Summary
Objective
Temporal lobe epilepsy (TLE) is often associated with memory deficits. Reactivation of memory traces in the hippocampus occurs during sharp‐wave ripples (SWRs; 140‐250 Hz). To better understand the mechanisms underlying high‐frequency oscillations and cognitive comorbidities in epilepsy, we evaluated how rigorously identified deep CA1 pyramidal cells (dPCs) discharge during SWRs in control and TLE mice.
Methods
We used the unilateral intraamygdala kainate model of TLE in video–electroencephalography (EEG) verified chronically epileptic adult mice. Local field potential and single‐cell recordings were performed using juxtacellular recordings from awake control and TLE mice resting on a spherical treadmill, followed by post hoc identification of the recorded cells.
Results
Hippocampal SWRs in TLE mice occurred with increased intraripple frequency compared to control mice. The frequency of SWR events was decreased, whereas the overall frequency of SWRs, interictal epileptiform discharges, and high‐frequency ripples (250‐500 Hz) together was not altered. CA1 dPCs in TLE mice showed significantly increased firing during ripples as well as between the ripple events. The strength of ripple modulation of dPC discharges increased in TLE without alteration of the preferred phase of firing during the ripple waves.
Significance
These juxtacellular electrophysiology data obtained from identified CA1 dPCs from chronically epileptic mice are in general agreement with recent findings indicating distortion of normal firing patterns during offline SWRs as a mechanism underlying deficits in memory consolidation in epilepsy. Because the primary seizure focus in our experiments was in the amygdala and we recorded from the CA1 region, these results are also in agreement with the presence of altered high‐frequency oscillations in areas of secondary seizure spread.
Keywords: epilepsy, hippocampus, kainate, memory, oscillation, ripple
1.
Key Points.
CA1 ripples in the normal frequency range (<250 Hz) are persistently altered in the intraamygdala kainate mouse model of chronic temporal lobe epilepsy (TLE)
In vivo juxtacellular recordings were made from post hoc identified deep layer pyramidal cells in CA1
The pyramidal cells fired significantly more during ripples in TLE
The strength of modulation of discharges during ripples increased in TLE, without a change in the preferred phase of firing
The results support distortion of principal cell firing during offline ripples as a potential mechanism underlying memory disturbance in TLE
2. INTRODUCTION
Cognitive comorbidities in individuals with epilepsy often include memory problems.1, 2, 3, 4, 5, 6, 7 However, the mechanisms underlying memory deficits in epilepsy are not understood. Sharp‐wave ripples (SWRs) are high‐frequency (140‐250 Hz) oscillations observed on the local field potential (LFP) recordings in the hippocampus during periods of immobility or slow‐wave sleep, that is, during “offline” behavioral states.8 The SWRs are cognitively important events, because reactivation of spatial and episodic memory traces takes place during SWRs in the form of replayed neuronal firing sequences,9, 10 and interference with SWRs impairs memory.11, 12 The selective replay of specific neuronal sequences is believed to contribute to the stabilization of a memory engram,13, 14, 15 and recent evidence indicates that increased and less‐selective neuronal firing in the hippocampus during SWRs may contribute to the problems of spatial and episodic memory consolidation in epilepsy.16
Given the intense interest and considerable clinical relevance of high‐frequency oscillations in epilepsy,17, 18, 19, 20, 21 and the paucity of data on SWR‐related firing from rigorously identified cells in the chronically epileptic hippocampus, we carried out a series of targeted experiments to investigate the SWR‐related discharges of CA1 pyramidal cells (PCs) in an experimental model of temporal lobe epilepsy (TLE) in mice. There has been increasing recognition that CA1 PCs are heterogeneous, with respect to both firing patterns during network oscillations and limbic circuit heterogeneity.22, 23 We chose to focus on the PCs located in the deep subdivision of the CA1 PC (dPC) layer in order to reduce variability and because these deep cells may be more vulnerable to seizure‐induced injury.24, 25 Compared to superficial PCs (sPCs), dPCs are more likely to have place cells and fire at higher rates, and are more strongly modulated by slow oscillations during rapid eye movement (REM) sleep, and receive stronger innervation from the CA2 area that is thought to be important for SWR initiation.23 To avoid confounds from direct damage to hippocampal cells within the CA1 from chemoconvulsants or seizure‐induced apoptosis, we performed a unilateral injection of kainic acid in the amygdala to generate TLE in mice. In this model of TLE, neuronal cell loss and resultant gliosis occurs in the amygdala and the CA3 subfield of the hippocampus, with relative sparing of the CA1 region.26, 27, 28, 29, 30 Chronic TLE was verified with video–electroencephalography (EEG) for each mouse, and non‐invasive (ie, without disturbance of the intracellular milieu) juxtacellular electrophysiologic recording techniques31 were employed in awake control and TLE mice followed by post hoc identification of the recorded dPCs in CA1.
The results show that in this model of TLE, the frequency of intraripple oscillations are increased, while the frequency of occurrence of ripples is decreased. There is no change in the high‐frequency oscillations when SWRs, epileptiform interictal discharges, and high‐frequency, so‐called fast ripples (FRs; >250 Hz32, 33) are considered together. These results are similar to previous results from kindled rats34 indicating a potential conversion of normal‐frequency ripples to pathologic LFP events. During SWRs, CA1 dPCs fired significantly more, and the enhanced discharges could also be found outside SWRs during periods of immobility. These results obtained from an animal model where the primary epileptogenic insult is outside of the hippocampus (ie, in the amygdala) support the recent findings of Valero et al16 indicating a persistent disturbance of SWR‐related firing of CA1 deep and sPCs in the intraperitoneal injection of kainate model of chronic TLE in rats. Given the known association of TLE with cognition and memory,6, 35, 36, 37, 38, 39, 40 the crucial role of SWRs in the reactivation of memory engram‐related neuronal ensembles offline, and the role of inhibitory interneuron–principal cell interactions in SWR generation,8 our results are consistent with the critical importance of appropriate patterns of network activity for hippocampal memory functions.
