Abstract
Purpose:
To describe and quantify the rate of detection of renal cancer on unenhanced CT.
Methods:
This retrospective, HIPAA-compliant study was approved by the Institutional Review Board. Electronic health records for all patients who underwent unenhanced abdominal CT at our institution between 2000 and 2005 were reviewed to identify patients subsequently diagnosed with renal cancer during a follow-up period of up to 12 years. Images were reviewed to determine if the cancer was visible at index (first) unenhanced CT, and their findings recorded. Original radiology reports were reviewed to determine whether the renal cancer was reported; Fisher’s Exact Test compared imaging features of detected and missed cancers. Clinical outcomes including time until diagnosis and stage at diagnosis assessed potential impact of missed cancers.
Results:
Of 15,695 patients, 82 (0.52%) were diagnosed with renal cancer. Of these, 43/82 (52%) cancers were retrospectively detectable on index unenhanced CT. Among retrospectively detectable cancers, 63% (27/43) were originally detected and reported on index CT and 37% (16/43) were missed. Size was the only feature associated with detection; 83% (20/24) of cancers >3.0 cm were detected versus 37% (7/19) of cancers ≤3.0 cm (p=0.0036). Although none of the 16 missed renal cancers developed metastases between index CT and time of diagnosis (median 33.5 months), 4 (25%) progressed in stage.
Conclusions:
Renal cancer was rare in patients undergoing unenhanced abdominal CT. Over one-third of potentially detectable cancers were missed prospectively. However, missed cancers did not metastasize and infrequently progressed in stage before being diagnosed.
Keywords: Renal mass, Renal cell carcinoma, Renal cancer, Unenhanced CT, CT
Introduction
Most renal cancers are detected incidentally with cross sectional imaging (1–4). Multiphase IV contrast-enhanced CT and MRI increase the conspicuity of renal masses and help characterize them fully. However, these exams are typically performed only when a renal cancer is suspected or when an indeterminate renal mass has been previously identified (5–7). When evaluating unenhanced CT of the abdomen for various indications unrelated to renal mass detection and characterization, radiologists are challenged with recognizing renal cancers, and distinguishing them from more common benign etiologies (8).
The evidence supporting radiologists’ ability to render a confident diagnosis of renal masses at unenhanced CT has been building. Classic renal angiomyolipomas can be diagnosed with confidence at unenhanced CT by identifying fat in non-calcified renal masses (5,9). Recent studies have shown that unenhanced CT can also be used to diagnose benign renal cysts with confidence by identifying masses which are homogeneous and measure 20HU or less or 70HU or greater (10,11). The remainder include masses which might be cancers and are either heterogeneous, contain attenuations greater than 20 HU or less than 70 HU, or contain septa, thick walls, or calcification (8,12,13).
To our knowledge, there is no literature on how often renal cancers are recognized on unenhanced CT, and no data as to the clinical impact of not detecting them. Studies have shown that renal cancers can be detected at unenhanced CT due to a difference in attenuation relative to normal renal parenchyma or due to a deformation of the renal contour (14). In addition, use of narrow window settings may improve renal mass detection (15). As these prior studies utilized preselected cohorts of renal cancers, they cannot be used to determine the detection rate of renal cancer in clinical practice. Evaluation of patients brought to case review conferences or otherwise identified on an ad hoc basis has demonstrated errors in detecting non-stone renal pathology at unenhanced CT (16,17). However, the renal cancer detection rate cannot be determined with these study designs. Furthermore, the impact of not detecting a renal cancer is unknown. Therefore, the primary purpose of this study was to describe and quantify the rate of detection of renal cancer on unenhanced CT. As secondary objectives, we also assessed imaging features associated with the detection of renal cancer at unenhanced CT, and the clinical impact of not detecting them.
Materials and Methods
Study Site and Cohort
This retrospective study was compliant with the Health Insurance Portability and Accountability Act and was approved by our Institutional Review Board, which waived informed consent requirements. It was performed at a 777-bed urban tertiary academic medical center with more than 600,000 annual radiologic examinations. The study period was 1/1/2000 to 12/31/2005 to allow for up to 12 years of follow-up to diagnose renal cancer subsequent to the index unenhanced CT. During this period, a total of 17,213 adult patients underwent unenhanced abdominal CT, hereafter referred to as the index CT. As per institutional policy, 1,085 patients who were employees were excluded. Examinations including both an unenhanced acquisition and contrast-enhanced acquisition were also excluded. Each patient’s medical record was searched for a diagnosis of renal cancer of any type. We defined renal cancer as renal cell carcinoma, urothelial carcinoma, unspecified renal primary cancer, neoplasm “of uncertain behavior,” or metastatic cancer. Date of cancer diagnosis was compared with the date of the index CT. A total of 433 patients who were diagnosed with renal malignancy prior to their index CT were excluded. Of the remaining 15,695 patients, 8,870 with no diagnosis of renal cancer and less than 5 years follow-up were excluded. Excluded patients were considered lost to follow-up for the purposes of renal cancer prevalence estimation. A total of 82 patients with positive diagnosis of renal cancer after the index unenhanced CT comprised the final study cohort (Figure 1).
Figure 1.
