Abstract
Although the prevalence of anomalous coronary artery from the opposite sinus (ACAOS) in the general population is low, more frequent use of invasive and non-invasive imaging to rule out coronary artery disease has seen an increase in absolute numbers of ACAOS. ACAOS are traditionally classified as malignant (with an interarterial course) and benign variants. Malignant variants have been recognised in autopsy studies to be an underlying cause of sudden cardiac death in young athletes. Conversely, it seems that older people with ACAOS are less predisposed to adverse cardiac events. Non-invasive anatomic imaging is complementary to invasive imaging and helps to further identify high-risk anatomic features. Using functional non-invasive perfusion imaging can assess potential ischaemia induced by dynamic compression of malignant ACAOS. Information gained from clinical imaging guides the management of these patients.
Keywords: Anomalous coronary artery from the opposite sinus of Valsalva, coronary artery anomaly, sudden cardiac death, non-invasive imaging
Anomalous origin of the coronary artery from the opposite sinus of Valsalva (ACAOS) is a rare inborn disease that is characterised by an anomalous course and/or termination of a native coronary vessel.[1,2] The traditional definition of ACAOS differentiates between benign and malignant variants. Malignant ACAOS has an interarterial course (IAC) of the anomalous vessel between the pulmonary artery and the aorta. Benign variants represent all other courses, such as in front of the pulmonary artery (prepulmonic) or a retroaortic course.[2] The term malignant originates from autopsy studies in young athletes, where ACAOS with IAC was found to be a potential underlying cause of sudden cardiac death (SCD).[3] It is suggested that people with ACAOS and IAC may be at risk for myocardial ischaemia and consecutive arrhythmia, even in the absence of atherosclerotic lesions, as the anomalous vessel is prone to dynamic compression during physical exercise. Beside the classical malignant variant of ACAOS with IAC, other anatomic high-risk features are known, such as a slit-like ostium, acute take-off angle, or an intramural course where the proximal part of the anomalous vessel passes in the tunica media of the aortic wall, with proximal narrowing and an elliptic vessel shape.[1,4]
The prevalence of ACAOS in the general population is about 1%.[5] A recent study from 2018 screened 5,169 children aged between 11 and 18 years old with cardiac MRI (CMR) and showed a prevalence of ACAOS with intramural course of 0.44%.[6] In a study from our centre evaluating mainly middle-aged and older patients with suspected coronary artery disease (CAD) undergoing coronary CT angiography (CCTA), 66 of the 5,634 evaluated patients showed an ACAOS. Of these, 36 patients had an ACAOS with IAC – a prevalence of 0.64%.[2] Shi et al. demonstrated that CCTA outperforms invasive angiography to identify the origin and course of ACAOS due to its high spatial and temporal resolution.[7] The 3D visualisation enabled by CCTA makes this imaging modality superior to other techniques such as echocardiography and it has become the primary investigative tool in patients with ACAOS.[4]
Doctors are seeing more patients with ACAOS because it is detected incidentally with the increasing use of CCTA, echocardiography and CMR to evaluate symptomatic patients. Accurate assessment is crucial to guide the management of affected patients and determine their sports activity, invasive treatment versus surgical correction and medical treatment.
