Abstract
We present the design, synthesis, and applications of a new class of voltage-sensitive fluorescent indicators built on a modified carbofluorescein scaffold. Carbofluoresceins are an attractive target for responsive probes because they maintain oxygen substitution patterns at the 3’ and 6’ positions, similar to fluorescein, while simultaneously possessing excitation and emission profiles redshifted nearly 50 nm compared to fluorescein. However, the high pKa of carbofluorescein dyes, coupled with their tendency to cyclize to non-fluorescent configurations precludes their use in voltage-imaging applications. Here, we overcome the limitations of carbofluores-ceins via chlorination to lower the pKa by 2 units to 5.2 and sulfonation to prevent cyclization to the non-absorbing form. To achieve this, we devise a synthetic route to halogenated sulfonated carbofluoresceins from readily available, inexpensive starting materials. New, chlorinated sulfone carbofluoresceins have low pKa values (5.2) and can be incorporated into phenylenevinylene molecular wire scaffolds to create carboVoltage-sensitive Fluorophores (carboVF dyes). The best of the new carboVF dyes, carboVF2.1(OMe).Cl, possesses excitation and emission profiles >560 nm, displays high voltage sensitivity (>30% ΔF/F per 100 mV), and can be used in the presence of other blue-excited fluorophores like green fluorescent protein (GFP). Because carboVF2.1 (OMe).Cl contains a phenolic oxygen, it can be incorporated into fluorogenic labeling strategies. Alkylation with a sterically bulky cyclopropylmethyl-derived acetoxymethyl ether renders carboVF weakly fluorescent; we show that fluorescence can be restored by the action of porcine liver esterase (PLE) both in vitro and on the surface of living cells and neurons. Together, these results suggest chlorinated sulfone carbofluoresceins can be promising candidates for hybrid chemical-genetic voltage imaging at wavelengths beyond typical fluorescein excitation and emission.
Table of Contents Graphic
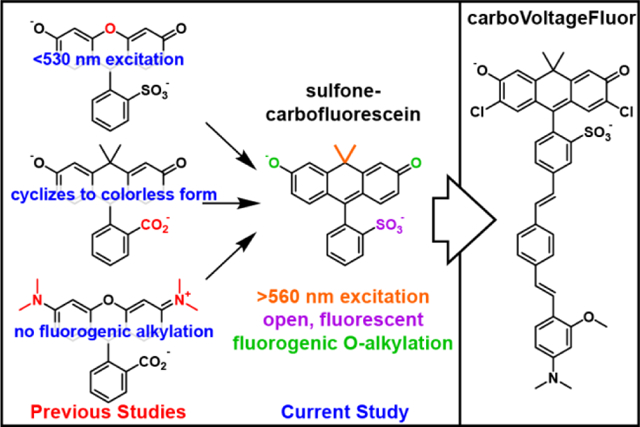
INTRODUCTION
Membrane potential is central to life. Cells exert an array of strategies to maintain tight control over the unequal ionic distribution across their plasma membranes. The millisecond changes in membrane potential associated with electrically excitable cells like neurons and cardiomyocytes are most traditionally studied using electrode-based techniques. While these methods transformed our ability to monitor membrane voltage, they remain low through-put and highly invasive. Optical approaches for measuring membrane voltage dynamics are attractive because they offer the opportunity to probe voltage changes in a minimally-invasive, high-throughput fashion.
Inspired by theoretical descriptions of electron transfer as a voltage-sensing mechanism,1 coupled with early demonstrations of molecule-scale electric fields altering the efficiency of electron transfer,2 we recently initiated a program to optically measure membrane voltage in living systems using fluorescent indicators.3–4 These voltage-sensitive fluorophores, or Volt-ageFluors, make use of photoinduced electron transfer, or PeT, as a voltage-sensing trigger. Changes in the membrane voltage alter the efficiency of PeT, allowing the fluorescent sensor to toggle between dim and bright states in a voltage-dependent manner. Initial VoltageFluors were based on sulfonefluores- cein5–7 and provide a fast (sub-microsecond responses)8–9 and sensitive (>20% ΔF/F per 100 mV) readout of membrane potential. The approach appears generalizable: both rhodamine (RhoVR)10 and silicon-rhodamine (BeRST)11 fluorophores can be co-opted to achieve voltage imaging in the >560 nm region of the visible spectrum. The ability to tune voltage sensing across a range of colors enables compatibility with other commonly-used fluorophores, such as the green fluorescent protein (GFP).12
A general drawback of chemically-synthesized voltage indicators is their inability to target to specific cells of interest.13–15 This complicates the interpretation of optical voltage signals, especially in the context of neurobiology, where cell membranes often overlap in the intricate arborization of neuronal processes. Recently, we showed that fluorescein-based Volt-ageFluors could be ligated directly to cells of interest using self-labeling enzymes like the SpyTag/SpyCatcher system.16–17 This approach should be generalizable to other colors of voltage-sensitive dye (like RhoVR and BeRST) and to other self-labeling enzymes.18–21 However, one potential problem is that labeling of cells of interest depends on expression of enzyme on the cell surface. The 1:1 stoichiometry of indicator and enzyme will limit the total number of indicators, and therefore fluorescence intensity, on the cell surface.