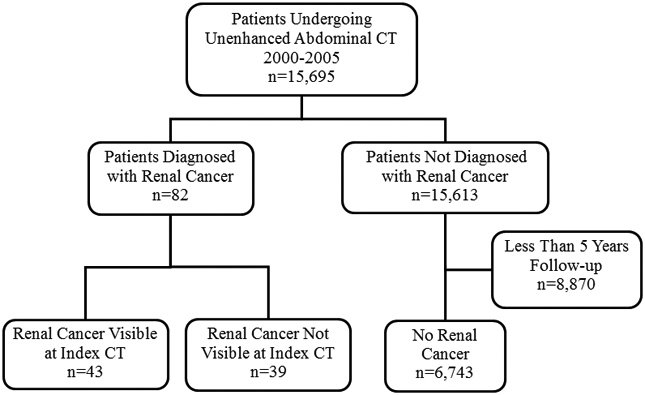
Renal Cancer at Unenhanced CT: Study Cohort (n=82) Flowchart
Image Evaluation
The index CT and any other relevant cross-sectional imaging for study patients were reviewed retrospectively by two abdominal radiologists with 18 and 28 years of practice experience, respectively (RK and SG). Relevant information extracted from clinical notes and radiology, surgery, and pathology reports was available during this review.
By means of consensus, the radiologists determined if the renal cancer was present and visible at the time of the index unenhanced CT. A third abdominal radiologist (SO) characterized the findings on CT (mass size, attenuation, presence of calcification, heterogeneity, and alteration of the renal contour).
Renal Cancer Detection
Radiology reports of patients with a positive diagnosis of renal cancer were reviewed to determine if the renal cancer was detected or missed in the original clinical radiology report.
Renal cancers were considered detected if the report included the renal mass finding and the finding was either considered diagnostic of or possibly representing a cancer, or follow-up renal imaging was recommended. Renal cancers were considered not detected, i.e., missed, if the report did not include the renal mass finding, or if the renal mass was reported but cancer was not included as a possible etiology and no follow-up renal imaging was recommended.
Outcomes
Radiology images and reports, pathology reports, operative notes and clinical notes were reviewed to assess renal cancer stage at index CT and at the time of diagnosis to determine clinical outcomes. Clinical outcomes included: a) estimated stage of renal cancer at index CT, b) stage of renal cancer at the time of pathologic diagnosis, and c) time from index CT to pathologic diagnosis of renal cancer. The American Joint Committee on Cancer (AJCC) staging system, also known as the TNM staging system (6th edition), was used (18); stage 1 and 2 renal cell carcinomas (RCCs) are organ-confined, without lymph node involvement or metastases. Stage 1 RCCs are less than 7 cm and stage 2 RCCs are 7 cm or greater. Stage 3 RCCs have spread into the major veins or perinephric tissues, but not into the adrenal gland or beyond Gerota’s fascia, or involve regional lymph nodes. Stage 4 RCCs have spread beyond Gerota’s fascia or have metastasized. Stage 0 urothelial carcinoma is considered carcinoma in situ if it has not spread through the lamina propria, without lymph node or metastatic disease. If stage at the time of index CT was uncertain due to lack of information, the lowest possible stage was assumed to estimate the greatest possible harm of failing to detect a renal cancer.
Statistical Analysis
The primary outcome was the detection of renal cancer, based on the original clinical radiology report of the index CT. Secondary outcomes included the association of imaging features with detection, which was evaluated with Fisher’s Exact test, and the impact of detection of a suspicious renal mass on index CT on time to diagnosis of renal cancer, which was evaluated with Wilcoxon Rank Sum test. P-values less than 0.05 were considered statistically significant.
Results
Study Cohort
Among the 15,695 patients, 82 renal cancers were diagnosed after index unenhanced CT, a prevalence of 0.52% (82/15,695). The remaining 15,613 patients did not have renal cancer. In the overall cohort, mean age was 55 years ± 18; 53% of patients were female. In the renal cancer patient cohort, mean age was 58 years ± 13 with 30% females.
Renal Cancer Cohort
Of the 82 renal cancers, 43 (52%) were retrospectively visible on the index CT. Of the 43 retrospectively detectable renal cancers, 63% (27/43) were detected and reported in the index CT original clinical radiology report. Median time to diagnosis for these cancers was 8 months after index CT (Inter-Quartile Range [IQR] 2, 46 months). They consisted of 38 renal cell carcinomas (RCCs), 4 metastases, and 1 urothelial carcinoma. Of the 38 RCCs, 26 were stage I, 2 were stage II, 6 were stage III and 4 were stage IV at diagnosis; the urothelial carcinoma was stage 0 at diagnosis (Table 1).
