Abstract
Objective
The aim of this study was to evaluate the clinical features and treatment outcomes of patients with juvenile myoclonic epilepsy (JME) in western China.
Method
We continuously reviewed one hundred and five outpatients with JME who were diagnosed and treated at the Epilepsy Registration Center of West China Hospital between October 2012 and July 2014. Seizure control stratified into different seizure types and by antiepileptic drugs (AEDs) was prospectively evaluated every 3‐6 months.
Results
Among 105 patients with JME, eighty‐five patients (81%) received monotherapy including valproate (VPA, 47%) and levetiracetam (LEV, 43%) treatment. The rates of seizure freedom 1, 3, and 5 years after the initiation of AED treatment were 64.8% (68/105), 29.5% (31/105), and 14.6% (12/82) in JME patients, respectively. Patients with myoclonic seizure (MS) and absence seizure (AS) were less frequently seizure‐free than those with MS and generalized tonic‐clonic seizure (GTCS) (P = 0.012). Patients on VPA monotherapy had better control of GTCS than patients on LEV monotherapy (P = 0.036). There is a trend of lower rates of seizure freedom in patients treated with LEV than in those treated with VPA after the first‐year treatment period.
Significance
Our data suggest that in JME, seizure control is linked to seizure type, possibly allowing a more individualized approach when counseling JME patients.
Keywords: juvenile myoclonic epilepsy, levetiracetam, seizure outcome, valproate
Key Points.
Seizure type and antiepileptic drugs (AEDs) was prospectively evaluated every 3‐6 months. AEDs were associated with seizure outcomes in juvenile myoclonic epilepsy (JME)
Valproate (VPA) had a better effect than levetiracetam (LEV) on generalized tonic‐clonic seizure (GTCS) control, and no seizure‐free difference between LEV and VPA was found in JME patients with all seizure types
A total of 42 female patients with JME were identified, of which 78.6% were treated with LEV monotherapy
During the first year of the treatment period, LEV‐treated patients showed better seizure control than VPA‐treated patients.
1. INTRODUCTION
Juvenile myoclonic epilepsy (JME) is the most common type of genetic generalized epilepsy (GGE), occurring around puberty. The first patient with JME was described as having no abnormality in intelligence and normal magnetic resonance imaging of the brain by Herpin in 1867.1 In 1985, JME was classified by the International League Against Epilepsy (ILAE) as epilepsies and epileptic syndromes.2 The seizure type of JME was myoclonic seizure (MS), which could be combined with generalized tonic‐clonic seizure (GTCS) observed in 80%‐95% of JME patients, or absence seizure (AS) observed in approximately one‐third of JME patients.3 The typical interictal electroencephalogram (EEG) is characterized by 3‐6 Hz generalized irregular spike‐wave or polyspike‐wave discharges of frontal predominance with a normal background.
Most patients with JME had a good response to appropriate antiepileptic drugs (AEDs), but a high rate of relapse upon AED withdrawal was identified.4, 5 Therefore, it was recommended that patients with JME receive long‐term AED treatment.6 Until recently, several studies have concentrated on the long‐term prognosis of JME.7, 8, 9, 10, 11, 12, 13 A study of 66 JME patients with a mean follow‐up time of 44.6 years reported seizure freedom for at least 5 years in 59.1% of patients.11 Valproate (VPA) was considered the most effective AED in 90% of JME patients despite the risk of teratogenesis and other side effects.14 Recently, the Medicines and Healthcare Products Regulatory Agency (MHRA) banned the antiepileptic drug sodium valproate in the UK in all women of childbearing potential who are not enrolled in a pregnancy prevention program.15 In summary, long‐term treatment must be carefully weighed because of the risks of high seizure relapse in JME.
The aim of our study was to evaluate the seizure outcomes of patients with JME in a large hospital. This was also the first described JME study in western China. Furthermore, the outcomes of JME patients treated with LEV and VPA monotherapy were analyzed.