The lower fluorescence intensity of the one enzyme, one indicator problem could be partially addressed by fluorogenic approaches in which either photo-uncaging light or cell surface enzyme could catalytically activate the fluorescence of a caged voltage indicator. We showed that both of these strategies, photoactivation and enzymatic uncaging,22–23 could be employed with fluorescein-based VoltageFluors to provide local contrast and voltage sensing in neurons. This strategy requires the presence of a phenolic oxygen in the fluorophore to enable alkylation-dependent reduction of fluorescence.24–25 As a result, none of the long-wavelength indicators (>560 nm excitation) developed in our lab, like RhoVR or BeRST can be readily adapted to this fluorogenic strategy, because they rely on rhodamintype xanthenes, which contain substituted nitrogens in place of oxygen at the 3’ and 6’ positions of the xanthene fluorophore (Scheme 1 ).
Scheme 1.
Red-shifted voltage-sensitive dyes for fluorogenic targeting to specific cells
We were attracted, therefore, to carbofluorescein-based dyes, which maintain the key phenolic oxygen framework characteristic of fluorescein, but achieve >560 nm excitation and emission by the substitution of the bridge-head oxygen of the xanthene fluorophore with a geminal dimethyl carbon fragment.26–27 Carbofluorescein scaffolds have been incorporated into PeT- based Ca2+ indicators,28 so we hypothesized that carbofluoresceins could be incorporated into a voltage-sensing platform via installation of a phenylenevinylene molecular wire for membrane localization and voltage sensing. To achieve this, however, would require breaking the propensity for carbofluoresceins to cyclize, in non-polar conditions, to non-absorbing, nonemissive states.26–27 We hypothesize the open/close equilibrium of carbofluorescein could be shifted to favor the open form by the inclusion of a sulfonate group.
We here report the design, synthesis, and application of a new suite of sulfone-carbofluoresceins to voltage imaging. We established an efficient, 7-step synthesis from readily available, inexpensive starting materials to gain access to novel sulfone-carbofluorescein and halogenated sulfone-carbofluoresceins. All of the new sulfone-carbofluoresceins show a decreased propensity for cyclization and can be combined with phenylene-vinylene molecular wires to yield voltage-sensitive indicators. The best of these, carboVF2.1(OMe).Cl, which is based on a never-before-reported chlorinated carbofluorescein scaffold, shows a voltage sensitivity of 31% ΔF/F per 100 mV in HEK cells with a SNR of 170:1, exceeding that of RhoVR (160:1)10 and rivaling that of BeRST.11 We show that carboVF2.1 (OMe).Cl can be adapted to fluorogenic targeting strategies for use in neurons, paving the way for fluorogenic targeting of voltage indicators to specific cells using multiple colors.
RESULTS
Synthesis of sulfone-carbofluorescein dyes
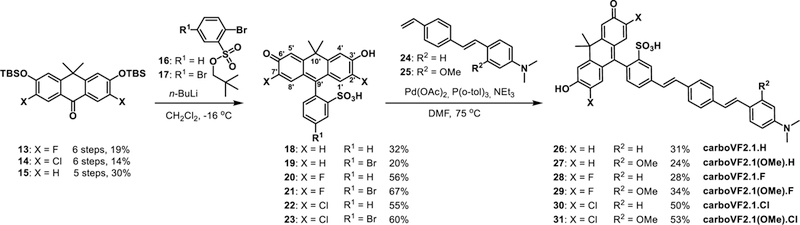
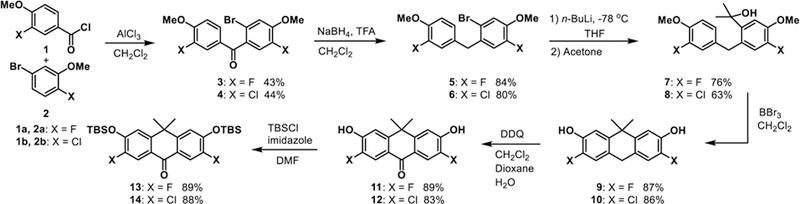
For the application of carbofluoresceins to voltage sensing, a key challenge is the installation of a sulfonic acid group on the meso aromatic ring, which we discovered was critical for both preventing passage of molecular-wire fluorescent voltage indicators through cellular membranes10 and for driving the correct alignment of the voltage-sensing phenylene vinylene molecular wire within the plasma membrane.4 Sulfone xanthene dyes with oxygen at the 10’ bridge-head position, such as fluorescein or rhodamines, can be readily accessed by the acid-catalyzed condensation of the corresponding resorcinols, in the case of fluorescein, or aminophenols, in the case of rhodamines, with carboxylic acids, anhydrides, or aldehydes. Carbofluoresceins and sulfone-carbofluoresceins, where a geminal dimethyl group replaces oxygen at the 10’ bridgehead of the xanthene (Scheme 3), cannot be easily synthesized by this analogous route. Previously reported carbofluorescein fluorophores were synthesized through the addition of an aryl-metal species to a protected anthracenyl ketone, yielding unsubstituted carbofluorescein26 and fluorinated carbofluorescein.27 We envisioned adapting our earlier synthesis of sulfone silicon-rhodamines11 to access both unsubstituted and halogenated, sulfone-carbofluoresceins. The short, 6-step synthesis to access reported fluorinated28 and novel chlorinated ketones 13 and 14 is outlined in Scheme 2. The overall yield for fluorinated ketone 13 is 19%, adding an additional two steps to the previously reported route27—with an overall yield of 46%—but beginning with starting materials that are 100-fold less expensive than the starting materials reported in the synthesis of 13.27 Additionally, our route provides access, for the first time, to ketone 14 in 14% overall yield, beginning from inexpensive starting materials.