3. MATERIALS AND METHODS
All experiments were carried out at the University of California, Irvine, and conducted in accordance with the policies of the Institutional Animal Care and Use Committee at that institution.
3.1. Intraamygdala kainate injection to induce epilepsy
We anesthetized 15‐week‐old male C57BL/6J mice with isoflurane for the surgery. The timeline in Figure S1 describes relative times of kainic acid (KA) injection, EEG recordings, and juxtacellular recordings. Localized KA injections (50‐100 nL, 20 mmol/L KA in saline) were delivered to the right dorsal amygdala (with respect to bregma, 0.94 mm posterior, 2.85 mm to the right; and 4.5 mm ventral). Control mice received a saline‐only injection. All KA‐injected animals developed behavioral status epilepticus, including tonic‐clonic seizures, which we terminated after 40 minutes with a single injection of diazepam (6 mg/kg, i.p.) for consistency and to avoid mortality. Note that electrographic seizures or even subclinical status epilepticus may have continued for a variable amount of time after diazepam administration.41, 42, 43 After the KA injection, mice were singly housed in enriched environments under a 12‐hour light/dark cycle.
3.2. EEG recordings
Twenty weeks after intracranial injection, we implanted a stainless steel head plate to allow for in vivo electrophysiologic recordings (day 1), with the opening 2 mm behind bregma. This was affixed to the skull with a thin layer of cyanoacrylate glue.44 On day 2, we implanted two twisted bipolar electrodes in the left dorsal hippocampus (contralateral to the KA injection site) to verify the presence of spontaneous seizures (with respect to bregma, 2.6 mm posterior, 1.75 mm to the right, and 1.4 mm ventral). Dental cement was used to fill in the head‐stage implant and secure the EEG electrode. Different electrodes were used for electrophysiology to record ripples and single cell spikes (see subsequent text). Postmortem analysis revealed the tip of these electrodes to be either in CA1 or CA3. Video‐EEG monitoring was performed from days 10‐14. EEG data were digitized by an NI USB‐6221‐BNC digitizer (National Instruments) sampled at 500‐1000 Hz.45 Mice were observed with video monitoring for 5 days, 24 hours a day, maintaining the 12‐hour light/dark cycles. Telemetry recording units were located above the cages and mice were allowed to move freely and accessed food and water ad libitum. Continuous EEG signals were analyzed with a custom‐written program in MATLAB for seizure detection only, and then seizures were verified manually by inspecting the EEG trace and the video footage.46 Seizure duration was measured on the EEG. Mouse behavior and severity of seizures were scored according to a modified Racine scale.47, 48 Only seizures reaching class 5 (rearing and falling) were utilized in the analysis. Of note, we have 5 days of full video‐EEG data (presented in Figure 1) for only 7 of 10 epileptic mice due to a software crash, but the three mice for which all of the data were not available were also confirmed to have spontaneous seizures.
Figure 1.
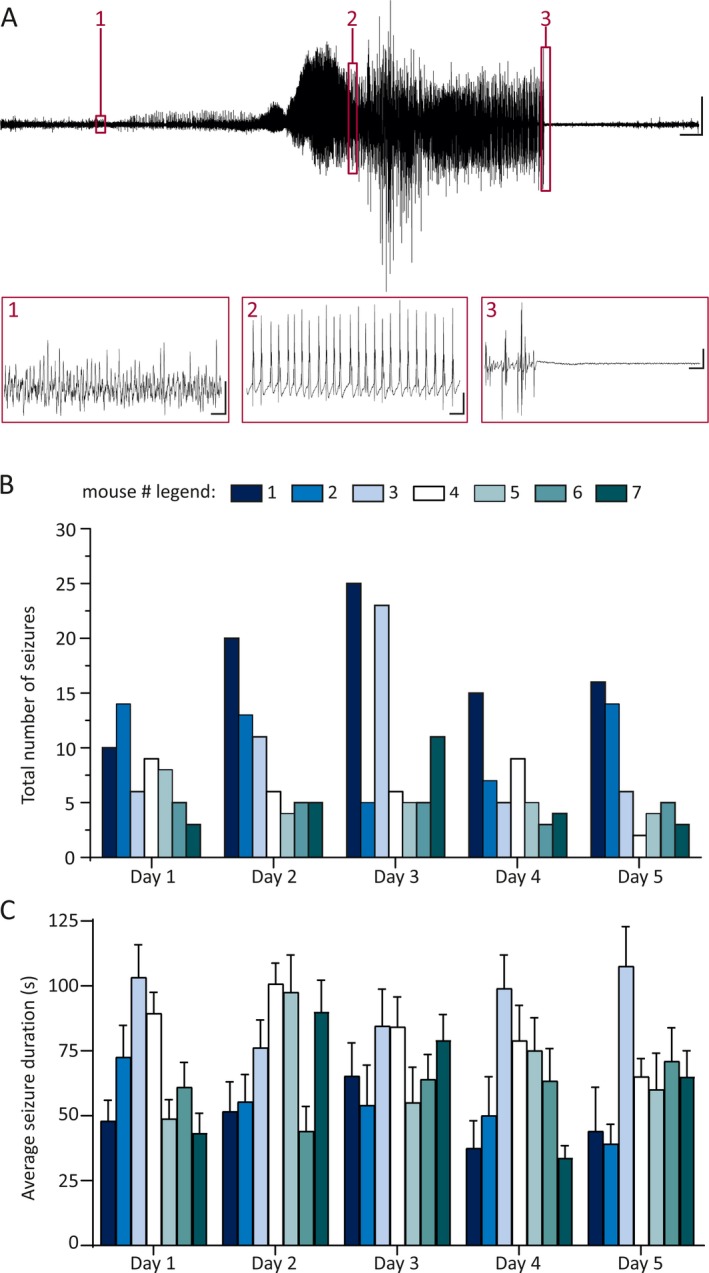
Microinjection of kainic acid (KA) into the amygdala produces frequent, chronic behavioral temporal lobe seizures. A, Top: Representative electroencephalography (EEG) trace during a behavioral (Racine stage V) seizure recorded with a hippocampal depth electrode, 21 weeks after KA injection. Bottom: Numbered and outlined regions of the top trace are enlarged to show detail, in respective numbered boxes. Scale bars: Top—5 s, 500 μV; Box 1—100 msec, 5 μV, Box 2—100 msec, 250 μV, Box 3—100 msec, 250 μV. B, C, Summary plots demonstrate the total number of seizures per day (B) and the mean length of a single seizure (C) in each individual mouse, for the 5 days preceding the in vivo juxtacellular recordings. These graphs refer only to Racine class V, generalized tonic‐clonic seizures. Color legend refers to panels B and C. Note that these graphs only include data from epileptic mice with 5 days of reliable EEG recordings. None of the control mice demonstrated any seizures
3.3. In vivo electrophysiology
On day 15, mice were placed atop a spherical treadmill consisting of a polystyrene ball suspended above a stainless steel bowl for training.49 On day 16, mice underwent concurrent in vivo LFP and juxtacellular recordings while resting on the polystyrene ball. For details of the juxtacellular recordings, please see Supplementary Methods and Figure S1.