2. MATERIALS AND METHODS
2.1. Patients
This was a large, single‐center, prospective study. All study populations consisted of epilepsy patients from the Department of Neurology at West China Hospital between October 2012 and July 2014. All patients in this study were followed up every 3‐6 months until July 2017. The diagnosis of patients with JME was decided by two neurologists according to clinical review, medical records, and EEG recordings. The inclusion criteria were as follows: (a) the diagnosis of JME on the basis of ILAE criteria, including patients with childhood absence epilepsy (CAE) emerging to JME16 and (b) patients who received AED treatment in our clinic based on patient and parent decision. The exclusion criteria were as follows: (a) abnormal CT/MRI presentation; (b) unexpected death occurring during the treatment period; (c) development of intracranial infection or injury before the onset of JME; (d) poor treatment compliance by patients; and (e) the presence of other epileptic syndromes, such as eyelid myoclonia with absence seizures. Two patients were excluded due to death: one died of drowning and the other died of status epilepticus. Five patients were excluded because of poor drug adherence (irregular medication or drug discontinuance against the doctor's advice). Finally, 105 JME patients were included in this study.
We reviewed the demographic and clinical data of the patients, including age, gender, age of seizure onset, age at diagnosis, duration of epilepsy, seizure type, AED treatment, family history, and social outcome, from our database. Seizure status and AED treatment and withdrawal were evaluated every 3‐6 months by clinic visits or telephone interview. Every time the study population visited the neurologists, new medical information was recorded on case‐record forms. When patients could not return to the hospital for follow‐up, they were followed up by telephone. In addition, we further analyzed the outcomes of patients treated with LEV and VPA monotherapy and carried out a subgroup analysis based on seizure type.
The enrolled patients were divided into a remission group and an uncontrolled group. The remission group was defined as having had no seizure for at least 12 months at the last follow‐up time, while the remaining patients were considered part of the uncontrolled group. The follow‐up period was determined from the recruitment date (their first clinic attendance) to the last follow‐up by clinic visit or telephone interview.
2.2. Statistical analysis
Microsoft Excel 2013® and SPSS version 20 software (SPSS Inc.) were used for statistical analyses. Chi‐square tests or Fisher's exact tests were used for group comparisons of categorical variables. Nonparametric data were analyzed by the Mann‐Whitney U and t tests. A P value less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Analysis of demographic and clinical data of patients
A total of 105 patients (age range, 16‐41) diagnosed with JME, 54.3% (57/105) of whom were female, were analyzed. The median age of seizure onset was 11.9 ± 4.3 years, and the median age of diagnosis was 14.5 ± 3.7 years. The median age at the last follow‐up was 21.5 ± 5.3 years, with a median epileptic period duration of 9.6 ± 5.6 years. The median follow‐up time was 47.9 ± 7.2 months. A total of 3.8% (4/105) of patients had a family history of epilepsy. The 1‐year, 3‐year, and 5‐year seizure‐free rates of the patients were 64.8% (68/105), 29.5% (31/105), and 14.6% (12/82), respectively.
In total, 81% (85/105) of patients with JME received monotherapy, of whom 40 patients (37 male, three female) were treated with VPA and 37 patients (four male, 33 female) were treated with LEV. In addition, five patients previously treated with VPA (three patients) or LEV (two patients) as monotherapy successfully stopped using AEDs in our study, two of whom did so without consulting a doctor. There was a significant difference in the seizure type of JME between the remission and uncontrolled groups (P = 0.027 < 0.05). A significant difference in the number of patients with MS + GTCS and with MS + AS was also found between the remission and uncontrolled groups (P = 0.012 < 0.05). The demographic and clinical data in patients with JME are summarized in Table 1.
Table 1.
