Abstract
Macrophages are present in most somatic tissues, where they detect and attack invading pathogens. Macrophages also participate in many nonimmune functions, particularly those related to tissue maintenance and injury response. The sensory organs of the inner ear contain resident populations of macrophages, and additional macrophages enter the ear after acoustic trauma or ototoxicity. As expected, such macrophages participate in the clearance of cellular debris. However, otic macrophages can also influence the long-term survival of both hair cells and afferent neurons after injury. The signals that recruit macrophages into the injured ear, as well as the precise contributions of macrophages to inner ear pathology, remain to be determined.
Macrophages are a class of leukocyte (“white blood cell”) that serve as key effectors of the innate immune system, an evolutionarily ancient cellular defense network that is present in all metazoans (e.g., Buchmann 2014). A primary task of macrophages is to recognize and eliminate invading microbes. To accomplish this, macrophages possess numerous membrane-bound receptors that detect molecules expressed by pathogens. Activation of these receptors initiates signaling cascades that cause macrophages to engulf invading cells and to produce and secrete chemicals that are toxic to these invaders. However, the role of macrophages extends beyond immune response to include normal tissue maintenance. Macrophages can recognize cells undergoing apoptosis and then quickly remove their remnants to maintain normal tissue structure. Cellular injury attracts both resident macrophages as well as additional myeloid-lineage cells from circulation. Together, these cells remove debris and also secrete growth factors and other biologically active molecules that promote repair and regeneration.
The labyrinthine structures of the inner ear are assembled from various types of epithelial and mesenchymal cells. Like most tissues, the inner ear also contains resident macrophages. The function of these cells is still under active investigation, but it is clear that otic macrophages play a role in the ear’s response to injury. Sensory hair cells can be killed by a variety of insults (e.g., noise exposure, ototoxicity) or die as a consequence of normal aging. Hair cell epithelia possess several distinct methods for removing cellular debris (reviewed by Hirose et al. 2017), including macrophage-mediated phagocytosis of dead or dying hair cells (e.g., Bohne 1971; Fredelius and Rask-Andersen 1990). Macrophages also participate in the inflammatory response of the inner ear, which can be triggered by infection or as a result of surgical intervention (i.e., the insertion of a cochlear prosthesis). Comprehensive overviews of inflammatory mechanisms in the cochlea have recently appeared elsewhere (Hirose et al. 2017; Jiang et al. 2017; Kalinec et al. 2017; Wood and Zuo 2017). The present review focuses on how macrophages interact with the sensory receptors and afferent neurons of the inner ear and how such interactions can influence the survival and repair of those cells.
ORIGIN AND DISTRIBUTION OF OTIC MACROPHAGES
The ontogeny of macrophages is complex and occurs at several times and locations in vertebrate embryos. The process has been best characterized in mice, where the earliest macrophages are produced by the division of yolk sac progenitors at ∼E8 (“primitive hematopoiesis”). These cells migrate into the forming embryo where they continue to proliferate and establish residency in numerous tissues. The developmental process culminates in the production of additional tissue macrophages from hematopoietic stem cells that are located in the fetal liver and bone marrow (“definitive hematopoiesis”). Microglia, the resident macrophages of the central nervous system (CNS), are produced slightly earlier; they originate from precursors that leave the yolk sac at ∼E7.5 and migrate into the forming brain (Ginhoux et al. 2010). Once they arrive in the CNS, these precursors continue to divide to produce the entire population of microglia.
The developmental lineage of otic macrophages has not been characterized. Macrophages are observed in contact with the mouse otic vesicle as early as ∼E10 (Hirose et al. 2017), but it is not certain whether these cells go on to colonize the inner ear. It is also not known whether the earliest otic macrophages are produced directly from yolk sac progenitors or from hematopoietic stem cells. The macrophage population in the ear appears to change during embryonic development. In mice, macrophage density in both the organ of Corti and in the spiral ganglion peaks at P7-10 and then declines to mature levels by ∼P30 (Brown et al. 2017). The role of macrophages in cochlear development is not clear. Macrophages in the immature cochlea are likely to participate in debris clearance following programmed cell death (Brown et al. 2017; Liu et al. 2018), but they may also help sculpt the developing sensory tissue. Considerable synaptic remodeling occurs during cochlear development (e.g., Bulankina and Moser 2012; Coate and Kelley 2013), and studies of CNS development have shown a role for microglia in the elimination of excess synapses (reviewed by Schafer and Steven 2015). Microglia-mediated synaptic modification in the CNS relies on the complement pathway (Schafer et al. 2012), whereas cochlear innervation develops normally in the absence of complement signaling (Calton et al. 2014). Thus, any role for macrophages in the refinement of cochlear innervation is likely to use different signals than those operative in the developing brain.
MACROPHAGES IN THE COCHLEA
The mature cochlea contains resident macrophages that are distributed throughout its sensory, neuronal, and supporting tissues (e.g., Hirose et al. 2005; Kaur et al. 2015a; O’Malley et al. 2016; Liu et al. 2018). The dynamics of this resident population have not been carefully studied. In many tissues, resident macrophages are maintained by the proliferation of existing macrophages (Ginhoux and Guilliams 2016). It is likely that cochlear macrophages display a low level of cell death and cell replacement (Okano et al. 2008), but it is not clear how many new macrophages arise from local proliferation versus recruitment from circulation. In any case, destruction of the entire population of otic macrophages (e.g., by irradiation) leads to the recruitment of circulating myeloid-lineage cells, which then reestablish the resident macrophage population (Okano et al. 2008; Sato et al. 2008; Tan et al. 2008). Such observations demonstrate that bone-marrow-based hematopoietic precursors have the ability to generate otic macrophages in mature animals.