3.4. Statistics and data analysis
Statistical significance was determined using GraphPad Prism. Data that met assumptions of normality and equal variance underwent parametric tests. Otherwise, nonparametric equivalents were used. Specific statistical tests used are indicated in the Results section. All data are presented as mean ± standard deviation. We consider P < 0.05 to be significant. Exact P‐values are reported unless P < 0.0001. Calculation of circular statistics was performed in Excel (Microsoft Corp.) via the Watson‐Williams method.50, 51 Analysis of oscillations, interictal discharges (IEDs), and spike timing within the oscillations was performed using custom MATLAB software; for details see Supplementary Methods and Figure S1.
3.5. Immunohistochemical analysis
Two to four hours after labeling of the recorded cell, mice were deeply anesthetized and intracardially perfused (4% paraformaldehyde, 0.05% glutaraldehyde, and 0.2% picric acid). After fixation overnight, brains were sectioned to a thickness of 60‐70 μm using a Leica Vibratome (VT1000s). To visualize filled neurons from juxtacellular recordings, we employed the avidin‐biotin‐peroxidase method (Vectastain elite ABC). All cells used in the analysis were verified as dPCs through this method. The neuron and neurites were traced with a 63× objective and a drawing tube. Digital reconstructions were finalized with Adobe Photoshop CS5. Nissl staining was performed with incubation in 1:100 avidin‐biotinylated‐peroxidase followed by fluorescein isothiocyanate and avidin‐D.
4. RESULTS
4.1. Kainate injection into the amygdala induces chronic spontaneous seizures in mice
Because we were interested in network effects of chronic epilepsy, we performed all experiments 21 weeks after the intraamygdala injection of kainate. Prior studies have shown that spontaneous seizures persist at least up to 3 months after status epilepticus.52, 53 We therefore first obtained EEG recordings to verify that the mice continued to experience spontaneous electroclinical seizures at the 21‐week time point (Figure 1A). Each of the epileptic mice had at least two seizures/d (Figure 1B, 8.5 ± 5.8 seizures/d averaged over 35 mouse‐days of recording). The mean duration of all seizures was 65.5 ± 22.5 seconds (Figure 1C, n = 297 seizures in seven mice). Nissl stains demonstrated profound neuronal loss confined to the ipsilateral amygdala and hippocampal CA3 area, with minimal damage in CA1, similar to prior reports (data not shown27, 28, 29, 54, 55).
4.2. Chronic seizures alter the network properties of sharp wave ripples
We next investigated the characteristics of SWRs in the CA1 region of chronically epileptic mice. SWRs are brief, high‐frequency oscillations (defined as 140‐250 Hz in this article; note that the exact frequency band varies somewhat between studies), which occur in the hippocampus and are critical for memory consolidation.8 In humans, ripples of similar frequency are associated with the seizure‐onset zone in patients undergoing epilepsy surgery.56 Nevertheless, the behavior of SWRs in vivo in animal models of TLE has not yet been elucidated.
We performed LFP recordings in the CA1 of awake, head‐fixed mice during quiet wakefulness (walking/resting). We fine‐tuned the exact location of the recording electrode within the CA1 layer to maximize the amplitude of SWRs. Representative SWRs from control and TLE mice are illustrated in Figure 2A. The frequency of occurrence of SWRs was significantly decreased in TLE mice as compared to controls (Figure 2B, CON: 0.18 ± 0.068 ripples/s, TLE: 0.095 ± 0.036 ripples/s; P = 0.006). The overall duration of each ripple was also shorter in the mice with chronic seizures (Figure 2C, CON: 61.85 ± 4.94 msec, TLE: 53.98, ± 7.92 msec; P = 0.035, unpaired t test). However, the frequency of oscillations within the SWRs was increased in epileptic mice (Figure 2D, CON: 155 ± 9.86 Hz, TLE: 179.2 ± 17.98 Hz; P = 0.006, unpaired t test). The two experimental groups did not demonstrate differences in the number of oscillatory cycles per ripple (Figure 2E, CON: 8.74 ± 0.76 cycles/ripple, TLE: 8.79 ± 1.72 cycles/ripple; P = 0.94, unpaired t test). Given the decrease in the frequency of SWR occurrence, we postulated that this might have been accompanied by the appearance of pathologic oscillations and discharges. Indeed, we detected the occurrence of IEDs and FRs in epileptic, but not control, animals (Figure 2F, CON IED: 0 Hz, 0/7 mice, TLE IED: 0.05 ± 0.05 Hz, 10/10 mice, P = 0.0001; CON FR: 0 Hz, 0/7 mice, TLE FR: 0.024 ± 0.047 Hz, 3/10 mice, mean includes 0 values, P = 0.23, Mann‐Whitney test, example traces Figure 2H). We then compared the overall frequency of all tested oscillations and discharges SWRs, FRs, and IEDs (SFIs) between control and epileptic animals and found no change in the frequency of these groups of events (Figure 2G; CON: 0.177 ± 0.068 Hz; TLE: 0.169 ± 0.089 Hz, P = 0.85, unpaired t test).
