Summary
Febrile infection–related epilepsy syndrome (FIRES) is a severe epileptic encephalopathy with presumed inflammatory origin and lacking effective treatments. Anakinra is the human recombinant interleukin 1 receptor antagonist clinically used in autoinflammatory or autoimmune conditions. We report a case of FIRES for which the spatial and temporal match between electroencephalography (EEG) and magnetic resonance imaging (MRI) focal alterations provides support for the detrimental synergic interplay between seizures and inflammation that may evolve to permanent focal lesions and progressive brain atrophy in weeks to months. Brain biopsy showed aspects of chronic neuroinflammation with scarce parenchymal lymphocytes. We report the novel evidence that anakinra reduces the relapse of highly recurrent refractory seizures at 1.5 years after FIRES onset. Our evidence, together with previously reported therapeutic effects of anakinra administered since the first days of disease onset, support the hypothesis that interleukin 1β and inflammation‐related factors play a crucial role in seizure recurrence in both the acute and chronic stages of the disease.
Keywords: epileptic encephalopathy, febrile infection–related epilepsy syndrome, IL‐1β, neuroinflammation
1. INTRODUCTION
Febrile infection–related epilepsy syndrome (FIRES) is a severe epileptic encephalopathy that occurs in previously healthy subjects. Although it was initially described in children, adults may also be affected.1 The pathogenesis remains unknown, although a presumed immune and inflammatory‐mediated etiology is suspected.1 FIRES is characterized by an explosive superrefractory status epilepticus, a preceding history of fever, and presumable infection without evidence of infective encephalitis. Seizures are extremely difficult to control and the outcome is poor. Interleukin 1β (IL‐1β) is a proinflammatory cytokine chiefly involved in both infections and febrile seizures,2 and recent evidence has shown that the human recombinant interleukin 1 (IL‐1) receptor antagonist (anakinra), which blocks IL‐1β actions, reduces seizures in the acute phase of FIRES.3 Anakinra effectively reduced drug‐resistant seizures also in some cases of inflammatory chronic epilepsy.4, 5
We report the novel evidence of a beneficial use of anakinra in the chronic phase of FIRES when seizure clusters were refractory to multiple antiepileptic drugs. Our data support an underlying pathogenic role of neuroinflammation and the therapeutic potential of a specific antiinflammatory intervention in the chronic phases of FIRES.
2. CASE REPORT
2.1. History of the acute epileptic phase
After 4 days of fever and upper respiratory tract infection, a previously healthy 10‐year‐old child presented recurrent focal and generalized seizures and developed since the first day a status epilepticus refractory to midazolam and phenytoin. The family belongs to a mixed mulatto‐mestizo ethnicity and the family history was unremarkable for seizures and epilepsy. Status epilepticus was partially controlled, inducing burst‐suppression activity using midazolam, propofol, and then thiopental. Also an antimicrobial empiric treatment with acyclovir and ceftriaxone was started. Each attempt to reduce sedation in the first weeks caused relapse of severe status epilepticus with seizures and hypertension (secondary to seizures). Treatment with phenobarbital, levetiracetam, topiramate, lacosamide, and clobazam was unsuccessful. High‐dose steroids (methylprednisolone, 1 g, i.v., for 5 days, followed by enteral prednisone 2 mg/kg) and i.v. immunoglobulins were administered without efficacy (see therapy and seizure course in Figure 1). Protracted anesthetic coma with propofol caused important systemic complication (hypotension, hypertransaminemia, increase of serum pancreatic enzymes). Consequently, ketamine was introduced, attaining partial control of seizures, which nonetheless relapsed after a short time. Therefore, we introduced ketogenic diet, which was continued for 3 weeks without benefit. After 2 months, the patient was weaned from sedation and tracheal intubation, and was maintained under treatment with phenobarbital, topiramate, and lacosamide. He continued to manifest highly recurring seizures without clear consciousness recovery, but with a trend toward seizure reduction over the following months. Trend of seizures and responses to therapy during the first 3 years after disease onset are shown in Figure 1A.
Figure 1.