Macrophages in the Sensory Region of the Organ of Corti
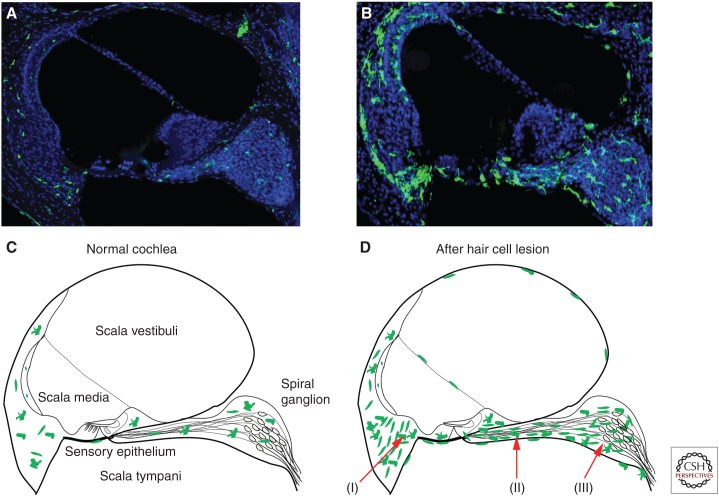
The sensory epithelium of the cochlea is comprised of inner and outer hair cells, as well as several distinct types of supporting cells. Macrophages are rarely observed within the sensory epithelium of the uninjured cochlea but are present in the surrounding tissues (Hirose et al. 2005). Moderate numbers of macrophages reside in the spiral ligament of the lateral wall, near the distal processes of cochlear neurons, and are distributed among the cell bodies of the spiral ganglion (Fig. 1A,C). Some macrophages are also present below the basilar membrane (Hu et al. 2018). Curiously, the morphology of basilar membrane-associated macrophages appears to vary systematically with their tonotopic location (Hu et al. 2018).
Figure 1.
Macrophages in cross sections of normal and injured cochleae. Images in A and B are frozen sections of cochleae from CX3CR1+/GFP transgenic mice, which express green fluorescent protein (GFP) (green) in all macrophages, monocytes, and microglia. Sections were also labeled with DAPI (blue). (A,C) The uninjured cochlea contains resident macrophages, which are distributed throughout the nonsensory supporting tissues. (B,D) After loss of cochlear hair cells, increased numbers of macrophages are present in all regions of the cochlea. Arrows indicate locations where macrophage numbers are particularly enhanced after acoustic trauma or aminoglycoside ototoxicity: (I) lateral wall, (II) osseous spiral lamina, and (III) the spiral ganglion.
Although the majority of published data on cochlear macrophages were obtained from studies of rodents, very similar patterns of macrophage distribution are observed in the cochleae of adult humans (O’Malley et al. 2016; Liu et al. 2018).
Macrophages in Supporting Tissues of the Cochlea
The main body of the cochlea is formed from three adjoining fluid chambers: scala vestibuli, scala tympani, and scala media. Scalae vestibuli and tympani contain perilymph, which is similar in composition to cerebrospinal fluid. Scala media contains potassium-rich endolymph, which is maintained by specialized ion-transporting cells in the stria vascularis. In the uninjured cochlea, macrophages are occasionally observed below the basilar membrane, but are otherwise not present in the cochlear fluid spaces (Hirose et al. 2005; Kaur et al. 2015a; Hu et al. 2018). Macrophages also reside in the tissues of the lateral border of scala media (the spiral ligament and the stria vascularis). Strial macrophages are often observed in close association with capillaries and form part of the blood–labyrinth barrier (Shi 2010; Zhang et al. 2012). The number of macrophages in the stria is increased following lipopolysaccharide-induced inflammation, and this is accompanied by enhanced permeability of the blood–labyrinth barrier (Hirose et al. 2014a).
MACROPHAGE RESPONSE TO COCHLEAR INJURY: THE SENSORY REGION
Cochlear hair cells and the synapses between inner hair cells and afferent neurons are highly vulnerable to insult and can be damaged or lost after exposure to loud sounds (reviewed by Liberman 2016). Numerous studies have reported increased numbers of macrophages within the cochlea following noise exposure (Bohne 1971; Fredelius and Rask-Andersen 1990; Hirose et al. 2005; Tornabene et al. 2006; Tan et al. 2008; Yang et al. 2015; Frye et al. 2017; Kaur et al. 2018). The majority of published data on macrophage response to noise injury are derived from studies of mice, in which animals are typically exposed for 1–2 hours to octave-band sound stimuli of ∼100–120 dB sound pressure level (SPL) intensity (characterized in Wang et al. 2002; Hirose and Liberman 2003). Following such regimens, enhanced numbers of macrophages are observed in the cochlear tissues within 3 days of exposure (Hirose et al. 2005). High densities of macrophages are particularly notable in the osseous spiral lamina, the scala tympani surface of the basilar membrane, and the lower portion of the lateral wall (Fig. 1B,D; also see Hirose et al. 2005; Tornabene et al. 2006; Yang et al. 2015; Kaur et al. 2018). In addition to hair cell injury, acoustic trauma also causes considerable damage to the fibrocytes of the spiral limbus, and this is accompanied by macrophage recruitment into the limbus (Hirose et al. 2005). Finally, noise injury leads to enhanced numbers of macrophages lining the cellular boundaries of scalae tympani and vestibuli (Hirose et al. 2005). Macrophages are rarely observed in scala media, probably because the potassium content of endolymph is incompatible with macrophage survival.
Several lines of evidence suggest that the initial increase in cochlear macrophages observed after injury is a result of recruitment from circulation. After noise, high densities of macrophages are first evident at sites near cochlear blood vessels, as would occur if they were leaving the circulatory system. Also, injection of BrdU shortly after acoustic trauma results in very few labeled leukocytes, even though the number of cochlear macrophages has substantially increased (Hirose et al. 2005). The enhanced numbers of macrophages observed within the sensory regions of the injured cochlea is transitory; their numbers peak at ∼2 weeks after injury and then subside (Hirose et al. 2005; Kaur et al. 2015a). Macrophages in the normal and injured organ of Corti also exhibit diverse morphologies, ranging from amoeboid to ramified (e.g., Ladrech et al. 2007).
The macrophage response to aminoglycoside ototoxicity is similar to that observed after noise exposure. Macrophage dynamics have been characterized following multiple systemic injections of aminoglycosides and in response to the coadministration of aminoglycosides and loop diuretics (e.g., furosemide). In both cases, enhanced numbers of macrophages migrate into the spiral ganglion, the habenular region (near the distal processes of the afferent neurons), and the lateral wall (Ladrech et al. 2007; Sato et al. 2010; Hirose and Sato 2011; Kaur et al. 2018). As with noise, the high concentrations of macrophages observed at these locations probably reflect their entry points into the injured cochlea. Macrophages also respond to age-related loss of hair cells in the cochleae of mice (Frye et al. 2017). This process requires further study, as it is not clear whether macrophages actively influence the process of cochlear degeneration with age.
