Abstract
Patient hand hygiene is a commonsense measure that has been associated with reductions in colonization or infection with bacterial and viral pathogens in quasi-experimental studies. We conducted a nonblinded pilot randomized trial to assess the impact of an educational patient hand hygiene intervention on acquisition of colonization by selected health care-associated pathogens in hospitalized patients. For patients with negative admission cultures, the intervention did not reduce the new acquisition of colonization by pathogens compared with that of standard care.
Keywords: Methicillin-resistant, Staphylococcus aureus, Vancomycin-resistant enterococci, Fluoroquinolone-resistance, Candida species
Hospitalized patients and long-term care facility residents frequently have hand contamination with health care–associated pathogens.1–3 Therefore, patient hand hygiene has been advocated as a practice to possibly prevent the acquisition of pathogens and reduce the risk of transmission by colonized patients.1–8 Interventions that improve access to hand hygiene products and engage health care personnel have been effective in increasing patient hand hygiene.4–9 In a nonblinded randomized trial, such an intervention resulted in a significant reduction in the recovery of pathogens from the hands of patients compared with standard care.4 Moreover, in quasi-experimental studies, patient hand hygiene interventions have been associated with reduced colonization or infection with bacterial pathogens and respiratory viruses.2,7–9
Given the potential benefits of patient hand hygiene and the minimal risk for harm, there is a need for high-quality studies to determine whether patient hand hygiene interventions are effective in reducing the acquisition of colonization and infection with health care–associated pathogens. Thus, to obtain preliminary information for future large-scale trials, a pilot study was conducted to assess the impact of an educational patient hand hygiene intervention on the acquisition of colonization with selected health care–associated pathogens in hospitalized patients.
METHODS
The Louis Stokes Cleveland Veterans Affairs Medical Center is a 210-bed acute-care facility. At the time of the study, all hospitalized patients received a bottle of alcohol-based hand sanitizer supplied with other toiletry items but were given no instruction on proper hand hygiene. In previous studies, patients in our facility often were unaware of the bottle of hand sanitizer or rarely used it owing to lack of instruction.4,6,10 All patients were screened for methicillin-resistant Staphylococcus aureus (MRSA) nasal carriage on admission and at discharge.
The center’s institutional review board approved the study protocol. Between October 2016 and March 2017, we conducted a pilot study designed as a nonblinded parallel randomized trial of a patient hand hygiene intervention versus standard care for a convenience sample of patients admitted to 4 medical-surgical wards with an anticipated length of stay of ≥ 2 days. Patients with dementia, a condition that prevented performance of hand hygiene, known MRSA colonization, or anticipated length of stay of < 2 days were excluded. Patients were randomized based on a coin toss.
For patients in the intervention group, education was based on a Five Moments for Patient Hand Hygiene model.10 All education was provided by research personnel. In addition to a Five Moments for Patient Hand Hygiene poster, intervention patients received illustrations showing the effectiveness of alcohol hand sanitizer in removing MRSA. A bottle of alcohol hand sanitizer provided by research personnel was placed at the bedside, and frequent hand hygiene was encouraged; the bottle of hand sanitizer was in addition to the bottle of hand sanitizer provided with toiletry items. Patients received reeducation during daily follow-up visits for up to 5 days or until discharge. During each visit, research personnel directly facilitated use of the alcohol hand sanitizer by applying sanitizer to the patient’s hands and observing the hand rubbing technique. To assess hand sanitizer use, alcohol bottles were weighed before distribution and on the second day of the intervention. Patients with a length of stay < 2 days received the educational intervention but were excluded from the analysis. Patients randomized to the control group did not receive education or the extra bottle of hand sanitizer.
For intervention and control patients, perirectal swabs were collected (BBL CultureSwabs; BD, Cockeysville, MD) on admission and either at day 5 of the hospital stay or on discharge. The swabs were cultured for vancomycin-resistant enterococci (VRE), fluoroquinolone-resistant gram-negative bacilli, and Candida spp as described previously.4 The microbiologist processing the cultures was blinded to study group assignment.
Medical records were reviewed to obtain information on MRSA surveillance results, medical conditions, medications, devices, mobility, long-term care facility residence, and length of stay. At 3 months after discharge, medical records were reviewed to assess for new colonization or infection with the pathogens. The primary outcome was new acquisition of colonization with ≥ 1 of the pathogens (ie, MRSA, VRE, fluoroquinolone-resistant gram-negative bacilli, and Candida spp). The study was powered to detect a medium to large effect size. Based on preliminary data showing that ~33% of new admissions acquire colonization with ≥ 1 of the pathogens, a power calculation indicated that 40 patients per group would provide 75% power to detect a medium to large effect size of the Cohen h = 0.6. It was anticipated that approximately one-third of patients enrolled either would not be hospitalized for ≥ 2 days or would not have discharge swabs collected because they were discharged when coordinators were unavailable. Thus, the goal was to enroll sufficient patients to provide ≥ 40 participants with discharge swabs in each group. Bivariate analyses were conducted to compare characteristics of groups. The Fisher exact test was used for categorical data, and the Student paired t test was used for normally distributed data. Data were analyzed using SPSS version 10.0 (SPSS, Chicago, IL).