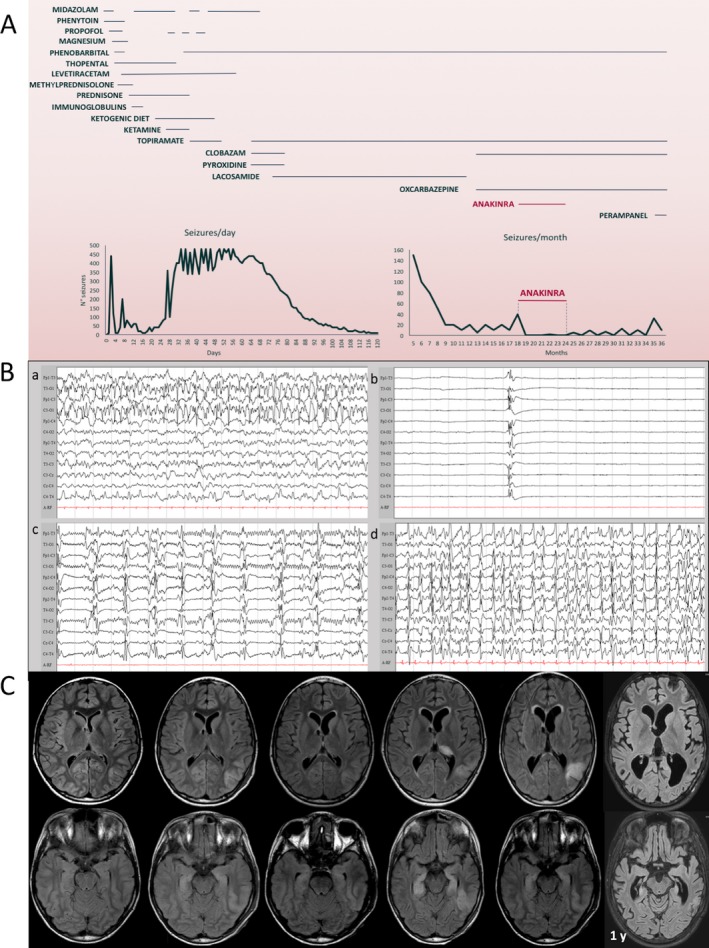
Evolution of the disease and treatment effect. A, Trend of seizures and treatments during the 3 years after disease onset. Thiopental sedation in the acute phase induces burst‐suppression with status epilepticus rebound at thiopental withdrawal. During the chronic phase, seizure reduction is attained by anakinra. B, Representative electroencephalography (EEG) tracings acquired during the first month from disease onset. a: EEG tracing of the second day during a left temporal‐occipital epileptic activity; b: burst‐suppression EEG pattern induced by high‐dose thiopental used to control the aggressive status epilepticus; c: relapse of epileptic abnormalities upon reduction of thiopental sedation; d: status epilepticus after interruption of continuous pharmacologic burst‐suppression (26 days from disease onset). C, Brain magnetic resonance imaging (MRI) evolution during the first year from disease onset. The first brain MRI performed at 2 days (d) from the onset of status epilepticus was unremarkable. The second and third MRI studies at 9 and 21 days showed a stable cortical hyperintensity in the left temporal‐occipital region, with a slight hyperintensity of both hippocampi. The fourth MRI (41 day) was performed after more than 10 days from burst‐suppression interruption (indicated by dotted vertical line in C) with the patient experiencing rebound of aggressive ongoing multifocal and generalized seizures. A more evident left temporal‐occipital hyperintensity involved not only the cortical region, but also the subcortical white matter. A diffuse hyperintensity of the bilateral hippocampal region and of the pulvinar region of the left thalamus became evident. In the fifth MRI study (78 days) after gradual reduction of epileptic activity, the hippocampal alteration was slightly reduced and the pulvinar alteration was no longer present, whereas the temporal‐occipital lesion was still present and a progressive increase in ventricular size became evident. At 1‐year follow‐up, the MRI showed a global brain atrophy with an increased enlargement of the posterior temporal horn. The left temporal‐occipital hyperintensity as well as the hippocampal hyperintensity were no longer visible, but these structures were deeply atrophic
2.2. Laboratory tests