The demographic and clinical characteristics of the two groups
| Variable | Overall | Remission group | Uncontrolled group | P value |
|---|---|---|---|---|
| Number of patients, n (%) | 105 | 68 (64.8%) | 37 (35.2%) | ‐ |
| Age (y) | 21.5 ± 5.3 | 21.2 ± 5.2 | 22.1 ± 5.3 | 0.375 |
| Gender (female to male) | 57/48 | 37/31 | 20/17 | 0.972 |
| Age of seizure onset (y) | 11.9 ± 4.3 | 11.8 ± 4.6 | 12.1 ± 3.8 | 0.778 |
| Age at diagnosis (y) | 14.5 ± 3.7 | 14.3 ± 3.8 | 14.6 ± 3.7 | 0.683 |
| Duration of epilepsy (y) | 9.6 ± 5.6 | 9.3 ± 5.0 | 10.1 ± 6.7 | 0.490 |
| Follow‐up time (mo) | 47.9 ± 7.2 | 48.2 ± 6.6 | 47.4 ± 8.2 | 0.255 |
| Seizure type | 0.027 | |||
| MS only | 11 | 7 | 4 | |
| MS+GTCS | 72 | 52 | 20 | 0.012 |
| MS+AS | 6 | 1 | 5 | |
| MS+GTCS+AS | 16 | 8 | 8 | |
| Monotherapy/polytherapy | 85/20 | 57/12 | 28/8 | 0.550 |
| Monotherapy (female to male) | ||||
| LEV | 37 (33/4) | 28 | 9 | ‐ |
| VPA | 40 (3/37) | 25 | 15 | ‐ |
| LTG | 3 (2/1) | 1 | 2 | ‐ |
| CBZ | 1 (1/0) | 1 | 0 | ‐ |
| TPM | 4 (3/1) | 2 | 2 | ‐ |
| Polytherapy (female to male) | ||||
| VPA+LEV | 6 (5/1) | 4 | 2 | ‐ |
| LEV+LTG | 1 (1/0) | 1 | 0 | ‐ |
| VPA+LTG | 4 (2/2) | 3 | 1 | ‐ |
| LEV+TPM | 4 (3/1) | 3 | 1 | ‐ |
| LEV+OXC | 3 (3/0) | 0 | 3 | ‐ |
| TPM+VPA+LTG | 1 (0/1) | 0 | 1 | ‐ |
| TPM+VPA+OXC | 1 (1/0) | 0 | 1 | ‐ |
| Median dose (mg, d) | ||||
| VPA | 556.8 | 1250.0 | ‐ | |
| LEV | 629.1 | 1263.9 | ‐ | |
| Seizure‐free, n (%) | ||||
| 3 y | 31/105 (29.5%) | ‐ | ||
| 5 y | 12/82(14.6%) | ‐ | ||
| Family history of epilepsy, n (%) | 4 | 3 (2.9%) | 1 (0.9%) | ‐ |
Remission group = no seizure for at least 1 year.
Abbreviations: AS, absence seizure; CBZ, carbamazepine; GTCS, generalized tonic‐clonic seizure; LEV, levetiracetam; LTG, lamotrigine; MS, myoclonic seizure; OXC, oxcarbazepine; TPM, topiramate; VPA, valproate.
3.2. Analysis of the social outcome of patients
The social outcomes of the remission and uncontrolled groups are shown in Table 2. No difference was found in all variables related to the social outcomes of patients with JME between the two groups. It should be noted that 48.5% of patients with JME were students.
Table 2.