Scheme 3.
Synthesis of carboVoltageFluor dyes
Scheme 2.
Synthesis of carbofluorescein precursors
Friedel-Crafts acylation of 2a or 2b with in situ-generated acid chlorides derived from 1a or 1b provides fluorinated benzophenone 3 in 43% yield and the analogous chlorinated benzophenone 4 in 44% yield. Reduction of the ketone with NaBH4 provides benzylic intermediates 5 and 6 in 84% and 80% yield. Intermediates 5 and 6 undergo lithium-halogen exchange mediated by n-butyllithium, and treatment with acetone furnishes tertiary alcohols 7 and 8 in 76% and 63% yield. Lewis-acid catalyzed cyclization and simultaneous deprotection of methyl ethers with BBr3 in CH2Cl2 gives anthrone precursors 9 and 10 in 87% and 86% yield. Following column chromatography, these air-sensitive compounds were immediately oxidized with DDQ in a mixture of CH2Cl2, dioxane, and water to give 11 and 12. Subsequent TBS-protection of the alcohols gave the required halogenated, TBS-protected anthrones in 89% (13, fluoro) and 88% (14, chloro) yield. Unsubstituted anthrone 15 (X = H, Scheme 3) was prepared according to the reported procedure.26
Reaction of aryl lithium species generated from 16 or 17 (R1 = H or Br) with silyl-protected anthrones 13–15 gave sulfone carbofluorescein dyes 18–23 in yields ranging from 20% to 67%, with halogenated anthrones giving higher yields (> 55%). We found that using the neopentyl sulfonate ester,29 rather than the corresponding isopropyl ester for 16 and 17, led to improved yields of sulfone carbofluoresceins (18–23). Lithiation of 16 or 17 and addition to the anthrone gave the best results when performed in CH2Cl2 at −20 °C; attempts in THF at a range of temperatures (−78 °C, −40 °C, and −20 °C) gave a complicated mixture of products or low yields. Sulfone carbofluorescein fluorophores 19, 21, and 23 were combined with styrenes 24 or 25 in a Pd-catalyzed Heck reaction to yield carbofluorescein-based voltage indicators 26–31 in 24% to 53% yields after reversephase HPLC purification.
Spectroscopic properties of sulfone carbofluoresceins
Sulfone carbofluoresceins display excitation and emission profiles significantly red-shifted from sulfonated fluoresceins. Sulfone carbofluorescein 18 possesses an absorbance maximum at 550 nm and emission at 576 nm (Fig. 1a,b), while sulfone fluorescein absorbs and emits at 499 and 532 nm.4 This represents a bathochromic shift of 51 nm and 44 nm for absorption and emission, respectively. This is in good agreement with the 53 and 57 nm shift in absorption and emission observed for traditional fluorescein to carbofluorescein.26 Sulfonation of carbofluorescein 18 also shifts the absorption and emission profile by 6 and 9 nm, respectively, relative to the parent carbofluorescein (Fig. 1a,b), which absorbs maximally at 544 nm and emits at 567 nm. Again, this is similar to the shift observed when moving from fluorescein to sulfone fluorescein (491 nm to 499 nm for absorption; 510 nm to 532 nm for emission). We observe a similar spectroscopic shift for fluorinated, sulfone carbofluorescein 20 (562/593 nm, abs/em), when compared to the corresponding fluorinated carbofluorescein (555/581 nm, abs/em).27
Figure 1.
