Abstract
One of the defining features of Acute Respiratory Distress Syndrome (ARDS) is non-cardiogenic pulmonary edema, which results from increased permeability of the alveolar-capillary barrier and passage of protein-rich fluid into the interstitium and alveolar spaces. The loss of protein from the intravascular space disrupts the normal oncotic pressure differential and causes patients with ARDS to be particularly sensitive to the hydrostatic forces that correlate with intravascular volumes. Conservative fluid management, in which diuretics are administered and intravenous fluid administration is minimized, may decrease hydrostatic pressure and increase serum oncotic pressure, potentially limiting the development of pulmonary edema. However, the cause of death in most patients with ARDS is multiorgan system failure, not hypoxemia, and the impact of conservative fluid management on the incidence of extra-pulmonary organ failure during ARDS is unclear. These physiologic observations have led to a series of studies examining the impact of fluid management on the development of ARDS, resolution of ARDS, survival from ARDS, and long-term outcomes from ARDS. While questions remain, the current literature makes it clear that fluid management is an integral part of the care of patients with ARDS.
More than 30 years ago it was observed that ARDS survivors had a significantly lower cumulative fluid balance than patients who did not survive their illness.1 This clinical observation has generated decades of debate and research on the role of fluid management during ARDS.
The basic pathophysiology of ARDS suggests that fluid management may be important. While ARDS can be triggered by a variety of insults, including pulmonary (pneumonia, aspiration of gastric contents, pulmonary contusion) and extrapulmonary (trauma, nonpulmonary sepsis, transfusion of blood products) sources,2–7 the final common pathway of ARDS involves increased permeability of the alveolar-capillary barrier and accumulation of protein-rich edema fluid in the interstitial and alveolar spaces.8,9 The amount of edema fluid, quantified by measurement of extravascular lung water (EVLW), has been shown to be higher in patients with ARDS than similar patients with cardiogenic pulmonary edema.10 The passage of protein-rich fluid into the interstitium results in loss of the normal oncotic pressure differential between the intravascular and interstitial spaces. Patients with ARDS are, therefore, more sensitive to the hydrostatic forces that accompany increases in pulmonary capillary wedge pressure (PCWP) than patients with cardiogenic pulmonary edema (Figure 1).11
Figure 1. Effect of pulmonary capillary wedge pressure (PCWP) on extravascular lung water (EVLW).
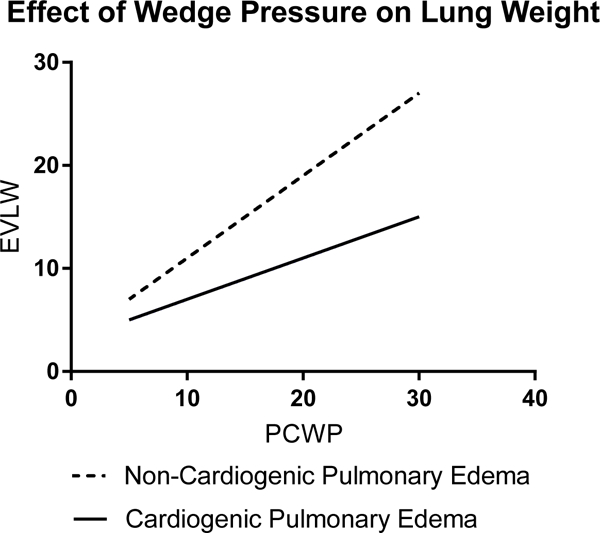
The non-cardiogenic pulmonary edema of ARDS is caused by increased permeability of the alveolar-capillary barrier and passage of protein-rich fluid into the interstitium and alveolar spaces. EVLW, a measurement of the volume of pulmonary edema fluid, has been shown to be higher in ARDS than in cardiogenic pulmonary edema.10 In ARDS, loss of protein from the intravascular space disrupts the normal oncotic pressure differential between the intravascular and interstitial spaces. This makes patients with ARDS very sensitive to the effects of intravascular volume. Experiments have shown that the same increase in PCWP will lead to a greater accumulation of pulmonary edema fluid in patients with ARDS than in those with cardiogenic pulmonary edema.11
Patients with ARDS, by definition, have a low clinical suspicion for cardiac failure or volume overload as the primary cause of their pulmonary edema, but studies have shown that elevations of PCWP are common in patients with confirmed ARDS. A retrospective analysis of patients with pulmonary artery catheters from a trial of ventilator management in ARDS showed that half of the ARDS patients spent significant portions of time with a PCWP above 18 mmHg.6 In a large, prospective trial of fluid management strategies in ARDS, nearly one third of patients had a PCWP greater than 18 mm Hg at enrollment (Figure 2).12,13 There are various mechanisms that predispose patients with ARDS to volume overload. In addition to the iatrogenic volume overload that may occur with treatment of the initial systemic insult (particularly sepsis), positive pressure ventilation decreases ventricular preload and also causes increases in endogenous levels of antidiuretic hormone, renin, aldosterone, and angiotensin II, all of which predispose to volume overload.14–16
Figure 2.