MACROPHAGE RESPONSE TO COCHLEAR INJURY: THE SPIRAL GANGLION
Sensory information from the cochlea is conveyed to the brain via the neurons of the spiral ganglion. The cell bodies of these afferents reside in Rosenthal’s canal, which spirals through the bony capsule of the cochlea along the modiolar axis. Within the spiral ganglion, the afferent cell bodies are associated with both glial cells and resident macrophages. Macrophages are also present along the distal processes of afferent neurons, extending through the osseous spiral lamina to near the base of the inner hair cells. The number of macrophages associated with auditory neurons increases after hair cell injury. At early time points, some of this increase probably reflects macrophages that are migrating toward the injured sensory epithelium. However, unlike the transient increase that is observed in the sensory epithelium, macrophage numbers within the ganglion remain elevated for at least several months after injury (Kaur et al. 2015a). What recruits these macrophages into the ganglion and why do they persist? One possible factor is neuronal injury. Certain types of cochlear insult (e.g., noise, ototoxicity) cause a concomitant loss of afferent neurons, and it has been shown that experimentally induced lesion of spiral ganglion neurons (SGNs) leads to macrophage recruitment (Sekiya et al. 2001; Lang et al. 2016). However, current evidence suggests that neuronal injury alone cannot fully account for the macrophage dynamics observed in the spiral ganglion after hair cell injury. For example, diphtheria toxin-induced hair cell lesion in Pou4f3-huDTR mice does not cause the loss of SGNs until >3 months after hair cell death (Tong et al. 2015; Kurioka et al. 2016). Yet such injury still leads to a rapid and long-term increase in macrophages within the ganglion (Kaur et al. 2015a). It is possible that the cessation of afferent discharge activity, which would occur after the loss of inner hair cells, might cause SGNs to release a signal that attracts macrophages. Also, the cell bodies of SGNs are immunoreactive for fractalkine (CX3CL1, a known macrophage chemoattractant) (Kaur et al. 2015a; Liu et al. 2018), and genetic deletion of the fractalkine receptor (CX3CR1) on macrophages reduces the recruitment of macrophages into the spiral ganglion after diphtheria toxin-mediated hair cell injury (Kaur et al. 2015a). As such, it appears that fractalkine signaling is one factor that regulates macrophage numbers in the spiral ganglion, but it is very likely that other—as-yet-unidentified—signals are also involved.
MACROPHAGES IN THE VESTIBULAR ORGANS
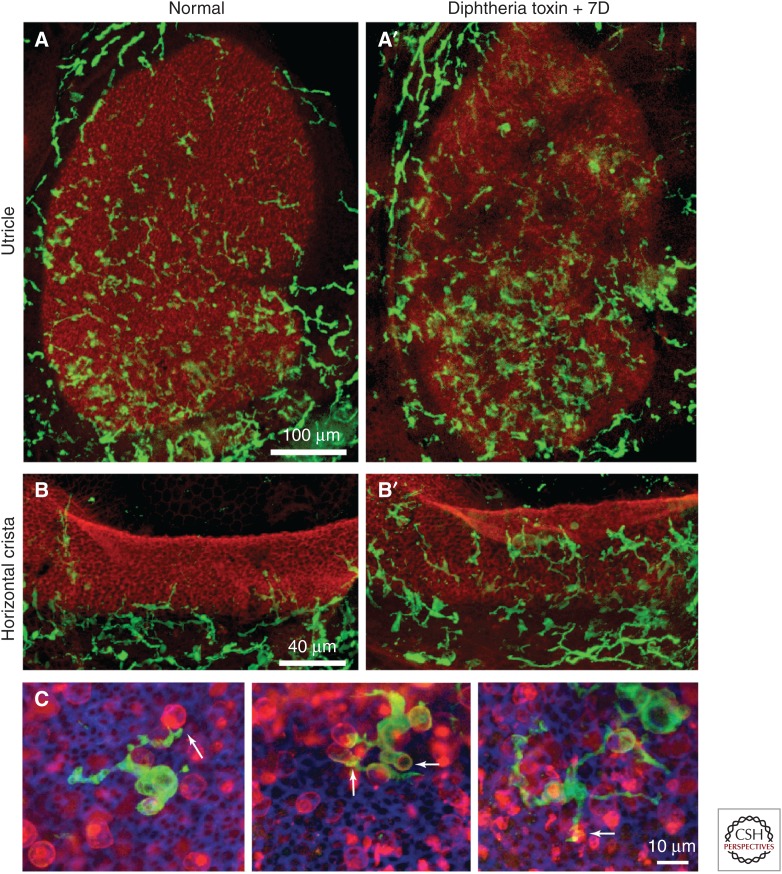
Like the cochlea, all of the vestibular sensory organs possess resident macrophages (Zhang et al. 2013). In normal (uninjured) tissue, resident vestibular macrophages are mainly confined to the mesenchymal/stromal tissues and are rarely observed within the sensory epithelium (Kaur et al. 2015b). It is difficult to create ototoxic lesions in the vestibular organs of mice, which remain the standard mammalian model for the study of innate immunity. However, vestibular hair cells can be killed via injection of diphtheria toxin in Pou4f3-huDTR transgenic mice (Golub et al. 2012) and this has permitted an assessment of macrophage activity after peripheral vestibular injury (Kaur et al. 2015b). Such studies indicate that the loss of hair cells results in a moderate increase in macrophages within the stromal tissues of the sensory organs, as well as the entry of macrophages into the sensory epithelium (Fig. 2). Macrophages within the injured epithelium exhibit a variety of morphologies, some of which appear to be actively involved in the phagocytosis of cellular debris (Fig. 2C). It is clear, however, that macrophages are not the sole phagocyte responsible for the removal of dying cells from the vestibular organs. Studies of utricles from both chicks and mice have shown that supporting cells can form actin-based “phagosomes,” which then engulf portions of dead hair cells (Bird et al. 2010; Monzack et al. 2015). Together, such observations suggest that macrophages and supporting cells play complementary roles in debris clearance after vestibular injury (Hirose et al. 2017).
Figure 2.
Distribution of macrophages in the vestibular organs of mice. Shown are whole mount images of the utricle (A,A′) and horizontal cristae (B,B′) of CX3CR1+/GFP transgenic mice, in which macrophages express green fluorescent protein (GFP) (green). Sensory epithelia are also labeled with phalloidin (red). (A,B) Moderate numbers of macrophages are present in the uninjured utricle and cristae, but are confined to the stromal/connective tissue, below the sensory epithelium. (A′,B′) Injection of diphtheria toxin in Pou4f3-huDTR mice results in the loss of most sensory hair cells, and this is accompanied by an increase in macrophage numbers in the maculae and cristae. (C) Macrophages enter the vestibular sensory epithelia after hair cell injury, where they are observed contacting and engulfing hair cell debris (arrows, C). Labels in C: green, GFP (macrophages); red, otoferlin (hair cells).