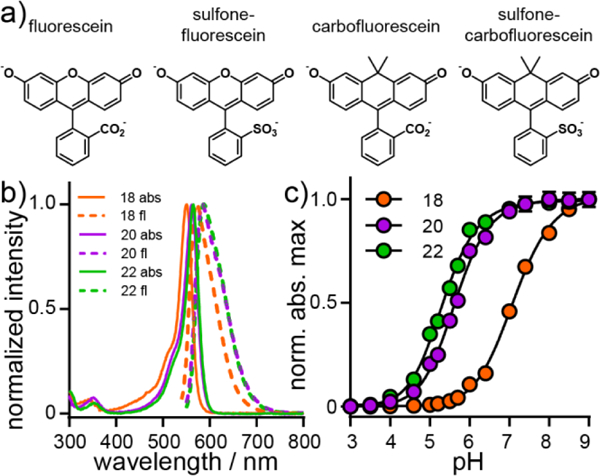
Spectroscopic characterization of sulfonated carbofluo-resceins. a) Structures of sulfonecarbofluorescein and related derivatives. b) Plot of normalized absorbance (solid line) and fluorescence emission intensity (dashed line) for 18 (H, orange), 20, (F, purple), and 22 (Cl, green). Spectra were acquired in HBSS. All dyes were measured at a concentration of 250 nM. c) Plot of normalized absorbance maximum vs. pH for 18 (H, orange), 20, (F, purple), and 22 (Cl, green). Error bars are ± S.E.M. for n = 3 independent determinations. If not visible, error bars are smaller than the marker.
Sulfonation of the meso aromatic ring of carbofluorescein derivatives (18, 20, and 22) results in a drop in the pKa relative to carboxy-substituted carbofluoresceins. Sulfone carbofluorescein 18 has a pKa of 7.11 (± 0.02, Fig. 1c, S1, Table S1), approximately 0.3 log units lower than prototypical carbofluorescein (values ranging from 7.4426 to 7.5427). Fluorine substitution on the 2’ and 7’ positions of sulfone carbofluorescein 20 results in a pKa of 5.63 (± 0.02, Fig. 1c, S1, Table S1), a full pH unit lower than 2’,7’-difluorocarbofluorescein without a sulfonate (pKa = 6.75).27 Chlorine substitution at the 2’ and 7’ positions further lowers the pKa of sulfone carbofluorescein 22 to 5.31 ± 0.02 (Fig. 1c, S1, Table S1), compared to 5.0 for dichlorofluorescein.30 Because halogenated sulfone carbofluoresceins possess pKa values approximately 1.5–2 units lower than physiological pH, we thought that halogenated sulfone carbofluoresceins would be most useful for cellular imaging, owing to their largely deprotonated and fluorescent state at physiological pH.
A defining characteristic of typical carbofluoresceins is their semi-cooperative transition from a colored, xanthene form to a cyclized and colorless form (Scheme 1), as indicated by Hill coefficients greater than unity for carbofluorescein (1.33 ± 0.03)26 and fluorinated-carbofluorescein (1.46 ± 0.02).26 Exchanging carboxylate for sulfonate breaks the cooperativity of carbofluoresceins: both unsubstituted and fluorine-containing, sulfone carbofluoresceins 18 (H) and 20 (F) display Hill coefficients near unity, 0.92 ± 0.01 and 1.02 ± 0.04, respectively. Chlorine-substituted sulfone carbofluorescein 22 (Cl) displays a similar Hill coefficient of 0.99 ± 0.01 (Fig. S1). Sulfonation of the meso aromatic ring decreases the propensity of carbofluoresceins to cyclize.
Carbofluorescein VoltageFluors 26–31, or carboVF dyes, display absorbance and emission profiles similar to their corresponding sulfone carbofluoresceins (18, 20, or 22, Table 1, Fig. 2a, Fig. S2). The carboVF dyes exhibit lower quantum yields (Table 1) than the corresponding free fluorophores (Table S1), indicating efficient PeT from the aniline donor to the fluorophore.
Table 1.
Properties of carboVoltageFluor indicators
| compound | X | R2 | λmax / nma | λem / nma | Φb | ΔF/Fc,d | relative brightnessd | SNRd,e |
|---|---|---|---|---|---|---|---|---|
| carboVF2.1.H (26) | H | H | 552 | 576 | 0.015 | 3 ± 1% | 0.27 ± 0.03 | 2.2 ± 0.4 |
| carboVF2.1(OMe).H (27) | H | OMe | 554 | 576 | 0.037 | 12 ± 1% | 0.58 ± 0.07 | 21 ± 3 |
| carboVF2.1.F (28) | F | H | 562 | 593 | 0.018 | 6 ± 0.4% | 0.84 ± 0.03 | 19 ± 2 |
| carboVF2.1(OMe).F (29) | F | OMe | 562 | 593 | 0.009 | 26 ± 2% | 0.93 ± 0.04 | 63 ± 10 |
| carboVF2.1.Cl (30) | Cl | H | 562 | 593 | 0.008 | 7 ± 1% | 0.33 ± 0.01 | 43 ± 8 |
| carboVF2.1(OMe).Cl (31) | Cl | OMe | 562 | 593 | 0.012 | 31 ± 2% | 1.00 ± 0.05 | 171 ± 10 |
Determined in HBSS with 0.01% SDS.