Figure 2.
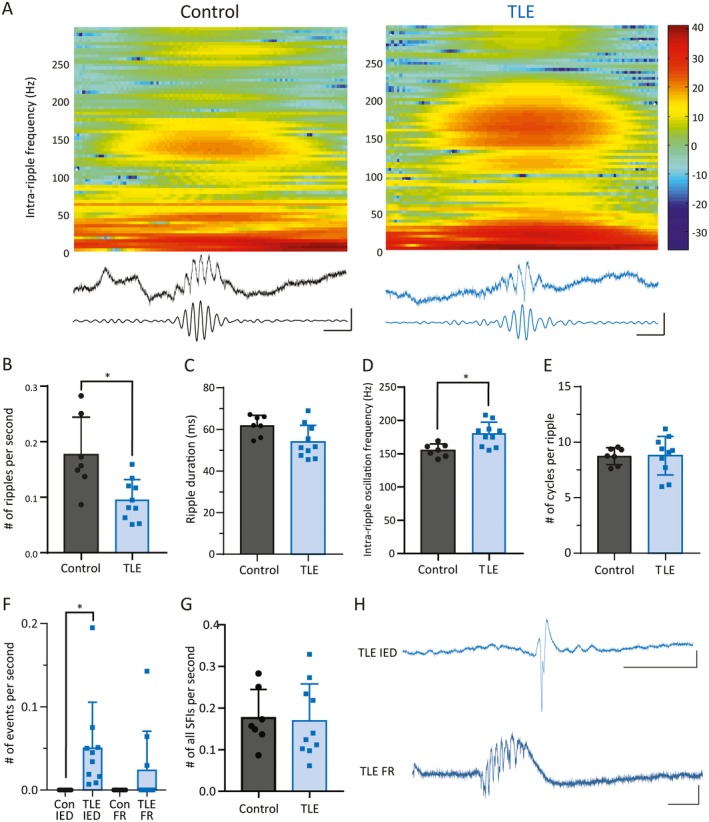
Altered properties of sharp‐wave ripples (SWRs) in temporal lobe epilepsy (TLE). A, A single SWR is demonstrated from a control (left) and a TLE (right) mouse. These are displayed with a spectrogram (top), unfiltered local field potential (LFP) recording (middle), and filtered LFP recording (bottom; filtered at 140‐250 Hz). Bar represents heat‐colored z‐scored power. Scale bars: 50 msec, 0.5 mV. There are fewer ripples in epileptic animals (B), and each ripple is shorter (C). The frequency within the SWR oscillation is significantly higher in the kainate‐injected mice (D). Nevertheless, there is no difference in the number of oscillatory cycles per ripple between genotypes (E). F, Epileptic mice, but not control mice, display interictal epileptiform discharges (IEDs) and fast ripples (FRs; both detected with filtration of 250‐500 Hz). No control animals demonstrated any IEDs or FRs. All 10 epileptic mice demonstrated IEDs, and 3 of 10 epileptic mice demonstrated FRs (note zero values in the TLE FR bar). G, When all high‐frequency oscillations are counted SWRs, FRs, and IEDs (SFIs), there is no difference in the frequency of oscillations between control and epileptic mice. Representative traces of an IED (H, upper panel) and a fast ripple (H, lower panel), each from an epileptic mouse. Scale bars: IED 100 msec, 1 mV, FR 50 msec, 1 mV (* indicates P < 0.05)
4.3. Individual deep CA1 pyramidal cells fire more during sharp‐wave ripples
The preceding results demonstrate that epilepsy alters the network properties of SWRs. Due to the important contribution of CA1 PC firing to the generation of SWRs, we hypothesized that single CA1 cells might fire differently during SWRs after chronic seizures. We chose to specifically record from the deep CA1 pyramidal cells (dPCs; located 20‐40 μm from the stratum radiatum25) in order to have a homogenous cell type to study.
We performed juxtacellular recordings in dPCs simultaneously with the above LFP recordings in 7 control and 10 TLE mice (Figure 3A). CA1 dPCs from epileptic animals demonstrated aberrant sprouting into the stratum pyramidale (Figure 3B: CON: n = 0/7, TLE: n = 4/10 with axonal arborization into stratum pyramidale and stratum radiatum; note that dendritic sprouting may also have taken place but was not quantified). Individual dPCs fired more frequently during these events in the epileptic mice as compared to controls (Figure 3C, CON: 8.72 ± 6.0 Hz, TLE: 23.55 ± 14.81 Hz, P = 0.025, unpaired t test). In addition, the dPC neurons in epileptic animals fired more frequently also between SWRs in epileptic animals (Figure 3D, CON: 0.90 ± 0.79 Hz, TLE: 4.06 ± 4.06 Hz, P = 0.005, Mann‐Whitney test). Thus, there is a general increase in firing of dPCs both during and between SWRs.
Figure 3.