Evaluation of cerebrospinal fluid (CSF) showed elevated protein level (60 mg/dL), normal glucose level (83 mg/dL), normal cells (<1/mm3), and negative viral and bacterial polymerase chain reaction (PCR) studies. Extensive blood investigations excluded ongoing infections, autoimmune disease, and antibody‐mediated encephalitis. Serum testing for anti‐basal ganglia antibodies (ABGAs) showed the presence of antibodies against a still unidentified 150 kDa antigen. An immunoblot stain on rat brain section6 showed a positive reaction, particularly in the neocortex and basal ganglia as compared to serum from a healthy control (Figure 2A). The significance of these findings remains unknown; they may represent an autoantibody‐mediated form of the disease or, as the titer of antibodies is low, a nonspecific reaction induced by exposure of the immune system to autoantigens released by injured brain cells. Mitochondrial pathology was considered through screening of POLG mutation and muscle biopsy, both resulting in negative findings. Next‐generation sequencing panel for genetic epilepsy did not show significant variants. Based on evidence of IL1RN haplotype containing RN2 in a group of Japanese patients with FIRES and an association of IL1RN rs4251981 G>A and SCN2A rs1864885 A>G,7 we tested for but we did not find these polymorphisms in the two genes in our patient.
Figure 2.
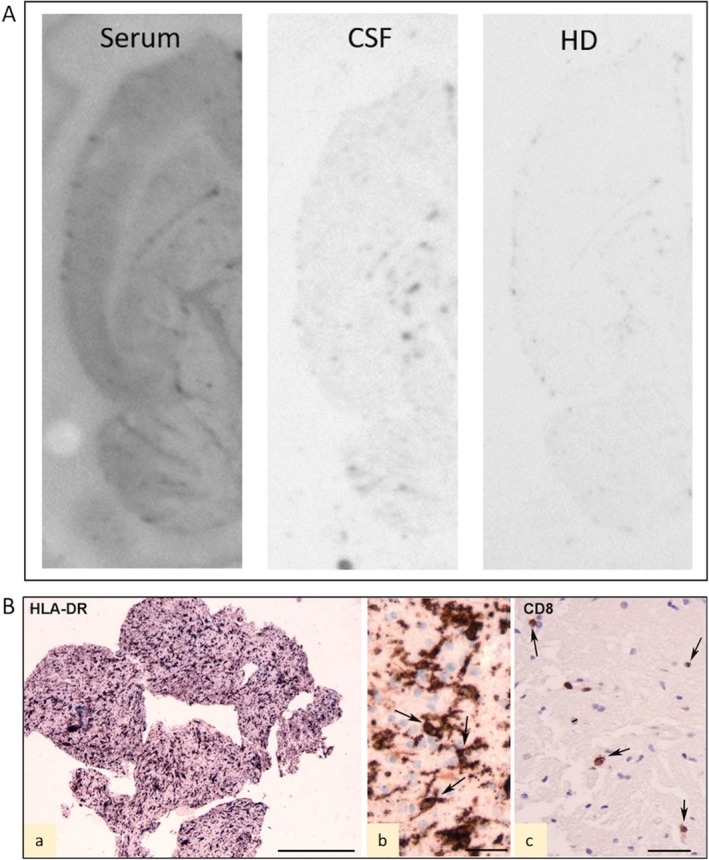
Immunoblots and histopathology. A, Immunohistoblots showing the labeling of horizontal transversal hemisections of rat brain with patient's serum and cerebrospinal fluid (CSF). No labeling was observed in sections incubated with a pool of healthy donor (HD) sera. The principal brain structure identified by patient's serum is neocortex (see Ref. 8). B, Histopathology of biopsy of left posterior temporal lobe. The biopsy consists of small fragments of predominant white matter (Wm) with presence of activated HLA‐DR‐positive cells (a); high magnification photographs of HLA‐DR‐positive reactive microglia within the Wm (arrows in b); few scattered CD8‐positive T lymphocytes (arrows in c). Scale bars: A, 1 mm; B, 25 μm; C, 40 μm. Anti‐human leukocyte antigen (HLA)‐DP, DQ, DR (HLA‐DR; clone CR3/43) anti‐CD8 (mouse monoclonal, clone C8/144B)
2.3. Continuous electroencephalography monitoring
Seizures were multifocal in both hemispheres, but with two prominent active foci, that is, a left posterior temporal focus and a less‐prominent right frontotemporal focus. Seizures stopped only during deep sedation with anesthetic (thiopental) but returned upon anesthetic drug reduction. The highly recurrent seizures had migrating focal seizure‐like features beyond the above‐described foci (Figure 1B).