SIGNALS THAT RECRUIT MACROPHAGES TO INJURED HAIR CELLS
Injury to hair cells leads to rapid recruitment of macrophages, but identification of the signals that mediate this response has proven to be challenging. One complicating factor is that many forms of cochlear injury (e.g., noise exposure, aminoglycoside ototoxicity) also damage other cells types within the ear (e.g., lateral wall fibrocytes, neurons of the spiral ganglion, etc.) and it is possible that those other injured cells may release their own signals for macrophage recruitment. The recent development of transgenic mice that express the human form of the diphtheria toxin receptor specifically on hair cells has permitted the selective ablation of hair cells from the cochlea and vestibular organs, with no other apparent cellular pathology (Golub et al. 2012; Tong et al. 2015). As noted previously, induced hair cell lesions in such mice still leads to increased macrophage numbers in both the cochlea and vestibular organs, indicating that hair cell death alone is sufficient to recruit macrophages toward sensory tissue (Kaur et al. 2015a,b). Identification of the recruitment signal is a priority for future studies. One possible candidate is ATP, which is released from cells in the injured cochlea (Lahne and Gale 2008) and activates leukocytes via purinergic receptors (Di Virgilio et al. 2017). However, the trafficking of macrophages through the tissues of the inner ear is also likely to be regulated by an assortment of cytokines and chemokines. As noted above, fractalkine (CX3CL1) is expressed on inner hair cells, supporting cells and SGNs, and genetic deletion of its receptor (CX3CR1) can affect macrophage recruitment after cochlear injury (Sato et al. 2010; Kaur et al. 2015a). The effects of CX3CR1 deletion on macrophage activity vary with the specific form of cochlear injury. Noise or aminoglycoside injury to CX3CR1-null mice leads to enhanced numbers of macrophages entering the cochlea, particularly in the region near the lateral wall (Sato et al. 2010; Kaur et al. 2018). In contrast, deletion of CX3CR1 reduces macrophage entry in response to hair cell–specific injury in Pou4f3-huDTR mice (Kaur et al. 2015a). The reasons for these differences are not clear. Finally, it is worth noting that immunoreactivity for CX3CL1 is not observed in the mouse vestibular organs (ME Warchol, unpubl.) and genetic deletion of CX3CR1 does not affect macrophage recruitment into the mouse utricle after hair cell injury (Kaur et al. 2015b). Such observations point to differences in macrophage regulation in the auditory versus vestibular organs.
Other signals that may regulate the recruitment of otic macrophages include macrophage migration inhibitory factor (MIF) and macrophage chemoattractant protein-1 ([MCP1], also known as CCL2). Both molecules are present in the developing ear, where they appear to promote the survival of afferent neurons (Bank et al. 2012; Shen et al. 2012; Barald et al. 2018). Genetic deletion of MIF also increases hair cell susceptibility to noise injury, an effect that may be mediated by changes in macrophage recruitment (Kariya et al. 2015). Future studies are required to assess these candidate signals. Finally, numerous laboratories are currently using RNA-Seq methodology to characterize gene-expression changes in the injured mammalian ear and these studies are likely to identify many additional candidate recruitment factors.
MACROPHAGE INFLUENCES ON CELL SURVIVAL IN THE INJURED EAR
In addition to their role as scavengers of dead cells, macrophages can also influence hair cell survival after injury, even without clear evidence of direct cell–cell contact. The heat shock protein heme oxygenase-1 ([HO-1], also known as HSP32) can protect hair cells from ototoxic injury (Francis et al. 2011). In the mouse utricle, HO-1 is mainly produced by resident macrophages and eliminating those macrophages (via treatment with clodronate-containing liposomes) leads to enhanced hair cell death after exposure to ototoxins (Baker et al. 2015). Two other macrophage-associated signaling molecules have been shown to regulate cell survival in the injured cochlea. The chemokine CCL2, acting through its receptor CCR2, is a known attractant for monocytes (e.g., Deshmane et al. 2009). Macrophages expressing CCR2 are uncommon in the uninjured ear, and genetic deletion of CCR2 does not reduce macrophage recruitment into the cochlea after acoustic trauma (Sautter et al. 2006; Hirose et al. 2014b). Interestingly, however, deletion of CCR2—but not CCL2—increases hair cell susceptibility to noise injury (Sautter et al. 2006). Similarly, mice that lack the gene for the fractalkine receptor CX3CR1 show increased sensitivity to ototoxicity, when compared with wild-type or heterozygous littermates (Sato et al. 2010), and deletion of CX3CR1 also enhances the loss of SGNs following several forms of hair cell death (Kaur et al. 2015a, 2018). Macrophages are the only cells within the ear that express either CCR2 or CX3CR1, so it is likely that the increased vulnerability that occurs in the absence of these receptors is caused by changes in macrophage function. Together, these findings suggest a central role for macrophages in otic pathology, but the full implications remain to be explored.
MACROPHAGE–HAIR CELL INTERACTIONS IN NONMAMMALIAN VERTEBRATES
Most current research on auditory function and pathology focuses on mammalian models. However, both chickens and zebrafish are also used in studies of hair cell development, death, and regeneration. As a consequence, we possess a general outline of how macrophages respond to hair cell injury in the inner ears of birds and lateral line neuromasts of zebrafish. Many facets of hair cell biology are highly conserved, and it is likely that the signals that recruit macrophages toward injured hair cells will be similar in most vertebrates. Nonmammalian models can offer unique opportunities for exploring the nature of the interaction between hair cells, auditory neurons, and macrophages.
The Avian Inner Ear
The avian inner ear is a classic model for the study of otic development (e.g., Groves and Fekete 2012). In addition, the mature avian ear is capable of regenerating lost hair cells, and many cellular and molecular studies have been aimed at identifying the signaling pathways that underlie this process (Warchol 2011; Atkinson et al. 2015). Like their mammalian counterparts, the sensory organs of the avian inner ear contain resident macrophages (Warchol 1997, 1999; Bhave et al. 1998; O’Halloran and Oesterle 2004). As in mammals, macrophages in the avian basilar papilla and vestibular organs mainly reside in the associated connective tissues, and few macrophages are present in undamaged sensory epithelia. However, hair cell injury leads to enhanced numbers of macrophages and other leukocytes within the sensory regions (Warchol 1997; Bhave et al. 1998; O’Halloran and Oesterle 2004; Warchol et al. 2012). Macrophage recruitment to injury sites occurs quickly. Experiments employing organotypic cultures of the chick basilar papilla have shown that enhanced numbers of macrophages are present at lesion sites within 4 hours of hair cell death (Warchol 1997). Treatment with mitotic tracers has also revealed proliferation of macrophages in the avian ear (Warchol 1997; Bhave et al. 1998), but the dynamics of the resident population have not been carefully characterized.