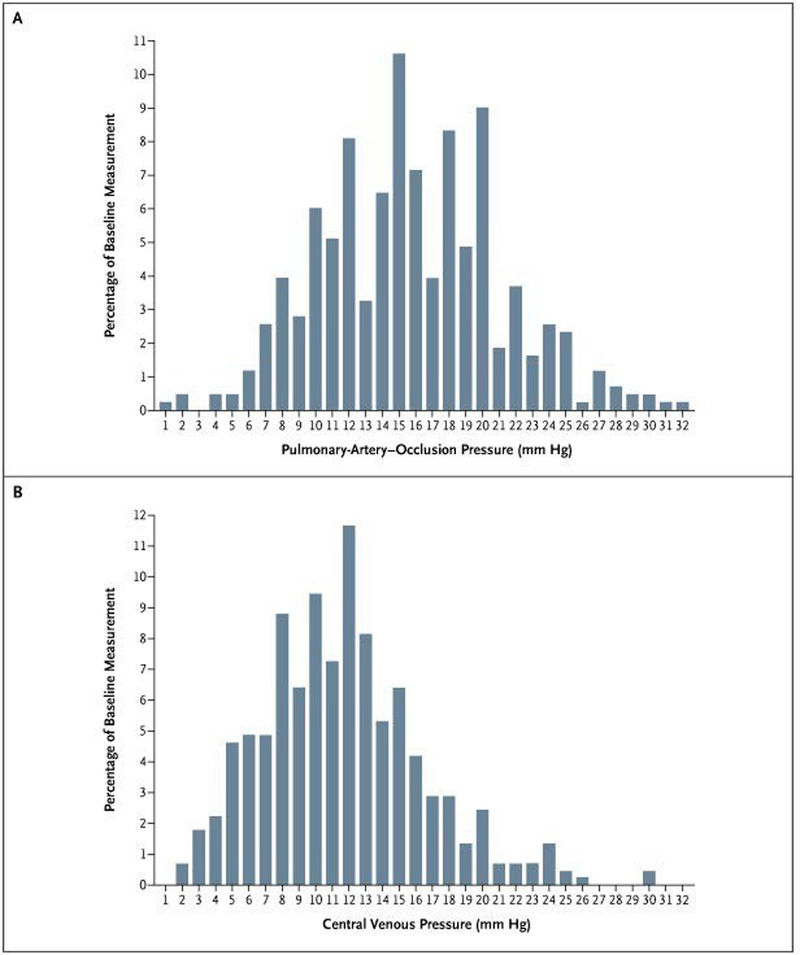
The distribution of baseline pulmonary artery occlusion pressure and central venous pressure measurements from patients in the FACTT trial.13 About one-third of patients had a baseline pulmonary artery occlusion pressure above 18 cm H2O although relatively few had pressures above 20 cm of H2O and/or low cardiac output.
Early animal models of ARDS suggested that reducing intravascular volume reduced the amount of pulmonary edema,17–22 but it remained unclear if reducing edema would improve clinical outcomes. Furthermore, most patients with ARDS do not die from hypoxemia, but from multiorgan system failure.23,24 Concerns have persisted that conservative fluid management in ARDS might increase the risk of other nonpulmonary organ failures, particularly shock and acute kidney injury.25 A great deal of research has examined the pulmonary and systemic effects of fluid management in patients at risk of ARDS and in patients diagnosed with ARDS. Additional studies have explored the long-term outcomes associated with different fluid management strategies and the effect of colloids in ARDS, while others have attempted to identify subpopulations of patients with ARDS with differential responses to fluid management strategies.
Fluid Management in Patients at Risk of ARDS
Most research on fluid management in ARDS has focused on patients who already meet criteria for ARDS. A provocative group of studies, however, have examined the impact of early fluid management in critical illness on the risk of developing ARDS.
In a retrospective analysis of 152 patients who developed ARDS following initiation of mechanical ventilation for other reasons, Xiaoming et al.26 identified positive fluid balance as a risk factor for the development of ARDS that remained significant after adjustment for other risk factors, such as tidal volume and plateau pressure. In a large case-control study of 414 patients with hospital-acquired ARDS during a 10-year period in the medical or surgical ICU at the Mayo Clinic, Ahmed et al.27 identified volume overload as a potentially preventable cause of hospital-acquired ARDS. Finally, an observational study of patients undergoing elective lung resections found that positive fluid balance was an independent risk factor for ARDS.28
Conversely, other studies have suggested that in patients at risk for ARDS from sepsis or pancreatitis, inadequate initial fluid resuscitation may be associated with worse clinical outcomes. In an observational study of patients with ARDS from septic shock in two large medical centers,29 patient’s illnesses were divided into two periods: resuscitation (within six hours of onset of vasopressors) and post-resuscitation. Patients were classified as having inadequate fluid resuscitation if they received less than 20 ml/kg fluid bolus or failed to achieve a central venous pressure (CVP) of at least 8 mm Hg during the resuscitation period. Their subsequent management was classified as conservative or liberal fluid management. Similar to previous studies, conservative fluid management during the post-resuscitation period was associated with improved survival. During the resuscitation period, however, it appeared that inadequate resuscitation was associated with worse survival. Though still uncertain, it is possible that inadequate fluid resuscitation may accentuate systemic inflammation, worsening the injury to the alveolar-capillary barrier and increasing the accumulation of protein-rich edema fluid in the interstitial and alveolar spaces.
To date, there are no prospective clinical trials controlling fluid management strategy in patients at risk of ARDS. The Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial30 is an ongoing, multicenter trial comparing a restrictive fluid strategy (prioritizing vasopressors early in resuscitation) to a liberal fluid strategy (prioritizing intravenous fluid administration) in the first 24 hours after development of sepsis-induced hypotension. The trial is expected to enroll 2,320 patients over three years. In addition to the primary outcome of all-cause 90-day mortality, the development of ARDS within the first 7 days of randomization will be examined as one of the prespecified secondary outcomes.
In addition to the volume, the type of fluid administered to patients may also influence the risk of developing ARDS. Massive transfusion of blood products has long been recognized as a risk factor for ARDS in trauma patients,31 and recent studies have suggested that any transfusion of blood products is an independent risk factor for ARDS, in medical and non-trauma surgical patients.26,27,32–34 In a study of 688 critically ill patients from Massachusetts General Hospital, Gong et al34 reported that transfusion of packed red blood cells was associated with both an increased risk of developing ARDS and an increased risk of mortality in patients with ARDS. The risk of ARDS correlates with the number of transfused blood products, and the transfusion of fresh frozen plasma or platelets is associated with an even greater risk of ARDS than the transfusion of packed red blood cells.32,33
Trials attempting to limit unnecessary transfusions have shown potential in reducing the incidence of ARDS. In the landmark Transfusion Requirements in Critical Care (TRICC) trial,35 which demonstrated that a transfusion threshold of 7.0 g/dl is safe for most critically ill adults, the incidence of ARDS was numerically lower in the restrictive transfusion group, compared to the liberal transfusion group (7.7% vs. 11.4%; P=0.06). In a double-blind placebo-controlled trial of recombinant Factor VIIa in trauma patients, Factor VIIa was associated with a reduction in transfusion requirements and a reduction in subsequent ARDS (16% v. 4%; P=0.03) in the subgroup of patients with blunt trauma. Similarly, Yilmaz et al.36, in a before-after study, reported that the institution of an interdisciplinary intervention designed to decrease the use of large tidal volumes and the administration of unnecessary transfusions in mechanically ventilated patients was associated with a significant decrease in the incidence of ARDS (10% vs. 28%; P<0.001), a relationship that remained significant in analyses that adjusted for differences in baseline characteristics (odds ratio 0.21; 95% confidence interval 0.10 to 0.40).