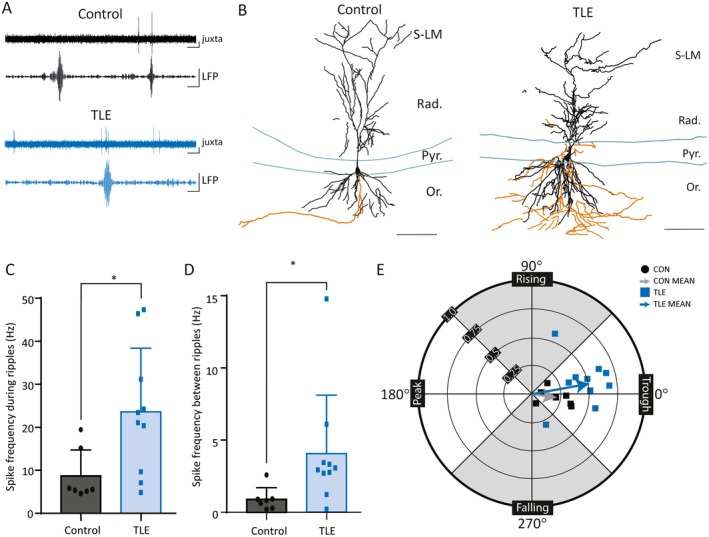
Increased firing of deep CA1 pyramidal cells during sharp‐wave ripples (SWRs) in temporal lobe epilepsy (TLE). A, Concurrent juxtacellular single‐cell activity (juxta) and local field potential network activity (LFP) demonstrating SWRs in control and epileptic mice. LFP traces filtered at 140‐250 Hz. Scale bars: 50 msec, 0.2 mV. B, Camera lucida reconstruction of a Neurobiotin‐filled deep pyramidal cell (dPC) (Vector Labs) in a control mouse (left) and in an epileptic mouse (right). Dendrites (black) and axon (orange) are reconstructed from three consecutive sections of 70 μm each. Layers of CA1 labeled as follows: Or, oriens; Pyr, pyramidal cell layer; Rad, radiatum; S‐LM, stratum lacunosum‐moleculare. Scale bars: 100 μm. C, D, Single cells exhibit increased firing frequency both during (C) and between (D) SWRs in epileptic mice when compared with control (* indicates P < 0.05). E, Wind rose plot in polar coordinates demonstrating the modulation strength (r) and preferred phase of firing (θ) of CA1 dPCs during SWRs in control and epileptic animals. The arrows are vectors representing the mean values. CA1 dPCs fire preferentially during the trough in both groups of animals, but the SWR exerts a higher degree of modulation on firing rate in the TLE animals
Several studies have unequivocally demonstrated that CA1 PCs preferentially fire spikes during the trough phase of SWRs.57, 58 Consistent with prior studies, the phase preference of dPCs in both control and epileptic animals occurs during the trough of the SWR oscillation (Figure 3E; CON: 351.0° ± 27, n = 7, TLE: 10.1° ± 30, n = 10, P = 0.34, Watson‐Williams test). We then determined the modulation strength, that is, the amount of change that the SWR oscillation made to the CA1 firing pattern using custom MATLAB scripts as described previously (see Methods).49 The modulation strength is dependent on the relative distributions of spikes during the oscillatory cycle, not only on the overall firing frequency. There was a significant increase in the strength of modulation of discharges during SWRs in CA1 dPCs from epileptic animals compared to those from control animals (Figure 3E, CON: 0.23 ± 0.12, n = 7; TLE: 0.52 ± 0.13, n = 10; P = 0.0003, unpaired t test). In addition, we found that each individual cell was more likely to fire during a particular SWR in epileptic mice (percent of SWRs in which a given neuron fired at least one spike, CON: 38.9 ± 26.9%, range 21.4%‐78.8%, n = 7; TLE 74.7 ± 30.8%, range 14.2%‐100%, n = 10, P = 0.039). Indeed, in 3 of 10 epileptic mice, but 0 of 7 control mice, the recorded CA1 dPC fired during every single ripple. These results indicate that individual CA1 dPCs are more entrained to the SWRs and more likely to fire during a particular SWR in epileptic animals.
5. DISCUSSION
In this study, we have shown that kainate injection into the amygdala leads to the development of chronic temporal lobe epilepsy in mice, lasting at least up to 21 weeks after the initial convulsive insult. We found that there are fewer SWRs in the hippocampus of amygdala‐kindled epileptic animals than controls, but these SWRs oscillate at higher frequency. The epileptic animals also develop IEDs and FRs that do not occur in control animals. We found that rigorously identified dPCs fire more frequently in epileptic than control animals, and that a given cell is more likely to fire during each ripple, without a change in the phase preferential firing of the recorded cells. Finally, we found that the SWR oscillation has a stronger modulatory effect on the firing of CA1 dPCs in epileptic than control animals.
We found that CA1 dPCs fire much more frequently in TLE. In these chronically epileptic animals, the firing rate is increased by a factor of 4.5 between SWRs and by a factor of 2.7 during SWRs. It has been shown that the timing of dPC spiking is precisely linked to the phase of SWR and is separate from the spike timing of sPCs.59, 60 Therefore, the increased firing of dPCs in TLE may be relaying incorrect sequences during ripple‐related memory replays, and in this manner contributing to memory‐related cognitive dysfunction. In addition, the increased baseline firing rate could have implications for the ability of these cells to function as place cells. DPCs are more likely to participate in the generation of place fields than sPCs,22, 61 and have distinct patterns of efferent projections to cortical and other subcortical areas.23 This alteration of limbic network function could contribute to the memory deficits seen in rodents and patients with chronic epilepsy.