Table 1:
Stage and grade of renal cancers at the time of diagnosis for patients with visible cancers on index CT (n=43)
| Full Stage at Diagnosis | Cases | Percent |
|---|---|---|
| Renal Cell Carcinomas (n=38) | ||
| I (T1N0M0) | 26 | 60.46% |
| II (T2N0M0) | 2 | 4.65% |
| III (T3N0M0) | 6 | 13.95% |
| IV (T2NxM1) | 1 | 2.33% |
| IV (T3NxM1) | 1 | 2.33% |
| IV (T4N1M1) | 1 | 2.33% |
| IV (T4NxM1) | 1 | 2.33% |
| Lymphoma | 1 | 2.33% |
| Metastases | 3 | 6.97% |
| Urothelial Carcinoma | 1 | 2.33% |
The 43 cancers that were retrospectively visible in index CT had a mean maximum diameter of 3.9 cm (range 1.0-9.7 cm); 24 (56%) were >3.0 cm; 22 (51%) were homogeneous and measured greater than 20 and less than 70 HU, and 21 (49%) were heterogeneous or contained one or more septa or thick walls (Table 2). Of the 43 cancers, 35 (81%) deformed the renal contour.
Table 2:
Imaging features of renal cancers at index unenhanced CT
| Detected (n=27) |
Missed (n=16) |
Total (n=43) |
Proportion Detected |
p-valuea | |
|---|---|---|---|---|---|
| Size | 0.0036 | ||||
| <= 3.0 cm | 7 | 12 | 19 | 37% | |
| >3.0 cm | 20 | 4 | 24 | 83% | |
| Heterogeneity | 0.3475 | ||||
| Heterogeneousb | 15 | 6 | 21 | 71% | |
| Homogeneous | 12 | 10 | 22 | 55% | |
| ≥70 HU | 0 | 0 | 0 | ||
| >20 but <70 HU | 12 | 10 | 22 | ||
| ≤20 HU | 0 | 0 | 0 | ||
| Calcification | 0.2824 | ||||
| Present | 3 | 0 | 3 | 100% | |
| Absent | 24 | 16 | 40 | 60% | |
| Contour Deformity | 0.4433 | ||||
| Yes | 23 | 12 | 35 | 66% | |
| Exophytic | 16 | 9 | 25 | 64% | |
| Endophyticc | 3 | 3 | 6 | 50% | |
| Whole/Majority of Kidney Involved | 4 | 0 | 4 | 100% | |
| No | 4 | 4 | 8 | 50% |
Values in bold are statistically significant
Heterogeneous includes masses with septations or wall thickening
Endophytic: projecting into the renal sinus and displacing the renal sinus fat
Detection
Of the 43 retrospectively visible renal cancers, 27 (63%) were detected and reported on index CT; in 16, the possibility of a renal cancer was included in the differential diagnosis and in 11, although the possibility of a renal cancer was not raised, follow-up renal imaging was recommended. Sixteen (37%) renal cancers, visible retrospectively by reviewing radiologists, were not detected at the time of the index CT; Of these 16 cancers, 10 were not included in the radiology report, presumably because they were not identified or were identified and deemed not needing comment. The remaining six cancers were reported, but the possibility of a renal cancer was not included in the differential diagnosis and no follow-up recommendation were made. The stage and grade of these 16 cancers are depicted in (Table 3).
Table 3:
Stage and grade for cancers visible retrospectively but not detected on index unenhanced CT
| Full Stage at Diagnosis | n=16 |
|---|---|
| I (T1N0M0) | 12 |
| II (T2N0M0) | 1 |
| III (T3N0M0) | 1 |
| Metastasis | 1 |
| Urothelial Carcinoma | 1 |
Size was the only feature associated with detection; 83% (20/24) of cancers greater than 3.0 cm were detected versus 37% (7/19) cancers less than 3.0 cm (p=0.0036).
Renal Cancer Outcomes
Detected renal cancers were diagnosed in a median of 4 months (IQR 1, 21) while retrospectively visible but originally not reported renal cancers were diagnosed in a median of 33.5 months (IQR 8.5, 59, p=0.0060). None of the 16 missed renal cancers developed metastases between the index CT and time of diagnosis. Four (25%) progressed in stage (Table 4). One increased in size but remained organ-confined, progressing from stage 1 to stage 2. Two renal cell carcinomas that were stage 1 progressed to stage 3 at diagnosis when they were found to invade perinephric tissues on surgical specimens. Finally, a renal cancer that was stage 2 at index CT progressed to stage 3 at diagnosis when pathology demonstrated renal vein invasion.
Table 4.
Renal cancers which progressed between index unenhanced CT and diagnosis
| Stage at Index CT |
Stage at Diagnosis |
Delay* (Months) |
Potential Progression |
|---|---|---|---|
| I | III | 46 | Growth (2.5 to 7.1 cm), perinephric fat invasion at diagnosis not visible on index CT |
| I | III | 69 | Growth (3.2 to 3.9 cm), perinephric fat invasion at diagnosis not visible on index CT |
| II | III | 8 | Growth (7.0 to 7.3 cm), renal vein invasion at diagnosis not visible on index CT |
| I | II | 41 | Growth (2.3 to 7.2 cm) |
Delay = Time between index CT and diagnosis in months
Discussion
Renal cell carcinoma is most commonly diagnosed incidentally in patients who are examined with cross-sectional imaging for a non-renal complaint (1–4). The role of contrast-enhanced CT in diagnosing renal cancer is well-established (5–7). Renal cancers may also present at unenhanced CT performed for various, unrelated indications, although our study shows this is rare. Of 15,695 patients examined with unenhanced CT, only 82 (82/15,695=0.5%) were diagnosed with renal cancer during the up to 12 years of follow-up, and of these cancers only 43 (43/15,695=0.3%) had a renal cancer visible in retrospect on the index unenhanced CT. The remaining patients may have had occult cancers that were not visible or they may have developed the cancer subsequent to the index CT. Although renal cancers at unenhanced CT are rare, and some may not be visible, we aimed to review how often the visible cancers were detected, describe their unenhanced CT features, and determine what impact not detecting them had on patient outcome.