The pathophysiologic connection between blood product transfusion and the development of ARDS is unclear. It has been proposed that transfusions are part of a “two-hit” model where neutrophils are primed by an initial insult, like sepsis, and then activated by components of transfused blood.37 A recent paper demonstrated that transfusion of packed red blood cells induces necrotic cell death of human lung endothelial cells and enhances susceptibility to lung inflammation through release of HMGB1.38 Several studies have proposed a relationship between the storage duration of packed red blood cells and the risk of ARDS. Prolonged storage has been shown to lead to the accumulation of proinflammatory cytokines, such as IL-1, IL-8, and TNF-α,39 and oxidants such as free hemoglobin and isoprostanes.40 In one retrospective study it was demonstrated that transfusion of blood with longer median storage duration was associated with a higher risk of subsequent ARDS in patients with sepsis.41 Recently, however, three large, multicenter trials enrolling thousands of patients failed to show any relationship between the age of transfused red blood cells and the outcomes of critically ill adults, calling these mechanisms into question.42–44
Fluid Management during ARDS
Most studies of fluid management during ARDS have focused on the days following the diagnosis of ARDS. Building on a series of observational studies showing that a negative fluid was associated with improved survival,45–47 a provocative observational study reported that patients with ARDS whose PCWP decreased during their hospitalization experienced improved survival,48 prompting interest in goal-directed diuresis protocols. In addition to decreasing the hydrostatic pressure associated with elevated PCWP, it was proposed that diuresis could increase the serum oncotic pressure, which might further limit the progression of pulmonary edema.
Early trials of fluid management in ARDS used a range of metrics to target diuresis, including fluid balance, CVP, PCWP, and EVLW. These studies were limited by small size and heterogeneity of interventions and outcomes. A single-center trial of 40 patients with ARDS compared a protocol that guided fluid management based on EVLW measurements to usual care and failed to show a difference in outcomes.49 Another trial of 89 patients with respiratory failure of any etiology from a single ICU compared a protocol of conservative fluid management (based on a goal EVLW) to a liberal strategy that targeted a PCWP of 10–17 mmHg and demonstrated that the conservative strategy was associated with a lower fluid balance, fewer ventilator days, and a shorter ICU length of stay.50 Finally, a study of 37 patients with ARDS and hypoproteinemia demonstrated that a five-day protocol of albumin and continuous furosemide lead to improvement in fluid balance (−3300 mls vs 500 mls), oxygenation, and hemodynamics, compared to dual placebo.51
The definitive trial evaluating fluid management during ARDS was the Fluid and Catheter Treatment Trial (FACTT).12 FACTT was a large, multicenter trial that randomized 1,001 ventilated patients within 48 hours of their ARDS diagnosis to conservative versus liberal fluid management. The liberal fluid strategy prioritized end-organ perfusion, and the conservative approach prioritized pulmonary function to facilitate weaning from mechanical ventilation.25 The trial used a sophisticated protocol that called for either diuresis or fluid boluses to target a PWCP < 8 mm Hg or CVP < 4 mm Hg in the conservative group, and a PWCP of 14–18 or a CVP of 10–14 in the liberal group. Importantly, the protocol only dictated management for patients who were not in shock, and diuretic administration was suspended until 12 hours after a fluid bolus or the reversal of shock. The trial achieved an impressive difference in cumulative fluid balance over the first seven days of the study with a mean fluid balance of −136 ml in the conservative group compared to 6992 ml in the liberal group. The authors noted that the fluid balance in the liberal group closely approximated that observed in two prior large randomized trials of ARDS, which controlled tidal volume and positive end-expiratory pressure but deferred fluid management to treating clinicians.52,53
Despite the impressive difference in fluid balance, the primary outcome of 60-day in-hospital mortality did not significantly differ between groups (25.5% in the conservative strategy group vs. 28.4% in the liberal-strategy group; 95% confidence intervals for absolute risk difference, −2.6 to 8.4%; P=0.30). The conservative-strategy group experienced more ventilator-free days (14.6 vs. 12.1; P < 0.001) and more ICU-free days (13.4 vs 11.2; P < 0.001). The difference in fluid balance also did not appear to affect the severity of shock or the incidence of any extra-pulmonary organ failures, including acute kidney injury. In fact, numerically fewer patients received renal replacement therapy in the conservative fluid management than in the liberal fluid management group (10% vs. 14%; P=0.06).
A recent meta-analysis of all randomized controlled trials of fluid management during ARDS mirrored the results of the FACTT trial, demonstrating no statically significant difference in mortality between patients receiving conservative and liberal fluid management strategies (relative risk 0.91; 95% confidence interval, 0.77 to 1.07), but an increase in the number of ventilator-free days and ICU-free days with conservative fluid management.12
Since the FACTT trial, efforts have continued to determine the effects of fluid management on kidney function. The original FACTT trial reported no difference in the incidence of dialysis or renal failure as defined by the Brussels Organ Failure criteria (serum creatinine > 2 mg/dL).12 An analysis using the more-recent Acute Kidney Injury Network consensus definition of acute kidney injury (an absolute rise in creatinine of >0.3 mg/dL or a relative change of >50% over 48 hrs), appeared to suggest that conservative fluid management may have been associated with a greater incidence of acute kidney injury (57% vs. 51%; P=0.04).54 Experts have pointed out, however, that fluid balance may affect the measurement of serum creatinine because creatinine is distributed throughout the intracellular and extracellular fluid compartments.55 This would suggest that conservative fluid management could artificially elevate creatinine measurements, leading to over-diagnosis of acute kidney injury from hemoconcentration. Conversely, liberal fluid management could cause hemodilution and under-diagnosis of acute kidney injury. In an interesting analysis, Liu et al.54 recalculated all of the creatinine measurements from the FACTT trial with adjustment for fluid balance and demonstrated that adjustment for fluid balance changed the classification of acute kidney injury in 185 patients (18.5%). It also appeared that the adjusted classification system was a better predictor of mortality than unadjusted creatinine measurements. Following adjustment for fluid balance, the incidence of acute injury was lower in the conservative fluid management group (58% vs. 66%; P=0.007).