Determined in HBSS.
Per 100 mV.
Determined in HEK cells.
Sampled at 500 Hz. Error is ± S.E.M. for n = at least 5 cells for ΔF/F and SNR determination and n = 3 coverslips (>100 cells per coverslip) for relative brightness.
Figure 2.
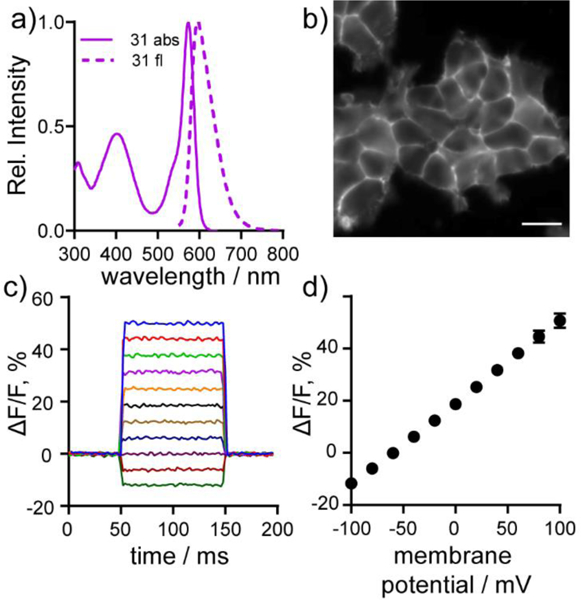
Cellular and in vitro characterization of carbo-VoltageFluor dyes. a) Normalized absorbance (solid line) and emission (dashed line) spectra of cVF2.1(OMe).Cl (31) in HBSS with 0.01% SDS. b) HEK cells stained with 500 nm cVF2.1(OMe).Cl (31). Scale bar is 10 μm. c) Plot of the fractional change in fluorescence of cVF2.1(OMe).Cl (31) vs time for 100 ms hyper-and depolarizing steps (±100 mV in 20 mV increments) from a holding potential of −60 mV for single HEK cells under whole-cell voltage-clamp mode. (d) Plot of % ΔF/F vs final membrane potential summarizing data from seven separate cells, revealing a voltage sensitivity of approximately 31% per 100 mV. Error bars are ± S.D.
Cellular characterization of carbo-VoltageFluor dyes
All of carboVF dyes (26–31) localize to the cellular membranes of HEK293T cells, as determined by fluorescence microscopy (Fig. 2b, Fig. S3). Generally, halogenated carboVFs (28–31) display higher cellular brightness than the unhalogenated derivatives (26 and 27), likely because the higher pKa of unhalogenated sulfone-carbofluoresceins (7.1 vs 5.6 and 5.3) decreases the fluorescence of unhalogenated carboVFs 26 and 27 at physiological pH. carboVF2.1(OMe).Cl (31) is the brightest dye, with relative fluorescence intensities up to 4-fold greater than carboVF2.1.H (26), the dimmest dye, and about 10% brighter than carboVF2.1(OMe).F (29) (Table 1, Fig. S3).
All of the carboVF dyes are voltage-sensitive. Patch clamp electrophysiology, coupled with fluorescence imaging, revealed that the carboVF dyes increased fluorescence upon membrane depolarization (more positive potentials) and decreased fluorescence upon hyperpolarization (less positive potentials). When the aniline donor of cVF dyes is unsubsituted (R2 = H, Scheme 3), carboVF dyes exhibit lower voltage sensitivity (in units of ΔF/F per 100 mV): carboVF2.1.H (26) shows a 3% ΔF/F per 100 mV, carboVF2.1.F (28) 6%, and car- boVF2.1.Cl (30) 7% (Table 1, Fig. S4). The voltage sensitivity of carboVF dyes improves with substitution of the hydrogen on the aniline ring for methoxy (R2 = OMe), moving to 12% for carboVF2.1 (OMe).H (27), 26% for carboVF2.1(OMe).F (29), and 31% for carboVF2.1(OMe).Cl (31) (Table 1, Fig. 2c,d, Fig. S4). Halogenated carboVF dyes 29 and 31 represent the most sensitive red-shifted, fluorescein-based voltage indicators to date. Dyes with methoxy substitution patterns on the aniline (R2 = OMe) display higher signal-to-noise ratios (SNR) per 100 mV and show brighter membrane-associated fluorescence in HEK cells—perhaps due to more efficient incorporation into cell membranes. Because of its low pKa (~5.3), high voltage sensitivity (31% ΔF/F per 100 mV), favorable brightness, and high SNR for detecting voltage changes in cells (~170:1), we carried carboVF2.1(OMe).Cl (31) forward for further characterization in neurons.