2.4. Brain magnetic resonance imaging evolution
The first brain magnetic resonance imaging (MRI) was performed 2 days after onset of status epilepticus and did not show remarkable signs (Figure 1C). Subsequent brain MRI studies were performed at 8, 21, 41, and 78 days, marking the topographic anatomic relationship between the evolving MRI alterations and the trend of electroencephalography (EEG) epileptic focal activities. During partial suppression of seizures by anesthetic drugs, brain MRI showed T2‐weighted hyperintensities in the left temporo‐occipital region, in the right insula, and both hippocampi. During aggressive uncontrolled ongoing multifocal seizures, the left temporo‐occipital hyperintensity increased, involving both cortical and subcortical regions. After reduction of epileptic activity, hippocampal and left pulvinar hyperintensities were clearly reduced; the temporo‐occipital lesion was still present. At 1 year, brain MRI showed an increased global atrophy with a temporo‐occipital focal malacia, where previously MRI hyperintensity and epileptic activity were more prominent (Figure 1C).
2.5. Histopathology
Brain biopsy was performed at 2 months from onset showing reactive astrogliosis (not shown), prominent microglia activation, and a few scattered CD8‐positive T lymphocytes (Figure 2B). No known or novel viruses could be identified based on a nucleotide and protein search (using a random DNA and RNA amplification approach in combination with next‐generation sequencing ‐ not shown).
2.6. History of chronic epilepsy phase and effect of anakinra
Four months after status epilepticus onset the patient was bedridden and poorly reactive to stimuli with recurrent daily seizures. He was then sent to an intensive rehabilitation unit where he stayed for 5 months. Seizures during both acute and chronic phases had multifocal onset and corresponding variable semiology, occasionally with secondary generalization. During the chronic phase the seizures were focal motor and nonmotor. In particular, the most frequent manifestations were behavioral arrest, ocular deviation, and facial and oral clonic movements. In the following months, seizures gradually decreased in frequency but continued with a tendency to a monthly clustering of seizures. EEG showed recurrent electric focal seizures (multifocal foci, predominantly in the posterior regions of brain). After discharge, during the rehabilitation period, new hospital admissions for cluster of clinical seizures were required for adjustments of the polytherapy (high‐dose phenobarbital and topiramate, oxcarbazepine, and clobazam at therapeutic doses) but without sustained efficacy. At 1 year and a half from the disease onset, during a new admission for frequent clusters of epileptic multifocal and generalized seizures unresponsive to phenobarbital 7 mg/kg, topiramate 11 mg/kg, clobazam 0.5 mg/kg, and oxcarbazepine 30 mg/kg daily, treatment with subcutaneous (s.c.) anakinra was introduced at the dosage of 2.5 mg/kg per day (100 mg) with partial efficacy and 3 days after increased to 2.5 mg/kg twice a day, finally reaching full seizure control. Anakinra is a recombinant of human IL‐1 receptor antagonist, approved for different neonatal and pediatric autoinflammatory acute and chronic conditions. Its safety profile has been established after long‐term administration at a dose from 1 to 10 mg/kg per day (maximum 200 mg/day).8 No adverse effects were reported in our patient. The occurrence of oppositional behavior made it sometimes problematic for caregivers to perform subcutaneous injections, but the drug was always regularly administered. During this treatment no comedication dosage was modified. Two months after introduction, anakinra was reduced to 100 mg per day (2.5 mg/kg per day). During the 7 months of anakinra treatment only two clinical seizures occurred and control EEG studies show no electric seizures, with some improvement of background activity. After anakinra withdrawal, seizures and EEG epileptiform abnormalities increased until a new admission for epileptic status occurred 11 months after anakinra interruption (Figure 1A). Status epilepticus was managed with adjunctive intravenous doses of phenobarbital after which oral perampanel was initiated. Anakinra reintroduction has been considered but not started yet due to insurance issues and patient's increased oppositional behavior toward injections.