Long-term Outcomes of Conservative Fluid Management during ARDS
One weakness of the majority of studies of ARDS, including the FACTT study, is the lack of systematic collection of long-term outcomes. In recent years the field of critical care has experienced a growing appreciation that survivors of critical illness experience high rates of prolonged physical and cognitive impairments,56–58 and there has been a movement to include routine assessment of long-term outcomes as part of many investigations into the effectiveness of an intervention in critical care.59 In this context, a follow-up study of cognitive function in survivors of the FACTT trial raised interesting questions about the long-term cognitive impacts of conservative fluid management.60 In the initial FACTT study, cognitive function was assessed during the index hospitalization via the Glasgow Coma Scale, a crude measurement at best of cognitive function. In this analysis, it appeared that conservative fluid management was associated with an increase in the number of days with a normal Glasgow Coma Scale (18.2 vs. 17.2; P=0.03). The authors postulated several possible explanations including that conservative fluid strategy might prevent cerebral edema or decrease sedation requirement as a consequence of earlier improvement in lung function and earlier removal from mechanical ventilation.12
In a follow-up study, Mikkelsen et al.60 assessed neuropsychological function at two and twelve months post–hospital discharge in survivors of the FACTT trial using a telephone battery of tests. The tests took 45 to 60 minutes to administer and had been previously validated against in-person assessments, specifically in survivors of ARDS.61–63 They found that lower PaO2, lower CVP, and enrollment in the conservative fluid-management arm were associated with cognitive impairment at 12 months, and lower PaO2 and conservative fluid-management strategy remained independently associated with cognitive impairment in multivariable analyses. The mechanism through which conservative fluid management might cause cognitive impairment remains unclear. They noted that lower CVP, one of the targets of conservative fluid management, was associated with cognitive impairment, but there was no evidence of reduced cerebral perfusion in the conservative fluid group as cardiac index and systolic blood pressure were similar between the two groups. The authors acknowledged that the major limitation of the study was the significant rate of attrition between the initial study and the measurement of long-term outcomes. Of the 1,001 patients enrolled in the FACTT trial, only 75 patients (7.5%) completed all testing. The possibility of selective loss to follow-up and lack of accounting for the competing risk of death by 12 months limits interpretation of these study results, and highlights the challenges of understanding the effects of fluid management on long-term outcomes among critically ill patients.
Choice of Intravenous Fluids in ARDS
Patients with ARDS frequently experience hypoproteinemia due to hemodilution and loss of protein through leakage into the interstitial and alveolar spaces. The loss of serum oncotic pressure that accompanies hypoproteinemia may worsen the non-cardiogenic pulmonary edema of ARDS and limit diuresis. These observations have driven interest in the effect of albumin administration in patients with ARDS.
In a follow-up to their trial of albumin and furosemide compared to dual placebo,51 Martin et al. examined the specific benefit of colloid administration in a multicenter study of 37 patients with ARDS and hypoproteinemia who were randomized to albumin and furosemide or furosemide alone.64 They found that the addition of albumin improved fluid balance, oxygenation, and hemodynamics, compared to placebo.
The SAFE study was a large multicenter trial of albumin vs. saline as the primary resuscitation fluid for 6,997 critically ill adults. 65 The SAFE study enrolled all adults admitted to the intensive care units of 16 academic hospitals in Australia and New Zealand. Overall, the study showed no difference in the rate of death, new organ failure, ICU length of stay, hospital length of stay, or duration of mechanical ventilation. In a prespecified subgroup analysis of the 127 patients with ARDS from the SAFE trial, the relative risk of death with albumin, compared to saline, was 0.93 (95% confidence intervals, 0.61 to 1.41; P=0.72). The ALBIOS study was designed to evaluate the effect of albumin administration in patients with sepsis, another subgroup that demonstrated a potential benefit from albumin in the SAFE study (relative risk for mortality, 0.93; 95% confidence intervals, 0.61 to 1.41; P= 0.09). The ALBIOS study randomized 1,818 patients with sepsis to 20% albumin and crystalloid solution or crystalloid solution alone.66 There was no significant difference in mortality (relative risk, 0.94; 95% CI, 0.85 to 1.05; P=0.29), or any of the pre-specified secondary outcomes. ARDS was not evaluated as a baseline characteristic or outcome of the ALBIOS trial.
A recent systematic review and meta-analysis highlighted the paucity of data on albumin in ARDS and called for a large, prospective clinical trial.67 It is noteworthy that while the relative risk for mortality was not significantly different in the meta-analysis (relative risk of death of 0.89; 95% confidence interval, 0.63 to 1.28; P=0.54) or any of the individual trials, the point estimate favored albumin in each of these analyses, and the 95% confidence interval includes clinically meaningful differences.
No studies have evaluated whether the choice of intravenous crystalloid solution affects the risk of ARDS or the outcomes of patients with ARDS. Two recent trials reported, however, that the administration of balanced crystalloids (such as lactated Ringer’s solution or Plasma-Lyte A) rather than saline (0.9% sodium chloride), improved outcomes in a broad population of critically and non-critically ill adults.68,69 The benefit in clinical outcomes with balanced crystalloids in these trials appeared to be strongest among patients with sepsis, many of whom may also have had ARDS. Further data on the relationship between intravenous crystalloid composition and outcomes in ARDS may be provided by the ongoing Plasma-Lyte 148 v Saline (PLUS) study and Balanced Solution versus Saline in Intensive Care Study (BaSICS).70,71
Identifying Groups Likely to Benefit from Conservative Fluid Management
One proposed reason for the difficulty in finding effective treatments for patients with ARDS is that ARDS may represent multiple distinct illnesses with differential responses to treatments.72 This recognition has prompted a series of examinations of previous trials for variables capable of separating “responders” from “non-responders”.