CarboVF2.1(OMe).Cl stains membranes of hippocampal neurons cultured from rat embryos and responds to spontaneous neuronal activity with clear, distinct action potentials (Fig. 3a,b). Optical recordings reveal recurrent, spontaneous activity in cultured hippocampal neurons, with action potentials and spiking events from multiple, neighboring cells (Fig. 3c,d). Field stimulation of neurons indicate that carboVF2.1(OMe).Cl responds to action potentials with 14% ± 1% ΔF/F (SNR = 28:1, n = 17 cells, Fig. S5): an improvement over sulfone-fluores-cein-based VoltageFluor2.1.Cl (7.5%, SNR = 20:1)23 and rho-damine-based RhoVR (9.5%, SNR = 12:1)10 and comparable to the performance of far-red silicon-rhodamine BeRST (18%).11
Figure 3.
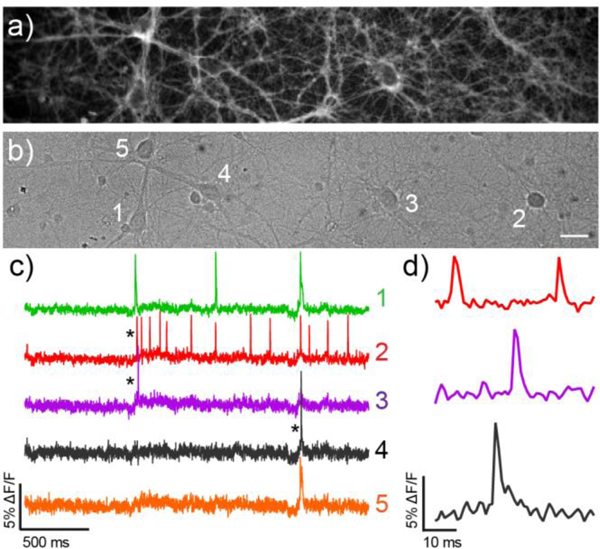
Voltage Imaging of spontaneous neuronal activity with cVF2.1(OMe).Cl a) Fluorescence and b) differential interference contrast (DIC) images of cultured rat hippocampal neurons stained with 500 nM cVF2.1(OMe).Cl. Scale bar is 20 μm. c) Optical traces of spontaneous activity of the neurons in panels a–b) recorded at 500 Hz. Activity is shown as ΔF/F vs time and d) shows highlighted and expanded ΔF/F traces.
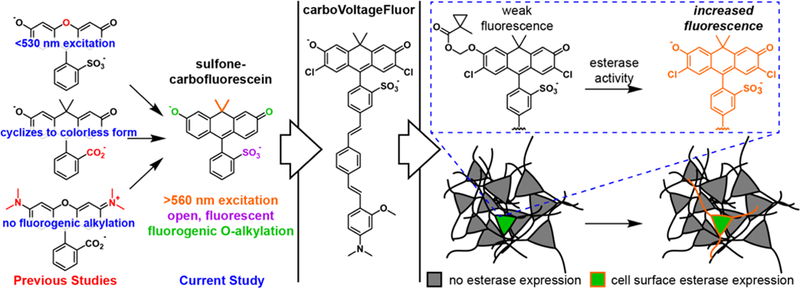
Fluorogenic targeting of carboVF dyes
Localization of synthetic voltage indicators to specific cells of interest remains an outstanding challenge.13–14, 17, 21–23, 31 The long-wavelength excitation and emission profile, high voltage sensitivity, ability to report on action potentials in mammalian neurons, and carbofluorescein molecular scaffold of carboVF derivatives make them an attractive choice for fluorogenic, cell-specific targeting strategies. We previously showed that fluorescein-based VoltageFluor dyes can be adapted to cell-specific targeting through a fluorogenic approach: alkylation of the phenolic oxygen of VF2.1.Cl with either a photolabile nitrobenzyl protecting group22 or a hydrolytically-stable cyclopropylmethyl acetoxy methyl ether23 results in decreased fluorescence, which can be restored by photo-uncaging or the action of an exogenously expressed esterase.32 Localization of porcine liver esterase (PLE) on the cell surface results in VF fluorescence restricted to cells that express PLE.
We hypothesized that combining esterase-mediated targeting with long-wavelength, phenol-containing, or fluorescein-like fluorophores, as opposed to aniline-containing, or rhodamine-like fluorophores, would provide an opportunity for imaging voltage dynamics from specific neurons using longer wavelengths of light. To accomplish this, we synthesized carbo-VoltageFluors targeted by esterase expression (carboVF-EX, Scheme 4). Current voltage-sensitive fluorophore scaffolds with excitation and emission profiles above 560 nm, like RhoVR (rhodamine) or BeRST (silicon-rhodamine) cannot be fluorogenically targeted using these strategies, because they lack the phenolic oxygen present in VoltageFluor and carboVoltageFluor dyes.
Scheme 4.