Analysis of the social outcomes of patients with JME
| Social outcome | Remission group (n = 68) | Uncontrolled group (n = 37) | P value |
|---|---|---|---|
| Profession, n (%) | |||
| Employed | 22 (32.4%) | 13 (35.1%) | 0.773 |
| Regular work | 13 (19.1%) | 8 (21.6%) | 0.759 |
| Temporary work | 9 (13.2%) | 5 (13.5%) | 0.968 |
| Unemployed | 11 (16.2%) | 8 (21.6%) | 0.489 |
| Students | 35 (51.5%) | 16 (43.2%) | 0.649 |
| Family status, n (%) | |||
| Married | 10 (14.7%) | 6 (16.2%) | 0.837 |
| Children | 8 (11.8%) | 4 (10.8%) | 0.883 |
| Single | 17 (25.0%) | 11 (29.7%) | 0.601 |
| Live‐in relationship | 6 (8.8%) | 2 (5.4%) | 0.71 |
| Monthly family earnings, CNY, n (%) | |||
| <5000 | 33 (48.5%) | 20 (54.1%) | 0.589 |
| 5000‐10 000 | 24 (35.3%) | 11 (29.7%) | 0.505 |
| >10 000 | 6 (8.8%) | 3 (8.1%) | 0.9 |
| Unknown | 5 (7.4%) | 3 (8.1%) | 0.889 |
| Current residence, n (%) | |||
| Countryside | 27 (39.7%) | 19 (51.3%) | 0.251 |
| Urban | 41 (60.3%) | 18 (48.6%) | |
| Driving license, n (%) | |||
| Yes | 15 (22.1%) | 3 (8.1%) | 0.070 |
| Driving | 3 (4.4%) | 1 (2.7%) | ‐ |
Abbreviations: CNY, China Yuan; remission group,no seizure for at least 1 year.
3.3. Outcome of antiepileptic drugs
For patients with all seizure types, there was no difference in the seizure‐free rate between the use of LEV and VPA (P = 0.417 > 0.05). VPA exhibited better GTCS control than LEV (P = 0.036 < 0.05). VPA and LEV showed no significant difference in MS control in patients with JME (P = 0.524). The seizure outcomes of AEDs are presented in Table 3. In addition, there is a trend of lower rates of seizure freedom in patients treated with LEV monotherapy than in those who received VPA monotherapy after the initial treatment period (Figure 1).
Table 3.
Analysis of patients who received levetiracetam (LEV) and valproate (VPA) monotherapy based on seizure type
| Seizure type | Antiepileptic drugs (AEDs | Remission group | Uncontrolled group | P value |
|---|---|---|---|---|
| ALLa | VPA | 21 | 19 | 0.417 |
| LEV | 16 | 21 | ||
| GTCSb | VPA | 27 | 9 | 0.036 |
| LEV | 15 | 15 | ||
| MSc | VPA | 22 | 18 | 0.524 |
| LEV | 23 | 14 |
Remission group = no seizure for at least 2 years.
No seizure for all seizure types in juvenile myoclonic epilepsy (JME) patients.
No seizure for only JME patients with generalized tonic‐clonic seizure.
No seizure for JME patients with MS.
Figure 1.
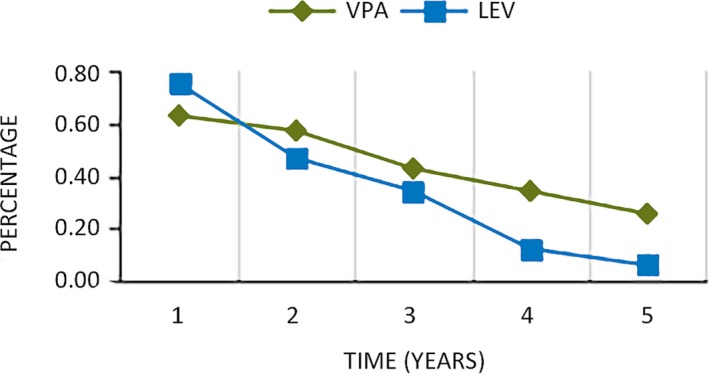
Remission rate of patients treated with valproate (VPA) and levetiracetam (LEV) vs follow‐up period. Time: years without seizure; percentage: remission rate
4. DISCUSSION
This was a prospective, single‐center study in which 105 patients with JME were identified on the basis of the inclusion and exclusion criteria. In total, 64.8%, 29.5%, and 14.6% of patients with JME were seizure‐free for more than 1, 3, and 5 years, respectively. The following are several important findings from this study.