Risk of Adverse Cardiovascular Events in ACAOS
It has been reported that strenuous physical exercise is associated with an increased risk of SCD in people with ACAOS. Autopsy studies from the US and Europe have shown an association of sports-related SCD with ACAOS in young athletes.[3,8–10] In most of the sports-related SCD cases, ACAOS showed an IAC of the anomalous vessel.[11] In a study analysing autopsy findings among young athletes with sports-related SCD in Switzerland, 7% of the cases had ACAOS.[12] In large study of young military recruits in the US, left-ACAOS (L-ACAOS) describing take-off of the left coronary artery from the right coronary sinus of Valsalva with IAC of the anomalous vessel, was a frequent underlying cause of SCD.[13] Although L-ACAOS is primarily associated with cardiac events, they also occur in people with right-ACAOS (R-ACAOS) with the right coronary artery originating from the left coronary sinus of Valsalva.[14] Although there is a risk for SCD among young athletes with ACAOS, the absolute calculated risk is low. Using data from Maron et al. and Brothers et al., the calculated cumulative risk of SCD over a 20-year period in children and young adults with ACAOS (aged 15–35 years) participating in competitive sports was 6.3% for L-ACAOS and 0.2% for R-ACAOS.[10,15,16] Even though these analyses are prone to ascertainment bias as well as underreporting, it does seem that the risk of SCD attributed to ACAOS is far lower than that reported in autopsy series.[3,8–10]
Pathophysiology of ACAOS
The underlying mechanisms of ACAOS leading to adverse cardiac events are not clearly understood. It has been suggested that high-risk anatomic features may contribute to repetitive coronary insufficiency under strenuous physical activity with consecutive ischaemia, scarring and ventricular arrhythmia. The pathomechanism of high-risk anatomic features during physical exercise (when tachycardia and hemodynamic overload induce increased myocardial demand while decreasing coronary flow) include kinking of the acute take-off angled anomalous vessel, lateral compression of the aorta on the intramural segment, intermittent closure of the slit-like orifice and compression of the interarterial segment by the aorta and pulmonary artery. Probably the most threatening feature is the intramural course and its length.[3,8,9,13] Therefore, using the differentiation between intramural versus non-intramural might be more accurate than using interarterial versus non-interarterial for the classification of malignant versus benign variants.
For some patients with L-ACAOS who have angina at rest and no relevant coronary artery stenosis, one commonly invoked hypothesis is that coronary spasm causes intermittent narrowing of the artery.[17] In clinical practice, spasm is not observed in L-ACAOS with intramural course unless cannulation during a procedure inadvertently results in trauma.[17] In ACAOS with intramural course, the artery is embedded in the aortic tunica media, which cannot cause coronary spasm because it has elastic tissue but no functional smooth muscle cells. Recently, Angelini et al. used acetylcholine to test for endothelial dysfunction in L-ACAOS patients with suggestive symptoms, especially in prepulmonic and retroaortic cases, which do not exhibit any identifiable mechanism of intrinsic ischaemia. When spasms occurred in such cases, they were usually diffuse, reproduced by intracoronary acetylcholine infusion and resolved quickly after administration of nitroglycerine.[17] Implementing the recent evidence, a coronary artery anomaly can therefore only be considered benign if beside the non-interarterial course other high-risk features are absent, namely there is no slit-like ostium, a normal take-off angle, no intramural course, no proximal elliptic vessel shape/proximal narrowing and no evidence of anomalous vessel-induced ischaemia, spasm or scar.
Whether the associated risk of ACAOS in young athletes is also applicable to symptomatic middle-aged or older people with newly diagnosed ACAOS among patients undergoing non-invasive imaging to rule out CAD, is unknown. Our group published data from 66 patients with a mean age of 56 (±11) years with unknown coronary anatomy and newly detected ACAOS found by CCTA and compared with their outcome to a cohort score-matched for age, gender, history of coronary revascularisation and segment stenosis. At a median follow-up of 4 years, the occurrence of major adverse cardiac events (MI, revascularisation and cardiac death) were not significantly different between the groups. The annual event rate of ACAOS versus controls was not significantly different and was 4.9% versus 4.8% with a hazard ratio of 0.94 (95%; CI: 0.39–2.28; p=0.89). Similarly, in a subgroup analysis, the annual event rate of ACAOS with IAC (n=40) was not significantly different compared with matched controls (5.2% versus 4.3%). The hazard ratio was 1.01 (95% CI [0.39–2.58]; p=0.99) of ACAOS with IAC.[18] Only three patients underwent surgery and the other patients were treated conservatively. It is unclear whether older patients with ACAOS are less susceptible to adverse cardiac events or whether a selection bias towards low-risk patients who survived beyond early adulthood may have influenced our results.