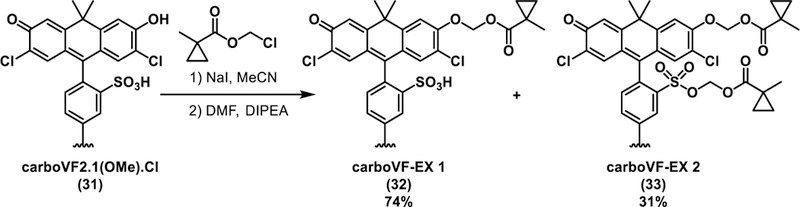
Synthesis of carboVoltageFluors targeted by esterse expression, carboVF-EX 1 and 2
CarboVF-EX 1 and 2 are synthesized in a single step from carboVF2.1(OMe).Cl (Scheme 4). Addition of stoichiometric (EX 1) or excess (EX 2) iodo-1-methylcyclopropane-carbox ylate in DMF using N,N-diisopropylethylamine as a base provides the singly and doubly protected carboVF-EX derivatives. Alkylation results in a hypsochromatic shift in the absorption spectrum, from a maximum at 550 nm for carboVF2.1 (OMe).Cl to 420 nm for carboVF-EX 1 (Fig. 4a,b, Fig. S6). Alkylation of the phenolic oxygen of carboVF2.1(OMe).Cl results in a 12-fold decrease in fluorescence quantum yield (Table S2).
Figure 4.
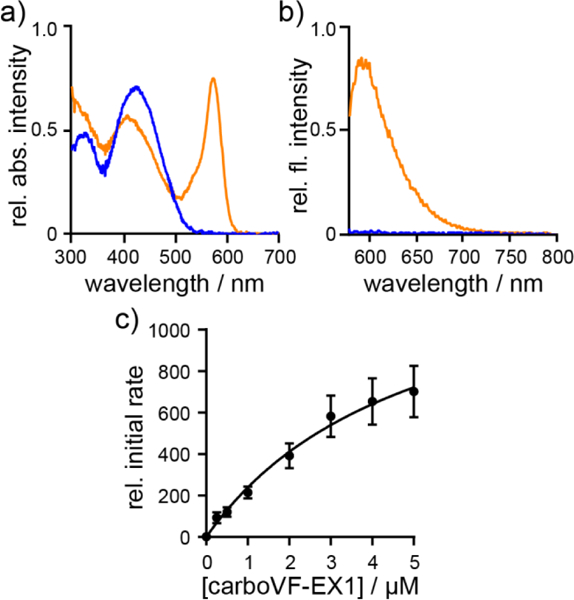
In vitro characterization of PLE-mediated uncaging of carboVF-EX 1. Normalized a) absorbance and b) emission spectra of carboVF-EX 1 (0.5 μM) in HBSS with 0.01% SDS. Spectra are acquired 2h after the addition of PLE (0.7 mg/mL, orange) or blank (blue). Excitation is provided at 572 nm. c) Plot of relative initial rate vs. carboVF-EX 1 concentration. Error bars are ± S.E.M. for n = 7 independent determinations. [PLE] is 168 ng/mL (1 nM) for saturation kinetics measurements.
The carboVF-EX dyes are substrates for purified PLE (Sigma, E2884). CarboVF-EX1 and EX2 are hydrolyzed by PLE, resulting in a 45- or 21- fold increase in fluorescence, respectively, after 2h. Release of the caged species results in carboVF2.1(OMe).Cl-like absorbance and emission profiles (Fig. 4a,b). HPLC analysis reveals carboVF2.1 (OMe).Cl as the product of PLE-mediated hydrolysis reactions (Fig. S7). Both carboVF-EX 1 and 2 show saturation-type enzymatic kinetics with PLE (Table S2, Fig. 4c, Fig. S6). The Michaelis constant, KM, for carboVF-EX 1 is 6.4 ± 1 μM and 0.16 ± 0.06 μM for carboVF-EX 2. These values correspond reasonably well with the values we obtained for the fluorescein-based VF-EX 1 (1.2 ± 0.5 μM) and VF-EX 2 (0.12 ± 0.03 μM). All of these values are within the range for the reaction of bis(cyclopropy-lacetoxymethy)-fluorescem, 0.5 μM.32 The double-protected carboVF-EX 2 is a better substrate for PLE than the singly-protected carboVF-EX 1: the kcat/KM for carboVF-EX 2 is 4.9 × 105 M–1s–1, nearly 5-fold larger than the value for carboVF-EX 1 (1.3 × 105 M–1s–1). Again, the measured kcat/KM values for car- boVF-EX dyes closely match the values obtained for fluorescein VF-EX dyes (Table S2).