Lateral Line Neuromasts of Zebrafish
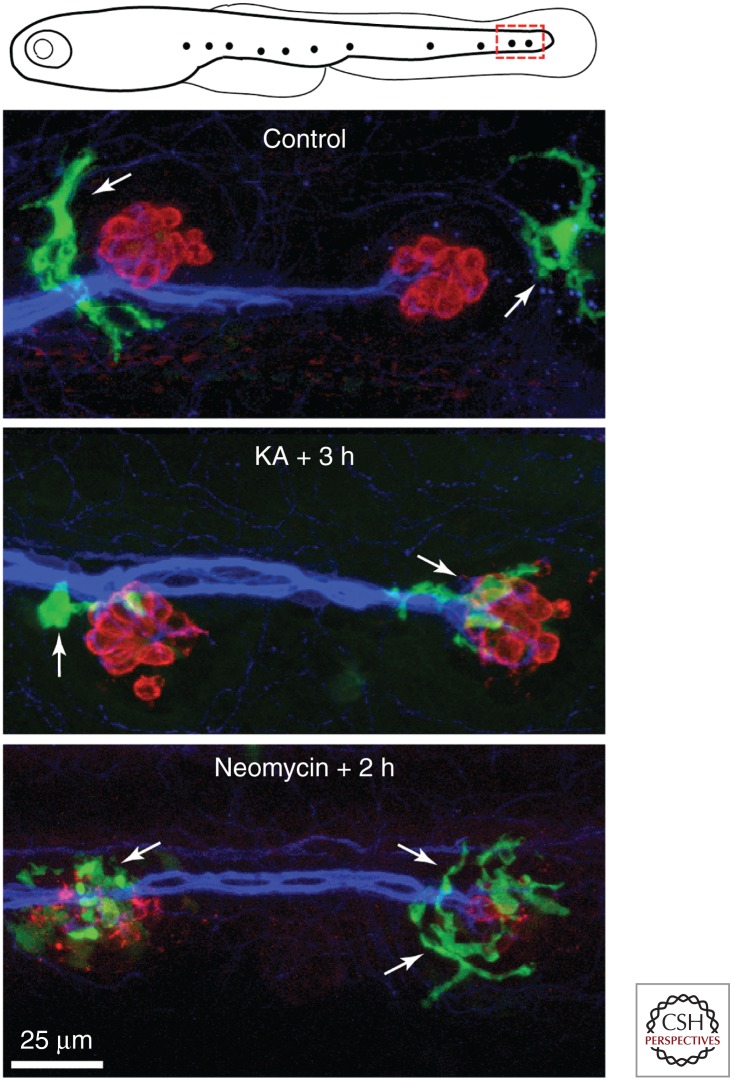
Fish and other aquatic vertebrates possess arrays of superficial hair-cell-containing sensory organs called neuromasts, which detect fluid flow near the animal's surface. Lateral line neuromasts of fish are typically small oval-shaped patches of ∼20 hair cells and associated supporting cells and receive both afferent and efferent innervation. The structure and function of lateral line hair cells appears nearly identical to the hair cells of the inner ear (e.g., Nicolson 2017). Because of the superficial location of neuromasts and the optical clarity of larvae, interactions between macrophages and hair cells are particularly easy to observe in zebrafish (Fig. 3). Studies of transgenic zebrafish in which macrophages express a fluorescent reporter indicate that neuromasts of the posterior lateral line usually possess one to two macrophages within a 50 µm radius. Such macrophages often extend fine processes (pseudopodia) into the sensory region of the neuromast, presumably to monitor cell viability (Hirose et al. 2017). Lateral line hair cells can be killed by incubating fish in water that contains aminoglycoside antibiotics, cisplatin, or excitotoxic agents (Ou et al. 2007; Harris et al. 2013; Sheets 2017). Once hair cells begin to die, the nearby macrophages converge onto the injured neuromasts and actively phagocytose debris (Hirose et al. 2017). As is the case for the inner ear, the signals that cause macrophages to enter injured neuromasts are not known.
Figure 3.
Macrophage interactions with lateral line neuromasts in larval zebrafish. Transgenic fish lines in which macrophages express fluorescent proteins (e.g., mpeg1: green fluorescent protein [GFP]) permit the observation of macrophage activity in living fish. Published data show that neuromasts of the posterior lateral line typically possess one to two macrophages within a 50 µm radius of each individual neuromast (Hirose et al. 2017). Images show macrophages near the two most-posterior neuromasts of the zebrafish lateral line at 5 days postfertilization (see red box in top schematic). Macrophages are commonly observed near neuromasts in fish with intact hair cells (Control). However, injury to neuromasts by application of kainic acid ([KA], 300 µm for 1 h) or neomycin (50 µm for 30 min) causes macrophages to enter neuromasts and contact both injured hair cells and their neurons. Labels: green (GFP, macrophages), red (HCS-1, hair cells), blue (ZN-12, afferent neurons).
CAN MACROPHAGES PROMOTE HAIR CELL REGENERATION?
Although the mammalian ear has a very limited capacity for sensory repair after injury, hair cell epithelia in nonmammalian vertebrates are able to regenerate, leading to nearly complete functional recovery (e.g., Warchol 2011). Most replacement hair cells are produced by the renewed division of supporting cells, although some new hair cells also arise from phenotypic conversion of supporting cells (Atkinson et al. 2015). Macrophages have been shown to play important stimulatory roles in repair and regeneration of numerous tissues (e.g., epidermis, heart, liver, and other tissues; reviewed by Vannella and Wynn 2017), and it has been suggested that macrophages may also help facilitate the regeneration of hair cells in nonmammalian species (Jones and Corwin 1996; Warchol 1997; Carrillo et al. 2016; Kniss et al. 2016). The time course of macrophage activation in response to hair cell injury is consistent with this notion. Macrophages enter lateral line neuromasts of zebrafish within 1 hour of ototoxic injury (Hirose et al. 2017), which is about 4 hours before the onset of regenerative proliferation (Romero-Carvajal et al. 2015). Macrophages are also recruited to lesioned neuromasts of axolotl salamanders within 1 hour of hair cell death, while the regenerative response begins >12 hours later (Jones and Corwin 1996). Laser microbeam ablation of hair cells in organotypic cultures has been used to quantify the injury response of macrophages in the chick basilar papilla. Enhanced numbers of macrophages are present at hair cell lesion sites within 4 hours after injury, whereas the onset of regenerative proliferation in this preparation occurs at ∼16 hours after injury (Warchol and Corwin 1996; Warchol 1997).