The design of the original FACTT trial itself evaluated one potential tool for distinguishing patients likely to benefit from conservative versus liberal therapy: invasive assessment of individual patients’ hemodynamics. Every patient enrolled in FACTT was randomized to conservative or liberal fluid management and to a second, factorialized, trial of hemodynamic assessment by central venous catheter or pulmonary-artery catheter. Pulmonary-artery catheters did not improve survival or organ function, and there was no interaction between the type of hemodynamic monitoring and the fluid management approach.13
In a secondary analysis of the 609 patients from FACTT who had a CVP available at enrollment and were not in shock, Semler et al.73 evaluated the effect of initial CVP on subsequent response to fluid management strategy. For patients with an initial CVP greater than 8 mmHg, the fluid management strategy did not affect mortality (18% versus 18%, P=0.93). For patients with a CVP less than or equal to 8 mmHg, conservative fluid management was associated with decreased mortality (17% versus 36%, p=0.005), and multivariable analysis confirmed that initial CVP modified the effect of fluid strategy on mortality (P value for interaction=0.031). The authors postulate several possible explanations for this relationship. Increases from a low filling pressure may cause pulmonary edema while changes from high filling pressures may have less effect, because clearance of pulmonary edema may be slower than its development. A low CVP could be a surrogate for other factors that portend a good response to conservative fluid management such as age, BMI, or nutritional status. Finally, the authors suggest that the difference could be mediated by the differential use of the two therapies dictated by the protocol: fluid boluses for patients with low CVPs and diuretics for patients with high CVPs.
Beyond examining whether hemodynamic measurements of volume status modify the effect of fluid strategy on outcome, a series of trial have evaluated biomarkers anticipated to modify the effect of fluid management on outcome. In a post-hoc study of 625 patients with adequate banked plasma from the FACTT trial, Semler et al.74 measured B-type Natriuretic Peptide (BNP) and aldosterone levels, two potential markers of intravascular volume status, to determine if they modified the effect of fluid management strategy on clinical outcomes. BNP levels were significantly elevated and broadly distributed in patients with ARDS, but did not predict mortality, correlate with fluid balance, or modify the effect of fluid management strategy. By contrast, aldosterone levels were clustered near the normal range but seemed to modify the effect of fluid management strategy on mortality (P value for interaction = .01). In patients with low aldosterone concentrations, conservative fluid management increased ventilator-free days (17.1 vs. 12.5; P < 0.001) and decreased mortality (19% vs 30%, P = 0.03).
An approach to differentiating potential responders from non-responders that has generated significant recent excitement is the use of latent class analysis.75 Calfee et al have applied latent class analysis to severe ARDS cohorts to identify a subpopulation of ARDS patients with a “hyperinflammatory” subphenotype. This subphenotype appears to be reproducible across trials and represents approximately one third of all patients with ARDS. Patients with the “hyperinflammatory” subphenotype are more likely to have sepsis, acidosis, require vasopressors, and have increased plasma levels of interleukin-6, interleukin-8, and tumor necrosis factor α. The two subphenotypes have been shown to have a differential response to PEEP with the “hyper-inflammatory” phenotype experiencing improved mortality with a high PEEP strategy, while the less inflammatory phenotype experienced worsened mortality with a high PEEP strategy. Recently, Calfee et al.76 performed a similar reanalysis of the FACTT trial and demonstrated that the hyper-inflammatory phenotype had worse survival with conservative fluid management (60-day mortality, 49% vs 39%) while the less inflammatory phenotype experienced improved mortality with conservative fluid management (60-day mortality, 17% vs. 24%) (P value for interaction=0.009).
Finally, in a recent, provocative analysis, Jolley et al77 re-examined patients who had participated in a prospective follow-up study to the FACTT trial, the Economic Analysis of Pulmonary Artery Catheters study. This study collected 1-year follow up data on 655 of the 1,000 patients enrolled in the FACTT trial. Using predictive modeling to estimate the 1-year mortality for all non-Hispanic black and white patients randomized in the FACTT trial, the authors reported a significant interaction between race and fluid treatment (P value for interaction = 0.01). One-year mortality was significantly lower for black subjects assigned to conservative fluid management (38% vs. 54%; P=0.03). In white patients, conversely, 1-year mortality was not significantly different between the conservative and liberal fluid management groups (35% vs. 30%; P=0.23).
Research Priorities for Fluid Management in ARDS
In the last several decades, basic science, translational, and clinical research has dramatically improved our understanding of fluid management in critical illness, generally. Although some of these studies have specifically addressed fluid management for patients with ARDS (or provide insights applicable to ARDS from related diseases like sepsis) major questions remain unanswered regarding the optimal fluid management for patients at risk for or diagnosed with ARDS (Table 1).78–80 Whether conservative fluid management early in sepsis resuscitation reduces the risk of ARDS development may be informed by the ongoing CLOVERS trial, or other similar studies. While the FACTT trial demonstrated that conservative fluid management increases ventilator-free days for patients with ARDS, the effect on mortality remains unknown – and whether any benefit are mediated by use of diuretics, limitation of intravenous fluid administration, or both requires further investigation. The FACTT trial initiated conservative fluid management after the resolution of shock, and whether limiting fluid administration and initiation diuretics during shock would be safe or effective remains unclear. Whether use of human albumin solution rather than crystalloids (with or without diuretics) improves pulmonary function and clinical outcomes urgently requires further evaluation. The effects of fluid choice, fluid dose, and fluid balance during each phase of ARDS on long-term outcomes require careful, prospective evaluation in rigorously designed randomized clinical trials. Similarly, whether pre-specified approaches to ARDS subphenotyping can be used to prospectively identify patients that will benefit from conservative or liberal fluid management merits evaluation in a prospective clinical trial.