Seizure type was associated with seizure outcome in patients with JME (P = 0.027 < 0.05). A significant difference was found between patients with MS + GTCS and patients with MS + AS in JME (P = 0.012). MS + GTCS in patients with JME was the optimal combination of seizure types for seizure control, which is in line with the results of a previous study.10 In addition, AS showed a negative effect on seizure outcome in JME. A study stated that patients with JME and additional absence seizures might represent a different JME subtype with a worse outcome.11 Another retrospective study reported that patients with all three seizure types were less likely to be seizure‐free in JME.17 However, no difference was found between seizure type and seizure outcome in JME in two other studies.7, 9 The different results of these studies may be due to small sample sizes, different study approaches, and so on.
Another finding in this study showed that patients with GTCS + MS who received VPA were more likely to be seizure‐free than those treated with LEV in JME (P = 0.036 < 0.05). However, for patients with all seizure types, there was no difference between treatment with LEV and treatment with VPA (P = 0.417 > 0.05). Although VPA is the first‐line treatment for patients with JME, the number of patients who received LEV (35.2%) monotherapy was nearly equal to that of patients treated with VPA (38.1%) monotherapy in our study. In addition, most of the female patients (57.9%) received LEV monotherapy in our study because of the link between VPA and teratogenicity. It is noteworthy that the MHRA, the ILAE, and the European Academy of Neurology (EAN) have banned the use of VPA in women and girls.15, 18 LEV, TPM, and LTG were studied as substitutes for VPA in JME treatment in some small randomized studies.19, 20, 21, 22 However, few patients received TPM and LTG in our study. This study confirmed that LEV had a certain effect on patients with JME. Although VPA had a better effect on GTCS control, which was consistent with another study,23 a recent study found that LEV and VPA showed similar retention rates, and LEV showed a good trend for MS control (36.7% [18/49] for VPA, 63.6% [14/22] for LEV, log rank P = 0.085).24 In addition, a study reported the efficacy of LEV in GGE with MS.25 Some studies also reported a good efficacy and tolerability profile for LEV in the treatment of JME.26, 27 Above all, LEV may be a better option for women with JME in view of the side effects of VPA. However, there are still no prospective and controlled studies that directly compare VPA and other AEDs in patients with JME.
It is worth noting that a trend of lower rates of seizure freedom was found in patients who received LEV than in those who took VPA after the initial treatment period (Figure 1). We speculated that patients who received LEV may more easily develop resistance than those who took VPA in JME. The long‐term efficacy of LEV as an add‐on therapy has been confirmed in patients with refractory partial epilepsy.28 However, the effect of LEV as a monotherapy for generalized epilepsy has not yet been identified. Although several studies reported that LEV was effective both as a monotherapy and as an adjunctive therapy in generalized epilepsy, these studies involved a small number of patients and short‐term follow‐up.22, 25, 26, 27, 28, 29 Large samples and long‐term studies are still needed to test LEV monotherapy in generalized epilepsy.
4.1. Limitations
There were several limitations to this study. (a) This was a single‐center, prospective study with a relatively small sample. (b) Side effects, reflex mechanisms, and psychological outcomes of patients with JME were not assessed. (c) The study lacks a control group consisting of patients with a different epilepsy syndrome, the use of which would improve our findings. Therefore, a large multicenter cohort study or a prospective controlled study is still needed to confirm these results.
5. CONCLUSION
We found that seizure type was associated with seizure outcome, that MS + GTCS were the optimal combination of seizure types for seizure control and that AS showed a negative effect on seizure outcome in JME. VPA had a better effect on GTCS control than LEV. These results may allow a more individualized approach when counseling JME patients.
CONFLICT OF INTERESTS
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non‐financial interest in the subject matter or materials discussed in this manuscript. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENT
We sincerely thank all JME patients who participated in this study. This study was supported by the National High Technology Research and Development Program of China (863 Program), (Item Number: 0040205403097).