The suggestion that macrophages may promote otic repair is appealing, but direct experimental evidence is equivocal. In one study, liposomally encapsulated clodronate was used to ablate resident macrophages in cultures of the chick basilar papilla, permitting an assessment of regeneration in the absence of macrophages (Warchol et al. 2012). Depletion of macrophages resulted in no observable deficits in debris clearance, regenerative proliferation, or hair cell recovery. This result suggests that macrophages are not essential for regeneration, but they do not rule out a modulatory role. Also, elimination of macrophages led to reduced proliferation among the mesothelial cells (or tympanic border cells) of the basilar membrane (Warchol et al. 2012). Macrophages in the injured auditory organs of both birds and mammals are often observed in direct contact with the lower (scala tympani) surface of the basilar membrane (Warchol 1997; Frye et al. 2017), and they may promote the repair or maintenance of this structure.
Zebrafish have become a common model system in regenerative biology (e.g., Gemberling et al. 2013), and macrophages have been shown to promote regeneration in many zebrafish tissues (Petrie et al. 2014; Lai et al. 2017). Hair cells in lateral line neuromasts of zebrafish can regenerate within 48–72 hours of ototoxic injury (Harris et al. 2013; Kniss et al. 2016) and, as noted above, macrophages are recruited into injured neuromasts shortly after hair cell death (Hirose et al. 2017). As is the case with the chick ear, it is not clear whether macrophages help stimulate regeneration of lateral line hair cells. Clodronate liposomes have been used to deplete macrophages in the vicinity of neuromasts and this appears to slow the rate of hair cell regeneration (Carrillo et al. 2016). However, these methods have limitations and the magnitude of the reported effect is quite small. Instead, the use transgenic zebrafish lines that permit the selective ablation of tissue macrophages (e.g., Petrie et al. 2014) will probably be required for a full evaluation of the role of macrophages in hair cell regeneration.
CONCLUDING REMARKS
The study of otic macrophages is still in its early stages and much remains to be learned about the function of macrophages in the normal and injured ear. One key uncertainty concerns the developmental origin of otic macrophages. During embryogenesis, microglia (the resident macrophages of the brain) are produced separately from the resident macrophages of other tissues (Ginhoux and Prinz 2015). It would be of interest to know whether the production of otic macrophages occurs in a similarly distinct fashion. The degree of phenotypic diversity among the resident macrophages of the inner ear also awaits careful study. The morphology of tissue macrophages can appear homogeneous, but genomic studies have revealed considerable differences in both the chromatin landscape and in gene expression among resident macrophages from different tissues (Gosselin et al. 2014, 2017; Lavin et al. 2014). Does the epigenetic structure of otic macrophages differ from that of resident macrophages in other tissues? Hair cell injury also attracts additional (nonresident) macrophages into the ear. How does the phenotype and activity of those new recruits differ from that of resident macrophages?
It also would be of great interest to identify the signals that attract macrophages into the ear following injury or pathology. It is likely that several partially redundant signals mediate such recruitment, with different injured cell types releasing their own signals. However, hair cell death alone is sufficient to attract macrophages into the spiral ganglion and toward the sensory epithelium (Kaur et al. 2015a,b), and this may depend on a small number of chemoattractants. Finally, the contribution of macrophages to pathology in both the injured and inflamed ear requires further clarification. It is clear that macrophages can influence the long-term survival of hair cells and afferent neurons after various types of cochlear lesions, but the underlying cellular mechanisms are largely unknown. Macrophages are also involved in diseases of the inner ear, such as primary infection or autoimmunity, and are likely to influence the pathogenesis of hearing loss caused by these conditions. Enhanced knowledge of the roles of macrophages in these disease processes is likely to lead to the development of clinical methods for improving outcomes following inner ear injury and infection.
ACKNOWLEDGMENTS
I am grateful to Dr. Keiko Hirose and Dr. Tejbeer Kaur for helpful discussions and comments on portions of the manuscript. Dr. Kaur also provided the images in Figure 2A. M.E.W. is supported by Grant R01DC006283 from the National Institute on Deafness and Other Communication Disorders (NIDCD)/National Institutes of Health (NIH).
Footnotes
Editors: Guy P. Richardson and Christine Petit
Additional Perspectives on Function and Dysfunction of the Cochlea available at www.perspectivesinmedicine.org
REFERENCES
- Atkinson PJ, Huarcaya Najarro E, Sayyid ZN, Cheng AG. 2015. Sensory hair cell development and regeneration: Similarities and differences. Development 142: 1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TG, Roy S, Brandon CS, Kramarenko IK, Francis SP, Taleb M, Marshall KM, Schwendener R, Lee FS, Cunningham LL. 2015. Heat shock protein-mediated protection against cisplatin-induced hair cell death. J Assoc Res Otolaryngol 16: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank LM, Bianchi LM, Ebisu F, Lerman-Sinkoff D, Smiley EC, Shen YC, Ramamurthy P, Thompson DL, Roth TM, Beck CR, et al. 2012. Macrophage migration inhibitory factor acts as a neurotrophin in the developing inner ear. Development 139: 4666–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barald KF, Shen YC, Bianchi LM. 2018. Chemokines and cytokines on the neuroimmune axis: Inner ear neurotrophic cytokines in development and disease. Prospects for repair? Exp Neurol 301: 92–99. [DOI] [PubMed] [Google Scholar]
- Bhave SA, Oesterle EC, Coltrera MD. 1998. Macrophage and microglia-like cells in the avian inner ear. J Comp Neurol 398: 241–256. [DOI] [PubMed] [Google Scholar]
- Bird JE, Daudet N, Warchol ME, Gale JE. 2010. Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian inner ear. J Neurosci 30: 12545–12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne BA. 1971. “Scar formation in the inner ear following acoustical injury.” Doctoral dissertation, Washington University, St. Louis, MO. [Google Scholar]
- Brown LN, Xing Y, Noble KV, Barth JL, Panganiban CH, Smythe NM, Bridges MC, Zhu J, Lang H. 2017. Macrophage-mediated glial cell elimination in the postnatal mouse cochlea. Front Mol Neurosci 10: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann K. 2014. Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front Immunol 5: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulankina AV, Moser T. 2012. Neural circuit development in the mammalian cochlea. Physiology 27: 100–112. [DOI] [PubMed] [Google Scholar]