Although our analysis represents the largest study of outcomes, the patient number is still small. Nevertheless, it may be hypothesised that with ageing for cardiovascular adverse events due to ACAOS becomes less relevant against the background of the increasing risk of CAD related cardiac events.[19] Therefore surgical treatment of ACAOS in this age group might not be mandatory in all cases.[20] When analysing a subgroup of this middle-aged population with ACAOS who underwent hybrid imaging with CCTA and single photon emission CT (SPECT), MI or myocardial scar due to ACAOS was rare and was more likely attributable to concomitant CAD.[19] When analysing sports behaviour in this age group, we found that in those with newly detected ACAOS, sporting activity at the time of diagnosis and after a median follow-up of 4.2 years did not differ significantly. This was not the case in patients engaged in competitive sports (17.5% versus 12.7%, p=0.62) nor those engaged in recreational sports (58.7% versus 61.9%, p=0.86).[21] The IAC of the anomalous vessel did not have an impact either on sport-related symptoms, and outcomes were favorable in all athletes irrespective of surgical correction.[21] This emphasises that middle-aged or older people with newly detected ACAOS do not automatically need to restrict sports activity and should be assessed differently compared with younger people.
Diagnosis of ACAOS
There is not a typical clinical presentation for ACAOS and in some cases, SCD or aborted SCD is the first presentation. While some patients present with chest pain, syncope or arrhythmia, most are asymptomatic and ACAOS will be detected incidentally during invasive or non-invasive cardiac imaging performed for other reasons, for example because of electrocardiogram changes or heart murmurs. Screening people for ACAOS is not recommended. However, CCTA is the primary imaging tool to evaluate high-risk anatomic features in patients with either a suspected or incidental finding of ACAOS followed by non-invasive ischaemic testing (Figure 1).[4] The different anatomic high-risk features, possible pathophysiology and preferred imaging modalities to evaluate ACAOS can be found in Table 1. For non-invasive testing for ischaemia, physical exercise with maximal heart rate is preferred to mimic real-life conditions. Alternatively, an inotropic and chronotropic agent, such as dobutamine stress testing, with SPECT, PET, CMR or echocardiography, can be performed to imitate physical exercise. CMR can be used as an alternative to CCTA to describe high-risk anatomic features. It has a slightly lower spatial resolution than CCTA and it can assess valvular function, ventricular function, regional contractility, and myocardial viability.[22] Cardiac catheterisation can be performed in people with ACAOS if the anatomy cannot be defined with non-invasive imaging and in adults with high probability for coexistent CAD. As the standard distribution models of myocardial perfusion territories subtended by coronary arteries do not correspond well with individual anatomy in patients with ACAOS, hybrid imaging, using CCTA/SPECT or CCTA/PET may help to non-invasively discriminate the impact of ACAOS on myocardial ischaemia from concomitant CAD (Figure 2).[19,23]
Figure 1: A Case of Atypical Chest Pain.
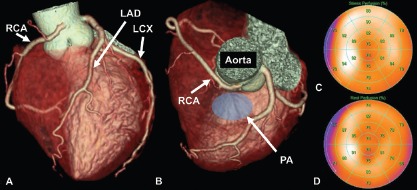
This is a case of a middle-aged patient with atypical chest pain. A coronary CT angiography was performed to rule out coronary artery disease. It showed a RCA originating from the left coronary sinus with an interarterial course of the anomalous vessel between the aorta and the pulmonary artery (A, B). A physical stress test using single-photon emission CT was performed to rule out ischaemia due to possible dynamic compression of the anomalous coronary vessel during exercise. There was no perfusion defect detected under maximal stress conditions (heart rate was 112% of maximal predicted heart rate; C) and under rest conditions (D). The anomalous coronary artery was interpreted as a coincidental finding and the patient was conservatively managed. No symptoms were reported at 1-year follow-up. LAD = left anterior descending artery; LCX = left circumflex artery; PA = pulmonary artery; RCA = right coronary artery.
Table 1: Different Anatomic Features and Imaging Modalities for Evaluation of ACAOS.