As to the neurofunctional outcome, the patient shows a cognitive disability with frontal behavior, aphasia, and a right spastic gait.
3. DISCUSSION
Clinical, neurophysiologic, and MRI features of the patient were consistent with FIRES.1 In accordance, EEG frequently shows global slowing and multifocal seizures and brain MRI in the initial stages of FIRES is often normal or with minor abnormalities, whereas later evolution typically leads to brain atrophy.9 Whether these changes are due to prolonged intractable seizures and involve brain inflammation remains uncertain.10 A series of brain MRI studies was collected in our patient and matched to continuous EEG monitoring, showing the topographic correspondence of the epileptic multiple focuses with the alterations on brain MRI. MRI lesions may be due to excitotoxic changes, even if the mechanism is not completely understood. The observation of new MRI alterations after stopping drug‐induced burst‐suppression, related to the increased seizure burden (Figure 1A‐C), suggests a vicious cycle between inflammation and seizure.11 This hypothesis suggests that novel therapies should be rapidly applied to efficiently control both inflammation and seizures. FIRES is a condition that may be sustained by brain inflammation, possibly as mirror focus of a systemic inflammatory response to a common infection in predisposed patients. In support, neuroinflammation in animal models is associated with long‐term reduction in seizure threshold together with lasting alterations of brain physiology and gene expression.12, 13 Moreover, specific antiinflammatory drugs reduce ongoing seizures and inhibit epileptogenesis.13 Early brain biopsy is performed rarely, but it could help to clarify FIRES pathogenesis: as in our case, brain biopsy indicated a sustained activation of the innate immune response. Overproduction of proinflammatory cytokines and chemokines by microglia in FIRES could contribute to epileptogenesis.14 In particular, the proinflammatory cytokine IL‐1β released by glial cells promotes neuroinflammation, enhances neuronal excitability, and contributes to seizures in animal models.2 Notably, the balance between brain IL‐1β and IL‐1Ra represents a crucial mechanism to control seizure recurrence and generalization, and anakinra exerts anticonvulsive effects in preclinical models and enhances the therapeutic response to otherwise ineffective drugs.15, 16
Based on a recent case report where anakinra was effective in the acute phase of FIRES3 and additional evidence of its therapeutic effects in inflammatory refractory epilepsy,4, 5, 11 we started anakinra treatment in a patient already in the chronic phase of FIRES during a period of intractable seizure relapse with multiple seizures per day. We observed a rapid and significant improvement after anakinra treatment (as seizures became much rarer than before treatment) and then again seizure increase after anakinra interruption. This observation supports the efficacy of anakinra in the chronic phase of FIRES, although the possibility that anakinra is just coincidentally related to seizure frequency reduction cannot be formally ruled out. This observation also might suggest that neuroinflammation could be involved in seizure generation and seizure recurrence in FIRES.11 Our data raise the need to design treatment protocols suitable for interrupting the vicious cycle between neuroinflammation and recurrent seizures to maximize the therapeutic effects and prevent long‐term outcomes.
The duration of anakinra treatment in FIRES for attaining a therapeutic effect persisting after drug withdrawal is a key aspect that requires further clinical studies. Multicenter studies are urgently needed to clarify the physiopathology and to ascertain efficacy, dose, and tolerance of drugs regulating innate immunity in severe epileptic conditions such as FIRES.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. All co‐authors have been substantially involved in the study and/or the preparation of the manuscript; no undisclosed groups or persons have had a primary role in the study and/or in manuscript preparation. All co‐authors have seen and approved the submitted version of the paper and accept responsibility for its content. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank the family members for their cooperation.