The imaging features of renal cancers were commensurate with prior studies: all cancers were either heterogeneous, contained one or more septa or wall thickening, or were homogeneous with attenuations greater than 20 and less than 70 HU. The majority (51%) of cancers in our cohort were homogeneous, in contrast to only 9% of renal cancers in a prior study (12). This is likely due to differences in source populations, as the cancers in our study were smaller and lower stage. This highlights the need to visualize attenuation differences between a possible renal cancer and the adjacent normal renal parenchyma. This can be aided by using narrow window settings (15). Also, renal cancers which deformed the outer renal contour were more likely to be detected at unenhanced CT than those that did not (14). We found this to be a common feature, seen in 35 (81%) of the renal cancers: 25 cancers deformed the outer contour, 6 protruded into the renal sinus and displaced the renal sinus fat, and 4 altered the contour of most or all the kidney. Using multiplanar images can help recognize a renal contour abnormality (17).
To our knowledge, our study is the first to report radiologists’ performance at detecting renal cancer on unenhanced CT in clinical practice. Our review of both radiology reports and images allowed us to calculate a detection rate, in contrast to prior studies which relied on peer review or case conference data to describe errors (16,17). False negative errors can increase with targets of low prevalence, for example sensitivity for detecting breast cancer at mammography dropped from 88% to 70% when prevalence dropped from 50% to nearly 1% (19). Our study was of patients encountered during routine clinical practice undergoing unenhanced CT for a multitude of clinical indications, where the prevalence of incidental renal cancer was less than 1% of patients. Thus our detection rate of 63% is likely more realistic than those based on enriched cohorts which had renal cancer rates as high as 50% (14,15). Finally, as radiologists in our cohort were challenged with not only perceiving renal cancers, but also differentiating them from benign renal masses, our detection rate is likely more accurate than studies which had excluded non-malignant renal findings such as cysts (14,15).
Of the 43 renal cancers retrospectively visible at unenhanced CT, 16 (37%) were not detected clinically prospectively. Cancers larger than 3.0 cm were more frequently detected than smaller ones. There were no differences in the detection of renal cancer based on heterogeneity, or calcification. Therefore, radiologists will likely need to rely on all imaging features to detect a renal cancer at unenhanced CT.
Over a third of the retrospectively visible renal cancers were not detected on the index CT and remained undiagnosed for several years (33.5 months median, 8.5-59 months IQR time to diagnosis). Despite this, none became metastatic in the interval between the index CT and diagnosis; 4 (25%) progressed due to growth or extension into the perirenal tissues. Although most small renal cancers are indolent with low metastatic potential, some may progress beyond an organ-confined stage and potentially affect prognosis (20–23).
Our definition of renal cancer detection required the radiologist to not only perceive that a mass existed within the kidney but also interpret the mass as potentially malignant (as evidenced by including cancer in the differential diagnosis or making a follow-up recommendation). Some authors have distinguished errors in perception and interpretation of imaging findings to better understand and attempt to diminish such errors (24,25). We chose to combine them, as both correct perception and interpretation are needed to produce clinically useful radiology reports. Our definition did not classify as ‘detected’ six renal masses that were described in the original reports because there was neither a mention of cancer in the differential diagnosis nor a follow-up imaging recommendation. As a result, one might consider our data as a reflection of ‘diagnosis rate’ rather than ‘detection rate’. Recommending a follow-up imaging examination when a renal mass had indeterminate features, even if the possibility of a cancer was not mentioned, would have eliminated six of the 16 missed cancers in our cohort and would have likely led to improved renal cancer detection.
We recognize the limitations of our study. First slightly more than half of our source population was excluded. Specifically, we excluded patients who did not have a diagnosis of renal cancer and less than 5 years of follow-up. Therefore, our estimate for renal cancer prevalence could range between 0.52% and 1.2% (excluding patients with less than 5 years of follow-up to reach the latter estimate). However, it is unlikely that patients with less than 5 years of follow-up had a higher risk of developing renal cancer than patients with longer follow-up and thus the prevalence is unlikely to exceed 1.2%. Second, consensus review to determine which renal cancers were retrospectively visible at index CT was performed with the knowledge of the location of the subsequently diagnosed renal cancer by two abdominal radiology subspecialists with extensive clinical experience. Therefore, we may have overestimated the proportion of cancers that may have been detectable by radiologists during clinical practice prospectively. This would falsely elevate both the prevalence of visible renal cancers at unenhanced CT as well as the rate that cancers were not detected. Even so, because renal cancers were rare findings, missing renal cancers at unenhanced CT was also rare (though over a third of the potentially detectable renal cancers were missed). Only 16 renal cancers were missed at index CT over a 5-year period with six of these ‘missed’ cancers observed prospectively but ignored (presumably as benign) by the radiologists at the time of reporting. Finally, assessing stage progression was limited by the fact that the index CT was unenhanced and therefore stages higher than 1 could have been missed. For example, it is possible that one of the three cancers which we determined to have progressed from stage 1 or 2 to stage 3 was already stage 3 at the time of the index CT. This is a known problem, as even with the use of contrast enhanced CT, a substantial proportion of renal cancers are upstaged from organ-confined (stage 1 or 2) to locally invasive (stage 3) after resection (26). Therefore, we may have overestimated the frequency at which missed renal cancers progressed prior to diagnosis.