| Anatomic feature | Rating of imaging methods used to detect different high-risk features | Possible mechanism of ischaemia | How to diagnose physiological consequence | Correction options |
|---|---|---|---|---|
| Interarterial course | CCTA/CMR>ICA (IVUS)>TTE | Dynamic compression | Re-implantation, pulmonary artery dislocation, unroofing (if intramural segment is present) | |
| Slit-like ostium | CCTA>ICA (IVUS)>CMR | Valve-like occlusion | Unroofing (if intramural segment is present), re-implantation, potentially PCI | |
| Acute take-off angle | CCTA>CMR>TTE>ICA | Kinking | SPECT/PET/CMR (using physical stress or dobutamine), alternatively ICA (FFR) using dobutamine or adenosine | Unroofing (if intramural segment is present), re-implantation, potentially PCI |
| Intramural course | ICA (IVUS)/CCTA>CMR>TTE | Dynamic compression | ||
| Intramural length | ICA (IVUS)/CCTA>CMR>TTE | Dynamic compression | ||
| Diastolic proximal narrowing/elliptic vessel shape | CCTA/ICA (IVUS)>CMR | Dynamic compression under stress | Unroofing, re-implantation, potentially PCI | |
| Systolic proximal narrowing/elliptic vessel shape | ICA (IVUS)>CCTA>CMR | ‘Milking’ and dynamic compression in rest and stress | ICA (IVUS) using dobutamine | |
| Arrhythmogenic substrate | _ | Recurrent intermittent ischaemia leading to myocardial scarring | Fibrosis possibly assessable using CMR | Unclear, potentially medication (beta-blocker) |
CCTA = coronary CT angiography; CMR = cardiac MRI; FFR = fractional flow reserve; ICA = invasive coronary angiography; IVUS = intravascular ultrasound; PCI = percutaneous coronary intervention; SPECT = single photon emission CT; TTE = transthoracic echocardiography.
Figure 2: A Case of Anomalous Origin of Left Circumflex and Left Anterior Descending Arteries.
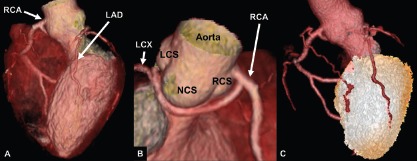
This is a case of an anomalous origin of the LCX and LAD from the RCA with a retroaortic benign vessel course. Hybrid coronary CT angiography with stress single-photon emission CT showed no perfusion defect in the anomalous vessel distribution territory. LAD = left anterior descending artery; LCS = left coronary sinus; LCX = left circumflex artery; NCS = non-coronary sinus; RCA = right coronary artery; RCS = right coronary sinus.
Linsen et al. performed dobutamine stress perfusion CT in a patient with L-ACAOS, but whether this functional imaging modality is a valid alternative for ACAOS needs to be investigated in a larger series with an established standard of reference.[24] As an invasive option, the cross-sectional area of stenosis can be most accurately assessed by using intravascular ultrasound (IVUS) or optical coherence tomography (OTC) to depict the exact proximal anatomy of the anomalous vessel.[25–27] IVUS is highly sensitive and reveals fundamental information on delicate changes of stenosis severity that phasically change during the cardiac cycle and with exercise. Furthermore, fractional flow reserve (FFR) measurement, with the ratio of mean distal (non-ectopic coronary) pressure to mean aortic pressure using adenosine and/or dobutamine and adrenaline, can help to invasively assess the functional severity of stenosis and ischaemia in patients with ACAOS.[25,27,28] However, it must be emphasised that a normal stress test is unable to definitively rule out ACAOS; these tests have a low negative predictive value and are only helpful if they are positive.[16] More studies are needed that compare invasive findings made with IVUS with non-invasive findings as this will help us better understand the pathophysiological consequences of ACAOS, which in turn will affect management options.
Management of Patients with ACAOS
Overview
Treatment options for patients with ACAOS include surgical correction, percutaneous coronary intervention, and medical and conservative treatment with or without sports restriction. The risk calculation and decision for treatment are difficult as there is no uniform way to stratify these patients. In patients with ACAOS and IAC who have symptoms such as chest pain, syncope, ventricular arrhythmia, SCD, or aborted SCD, and/or the presence of ischaemia on stress testing should be restricted from competitive sports and should undergo surgical or percutaneous correction. Resumption of competitive sports should usually be allowed 3 months after the intervention if they have no inducible ischaemia and no symptoms.[29]
The treatment dilemma occurs when asymptomatic patients are newly diagnosed with ACAOS, especially when the patient presents with R-ACAOS and IAC and negative stress testing. The dilemma is even more aggravated if the patient presents with a small R-ACAOS and IAC and left coronary artery dominance with only a small amount of left ventricular myocardium at risk.[30] In these cases, stress testing would most probably not detect any left ventricular ischaemia, and as right ventricular ischaemia is difficult to assess, clinical presentation and occurrence of stress-induced arrhythmias should be included in the decision-making. In these cases, IVUS and FFR measurement can help to evaluate stenosis and ischaemia.[30]
The American Heart Association and American College of Cardiology Scientific Statement differentiates between the much higher-risk L-ACAOS with IAC and the lower-risk R-ACAOS with IAC and states that those with R-ACAOS who are asymptomatic can return to competitive sports after exclusion of ischaemia.[31] L-ACAOS without IAC and R-ACAOS with intraseptal, prepulmonic or retroaortic courses are generally considered benign with scarce reports of cases with ischaemia.[32] These patients are usually not referred for surgery and are not restricted from competitive sports, but stress testing should be negative before allowing them to participate in sports. An extensive discussion regarding sports and the risk and benefits of surgical treatment versus conservative management should take place between the patient, their carers and parents if they are children, and the treating physicians.