Table 1.
Priorities for Fluid Management Research in ARDS.
| Important Ongoing Trials |
Phase of ARDS |
|
Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial Multicenter trial (NCT03434028) comparing early vasopressors to a liberal fluid strategy in the first 24 hours after development of sepsis-induced hypotension. This trial may provide definitive evidence regarding the effect of early fluid management on the development of ARDS. |
Development |
| High Priority Topics Needing Further Study | |
|
Timing of Initiation of Diuresis The FACTT trial began fluid restriction and diuresis 12 hours after the resolution of shock. Future trials should evaluate the impact of conservative fluid management beginning early after ARDS diagnosis, even among patients who remain in shock. CLASSIC2 (NCT NCT03668236) will investigate use of conservative fluid management during shock, but not specifically for patients with ARDS. |
Acute Illness |
|
Extravascular Lung Water (EVLW) Numerous small trials have demonstrated that measurement of EVLW may predict outcomes of patients with ARDS. Future trials should evaluate the use of EVLW as a bedside measurement to guide volume management (resuscitation and diuresis). |
Development and Acute Illness |
|
Albumin Several small trials have suggested that albumin may improve outcomes in ARDS, particularly when paired with diuretics. Future large trials are needed to evaluate the impact of albumin administration in patients with ARDS. This question could be factorialized with a trial examining timing of diuresis. |
Acute Illness |
|
Subphenotyping Several methods have shown potential in differentiating patients likely to benefit from conservative or liberal fluid management. Prospective trials are needed to validate the use of these markers in guiding fluid management strategy. |
Acute Illness |
|
Long-term Outcomes Recent secondary analyses of the FACTT trial have raised concerns about the long-term impact on cognitive and renal outcomes of conservative fluid management. Future trials of fluid management in ARDS should plan to rigorously and systematically collect these outcomes using valid long-term outcome measures. |
Recovery |
Conclusions
ARDS is characterized by decreased pulmonary vascular permeability and non-cardiogenic pulmonary edema, making fluid management a natural therapeutic target to improve patient outcomes. Currently, limited data inform the optimal approach to early fluid resuscitation for patients at risk for ARDS or the optimal fluid choice for patients with ARDS. A single, large, high-quality randomized trial suggested conservative fluid management should be the standard approach to patients with known ARDS who are no longer in shock, but the long-term cognitive outcomes of such an approach should be more rigorously evaluated. Recent research has proposed baseline variables that may be able to differentiate subpopulations within ARDS which will respond better to conservative or liberal fluid management – additional work is urgently needed to develop and validate such personalized approach to fluid management in ARDS. Today, appropriate management of patients with ARDS should include a careful, daily assessment of volume status, fluid balance, and potential for diuresis. In the future, fluid management research in ARDS should seek to definitively address the fundamental and unanswered questions of: Which fluid?...when?...how much?...and to whom?
Acknowledgments
Sources of Funding: J.D.C. was supported in part by the NIH (T32HL087738–12). M.W.S. was supported in part by the NHLBI (K23HL143053). T.W.R. was supported in part by the NIH (R34HL105869).
Footnotes
Conflicts of Interest: All authors completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors declared no potential conflicts of interest with the current work. T.W.R. reported serving as a consultant for Avisa Pharma, LLC, and as the Director of Medical Affairs for Cumberland Pharmaceuticals, Inc.
REFERENCES
- 1.Simmons RS, Berdine GG, Seidenfeld JJ, et al. Fluid Balance and the Adult Respiratory Distress Syndrome 1– 3. American Review of Respiratory Disease 1987;135(4):924–9. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. New England Journal of Medicine 2000;342(18):1334–49. [DOI] [PubMed] [Google Scholar]
- 3.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;151(2 Pt 1):293–301. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012;122(8):2731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. The American Journal of Surgery 1982;144(1):124–30. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Critical Care 2007;11:R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware LB, Bastarache JA, Bernard GR. Acute Respiratory Distress Syndrome In: Textbook of Critical Care. Elsevier; 2017. p. 413–24. [Google Scholar]
- 8.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 2006;27(4):337–49. [DOI] [PubMed] [Google Scholar]
- 9.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 2011;6:147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibbald WJ, Warshawski FJ, Short AK, Harris J, Lefcoe MS, Holliday RL. Clinical studies of measuring extravascular lung water by the thermal dye technique in critically ill patients. Chest 1983;83(5):725–31. [DOI] [PubMed] [Google Scholar]
- 11.Sibbald WJ, Short AK, Warshawski FJ, Cunningham DG, Cheung H. Thermal Dye Measurements of Extravascular Lung Water in Critically III Patients: Intravascular Starling Forces and Extravascular Lung Water in the Adult Respiratory Distress Syndrome. Chest 1985;87(5):585–92. [DOI] [PubMed] [Google Scholar]
- 12.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354(24):2564–75. [DOI] [PubMed] [Google Scholar]
- 13.Pulmonary-Artery versus Central Venous Catheter to Guide Treatment of Acute Lung Injury. New England Journal of Medicine 2006;354(21):2213–24. [DOI] [PubMed] [Google Scholar]
- 14.Kaczmarczyk G, Vogel S, Krebs M, Bünger H, Scholz A. Vasopressin and renin-angiotensin maintain arterial pressure during PEEP in nonexpanded, conscious dogs. Am J Physiol 1996;271(5 Pt 2):R1396–1402. [DOI] [PubMed] [Google Scholar]
