenson LW, Bourge RC et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–61. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 37.Maurer MS, Adamson PB, Costanzo MR et al. Rationale and design of the Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy Study (LAPTOP-HF) J Card Fail. 2015;21:479–88. doi: 10.1016/j.cardfail.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Abraham WT, Adamson PB, Costanzo MR et al. Hemodynamic monitoring in advanced heart failure: results from the LAPTOP-HF trial. J Card Fail. 2016;22:940. doi: 10.1016/j.cardfail.2016.09.012. [DOI] [Google Scholar]
- 39.Bonato P. Advances in wearable technology and its medical applications. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2021–4. doi: 10.1109/IEMBS.2010.5628037. [DOI] [PubMed] [Google Scholar]
- 40.Cajita M, Gleason KT, Han HR. A systematic review of mHealth-based heart failure interventions. J Cardiovasc Nurs. 2016;31:E10–22. doi: 10.1097/JCN.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrès E, Talha S, Zulfiqar AA et al. Current research and new perspectives of telemedicine in chronic heart failure: narrative review and points of interest for the clinician. J Clin Med. 2018;7:E544. doi: 10.3390/jcm7120544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonderman D. Artificial intelligence in cardiology. Wien Klin Wochenschr. 2017;129:866–8. doi: 10.1007/s00508-017-1275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.www.euro.who.int/en/publications/abstracts/from-innovation-to-implementation-ehealth-in-the-who-european-region-2016 WHO Regional Office for Europe. From Innovation to Implementation: eHealth in the WHO European Region. Denmark; WHO, 2016. Available at: (accessed 6 May 2019)
- 44.https://www.mydiabetesmyway.scot.nhs.uk My Diabetes My Way. About us. Available at: (accessed 6 May 2019)
- 45.https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130007S016B.pdf Food and Drug Administration. Summary of safety and effectiveness data (SSED). OneTouch Vibe™ Plus system. Washington, DC: FDA, 2016. Available at: (accessed 6 May 2019)