Surgical Therapy
The most frequently used and established surgical correction-technique is ‘unroofing’, where the intramural segment in the aorta is opened and a neo-ostium is created (Figure 3). This technique is only appropriate for patients with an intramural segment and unroofing corrects the mechanism of dynamic lateral phasic systolic compression of the arterial wall to the anomalous coronary vessel. The goal of unroofing is not to release the possible compression between the great vessels, but to resect the inner wall of the intramural segment. Alternatively, re-implantation of the aberrant coronary artery can be performed, especially when there is little or no intramural component of the anomalous vessel and when two separate ostiae exist.[16]
Figure 3: A Case of Restrosternal Pressure and Burning.
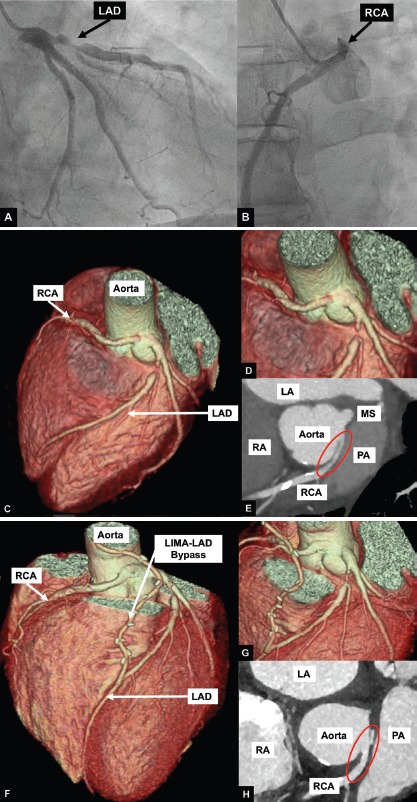
In this case, a 66-year-old man had retrosternal pressure and burning when playing volleyball, hiking or working in his garden. During the treadmill test, he had similar symptoms and the ECG showed non-significant ST depressions. Invasive coronary angiography (A) showed a subtotal occlusion of the proximal LAD and an anomalous origin of the RCA (B). Coronary CT angiography confirmed the subtotal occlusion of the LAD and an RCA originating from the left coronary sinus (C–E) with an interarterial course between the aorta and the pulmonary artery. There was an acute take-off angle (red circle), intramural course with proximal narrowing and elliptic proximal vessel shape. LIMA-LAD bypass grafting was performed (F–G) to treat the subtotal LAD occlusion. Further unroofing of the anomalous RCA vessel was also performed. The intramural segment (H) of the anomalous coronary was unroofed and the common wall debulked and the neo-orifice in the left sinus was created. Bypass grafting of the anomalous vessel was not performed as competitive flow of the native RCA could results in an unfavourable long-term patency of the bypass graft. The patient was symptom-free at follow-up after 2 years. LA = left atrium; LAD = left anterior descending artery; LIMA = left internal mammary artery; PA = pulmonary artery; RA = right atrium; RCA = right coronary artery.