In summary, renal cancer was a rare (in the 1% range) finding at unenhanced CT of the abdomen, and over one-third (37%) of potentially detectable cancers were missed prospectively or incorrectly interpreted; of those missed, none metastasized but a minority progressed in stage when ultimately diagnosed. Following established recommendations for the detection, evaluation and follow-up of renal masses at unenhanced CT will help minimize the number of missed cancers (8, 11–14).
Figure 2. Renal Cancers Detected at Unenhanced CT.
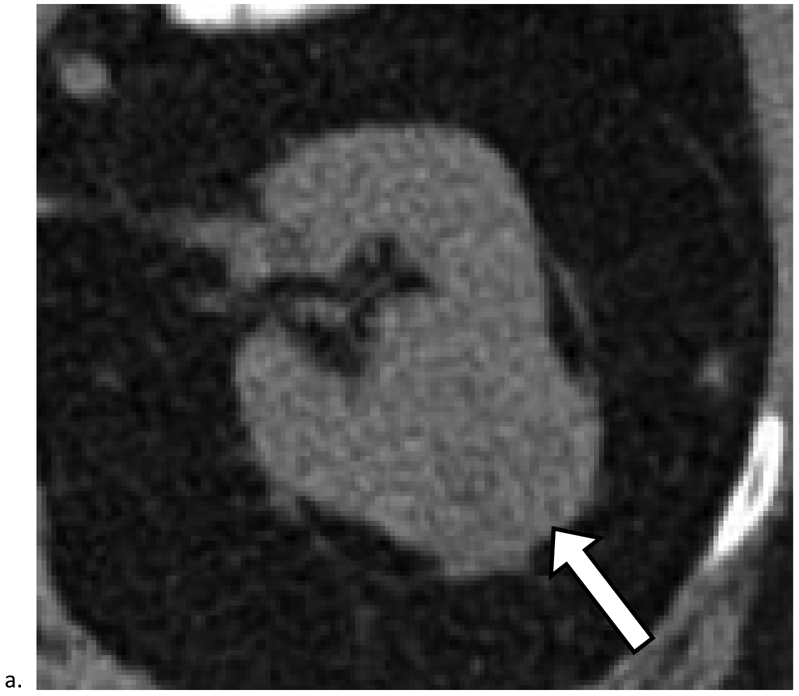
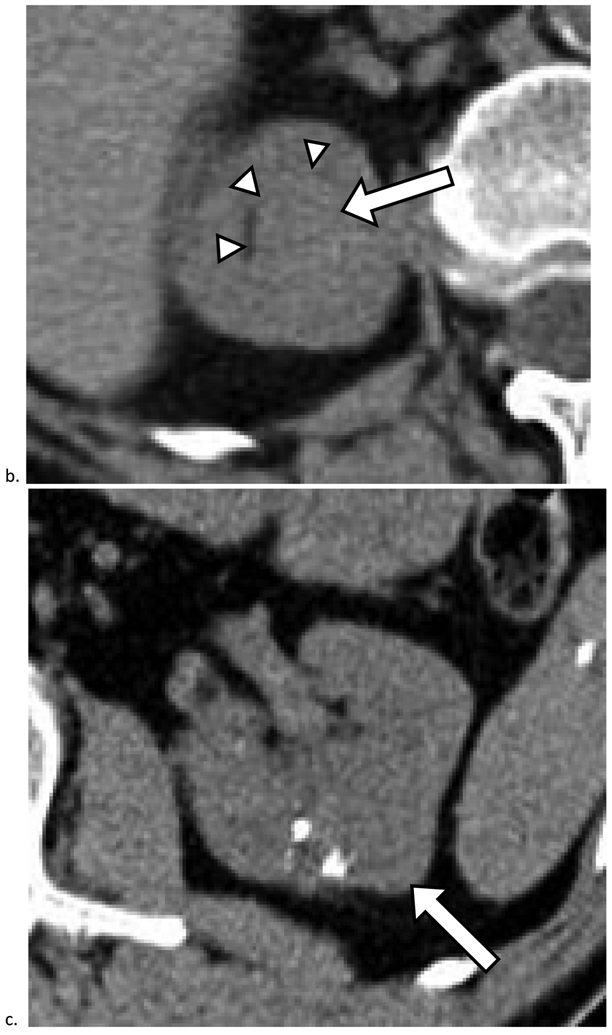
a). Mass heterogeneity and deformity of the outer renal contour aid in detection of renal cancer. Axial unenhanced CT image obtained in a 62-year-old female shows a 3.7 cm mass in the right kidney (arrow). The mass was detected and included in the radiology report as a possible cancer and diagnosed 6 months later as renal cell carcinoma (RCC). b). Deformation of the inner renal contour and displacement of the renal sinus fat (arrowheads) aid in detection. Axial unenhanced CT image obtained in a 67-year-old female shows a 2.4 cm homogeneous mass measuring 47 HU (arrow). The mass was detected and included in the radiology report as a possible cancer and diagnosed 6 months later as RCC. c). Calcifications aid in detection. Axial unenhanced CT image obtained in a 67-year-old male shows a 3.3 cm mass in the left kidney. The mass was detected and included in the radiology report as a possible cancer and diagnosed 2 months later as RCC.