Although we show an overall decrease in SWR frequency in epileptic mice, there are other studies that have shown the opposite. Notably, in the intrahippocampal kainate model of TLE, the rats that go on to develop epilepsy exhibit increased numbers of both ripples (similar to our SWRs) and FRs in the hippocampus.62 The latter experiments were performed during the latent period of epileptogenesis (1‐4 weeks after status). Of note, their results do show a slight down‐trend in the frequency of SWRs and FRs at the end of their recording period (week 5).62 Given that we are looking at dramatically different time points, and that our area of interest is outside of the origin of status (ie, the primary focus), we do not consider our results contradictory. Instead, we emphasize that further studies should investigate the properties of all SFIs together during the initial epileptogenic insult, the latent period, and over time during the phase of chronic seizures, both close to the primary focus and within the secondary seizure spread zone. For example, an interesting hypothesis that should be tested in the future is that the direction of the change in the frequency of ripple occurrence in TLE may be related to the degree of damage to the CA3 network, given that SWRs are thought to be triggered by the discharges of subpopulations of the CA3 pyramidal cells.8
It has been proposed that seizures may represent pathologic perturbations of physiologic brain oscillations.6, 8, 16, 32, 63, 64, 65, 66, 67 In support of this theory, the frequency of hippocampal SWRs decreases concomitantly with an increase in interictal discharges after kindling in rats.34 There is a resultant decrease in the coupling between hippocampal SWRs and neocortical sleep spindles, which may contribute to the deficits in memory and cognition seen in patients with TLE. A detailed analysis of the spectral properties of SWRs and FRs in the intraperitoneal kainate rat model of TLE also revealed a trend toward a decrease of ripple frequency in epileptic when compared to control rats in CA1.16 In the latter study, the ripples in the control rats were similar in frequency to our SWRs (ie, <250 Hz), and the ripples in the epileptic rats had a higher contribution of faster oscillations (250‐600 Hz), similar to our FRs. Juxtacellular recordings in CA1 pyramidal cells showed increased firing during ripples in epileptic compared to control rats,18 similar to our results. Finally, in a study of the Kv1.1α knockout mouse, the epileptic mice demonstrated a “pathologic” oscillation, which consists of a very high frequency oscillation superimposed on SWR‐like events, again suggesting that the pathologic oscillations are derived from alterations to physiologic oscillations.68 In the present study, we found a decrease in the rate of physiologic SWRs accompanied by the appearance of pathologic oscillations including IEDs and FRs and by increased firing of CA1 dPCs during the ripples, which is consistent with these three prior reports. Therefore, we propose that the network that generates physiologic SWRs is damaged during epileptogenesis in such a way that it is less likely to generate functional SWRs and more likely to generate nonfunctional pathologic oscillations, contributing to the cognitive impairment that is so prevalent in TLE.
6. LIMITATIONS
In this study, diazepam was used to terminate behavioral status epilepticus (SE); however, EEG was not performed to ensure cessation of electrographic seizures. If the duration of post‐SE ictal activity was different between animals, this may have been a source of variability in terms of seizure frequency and cell loss. Prior studies of the intraamygdala kainate model have demonstrated that seizures can originate from the amygdala or from the hippocampus,55, 69 but these studies were done less than 6 weeks after the initial insult (ie, SE). Therefore, in our animals, we are not certain whether the seizure focus resides solely in the amygdala, or also in one of the subfields of the hippocampus. Clinical studies suggest that high‐frequency oscillations (including FRs) represent a potential biomarker of epileptogenic tissue.70, 71, 72 Therefore, future studies should be done to elucidate the precise location of the seizure focus in our model and determine whether there is a correlation between seizure focus and the different classes of SFIs.
7. CLINICAL RELEVANCE
We have demonstrated in a chronic model of TLE that the occurrence of SWRs is significantly reduced. These ripples are crucial for memory formation.8 It is possible that the reduction of SWRs contributes to the cognitive problems seen in patients with TLE, whose cognition worsens over time with continued seizures.39 Future studies should determine whether IEDs and FRs are indeed pathologic versions of SWRs. Current antiepileptic drugs are not known to specifically affect the frequency of SWRs,73 but the ketogenic diet may be able to modulate pathologic oscillations.74 The restoration of SWRs to a physiologic state therefore represents an excellent target for the treatment of cognitive dysfunction, one of the most important comorbidities of epilepsy.75
DISCLOSURE
None of the authors has any conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
We would like to thank Dennis Jenkins for assistance with statistical data analysis. The work was supported by US National Institutes of Health (NIH) grants NS94668 (to I.S.). A.A. was supported by the University of Colorado Denver and Children's Hospital Colorado. M.O. was supported by the National Institutes of General Medical Sciences of the NIH (T34GM069337).