Pulmonary artery translocation is another technique used in patients with ACAOS without an intramural course and single coronary artery. The goal of this operation is to decompress the interarterial course of the anomalous vessel by repositioning the pulmonary artery confluence away from the anomalous artery either laterally or anteriorly.[16] Bypass surgery is usually not recommended as the rate of bypass graft patency is disappointingly low due to competing flow in the native vessel (Figure 3).[4] Although the risk of surgery for patients with ACAOS is very low with excellent outcome at follow-up, the long-term impact of surgery on the coronary arteries is unknown.[33,34]
Percutaneous Coronary Intervention
There are no trials available that compare surgery and percutaneous coronary intervention in patients with ACAOS. However, it appears that percutaneous coronary intervention is a safe and successful alternative in patients with R-ACAOS.[35] In a study including 42 patients with R-ACAOS, percutaneous coronary intervention was successfully performed in all cases and correlated with improved symptoms at more than one-year follow-up in the majority of patients. No ACAOS-related deaths occurred. The in-stent restenosis rate was four out of 30 (13%) at a mean follow-up time of 5 years.[35] Long-term follow-up data for this procedure in adults are limited. A percutaneous intervention of ACAOS is not advisable for children because they are still growing, which presents safety issues.
Medical Therapy
It is unclear whether beta-blockers can be used as an alternative therapy compared to surgical or interventional procedure. In case reports and in a series of 56 middle-aged/older people with ACAOS who were treated conservatively with beta-blockers, no SCD was observed at 5-year follow-up.[20,36] However, these were only R-ACAOS variants and no evidence is available for young people. It is unknown whether patients with an arrhythmogenic substrate due to an ACAOS may benefit from a beta-blocker therapy.
The treatment plan for people with ACAOS should be based on an interdisciplinary decision made between the treating physician, patient, cardiovascular imaging experts, cardiologists and heart surgeons depending on symptoms, age, sports behaviour, anatomic features of ACAOS and ischaemia testing. Follow-up for patients with ACAOS is recommended as short- and mid-term complications may occur, depending on the procedure that was performed. After surgical intervention, aortic valve regurgitation, aortic stenosis, re-stenosis of the anomalous vessel, and ischaemic changes on postoperative provocative testing may occur.[37,38]
The current evidence on therapeutic and follow-up recommendations in patients with ACAOS is scarce and is mainly based on case reports and expert opinions. Prospective randomised trials are lacking but remain difficult to perform due to ethical considerations. In addition, registries or observational trials comparing the different therapeutic techniques, including percutaneous coronary intervention versus surgical treatment are not available. The different therapeutic options should be evaluated in a prospective manner in multicentre studies so that current recommendations can be adapted.
Conclusion
ACAOS with IAC of the anomalous vessel is a potential underlying cause of SCD in young athletes. However, middle-aged and older people with newly detected ACAOS seem to be at lower risk for adverse cardiac events and in most cases may be treated conservatively. A precise anatomic description made using invasive and non-invasive imaging modalities and further functional perfusion assessment can help to stratify a patient’s risk. A recommendation to restrict participation in sports and the anatomical correction of the anomaly should only be made after incorporating all available information such as age, type of symptoms, sports behavior and imaging information, and should be individually balanced against the risk of an interventional procedure. If a decision is made towards anatomical correction, unroofing is a safe, established and preferred surgical procedure in patients with ACAOS and intramural course, whereas percutaneous coronary intervention represents an alternative method in selected cases. More evidence is needed to improve risk stratification in this patient group.