Figure 3. Missed Renal Cancers that Did Not Progress Prior to Diagnosis.
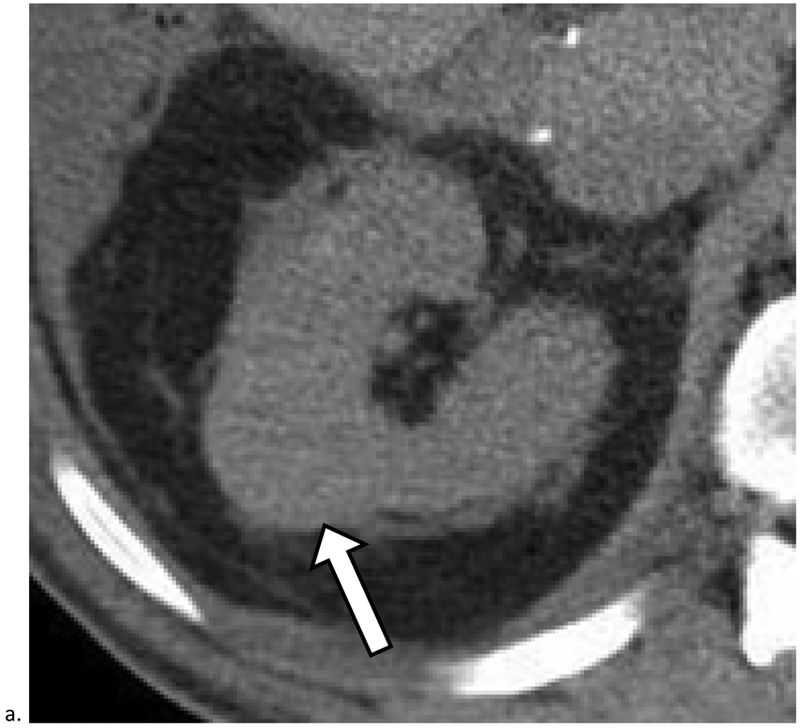
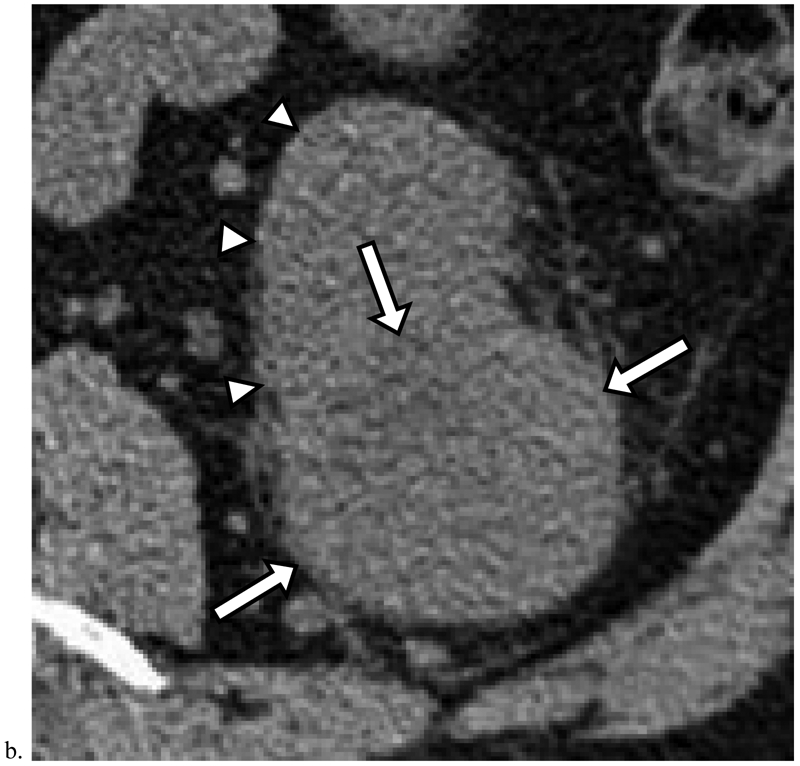
a). Homogeneous masses with attenuation greater than 20 HU and less than 70HU are indeterminate and may represent renal cancer. Axial unenhanced CT image obtained in a 72-year-old male shows a 2.9 cm homogeneous mass measuring 49 HU deforming the outer contour of the right kidney (arrow). Although this finding was identified, it was diagnosed as a cyst in the radiology report; it was diagnosed 9 months later as renal cell carcinoma (RCC). b). Heterogeneous masses which deform the renal contour may represent renal cancer. Axial unenhanced CT image obtained in a 57-year-old male shows a 7.1 cm heterogeneous mass (arrows) deforming the outer contour of the left kidney (arrowheads). Although this finding was identified, no diagnosis was offered and follow-up was not recommended. The mass was diagnosed 1 month later as RCC.