Marchionni I, Oberoi M, Soltesz I, Alexander A. Ripple‐related firing of identified deep CA1 pyramidal cells in chronic temporal lobe epilepsy in mice. Epilepsia Open. 2019;4:254–263. 10.1002/epi4.12310
REFERENCES
- 1. Aikia M, Salmenpera T, Partanen K, et al. Verbal memory in newly diagnosed patients and patients with chronic left temporal lobe epilepsy. Epilepsy Behav. 2001;2:20–7. [DOI] [PubMed] [Google Scholar]
- 2. Loiseau P, Strube E, Broustet D, et al. Learning impairment in epileptic patients. Epilepsia. 1983;24:183–92. [DOI] [PubMed] [Google Scholar]
- 3. Nickels KC, Zaccariello MJ, Hamiwka LD, et al. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat Rev Neurol. 2016;12:465–76. [DOI] [PubMed] [Google Scholar]
- 4. Pulliainen V, Kuikka P, Jokelainen M. Motor and cognitive functions in newly diagnosed adult seizure patients before antiepileptic medication. Acta Neurol Scand. 2000;101:73–8. [DOI] [PubMed] [Google Scholar]
- 5. Corcoran R, Thompson P. Epilepsy and poor memory: who complains and what do they mean? Br J Clin Psychol. 1993;32(Pt 2):199–208. [DOI] [PubMed] [Google Scholar]
- 6. Holmes GL. Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disord. 2015;17:101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long‐term forgetting and remote memory impairment. Brain. 2008;131:2243–63. [DOI] [PubMed] [Google Scholar]
- 8. Buzsaki G. Hippocampal sharp wave‐ripple: a cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diba K, Buzsaki G. Forward and reverse hippocampal place‐cell sequences during ripples. Nat Neurosci. 2007;10:1241–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–3. [DOI] [PubMed] [Google Scholar]
- 11. Ego‐Stengel V, Wilson MA. Disruption of ripple‐associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Girardeau G, Benchenane K, Wiener SI, et al. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–3. [DOI] [PubMed] [Google Scholar]
- 13. Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9. [DOI] [PubMed] [Google Scholar]
- 14. Nakashiba T, Buhl DL, McHugh TJ, et al. Hippocampal CA3 output is crucial for ripple‐associated reactivation and consolidation of memory. Neuron. 2009;62:781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valero M, Averkin RG, Fernandez‐Lamo I, et al. Mechanisms for selective single‐cell reactivation during offline sharp‐wave ripples and their distortion by fast ripples. Neuron. 2017;94:1234–47.e1237. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs J, LeVan P, Chander R, et al. Interictal high‐frequency oscillations (80‐500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiruska P, Alvarado‐Rojas C, Schevon CA, et al. Update on the mechanisms and roles of high‐frequency oscillations in seizures and epileptic disorders. Epilepsia. 2017;58:1330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dumpelmann M, Jacobs J, Kerber K, et al. Automatic 80‐250 Hz “ripple” high frequency oscillation detection in invasive subdural grid and strip recordings in epilepsy by a radial basis function neural network. Clin Neurophysiol. 2012;123:1721–31. [DOI] [PubMed] [Google Scholar]
- 20. Hussain SA, Mathern GW, Sankar R, et al. Prospective and “live” fast ripple detection and localization in the operating room: impact on epilepsy surgery outcomes in children. Epilepsy Res. 2016;127:344–51. [DOI] [PubMed] [Google Scholar]
- 21. Worrell GA, Parish L, Cranstoun SD, et al. High‐frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–506. [DOI] [PubMed] [Google Scholar]
- 22. Mizuseki K, Diba K, Pastalkova E, et al. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 2011;14:1174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soltesz I, Losonczy A. CA1 pyramidal cell diversity enabling parallel information processing in the hippocampus. Nat Neurosci. 2018;21:484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Towfighi J, Housman C, Brucklacher R, et al. Neuropathology of seizures in the immature rabbit. Brain Res Dev Brain Res. 2004;152:143–52. [DOI] [PubMed] [Google Scholar]
- 25. Lee SH, Marchionni I, Bezaire M, et al. Parvalbumin‐positive basket cells differentiate among hippocampal pyramidal cells. Neuron. 2014;82:1129–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mouri G, Jimenez‐Mateos E, Engel T, et al. Unilateral hippocampal CA3‐predominant damage and short latency epileptogenesis after intra‐amygdala microinjection of kainic acid in mice. Brain Res. 2008;1213:140–51. [DOI] [PubMed] [Google Scholar]
- 27. Araki T, Simon RP, Taki W, et al. Characterization of neuronal death induced by focally evoked limbic seizures in the C57BL/6 mouse. J Neurosci Res. 2002;69:614–21. [DOI] [PubMed] [Google Scholar]
- 28. Ben‐Ari Y, Lagowska Y, Le Gal La Salle G, et al. Diazepam pretreatment reduces distant hippocampal damage induced by intra‐amygdaloid injections of kainic acid. Eur J Pharmacol. 1978;52:419–20. [DOI] [PubMed] [Google Scholar]
- 29. Lee MC, Ban SS, Woo YJ, et al. Calcium/calmodulin kinase II activity of hippocampus in kainate‐induced epilepsy. J Korean Med Sci. 2001;16:643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levesque M, Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev. 2013;37:2887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinault D. A novel single‐cell staining procedure performed in vivo under electrophysiological control: morpho‐functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods. 1996;65:113–36. [DOI] [PubMed] [Google Scholar]
- 32. Engel J Jr, Bragin A, Staba R, et al. High‐frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. [DOI] [PubMed] [Google Scholar]
- 33. Kohling R, Staley K. Network mechanisms for fast ripple activity in epileptic tissue. Epilepsy Res. 2011;97:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gelinas JN, Khodagholy D, Thesen T, et al. Interictal epileptiform discharges induce hippocampal‐cortical coupling in temporal lobe epilepsy. Nat Med. 2016;22:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inostroza M, Brotons‐Mas JR, Laurent F, et al. Specific impairment of “what‐where‐when” episodic‐like memory in experimental models of temporal lobe epilepsy. J Neurosci. 2013;33:17749–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pearson JN, Rowley S, Liang LP, et al. Reactive oxygen species mediate cognitive deficits in experimental temporal lobe epilepsy. Neurobiol Dis. 2015;82:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rattka M, Brandt C, Loscher W. The intrahippocampal kainate model of temporal lobe epilepsy revisited: epileptogenesis, behavioral and cognitive alterations, pharmacological response, and hippoccampal damage in epileptic rats. Epilepsy Res. 2013;103:135–52. [DOI] [PubMed] [Google Scholar]