References
- 1.Angelini P et al. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115:1296–305. doi: 10.1161/CIRCULATIONAHA.106.618082. [DOI] [PubMed] [Google Scholar]
- 2.Grani C, Benz DC, Schmied C et al. Prevalence and characteristics of coronary artery anomalies detected by coronary computed tomography angiography in 5,634 consecutive patients in a single centre in Switzerland. Swiss Med Wkly. 2016;146:w14294. doi: 10.4414/smw.2016.14294. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Haas TS, Ahluwalia A et al. Demographics and epidemiology of sudden deaths in young competitive athletes: from the United States National Registry. Am J Med. 2016;129:1170–7. doi: 10.1016/j.amjmed.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Grani C, Buechel RR, Kaufmann PA, Kwong RY et al. Multimodality imaging in individuals with anomalous coronary arteries. JACC Cardiovasc Imaging. 2017;10:471–81. doi: 10.1016/j.jcmg.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka O, Hobbs RE et al. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 6.Angelini P, Cheong BY, Lenge De Rosen VV et al. High-risk cardiovascular conditions in sports-related sudden death: prevalence in 5,169 schoolchildren screened via cardiac magnetic resonance. Tex Heart Inst J. 2018;45:205–13. doi: 10.14503/THIJ-18-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H, Aschoff AJ, Brambs HJ, Hoffmann MH et al. Multislice CT imaging of anomalous coronary arteries. Eur Radiol. 2004;14:2172–81. doi: 10.1007/s00330-004-2490-2. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz EC, Mookadam F, Mookadam M et al. A systematic overview of anomalous coronary anatomy and an examination of the association with sudden cardiac death. Rev Cardiovasc Med. 2006;7:205–13. [PubMed] [Google Scholar]
- 9.Basso C, Maron BJ, Corrado D, Thiene G et al. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–501. doi: 10.1016/S0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Doerer JJ, Haas TS et al. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980 2006. Circulation. 2009;119:1085–92. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Shirani J, Poliac LC et al. Sudden death in young competitive athletes. Clinical demographic, and pathological profiles. JAMA. 1996;276:199–204. doi: 10.1001/jama.1996.03540030033028. [DOI] [PubMed] [Google Scholar]
- 12.Grani C, Chappex N, Fracasso T et al. Sports-related sudden cardiac death in Switzerland classified by static and dynamic components of exercise. Eur J Prev Cardiol. 2016;23:1228–36. doi: 10.1177/2047487316632967. [DOI] [PubMed] [Google Scholar]
- 13.Eckart RE, Scoville SL, Campbell CL et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–34. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 14.Jo Y, Uranaka Y, Iwaki H et al. Sudden cardiac arrest: associated with anomalous origin of the right coronary artery from the left main coronary artery. Tex Heart Inst J. 2011;38:539–43. [PMC free article] [PubMed] [Google Scholar]
- 15.Brothers J, Carter C, McBride M et al. Anomalous left coronary artery origin from the opposite sinus of Valsalva: evidence of intermittent ischemia. J Thorac Cardiovasc Surg. 2010;140:e27–9. doi: 10.1016/j.jtcvs.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 16.Brothers JA, Frommelt MA, Jaquiss RDB et al. Expert consensus guidelines: anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg. 2017;153:1440–57. doi: 10.1016/j.jtcvs.2016.06.066. [DOI] [PubMed] [Google Scholar]
- 17.Angelini P, Uribe C et al. Anatomic spectrum of left coronary artery anomalies and associated mechanisms of coronary insufficiency. Catheter Cardiovasc Interv. 2018;92:313–21. doi: 10.1002/ccd.27656. [DOI] [PubMed] [Google Scholar]
- 18.Grani C, Benz DC, Steffen DA et al. Outcome in middle-aged individuals with anomalous origin of the coronary artery from the opposite sinus: a matched cohort study. Eur Heart J. 2017;38:2009–16. doi: 10.1093/eurheartj/ehx046. [DOI] [PubMed] [Google Scholar]
- 19.Grani C, Benz DC, Schmied C et al. Hybrid CCTA/SPECT myocardial perfusion imaging findings in patients with anomalous origin of coronary arteries from the opposite sinus and suspected concomitant coronary artery disease. J Nucl Cardiol. 2017;24:226–34. doi: 10.1007/s12350-015-0342-x. [DOI] [PubMed] [Google Scholar]
- 20.Kaku B, Shimizu M, Yoshio H et al. Clinical features of prognosis of Japanese patients with anomalous origin of the coronary artery. Jpn Circ J. 1996;60:731–41. doi: 10.1253/jcj.60.731. [DOI] [PubMed] [Google Scholar]