Zhang Y, Chen J, Ren J, Liua W, Yanga T, Zhoua D. Clinical features and treatment outcomes of Juvenile myoclonic epilepsy patients. Epilepsia Open. 2019;4:302–308. 10.1002/epi4.12321
Contributor Information
Tianhua Yang, Email: yangth0306@yahoo.com.
Dong Zhou, Email: zhoudong66@yahoo.de.
REFERENCES
- 1. Eadie MJ. The epileptology of Theodore Herpin (1799‐1865). Epilepsia. 2002;43:1256–61. [DOI] [PubMed] [Google Scholar]
- 2. Anonymous . Proposal for classification of epilepsies and epileptic syndromes. Epilepsia. 1985;26:268–78. [PubMed] [Google Scholar]
- 3. Genton P, Thomas P, Kasteleijn‐Nolst TD, Medina MT, Salas‐Puig J. Clinical aspects of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S8. [DOI] [PubMed] [Google Scholar]
- 4. Delgadoescueta AV, Enrilebacsal F. Juvenile myoclonic epilepsy of Janz. Neurology. 1984;34:285–94. [DOI] [PubMed] [Google Scholar]
- 5. Panayiotopoulos C, Obeid T, Tahan A. Juvenile myoclonic epilepsy: a 5‐year prospective study. Epilepsia. 1994;35:285–96. [DOI] [PubMed] [Google Scholar]
- 6. Martínez‐Juárez IE, Alonso ME, Medina MT, Durón RM, Bailey JN, López‐Ruiz M, et al. Juvenile myoclonic epilepsy subsyndromes: family studies and long‐term follow‐up. Brain. 2006;129:1269–80. [DOI] [PubMed] [Google Scholar]
- 7. Baykan B, Altindag EA, Bebek N, Ozturk AY, Aslantas B, Gurses C, et al. Myoclonic seizures subside in the fourth decade in juvenile myoclonic epilepsy. Neurology. 2008;70:2123–9. [DOI] [PubMed] [Google Scholar]
- 8. Camfield CS, Camfield PR. Juvenile myoclonic epilepsy 25 years after seizure onset: a population‐based study. Neurology. 2009;73:1041–5. [DOI] [PubMed] [Google Scholar]
- 9. Geithner J, Schneider F, Wang Z, Berneiser J, Herzer R, Kessler C, et al. Predictors for long‐term seizure outcome in juvenile myoclonic epilepsy: 25‐63 years of follow‐up. Epilepsia. 2012;53:1379. [DOI] [PubMed] [Google Scholar]
- 10. Höfler J, Unterberger I, Dobesberger J, Kuchukhidze G, Walser G, Trinka E. Seizure outcome in 175 patients with juvenile myoclonic epilepsy – A long‐term observational study. Epilepsy Res. 2014;108:1817–24. [DOI] [PubMed] [Google Scholar]
- 11. Senf P, Schmitz B, Holtkamp M, Janz D. Prognosis of juvenile myoclonic epilepsy 45 years after onset: seizure outcome and predictors. Neurology. 2013;81:2128. [DOI] [PubMed] [Google Scholar]
- 12. Syvertsen M, Thuve S, Stordrange B, Brodtkorb E. Clinical heterogeneity of juvenile myoclonic epilepsy: follow‐up after an interval of more than 20 years. Seizure. 2014;23:344–8. [DOI] [PubMed] [Google Scholar]
- 13. Trinka E, Baumgartner S, Unterberger I, Unterrainer J, Luef G, Haberlandt E, et al. Long‐term prognosis for childhood and juvenile absence epilepsy. J Neurol. 2004;251:1235–41. [DOI] [PubMed] [Google Scholar]
- 14. Chowdhury A, Brodie M. Pharmacological outcomes in juvenile myoclonic epilepsy: support for sodium valproate. Epilepsy Res. 2016;119:62–6. [DOI] [PubMed] [Google Scholar]
- 15. Iacobucci G. MHRA bans valproate prescribing for women not in pregnancy prevention programme. BMJ. 2018;361:k1823. [DOI] [PubMed] [Google Scholar]