Figure 4. Missed Renal Cancers that Progressed Prior to Diagnosis.
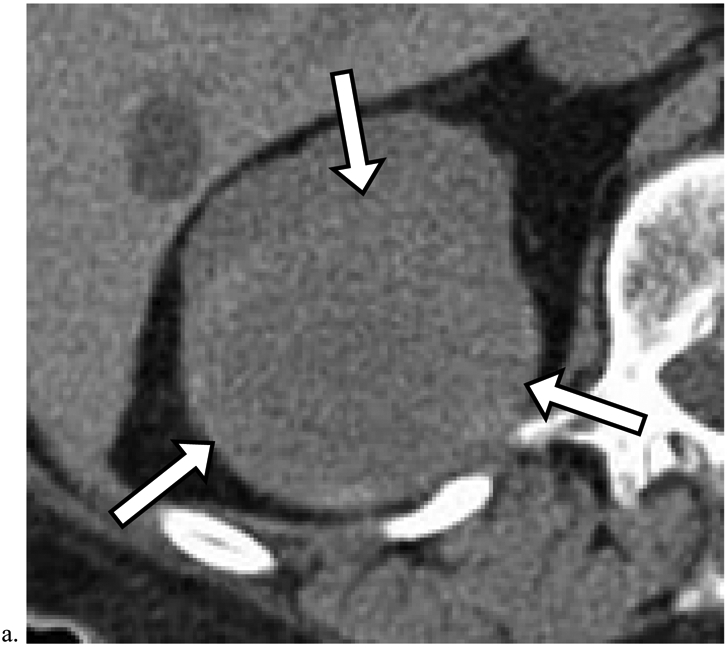
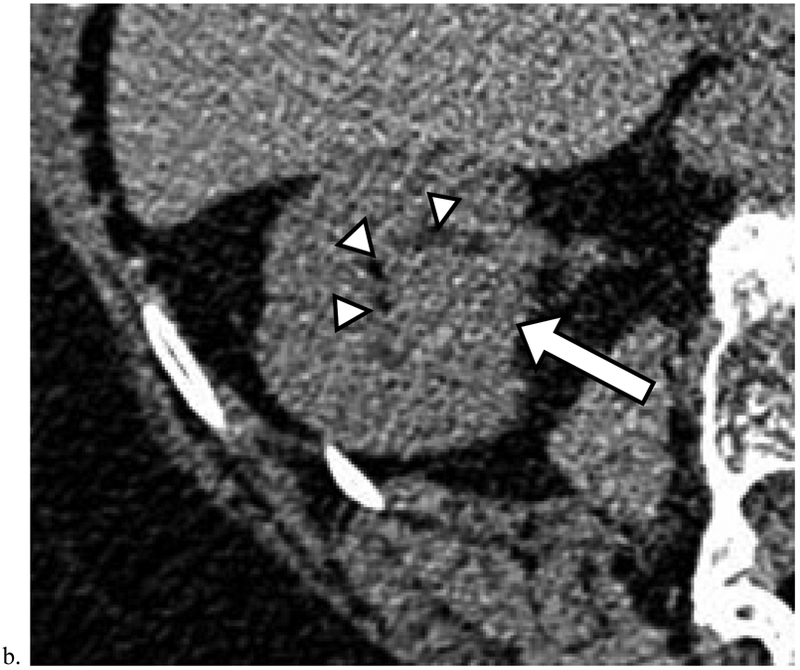
a). Heterogeneous masses which deform the renal contour may represent renal cancer. Axial unenhanced CT image obtained in a 74-year-old male shows a 7.0 cm heterogeneous mass in the right kidney (arrows). Although this finding was identified, no diagnosis or follow-up recommendation was offered in the radiology report. The mass was diagnosed 8 months later as renal cell carcinoma (RCC) (Table 5, row 3). The patient died of non-small cell lung cancer one year later. b). Deformity of the inner renal contour aids in detection of renal cancer. Axial unenhanced CT image obtained in a 78-year-old female shows a 2.5 cm homogeneous mass (arrow) measuring 40 HU deforming the inner renal contour and displacing the renal sinus fat (arrowheads). This finding was not included in the original radiology report. The mass was diagnosed 46 months later as RCC (Table 5, row 1).
Acknowledgments
Funding: This study received no direct funding. One of the authors was supported by National Library of Medicine training grant T15LM007092.
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: The requirement to obtain informed consent was waived by the study site’s Institutional Review Board.