Dilena R, Mauri E, Aronica E, et al. Therapeutic effect of Anakinra in the relapsing chronic phase of febrile infection–related epilepsy syndrome. Epilepsia Open. 2019;4:344–350. 10.1002/epi4.12317
REFERENCES
- 1. Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new‐onset refractory status epilepticus (NORSE), febrile infection‐related epilepsy syndrome (FIRES), and related conditions. Epilepsia 2018;59:739–744. [DOI] [PubMed] [Google Scholar]
- 2. Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL‐1 receptor/Toll‐like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 2011;25:1281–1289. [DOI] [PubMed] [Google Scholar]
- 3. Kenney‐Jung DL, Vezzani A, Kahoud RJ, LaFrance‐Corey RG, Ho ML, Muskardin TW, et al. Febrile infection‐related epilepsy syndrome treated with anakinra. Ann Neurol 2016;80:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jyonouchi H, Geng L. Intractable Epilepsy (IE) and Responses to Anakinra, a Human Recombinant IL‐1 Receptor Antagonist (IL‐1Ra): case Reports. J Clin Cell Immunol 2016;7:456–460. [Google Scholar]
- 5. DeSena AD, Do T, Schulert GS. Systemic autoinflammation with intractable epilepsy managed with interleukin‐1 blockade. J Neuroinflammation 2018;15:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernasconi P, Cipelletti B, Passerini L, Granata T, Antozzi C, Mantegazza R, et al. Similar binding to glutamate receptors by Rasmussen and partial epilepsy patients’ sera. Neurology. 2002;12:1998–2001. [DOI] [PubMed] [Google Scholar]
- 7. Saitoh M, Kobayashi K, Ohmori I, Tanaka Y, Tanaka K, Inoue T, et al. Cytokine‐related and sodium channel polymorphism as candidate predisposing factors for childhood encephalopathy FIRES/AERRPS. J Neurol Sci 2016;368:272–276. [DOI] [PubMed] [Google Scholar]
- 8. Kullenberg T, Löfqvist M, Leinonen M, Goldbach‐Mansky R, Olivecrona H. Long‐term safety profile of anakinra in patients with severe cryopyrin‐associated periodic syndromes. Rheumatol Oxf Engl 2016;55:1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rivas‐Coppola MS, Shah N, Choudhri AF, Morgan R, Wheless JW. Chronological evolution of magnetic resonance imaging findings in children with febrile infection‐related epilepsy syndrome. Pediatr Neurol 2016;55:22–29. [DOI] [PubMed] [Google Scholar]
- 10. Lee HF, Chi CS. Febrile infection‐related epilepsy syndrome (FIRES): therapeutic complications, long‐term neurological and neuroimaging follow‐up. Seizure 2018;56:53–59. [DOI] [PubMed] [Google Scholar]
- 11. Nabbout R, Vezzani A, Dulac O, Chiron C. Acute encephalopathy with inflammation‐mediated status epilepticus. Lancet Neurol 2011;10:99–108. [DOI] [PubMed] [Google Scholar]
- 12. Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Exp Neurol 2013;4:11–21. [DOI] [PubMed] [Google Scholar]
- 13. Aronica E, Bauer S, Bozzi Y, Caleo M, Dingledine R, Gorter JA, et al. Neuroinflammatory targets and treatments for epilpesy validated in experimental models. Epilepsia 2017;58:27–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakuma H, Tanuma N, Kuki I, Takahashi Y, Shiomi M, Hayashi M. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection‐related refractory status epilepticus. J Neurol Neurosurg Psychiatry 2015;86:820–822. [DOI] [PubMed] [Google Scholar]
- 15. Vezzani A, Dingledine R, Rossetti AO. Immunity and inflammation in status epilepticus and its sequelae: possibilities for therapeutic application. Expert Rev Neurother 2015;15:1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng‐Hao X, Wang Y, Tao AF, Yu J, Wang XY, Zu YY, et al. Interleukin‐1 receptor is a target for adjunctive control of diazepam‐refractory status epilepticus in mice. Neuroscience 2016;328:22–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