- 15.Annat G, Viale JP, Bui Xuan B, et al. Effect of PEEP ventilation on renal function, plasma renin, aldosterone, neurophysins and urinary ADH, and prostaglandins. Anesthesiology 1983;58(2):136–41. [DOI] [PubMed] [Google Scholar]
- 16.Koyner JL, Murray PT. Mechanical Ventilation and the Kidney. Blood Purif 2010;29(1):52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sznajder JI, Zucker AR, Wood LDH, Long GR. The Effects of Plasmapheresis and Hemofiltration on Canine Acid Aspiration Pulmonary Edema. Am Rev Respir Dis 1986;134(2):222–8. [DOI] [PubMed] [Google Scholar]
- 18.Prewitt RM, McCarthy J, Wood LDH. Treatment of Acute Low Pressure Pulmonary Edema in Dogs: RELATIVE EFFECTS OF HYDROSTATIC AND ONCOTIC PRESSURE, NITROPRUSSIDE, AND POSITIVE END-EXPIRATORY PRESSURE. J Clin Invest 1981;67(2):409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali J, Chernicki W, Wood LD. Effect of furosemide in canine low-pressure pulmonary edema. J Clin Invest 1979;64(5):1494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long R, Breen PH, Mayers I, Wood LD. Treatment of canine aspiration pneumonitis: fluid volume reduction vs. fluid volume expansion. Journal of Applied Physiology 1988;65(4):1736–44. [DOI] [PubMed] [Google Scholar]
- 21.Molloy WD, Lee KY, Girling L, Prewitt RM. Treatment of canine permeability pulmonary edema: short-term effects of dobutamine, furosemide, and hydralazine. Circulation 1985;72(6):1365–71. [DOI] [PubMed] [Google Scholar]
- 22.Reising CA, Chendrasekhar A, Wall PL, Paradise NF, Timberlake GA, Moorman DW. Continuous Dose Furosemide as a Therapeutic Approach to Acute Respiratory Distress Syndrome (ARDS). Journal of Surgical Research 1999;82(1):56–60. [DOI] [PubMed] [Google Scholar]
- 23.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest 2005;128(2):525–32. [DOI] [PubMed] [Google Scholar]
- 24.Wang CY, Calfee CS, Paul DW, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 2014;40(3):388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster DP. The case for and against fluid restriction and occlusion pressure reduction in adult respiratory distress syndrome. New Horiz 1993;1(4):478–88. [PubMed] [Google Scholar]
- 26.Xiaoming J, Malhotra A, Saeed M, Mark RG, Talmor D. Risk Factors for Acute Respiratory Distress Syndrome in Patients Mechanically Ventilated for Greater Than 48 Hours. Chest 2008;133(4):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed AH, Litell JM, Malinchoc M, et al. The Role of Potentially Preventable Hospital Exposures in the Development of Acute Respiratory Distress Syndrome: A Population-Based Study. Crit Care Med [Internet] 2014. [cited 2019 Jan 21];42(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97(6):1558–65. [DOI] [PubMed] [Google Scholar]
- 29.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest 2009;136(1):102–9. [DOI] [PubMed] [Google Scholar]
- 30.Self WH, Semler MW, Bellomo R, et al. Liberal Versus Restrictive Intravenous Fluid Therapy for Early Septic Shock: Rationale for a Randomized Trial. Ann Emerg Med 2018;72(4):457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopko PM, Holland PV. Transfusion-related acute lung injury. Br J Haematol 1999;105(2):322–9. [DOI] [PubMed] [Google Scholar]
- 32.Khan H, Belsher J, Yilmaz M, et al. Fresh-Frozen Plasma and Platelet Transfusions Are Associated With Development of Acute Lung Injury in Critically Ill Medical Patients. Chest 2007;131(5):1308–14. [DOI] [PubMed] [Google Scholar]
- 33.Gajic O, Rana R, Mendez JL, et al. Acute lung injury after blood transfusion in mechanically ventilated patients. Transfusion 2004;44(10):1468–74. [DOI] [PubMed] [Google Scholar]
- 34.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med 2005;33(6):1191–8. [DOI] [PubMed] [Google Scholar]
- 35.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340(6):409–17. [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz M, Keegan MT, Iscimen R, et al. Toward the prevention of acute lung injury: protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med 2007;35(7):1660–6; quiz 1667. [DOI] [PubMed] [Google Scholar]
- 37.Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med 2006;34(5 Suppl):S124–131. [DOI] [PubMed] [Google Scholar]
- 38.Qing DY, Conegliano D, Shashaty MGS, et al. Red blood cells induce necroptosis of lung endothelial cells and increase susceptibility to lung inflammation. Am J Respir Crit Care Med 2014;190(11):1243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med 2003;31(12 Suppl):S687–697. [DOI] [PubMed] [Google Scholar]
- 40.Grimshaw K, Sahler J, Spinelli SL, Phipps RP, Blumberg N. New frontiers in transfusion biology: Identification and significance of mediators of morbidity and mortality in stored red cell concentrates. Transfusion 2011;51(4):874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janz DR, Zhao Z, Koyama T, et al. Longer storage duration of red blood cells is associated with an increased risk of acute lung injury in patients with sepsis. Ann Intensive Care 2013;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacroix J, Hébert PC, Fergusson DA, et al. Age of Transfused Blood in Critically Ill Adults. New England Journal of Medicine 2015;372(15):1410–8. [DOI] [PubMed] [Google Scholar]
- 43.Cooper DJ, McQuilten ZK, Nichol A, et al. Age of Red Cells for Transfusion and Outcomes in Critically Ill Adults. New England Journal of Medicine 2017;377(19):1858–67. [DOI] [PubMed] [Google Scholar]
- 44.Heddle NM, Cook RJ, Arnold DM, et al. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. New England Journal of Medicine 2016;375(20):1937–45. [DOI] [PubMed] [Google Scholar]
- 45.Ferguson ND, Meade MO, Hallett DC, Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med 2002;28(8):1073–7. [DOI] [PubMed] [Google Scholar]
- 46.Sakr Y, Vincent J-L, Reinhart K, et al. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest 2005;128(5):3098–108. [DOI] [PubMed] [Google Scholar]
- 47.Schuller D, Mitchell JP, Calandrino FS, Schuster DP. Fluid Balance during Pulmonary Edema: Is Fluid Gain a Marker or a Cause of Poor Outcome? Chest 1991;100(4):1068–75. [DOI] [PubMed] [Google Scholar]