RESULTS
Of 180 patients assessed, 162 (90%) were eligible and agreed to participate. Of the 162 participants, 79 were randomized to control and 83 to intervention groups; 40 of the 79 controls (51%) and 42 of the 83 intervention patients (51%) had swabs collected at day 5 or on discharge. There were no significant differences in the baseline characteristics or carriage of the pathogens on admission for the intervention patients versus control patients who completed the study (Table 1). For patients in the intervention group, the median weight of hand sanitizer used after the first day of the intervention was 3.6 g (range, 1.2–17.7 g).
Table 1.
Characteristics of patients in the intervention and control groups
| Characteristic | Intervention group (n = 42) |
Control group (n = 40) |
P value |
|---|---|---|---|
| Male sex, n (%) | 41 (98) | 37 (93) | .35 |
| Age, y, median | 64 | 63 | .65 |
| Long-term care facility resident, | 7(17) | 8 (20) | .78 |
| n (%) | |||
| Medical condition, n (%) | |||
| Diabetes | 19 (45) | 14(35) | .06 |
| Cancer | 10(24) | 6(15) | .41 |
| Chronic lung disease | 12(29) | 11 (28) | 1.0 |
| Surgery in the past 3 mo | 13(31) | 8 (20) | .32 |
| Colonized on admission, n (%) | |||
| MRSA | 0(0) | 1 (2) | .49 |
| VRE | 7(17) | 4(10) | .52 |
| Fluoroquinolone-resistant | 6 (14) | 5(13) | 1.0 |
| gram-negative bacilli | |||
| Candida spp | 410) | 6(15) | .51 |
| Proton pump inhibitor, n (%) | 26 (62) | 25 (51) | 1.0 |
| Antibiotics within 90 d, n (%) | 22 (52) | 19(48) | .83 |
MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Figure 1 shows the percentage of patients in each group with negative admission culture results who acquired colonization with the pathogens. There were no significant differences in the percentages of patients acquiring colonization with ≥ 1 of the pathogens (10.0% [4 of 40] for control vs 9.5%, [4 of 42] for intervention; P =1.0). In addition, there were no significant differences in acquisition of the individual pathogens (P ≥ .49 for each comparison). None of the patients in either group developed an infection with MRSA, VRE, fluoroquinolone-resistant gram-negative bacilli, or Candida spp during admission or over the subsequent 3 months.
Fig 1.
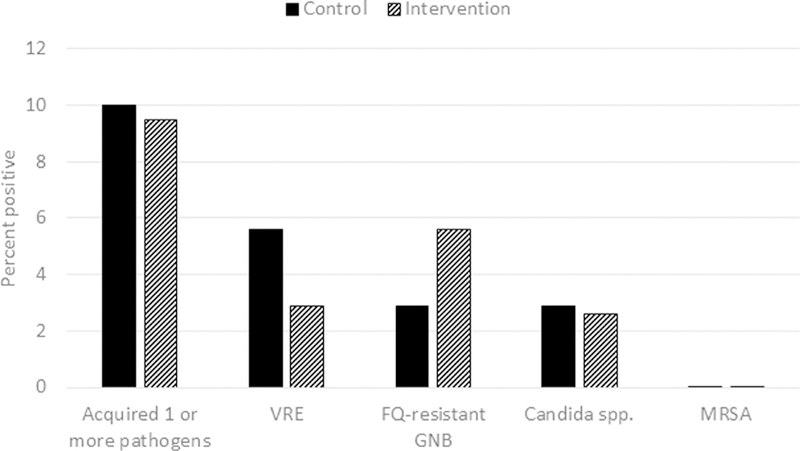
Percentage of patients in the control versus intervention groups who acquired colonization with the pathogens during their hospital admission. Patients with a positive admission culture for a pathogen were excluded from the analysis of acquisition for that pathogen. The control group received standard care; the intervention group received education on patient hand hygiene for up to 5 days or until discharge. Nares culture or polymerase chain reaction was performed for methicillin-resistant Staphylococcus aureus (MRSA); perirectal swabs were cultured for vancomycin-resistant enterococci (VRE), fluoroquinolone (FQ)-resistant gram-negative bacilli (GNB), and Candida spp.