- Calton MA, Lee D, Sundaresan S, Mendus D, Leu R, Wangsawihardja F, Johnson KR, Mustapha M. 2014. A lack of immune system genes causes loss in high frequency hearing but does not disrupt cochlear synapse maturation in mice. PLoS ONE 9: e94549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo SA, Anguita-Salinas C, Pena OA, Morales RA, Munoz-Sanchez S, Munoz-Montecinos C, Paredes-Zuniga S, Tapia K, Allende ML. 2016. Macrophage recruitment contributes to regeneration of mechanosensory hair cells in the zebrafish lateral line. J Cell Biochem 117: 1880–1889. [DOI] [PubMed] [Google Scholar]
- Coate TM, Kelley MW. 2013. Making connections in the inner ear: Recent insights into the development of spiral ganglion neurons and their connectivity with sensory hair cells. Semin Cell Dev Biol 24: 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. 2009. Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res 29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. 2017. The P2X7 receptor in infection and inflammation. Immunity 47: 15–31. [DOI] [PubMed] [Google Scholar]
- Francis SP, Kramarenko II, Brandon CS, Lee FS, Baker TG, Cunningham LL. 2011. Celastrol inhibits aminoglycoside-induced ototoxicity via heat shock protein 32. Cell Death Dis 2: e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredelius L, Rask-Andersen H. 1990. The role of macrophages in the disposal of degeneration products within the organ of Corti after acoustic overstimulation. Acta Otolaryngol 109: 76–82. [DOI] [PubMed] [Google Scholar]
- Frye MD, Yang W, Zhang C, Xiong B, Hu BH. 2017. Dynamic activation of basilar membrane macrophages in response to chronic sensory cell degeneration in aging mouse cochleae. Hearing Res 344: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemberling M, Bailey TJ, Hyde DR, Poss KD. 2013. The zebrafish as a model for complex tissue regeneration. Trends Genet 29: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Guilliams M. 2016. Tissue-resident macrophage ontogeny and homeostasis. Immunity 44: 439–449. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Prinz M. 2015. Origin of microglia: Current concepts and past controversies. Cold Spring Harb Perspect Biol 7: a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub JS, Tong L, Ngyuen TB, Hume CR, Palmiter RD, Rubel EW, Stone JS. 2012. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J Neurosci 32: 15093–15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, et al. 2014. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159: 1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, Jaeger BN, O’Connor C, Fitzpatrick C, Pasillas MP, et al. 2017. An environment-dependent transcriptional network specifies human microglia identity. Science 356: eaal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AK, Fekete DM. 2012. Shaping sound in space: The regulation of inner ear patterning. Development 139: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. 2013. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J Assoc Res Otolaryngol 4: 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. 2003. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol 4: 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Sato E. 2011. Comparative analysis of combination kanamycin-furosemide versus kanamycin alone in the mouse cochlea. Hearing Res 272: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Discolo CM, Keasler JR, Ransohoff R. 2005. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol 489: 180–194. [DOI] [PubMed] [Google Scholar]
- Hirose K, Hartsock JJ, Johnson S, Santi P, Salt AN. 2014a. Systemic lipopolysaccharide compromises the blood–labyrinth barrier and increases entry of serum fluorescein into the perilymph. J Assoc Res Otolaryngol 15: 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Li SZ, Ohlemiller KK, Ransohoff RM. 2014b. Systemic lipopolysaccharide induces cochlear inflammation and exacerbates the synergistic ototoxicity of kanamycin and furosemide. J Assoc Res Otolaryngol 15: 555–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Rutherford MA, Warchol ME. 2017. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hearing Res 352: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BH, Zhang C, Frye MD. 2018. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear Res 362: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Taghizadeh F, Steyger PS. 2017. Potential mechanisms underlying inflammation-enhanced aminoglycoside-induced cochleotoxicity. Front Cell Neurosci 11: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. 1996. Regeneration of sensory cells after laser ablation in the lateral line system: Hair cell lineage and macrophage behavior revealed by time-lapse video microscopy. J Neurosci 16: 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Lomberk G, Park C, Thein P, Kalinec F. 2017. Resolution of cochlear inflammation: Novel target for preventing or ameliorating drug-, noise-, and age-related hearing loss. Front Cell Neurosci 11: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya S, Okano M, Maeda Y, Hirai H, Higaki T, Noyama Y, Haruna T, Nishihira J, Nishizaki K. 2015. Macrophage migration inhibitory factor deficiency causes prolonged hearing loss after acoustic overstimulation. Otol Neurotol 36: 1103–1108. [DOI] [PubMed] [Google Scholar]
- Kaur T, Zamani D, Tong L, Rubel EW, Ohlemiller KK, Hirose K, Warchol ME. 2015a. Fractalkine signaling regulates macrophage recruitment into the cochlea and promotes the survival of spiral ganglion neurons after selective hair cell lesion. J Neurosci 35: 15050–15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Hirose K, Rubel EW, Warchol ME. 2015b. Macrophage recruitment and epithelial repair following hair cell injury in the mouse utricle. Front Cell Neurosci 9: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Ohlemiller KK, Warchol ME. 2018. Genetic disruption of fractalkine signaling leads to enhanced loss of cochlear afferents following ototoxic or acoustic injury. J Comp Neurol 526: 824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniss JS, Jiang L, Piotrowski. 2016. Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr Opin Genet Devel 40: 32–40. [DOI] [PubMed] [Google Scholar]
- Kurioka T, Lee MY, Heeringa AN, Beyer LA, Swiderski DL, Kanicki AC, Kabara LL, Dolan DF, Shore SE, Raphael Y. 2016. Selective hair cell ablation and noise exposure lead to different patterns of changes in the cochlea and the cochlear nucleus. Neuroscience 332: 242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladrech S, Wang J, Simonneau L, Puel JL, Lenoir M. 2007. Macrophage contribution to the response of the rat organ of Corti to amikacin. J Neurosci Res 85: 1970–1979. [DOI] [PubMed] [Google Scholar]