The final publication is available at link.springer.com
References
- 1.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998. February;51(2):203–5. [DOI] [PubMed] [Google Scholar]
- 2.Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982-1997). Urology. 2000. July;56(1):58–62. [DOI] [PubMed] [Google Scholar]
- 3.Schlomer B, Figenshau RS, Yan Y, Venkatesh R, Bhayani SB. Pathological features of renal neoplasms classified by size and symptomatology. J Urol. 2006. October;176(4 Pt 1):1317–1320; discussion 1320. [DOI] [PubMed] [Google Scholar]
- 4.Rabjerg M, Mikkelsen MN, Walter S, Marcussen N. Incidental renal neoplasms: is there a need for routine screening? A Danish single-center epidemiological study. APMIS. 2014. August;122(8):708–14. [DOI] [PubMed] [Google Scholar]
- 5.Israel GM, Bosniak MA. Pitfalls in Renal Mass Evaluation and How to Avoid Them. RadioGraphics. 2008. September;28(5):1325–38. [DOI] [PubMed] [Google Scholar]
- 6.Silverman SG, Israel GM, Herts BR, Richie JP. Management of the incidental renal mass. Radiology. 2008. October;249(1):16–31. [DOI] [PubMed] [Google Scholar]
- 7.Israel GM, Bosniak MA. An update of the Bosniak renal cyst classification system. Urology. 2005. September;66(3):484–8. [DOI] [PubMed] [Google Scholar]
- 8.Silverman SG, Israel GM, Trinh Q-D. Incompletely characterized incidental renal masses: emerging data support conservative management. Radiology. 2015. April;275(1):28–42. [DOI] [PubMed] [Google Scholar]
- 9.Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014. June;39(3):588–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonisch AI, Rubinowitz AN, Mutalik PG, Israel GM. Can High-Attenuation Renal Cysts Be Differentiated from Renal Cell Carcinoma at Unenhanced CT? Radiology. 2007. May;243(2):445–50. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor SD, Silverman SG, Ip IK, Maehara CK, Khorasani R. Simple cyst-appearing renal masses at unenhanced CT: can they be presumed to be benign? Radiology. 2013. December;269(3):793–800. [DOI] [PubMed] [Google Scholar]
- 12.Pooler BD, Pickhardt PJ, O’Connor SD, Bruce RJ, Patel SR, Nakada SY. Renal cell carcinoma: attenuation values on unenhanced CT. AJR Am J Roentgenol. 2012. May;198(5):1115–20. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor SD, Pickhardt PJ, Kim DH, Oliva MR, Silverman SG. Incidental Finding of Renal Masses at Unenhanced CT: Prevalence and Analysis of Features for Guiding Management. American Journal of Roentgenology. 2011;197(1):139–145. [DOI] [PubMed] [Google Scholar]
- 14.Jung SI, Park HS, Kim YJ, Jeon HJ. Unenhanced CT for the detection of renal cell carcinoma: effect of tumor size and contour type. Abdominal Imaging. 2014. April;39(2):348–57. [DOI] [PubMed] [Google Scholar]
- 15.Sahi K, Jackson S, Wiebe E, Armstrong G, Winters S, Moore R, et al. The Value of “Liver Windows” Settings in the Detection of Small Renal Cell Carcinomas on Unenhanced Computed Tomography. Canadian Association of Radiologists Journal. 2014. February;65(1):71–6. [DOI] [PubMed] [Google Scholar]
- 16.Donald JJ, Barnard SA. Common patterns in 558 diagnostic radiology errors: Common patterns of diagnostic errors. Journal of Medical Imaging and Radiation Oncology. 2012. April;56(2):173–8. [DOI] [PubMed] [Google Scholar]
- 17.Horton KM, Johnson PT, Fishman EK. MDCT of the Abdomen: Common Misdiagnoses at a Busy Academic Center. American Journal of Roentgenology. 2010. March;194(3):660–7. [DOI] [PubMed] [Google Scholar]
- 18.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. , editors. AJCC cancer staging manual. 6th Edition. New York: Springer; 2002. [Google Scholar]
- 19.Evans KK, Birdwell RL, Wolfe JM. If you don’t find it often, you often don’t find it: why some cancers are missed in breast cancer screening. PLoS ONE. 2013;8(5):e64366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DYT, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006. February;175(2):425–31. [DOI] [PubMed] [Google Scholar]
- 21.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma--a meta-analysis and review. J Urol. 2008. April;179(4):1227–1233-1234. [DOI] [PubMed] [Google Scholar]
- 22.Mues AC, Haramis G, Badani K, Gupta M, Benson MC, McKiernan JM, et al. Active surveillance for larger (cT1bN0M0 and cT2N0M0) renal cortical neoplasms. Urology. 2010. September;76(3):620–3. [DOI] [PubMed] [Google Scholar]
- 23.Haramis G, Mues AC, Rosales JC, Okhunov Z, Lanzac AP, Badani K, et al. Natural history of renal cortical neoplasms during active surveillance with follow-up longer than 5 years. Urology. 2011. April;77(4):787–91. [DOI] [PubMed] [Google Scholar]
- 24.Krupinski EA. Current perspectives in medical image perception. Atten Percept Psychophys. 2010. July;72(5):1205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzale JM, Kranz PG. Understanding errors in diagnostic radiology: proposal of a classification scheme and application to emergency radiology. Emerg Radiol. 2011. October;18(5):403–8. [DOI] [PubMed] [Google Scholar]
- 26.Tay MHW, Thamboo TP, Wu FMW, Zhaojin C, Choo TB, Ramaan L, et al. High R.E.N.A.L. Nephrometry scores are associated with pathologic upstaging of clinical T1 renal-cell carcinomas in radical nephrectomy specimens: implications for nephron-sparing surgery. J Endourol. 2014. September;28(9):1138–42. [DOI] [PubMed] [Google Scholar]