DISCUSSION
Patient hand hygiene is a commonsense measure that in quasi-experimental studies has been associated with reduced respiratory virus infections and reduced colonization or infection with bacterial pathogens, including MRSA, Clostridium difficile, and VRE.7–10 Our small sample size limits the conclusions that can be drawn from our pilot study. Nonetheless, there was no significant reduction in or trend toward reduction in new acquisition of the selected pathogens in the patient hand hygiene group compared with the standard care control group. Based on hand sanitizer consumption, the educational intervention was effective in promoting the performance of patient hand hygiene. Our findings suggest that patient hand hygiene as a single intervention might not have a substantial impact on acquisition of the target organisms, or may have only a relatively modest impact. These results have important implications for the design of randomized trials of patient hand hygiene interventions.
Our study has some limitations. First, the study was conducted in a single institution and was not blinded. Second, as noted previously, the study included only a small number of participants and was powered to detect only a medium to large effect size. Third, although the control group did not receive education on hand hygiene, it is possible that participation in the study may have resulted in increased hand hygiene among the control patients. Finally, the risk for acquisition of pathogens from the environment in the study hospital might be low compared with that in other hospitals owing to an ongoing environmental cleaning intervention that includes monitoring and feedback to environmental services personnel.
CONCLUSIONS
In summary, an educational patient hand hygiene intervention did not reduce the acquisition of colonization by selected health care–associated pathogens in hospitalized patients compared with that in standard care. Future studies are needed in other settings, with other target pathogens, and with more intensive hand hygiene interventions. It is also plausible that patient hand hygiene may be most beneficial when included as 1 component of a bundle of measures to prevent acquisition of pathogens. Thus, there is a need to evaluate strategies that combine patient hand hygiene with such interventions as patient bathing and enhanced environmental cleaning.
Acknowledgments
Funding/support: This work was supported by Merit Review Grant 1 I01 BX002944–01A1 from the US Department of Veterans Affairs (to C.J.D.).
Footnotes
Conflicts of interest: C.J.D has received research grants from Pfizer, Clorox, GOJO, PDI, and Avery Dennison. The other authors report no conflicts of interest.
References
- 1.Istenes N, Bingham J, Hazelett S, Fleming E, Kirk J. Patients’ potential role in the transmission of health care-associated infections: prevalence of contamination with bacterial pathogens and patient attitudes toward hand hygiene. Am J Infect Control 2013;41:793–8. [DOI] [PubMed] [Google Scholar]
- 2.Cheng VC, Tai JW, Chau PH, Lai CK, Chuang VW, So SY, et al. Successful control of emerging vancomycin-resistant enterococci by territory-wide implementation of directly observed hand hygiene in patients in Hong Kong. Am J Infect Control 2016;44:1168–71. [DOI] [PubMed] [Google Scholar]
- 3.Cao J, Min L, Lansing B, Foxman B, Mody L. Multidrug-resistant organisms on patients’ hands: a missed opportunity. JAMA Intern Med 2016;176:705–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunkesula VCK, Kundrapu S, Knighton S, Cadnum JL, Donskey CJ. A randomized trial to determine the impact of an educational patient hand-hygiene intervention on contamination of hospitalized patient’s hands with healthcare-associated pathogens. Infect Control Hosp Epidemiol 2017;38:595–7. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell M, Harris T, Horn T, Midamba B, Primes V, Sullivan N, et al. Sustained increase in resident meal time hand hygiene through an interdisciplinary intervention engaging long-term care facility residents and staff. Am J Infect Control 2015;43:162–4. [DOI] [PubMed] [Google Scholar]
- 6.Sunkesula VC, Knighton S, Zabarsky TF, Kundrapu S, Higgins PA, Donskey CJ. Four moments for patient hand hygiene: a patient-centered, provider-facilitated model to improve patient hand hygiene. Infect Control Hosp Epidemiol 2015;36:986–9. [DOI] [PubMed] [Google Scholar]
- 7.Gagné D, Bédard G, Maziade PJ. Systematic patients’ hand disinfection: impact on methicillin-resistant Staphylococcus aureus infection rates in a community hospital. J Hosp Infect 2010;75:269–72. [DOI] [PubMed] [Google Scholar]
- 8.Cheng VC, Wu AK, Cheung CH, Lau SK, Woo PC, Chan KH, et al. Outbreak of human metapneumovirus infection in psychiatric inpatients: implications for directly observed use of alcohol hand rub in prevention of nosocomial outbreaks. J Hosp Infect 2007;67:336–43. [DOI] [PubMed] [Google Scholar]
- 9.Pokrywka M, Buraczewski M, Frank D, Dixon H, Ferrelli J, Shutt K, et al. Can improving patient hand hygiene impact Clostridium difficile infection events at an academic medical center? Am J Infect Control 2017;45:959–63. [DOI] [PubMed] [Google Scholar]
- 10.Rai H, Knighton S, Zabarsky TF, Donskey CJ. A randomized trial to determine the impact of a 5 moments for patient hand hygiene educational intervention on patient hand hygiene. AmJ Infect Control 2017;45:551–3. [DOI] [PubMed] [Google Scholar]