- 16. Engel J. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. [DOI] [PubMed] [Google Scholar]
- 17. Guaranha MS, Filho GM, Lin K, Guilhoto LM, Caboclo LO, Yacubian EM. Prognosis of juvenile myoclonic epilepsy is related to endophenotypes. Seizure. 2011;20:42–8. [DOI] [PubMed] [Google Scholar]
- 18. Tomson T, Marson A, Boon P, Canevini MP, Covanis A, Gaily E, et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia. 2015;56:1006–19. [DOI] [PubMed] [Google Scholar]
- 19. Berkovic SF, Knowlton RC, Leroy RF, Schiemann J, Falter U; Levetiracetam N01057 Study Group . Placebo‐controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology. 2007;69:1751–60. [DOI] [PubMed] [Google Scholar]
- 20. Machado RA, García VF, Astencio AG, Cuartas VB. Efficacy and tolerability of lamotrigine in juvenile myoclonic epilepsy in adults: a prospective, unblinded randomized controlled trial. Seizure. 2013;22:846–55. [DOI] [PubMed] [Google Scholar]
- 21. Mantoan L, Walker M. Treatment options in juvenile myoclonic epilepsy. Curr Treat Options Neurol. 2011;13:355–70. [DOI] [PubMed] [Google Scholar]
- 22. Noachtar S, Andermann E, Meyvisch P, Andermann F, Gough WB, Schiemann‐Delgado J, et al. Levetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizures. Neurology. 2008;70:607–16. [DOI] [PubMed] [Google Scholar]
- 23. Calleja S, Salas‐Puig J, Ribacoba R, Lahoz CH. Evolution of juvenile myoclonic epilepsy treated from the outset with sodium valproate. Seizure. 2001;10:424–308. [DOI] [PubMed] [Google Scholar]
- 24. Sala‐Padró J, Toledo M, Santamarina E, González‐Cuevas M, Raspall‐Chaure M, Sueiras‐Gil M, et al. Levetiracetam and Valproate Retention Rate in Juvenile Myoclonic Epilepsy. Clin Neuropharmacol. 2016;39(6):302. [DOI] [PubMed] [Google Scholar]
- 25. Labate A, Colosimo E, Gambardella A, Leggio U, Ambrosio R, Quattrone A. Levetiracetam in patients with generalised epilepsy and myoclonic seizures: an open label study. Seizure. 2006;15:214–8. [DOI] [PubMed] [Google Scholar]
- 26. Specchio L, Gambardella A, Giallonardo A, Michelucci R, Specchio N, Boero G, et al. Open label, long‐term, pragmatic study on levetiracetam in the treatment of juvenile myoclonic epilepsy. Epilepsy Res. 2006;71:32–9. [DOI] [PubMed] [Google Scholar]
- 27. Specchio LM, Gambardella A, Giallonardo AT, Michelucci R, Specchio N, Boero G, et al. Effects of levetiracetam on EEG abnormalities in juvenile myoclonic epilepsy. Epilepsia. 2008;49:663–9. [DOI] [PubMed] [Google Scholar]
- 28. Bauer J, Ben‐Menachem E, Krämer G, Fryze W, Da SS, Kasteleijn‐Nolst TD. Levetiracetam: a long‐term follow‐up study of efficacy and safety. Acta Neurol Scand. 2006;114:169–76. [DOI] [PubMed] [Google Scholar]
- 29. Verrotti A, Cerminara C, Coppola G, Franzoni E, Parisi P, Iannetti P, et al. Levetiracetam in juvenile myoclonic epilepsy: long‐term efficacy in newly diagnosed adolescents. Dev Med Child Neurol. 2008;50:29–32. [DOI] [PubMed] [Google Scholar]