- 38. Coras R, Blumcke I. Clinico‐pathological subtypes of hippocampal sclerosis in temporal lobe epilepsy and their differential impact on memory impairment. Neuroscience. 2015;309:153–61. [DOI] [PubMed] [Google Scholar]
- 39. Hermann BP, Seidenberg M, Dow C, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60:80–7. [DOI] [PubMed] [Google Scholar]
- 40. Helmstaedter C. Effects of chronic epilepsy on declarative memory systems. Prog Brain Res. 2002;135:439–53. [DOI] [PubMed] [Google Scholar]
- 41. Vermoesen K, Smolders I, Massie A, et al. The control of kainic acid‐induced status epilepticus. Epilepsy Res. 2010;90:164–6. [DOI] [PubMed] [Google Scholar]
- 42. Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shinoda S, Araki T, Lan JQ, et al. Development of a model of seizure‐induced hippocampal injury with features of programmed cell death in the BALB/c mouse. J Neurosci Res. 2004;76:121–8. [DOI] [PubMed] [Google Scholar]
- 44. Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Armstrong C, Krook‐Magnuson E, Oijala M, et al. Closed‐loop optogenetic intervention in mice. Nat Protoc. 2013;8:1475–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Krook‐Magnuson E, Armstrong C, Oijala M, et al. On‐demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. [DOI] [PubMed] [Google Scholar]
- 48. Borges K, Gearing M, McDermott DL, et al. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. [DOI] [PubMed] [Google Scholar]
- 49. Varga C, Golshani P, Soltesz I. Frequency‐invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc Natl Acad Sci U S A. 2012;109:E2726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berens P. CircStat: a MATLAB toolbox for circular statistics. J Stat Softw. 2009;31: [cited 2018 May 25]. Available fromhttps://www.jstatsoft.org/article/view/v031i10. [Google Scholar]
- 51. Watson G, Williams E. On the construction of significance tests on the circle and the sphere. Biometrika. 1956;43:344–52. [Google Scholar]
- 52. Dunleavy M, Shinoda S, Schindler C, et al. Experimental neonatal status epilepticus and the development of temporal lobe epilepsy with unilateral hippocampal sclerosis. Am J Pathol. 2010;176:330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanaka S, Kondo S, Tanaka T, et al. Long‐term observation of rats after unilateral intra‐amygdaloid injection of kainic acid. Brain Res. 1988;463:163–7. [DOI] [PubMed] [Google Scholar]
- 54. Pollard H, Charriaut‐Marlangue C, Cantagrel S, et al. Kainate‐induced apoptotic cell death in hippocampal neurons. Neuroscience. 1994;63:7–18. [DOI] [PubMed] [Google Scholar]
- 55. Li T, Lytle N, Lan JQ, et al. Local disruption of glial adenosine homeostasis in mice associates with focal electrographic seizures: a first step in epileptogenesis? Glia. 2012;60:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nariai H, Nagasawa T, Juhasz C, et al. Statistical mapping of ictal high‐frequency oscillations in epileptic spasms. Epilepsia. 2011;52:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hulse BK, Moreaux LC, Lubenov EV, et al. Membrane potential dynamics of CA1 pyramidal neurons during hippocampal ripples in awake mice. Neuron. 2016;89:800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Csicsvari J, Hirase H, Czurko A, et al. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J Neurosci. 1999;19:274–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stark E, Roux L, Eichler R, et al. Pyramidal cell‐interneuron interactions underlie hippocampal ripple oscillations. Neuron. 2014;83:467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Valero M, Cid E, Averkin RG, et al. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp‐wave ripples. Nat Neurosci. 2015;18:1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Geiller T, Fattahi M, Choi JS, et al. Place cells are more strongly tied to landmarks in deep than in superficial CA1. Nat Commun. 2017;8:14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li L, Patel M, Almajano J, et al. Extrahippocampal high‐frequency oscillations during epileptogenesis. Epilepsia. 2018;59:e51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Soltesz I, Alger BE, Kano M, et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16:264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62:612–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Timofeev I, Bazhenov M, Seigneur J, et al. Neuronal synchronization and thalamocortical rhythms in sleep, wake and epilepsy In: Noebels JL, Avoli M, Rogawski MA. et al., editor. Jasper's basic mechanisms of the epilepsies. 4th ed Bethesda, MD: National Center for Biotechnology Information, 2012; [cited 2018 Sept 28]. Available from https://www.ncbi.nlm.nih.gov/books/NBK98144/ [PubMed] [Google Scholar]
- 66. Fogerson PM, Huguenard JR. Tapping the brakes: cellular and synaptic mechanisms that regulate thalamic oscillations. Neuron. 2016;92:687–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Broggini ACS, Esteves IM, Romcy‐Pereira RN, et al. Pre‐ictal increase in theta synchrony between the hippocampus and prefrontal cortex in a rat model of temporal lobe epilepsy. Exp Neurol. 2016;279:232–42. [DOI] [PubMed] [Google Scholar]
- 68. Simeone TA, Simeone KA, Samson KK, et al. Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices. Neurobiol Dis. 2013;54:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cavalheiro EA, Riche DA, Le Gal La Salle G. Long‐term effects of intrahippocampal kainic acid injection in rats: a method for inducing spontaneous recurrent seizures. Electroencephalogr Clin Neurophysiol. 1982;53:581–9. [DOI] [PubMed] [Google Scholar]
- 70. Zijlmans M, Jiruska P, Zelmann R, et al. High‐frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Frauscher B, Bartolomei F, Kobayashi K, et al. High‐frequency oscillations: the state of clinical research. Epilepsia. 2017;58:1316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pitkanen A, Loscher W, Vezzani A, et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016;15:843–56. [DOI] [PubMed] [Google Scholar]
- 73. Kudlacek J, Chvojka J, Posusta A, et al. Lacosamide and levetiracetam have no effect on sharp‐wave ripple rate. Front Neurol. 2017;8:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Simeone TA, Samson KK, Matthews SA, et al. In vivo ketogenic diet treatment attenuates pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices from epileptic Kv 1.1alpha knockout mice. Epilepsia. 2014;55:e44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kerr MP, Mensah S, Besag F, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia. 2011;52:2133–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