- 48.Humphrey H, Hall J, Sznajder I, Silverstein M, Wood L. Improved Survival in ARDS Patients Associated with a Reduction in Pulmonary Capillary Wedge Pressure. Chest 1990;97(5):1176–80. [DOI] [PubMed] [Google Scholar]
- 49.Eisenberg PR, Hansbrough JR, Anderson D, Schuster DP. A Prospective Study of Lung Water Measurements during Patient Management in an Intensive Care Unit. Am Rev Respir Dis 1987;136(3):662–8. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell JP, Schuller D, Calandrino FS, Schuster DP. Improved Outcome Based on Fluid Management in Critically III Patients Requiring Pulmonary Artery Catheterization. Am Rev Respir Dis 1992;145(5):990–8. [DOI] [PubMed] [Google Scholar]
- 51.Martin GS, Mangialardi RJ, Wheeler AP, Dupont WD, Morris JA, Bernard GR. Albumin and furosemide therapy in hypoproteinemic patients with acute lung injury. Crit Care Med 2002;30(10):2175–82. [DOI] [PubMed] [Google Scholar]
- 52.Higher versus Lower Positive End-Expiratory Pressures in Patients with the Acute Respiratory Distress Syndrome. New England Journal of Medicine 2004;351(4):327–36. [DOI] [PubMed] [Google Scholar]
- 53.Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine 2000;342(18):1301–8. [DOI] [PubMed] [Google Scholar]
- 54.Liu KD, Thompson BT, Ancukiewicz M, et al. Acute kidney injury in patients with acute lung injury: Impact of fluid accumulation on classification of acute kidney injury and associated outcomes*. Critical Care Medicine 2011;39(12):2665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bjornsson TD. Use of serum creatinine concentrations to determine renal function. Clin Pharmacokinet 1979;4(3):200–22. [DOI] [PubMed] [Google Scholar]
- 56.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA 2010;304(16):1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369(14):1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Girard TD, Dittus RS, Ely EW. Critical Illness Brain Injury. Annu Rev Med 2016;67:497–513. [DOI] [PubMed] [Google Scholar]
- 59.Hodgson CL, Watts NR, Iwashyna TJ. Long-Term Outcomes After Critical Illness Relevant to Randomized Clinical Trials [Internet] In: Vincent J-L, editor. Annual Update in Intensive Care and Emergency Medicine 2016. Cham: Springer International Publishing; 2016. [cited 2019 Jan 18]. p. 465–74.Available from: 10.1007/978-3-319-27349-5_37 [DOI] [Google Scholar]
- 60.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 2012;185(12):1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christie JD, Biester RCP, Taichman DB, et al. Formation and validation of a telephone battery to assess cognitive function in acute respiratory distress syndrome survivors. J Crit Care 2006;21(2):125–32. [DOI] [PubMed] [Google Scholar]
- 62.Mikkelsen ME, Shull WH, Biester RC, et al. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology 2009;14(1):76–82. [DOI] [PubMed] [Google Scholar]
- 63.Taichman DB, Christie J, Biester R, et al. Validation of a brief telephone battery for neurocognitive assessment of patients with pulmonary arterial hypertension. Respir Res 2005;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin GS, Moss M, Wheeler AP, Mealer M, Morris JA, Bernard GR. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med 2005;33(8):1681–7. [DOI] [PubMed] [Google Scholar]
- 65.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350(22):2247–56. [DOI] [PubMed] [Google Scholar]
- 66.Caironi P, Tognoni G, Masson S, et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. New England Journal of Medicine 2014;370(15):1412–21. [DOI] [PubMed] [Google Scholar]
- 67.Uhlig C, Silva PL, Deckert S, Schmitt J, de Abreu MG. Albumin versus crystalloid solutions in patients with the acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care 2014;18(1):R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Semler MW, Self WH, Rice TW. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med 2018;378(20):1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Self WH, Semler MW, Wanderer JP, et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N Engl J Med 2018;378(9):819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hammond NE, Bellomo R, Gallagher M, et al. The Plasma-Lyte 148 v Saline (PLUS) study protocol: a multicentre, randomised controlled trial of the effect of intensive care fluid therapy on mortality. Crit Care Resusc 2017;19(3):239–46. [PubMed] [Google Scholar]
- 71.Zampieri FG, Azevedo LCP, Corrêa TD, et al. Study protocol for the Balanced Solution versus Saline in Intensive Care Study (BaSICS): a factorial randomised trial. Crit Care Resusc 2017;19(2):175–82. [PubMed] [Google Scholar]
- 72.Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. New England Journal of Medicine 2017;377(6):562–72. [DOI] [PubMed] [Google Scholar]
- 73.Semler MW, Wheeler AP, Thompson BT, Bernard GR, Wiedemann HP, Rice TW. Impact of Initial Central Venous Pressure on Outcomes of Conservative versus Liberal Fluid Management in Acute Respiratory Distress Syndrome. Crit Care Med 2016;44(4):782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Semler MW, Marney AM, Rice TW, et al. B-Type Natriuretic Peptide, Aldosterone, and Fluid Management in ARDS. Chest 2016;150(1):102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2(8):611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Famous KR, Delucchi K, Ware LB, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med 2017;195(3):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jolley SE, Hough CL, Clermont G, et al. Relationship between Race and the Effect of Fluids on Long-term Mortality after Acute Respiratory Distress Syndrome. Secondary Analysis of the National Heart, Lung, and Blood Institute Fluid and Catheter Treatment Trial. Ann Am Thorac Soc 2017;14(9):1443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Investigators TP. A Randomized Trial of Protocol-Based Care for Early Septic Shock [Internet]. 10.1056/NEJMoa1401602. 2014 [DOI] [PMC free article] [PubMed]
- 79.Mouncey PR, Osborn TM, Power GS, et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock [Internet]. 10.1056/NEJMoa1500896. 2015 [DOI] [PubMed]
- 80.Group TAI and the ACT. Goal-Directed Resuscitation for Patients with Early Septic Shock [Internet]. 10.1056/NEJMoa1404380. 2014 [DOI] [PubMed]