- 21.Grani C, Benz DC, Steffen DA et al. Sports behavior in middle-aged individuals with anomalous coronary artery from the opposite sinus of Valsalva. Cardiology. 2018;139:222–30. doi: 10.1159/000486707. [DOI] [PubMed] [Google Scholar]
- 22.Ripley DP, Saha A, Teis A et al. The distribution and prognosis of anomalous coronary arteries identified by cardiovascular magnetic resonance: 15-year experience from two tertiary centres. J Cardiovasc Magn Reson. 2014;16:34. doi: 10.1186/1532-429X-16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grani C, Benz DC, Possner M et al. Fused cardiac hybrid imaging with coronary computed tomography angiography and positron emission tomography in patients with complex coronary artery anomalies. Congenit Heart Dis. 2017;12:49–57. doi: 10.1111/chd.12402. [DOI] [PubMed] [Google Scholar]
- 24.Linsen PVM, Kofflard MJM, Lam SW, Kock M et al. First in humans: dobutamine stress cardiac computed tomography to evaluate dynamic compression of an anomalous left coronary artery. Coron Artery Dis. 2018;29:607–8. doi: 10.1097/MCA.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal H, Molossi S, Alam M et al. Anomalous coronary arteries and myocardial bridges: risk stratification in children using novel cardiac catheterization techniques. Pediatr Cardiol. 2017;38:624–30. doi: 10.1007/s00246-016-1559-4. [DOI] [PubMed] [Google Scholar]
- 26.Unzue L, Garcia E, Lopez-Melgar B, Agudo-Quilez P. Percutaneous treatment of an anomalous left main arising from the opposite sinus with subpulmonic course. Cardiovasc Revasc Med. 2018;19((5 Pt B)):632–7. doi: 10.1016/j.carrev.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Driesen BW, Warmerdam EG, Sieswerda GT Anomalous coronary artery originating from the opposite sinus of Valsalva (ACAOS), fractional flow reserve-and intravascular ultrasound-guided management in adult patients. Catheter Cardiovasc Interv. 2018. [Epub ahead of print] [DOI] [PubMed]
- 28.Agrawal H, Qureshi AM, Alam M Anomalous aortic origin of a coronary artery with an intraseptal course: novel techniques in haemodynamic assessment. BMJ Case Rep. 2018. [DOI] [PMC free article] [PubMed]
- 29.Warnes CA, Williams RG, Bashore TM et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008;118:e714–833. doi: 10.1136/bcr-2018-225707. [DOI] [PubMed] [Google Scholar]
- 30.Angelini P, Uribe C et al. Symptomatic right coronary anomaly with dynamic systolic intramural obliteration and isolated right ventricular ischemia. Catheter Cardiovasc Interv. 2018;93:445–447. doi: 10.1002/ccd.28028. [DOI] [PubMed] [Google Scholar]
- 31.Van Hare GF, Ackerman MJ, Evangelista JA et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 4: Congenital Heart Disease: A Scientific Statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2372–84. doi: 10.1016/j.jacc.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 32.Kothari SS, Talwar KK, Venugopal P et al. Septal course of the left main coronary artery from right aortic sinus and ventricular tachycardia. Int J Cardiol. 1998;66:207–9. doi: 10.1016/S0167-5273(98)00218-6. [DOI] [PubMed] [Google Scholar]
- 33.Krasuski RA, Magyar D, Hart S et al. Long-term outcome and impact of surgery on adults with coronary arteries originating from the opposite coronary cusp. Circulation. 2011;123:154–62. doi: 10.1161/CIRCULATIONAHA.109.921106. [DOI] [PubMed] [Google Scholar]
- 34.Mery CM, De Leon LE, Molossi S et al. Outcomes of surgical intervention for anomalous aortic origin of a coronary artery: A large contemporary prospective cohort study. J Thorac Cardiovasc Surg. 2018;155:305–19.e4. doi: 10.1016/j.jtcvs.2017.08.116. [DOI] [PubMed] [Google Scholar]
- 35.Angelini P, Uribe C, Monge J et al. Origin of the right coronary artery from the opposite sinus of Valsalva in adults: characterization by intravascular ultrasonography at baseline and after stent angioplasty. Catheter Cardiovasc Interv. 2015;86:199–208. doi: 10.1002/ccd.26069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bixby MB et al. Successful medical management of a patient with an anomalous right coronary artery who declined surgery. Am J Crit Care. 1998;7:393–4. [PubMed] [Google Scholar]
- 37.Nees SN, Flyer JN, Chelliah A et al. Patients with anomalous aortic origin of the coronary artery remain at risk after surgical repair. J Thorac Cardiovasc Surg. 2018;155:2554–64.e3. doi: 10.1016/j.jtcvs.2017.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brothers JA, McBride MG, Seliem MA et al. Evaluation of myocardial ischemia after surgical repair of anomalous aortic origin of a coronary artery in a series of pediatric patients. J Am Coll Cardiol. 2007;50:2078–82. doi: 10.1016/j.jacc.2007.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]