- Lahne M, Gale JE. 2008. Damage-induced activation of ERK1/2 in cochlear supporting cells is a hair cell death-promoting signal that depends on extracellular ATP and calcium. J Neurosci 28: 4918–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Nishimoto E, Xing Y, Brown LN, Noble KV, Barth JL, LaRue AC, Ando K, Schulte BA. 2016. Contribution of mouse and human hematopoietic cells to remodeling of the adult auditory nerve after neuron loss. Mol Ther 24: 2000–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Marín-Juez R, Moura PL, Kuenne C, Lai JKH, Tsedeke AT, Guenther S, Looso M, Stainier DY. 2017. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. eLife 6: e25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159: 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. 2016. Noise-induced and age-related hearing loss: New perspectives and potential therapies. F1000Res 6: 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Molnar M, Garnham C, Benav H, Rask-Andersen H. 2018. Macrophages in the human cochlea: Saviors or predators—A study using super-resolution immunohistochemistry. Front Immunol 9: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzack EL, May LA, Roy S, Gale JE, Cunningham LL. 2015. Live imaging the phagocytic activity of inner ear supporting cells in response to hair cell death. Cell Death Differ 22: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson T. 2017. The genetics of hair-cell function in zebrafish. J Neurogenetics 31: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Halloran EK, Oesterle EC. 2004. Characterization of leukocyte subtypes in chicken inner ear sensory epithelia. J Comp Neurol 475: 340–360. [DOI] [PubMed] [Google Scholar]
- Okano T, Nakagawa T, Kita T, Kada S, Yoshimoto M, Nakahata T, Ito J. 2008. Bone marrow-derived cells expressing iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res 86: 1758–1767. [DOI] [PubMed] [Google Scholar]
- O’Malley JT, Nadol JB, McMc Kenna MJ. 2016. Anti CD163+, iba1+, and CD68+ cells in the adult human inner ear: Normal distribution of an unappreciated class of macrophages/microglia and implications for inflammatory otopathology in humans. Otol Neurotol 37: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Raible DW, Rubel EW. 2007. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res 233: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie TA, Strand NS, Yang CT, Rabinowitz JS, Moon RT. 2014. Macrophages modulate adult zebrafish tail fin regeneration. Development 141: 2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Keating MT, Nechiporuk A. 2003. Tales of regeneration in zebrafish. Dev Dyn 226: 202–210. [DOI] [PubMed] [Google Scholar]
- Romero-Carvajal A, Navajas Acedo J, Jiang L, Kozlovskaja-Gumbrienė A, Alexander R, Li H, Piotrowski T. 2015. Regeneration of sensory hair cells requires localized interactions between the Notch and Wnt pathways. Dev Cell 34: 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Shick HE, Ransohoff RM, Hirose K. 2008. Repopulation of cochlear macrophages in murine hematopoietic progenitor cell chimeras: The role of CX3CR1. J Comp Neurol 506: 930–942. [DOI] [PubMed] [Google Scholar]
- Sato E, Shick HE, Ransohoff RM, Hirose K. 2010. Expression of fractalkine receptor CX3CR1 on cochlear macrophages influences survival of hair cells following ototoxic injury. J Assoc Res Otolaryngol 11: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautter NB, Shick EH, Ransohoff RM, Charo IF, Hirose K. 2006. CC chemokine receptor 2 is protective against noise-induced hair cell death: Studies in CX3CR1+/GFP mice. J Assoc Res Otolaryngol 7: 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Steven B. 2015. Microglia function in central nervous system development and plasticity. Cold Spring Harb Perspect Biol 7: a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T, Tanaka M, Shimamura N, Suzuki S. 2001. Macrophage invasion into the injured cochlear nerve and its modification by methylprednisolone. Brain Res 905: 152–160. [DOI] [PubMed] [Google Scholar]
- Sheets L. 2017. Excessive activation of ionotrophic glutamate receptors induces apoptotic hair-cell death independent of afferent and efferent innervation. Sci Rep 7: 41102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YC, Thompson DL, Kuah MK, Wong KL, Wu KL, Linn SA, Jewett EM, Shu-Chien AC, Barald KF. 2012. The cytokine macrophage migration inhibitory factor (MIF) acts as a neurotrophin in the developing inner ear of the zebrafish, Danio rerio. Dev Biol 363: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. 2010. Resident macrophages in the cochlear blood–labyrinth barrier and their renewal via migration of bone-marrow derived cells. Cell Tissue Res 342: 21–30. [DOI] [PubMed] [Google Scholar]
- Tan BT, Lee MM, Ruan R. 2008. Bone-marrow-derived cells that home to acoustic deafened cochlea preserved their hematopoietic identity. J Comp Neurol 509: 167–179. [DOI] [PubMed] [Google Scholar]
- Tong L, Strong MK, Kaur T, Juiz JM, Oesterle EC, Hume C, Warchol ME, Palmiter RD, Rubel EW. 2015. Selective deletion of cochlear hair cells causes rapid age-dependent changes in spiral ganglion and cochlear nucleus neurons. J Neuro 35: 7878–7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene SV, Sato K, Pham L, Billings P, Keithley EM. 2006. Immune cell recruitment following acoustic trauma. Hearing Res 222: 115–124. [DOI] [PubMed] [Google Scholar]
- Vannella KM, Wynn TA. 2017. Mechanisms of organ injury and repair by macrophages. Ann Rev Physiol 79: 593–617. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. 2002. Dynamics of noise-induced injury and repair in the mouse cochlea. J Assoc Res Otolaryngol 3: 248–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME. 1997. Macrophage activity in organ cultures of the avian cochlea: Demonstration of a resident population and recruitment to sites of hair cell lesions. J Neurobiol 33: 724–734. [PubMed] [Google Scholar]
- Warchol ME. 1999. Immune cytokines and dexamethasone influence sensory regeneration in the avian vestibular periphery. J Neurocytol 28: 889–900. [DOI] [PubMed] [Google Scholar]
- Warchol ME. 2011. Sensory regeneration in the vertebrate inner ear: Differences at the levels of cells and species. Hearing Res 273: 72–79. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. 1996. Regenerative proliferation in organ cultures of the avian cochlea: Identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci 16: 5466–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Schwendener RA, Hirose K. 2012. Depletion of resident macrophages does not alter sensory regeneration in the avian cochlea. PLoS ONE 7: e51574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MB, Zuo J. 2017. The contribution of immune infiltrates to ototoxicity and cochlear hair cell loss. Front Cell Neurosci 11: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Vethanayagam RR, Dong Y, Cai Q, Hu BH. 2015. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience 303: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, et al. 2012. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid–blood barrier. Proc Natl Acad Sci 109: 10388–10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhang J, Neng L, Shi X. 2013. Characterization and inflammatory response of perivascular-resident macrophage-like melanocytes in the vestibular system. J Assoc Res Otolaryngol 14: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]