Cellular performance of carboVF-EX 1
We next sought to determine if we could elicit a similar turnon phenomenon in living cells. CarboVF-EX 1 (500 nM, 30 min or 1 h) was bath applied to HEK cells transfected with cell-sur-face PLE anchored via a glycophosphatidyl inositol, GPI, anchor (GPI sequence derived from decay-accelerating factor, DAF; Fig. S8).33 CarboVF-EX 1 was uncaged by PLE on the cell surface, displaying membrane-associated fluorescence in transfected cells (as indicated by nuclear-localized GFP fluorescence, Fig. 5a–c). CarboVF-EX2 did not elicit a turn-on response when bath-applied to PLE-expressing HEK cells (data not shown). We hypothesize that due to the extra carbon dimethyl group (C(CH3)2), carboVF-EX 2 is more lipophilic than VF-EX2 and does not properly load into cell membranes. Due to these results, we carried carboVF-EX 1 forward in our studies.
Figure 5.
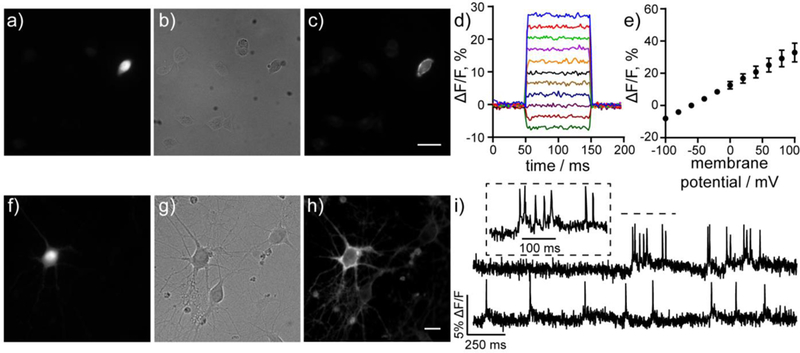
Cellular characterization of carboVF-EX 1. a–c) Wide-field fluorescence microscopy of HEK cells stained with carboVF-EX 1 (500 nM, 30 min) shows membrane labeling of the cell expressing cell surface PLE, as indicated by a) GFP fluorescence. b) DIC image of HEK cells and c) epifluorescence image showing carboVF-associated fluorescence in PLE-expressing cell. Scale bar is 10 μm. d) Voltage sensitivity of carboVF-EX1 in patch-clamped PLE-expressing HEK cells. e) Plot of AF/F vs membrane potential (in mV) for carboVF-EX 1. Data are mean ± S.D. for five cells. f–h) Live cell wide-field images of rat hippocampal neurons expressing PLE-DAF under the control of the synapsin promoter (Syn) and stained with carboVF-EX 1 (500 nM, 30 min). f) GFP fluorescence indicated PLE expression in neurons shown in the g) DIC image. h) CarboVF-associated fluorescence in PLE expressing neuron. Scale bar is 20 μm. i) Representative ΔF/F traces for spontaneous activity of neurons transfected with Syn-PLE and stained with carboVF-EX 1. Images were acquired at 500 Hz and represent single-trial acquisitions. Inset shows expanded time scale of indicated area of the upper trace.
HEK cells expressing PLE-DAF and stained with carboVF- EX 1 show a 8.9 (±0.7) and 5.0 (±0.4) fold turn-on after 30 mins and 1 h, respectively, when compared to non-transfected cells from the same culture (Fig. 5a–c, Fig. S9). These results match the observed 7-fold increase in fluorescence after uncaging of VF-EX 1 in HEK cells.23 As with fluorescein-based VF-EX 1, longer incubation times with carboVF-EX 1 increases the overall fluorescence associated with carboVF-EX 1 in PLE-expressing cells, but decreases the contrast due to accumulation of uncaged carboVF-EX 1 in untransfected cells (Fig. S9). Following enzymatic uncaging, carboVF-EX 1 was voltage sensitive in PLE-DAF expressing HEK cells, displaying an 18% ΔF/F per 100 mV, lower than the 31% ΔF/F displayed by carboVF2.1(OMe).Cl (Fig. 5d,e). The lower fractional voltage sensitivity may be a result of increased background staining with carboVF-EX 1 compared to carboVF2.1(OMe).Cl.
We observe selective staining of neurons expressing PLEDAF under the neuron-specific synapsin promoter (Syn) using carboVF-EX 1 (Fig. 5f–h, Fig. S10). Bath-application of carboVF-EX 1 using 500 nM, 1 pM, or 2 pM results in 2.4-, 2.3, and 1.9-fold turn-on after 30 minutes. We also loaded 500 nM carboVF-EX1 for 1 hr, but saw a decrease in contrast with a 1.8-fold turn-on (Fig. S11). Our results are comparable to those obtained with VF-EX1 (1 μM), which exhibits a 4.1-fold turn-on in neurons after 1 hr.23 The membrane fluorescence associated with carboVF-EX 1 in neurons is voltage-sensitive. CarboVF-EX 1 responds to field stimulation electrode-evoked action potentials with a voltage sensitivity of 8.4% ΔF/F (SNR = 12 ± 0.8, n = 9 cells, Fig. S12) and was able to record spontaneous spiking events in cultured neurons transfected with PLE-DAF (Fig. 5i) - comparable to the values of VF-EX2 / PLE in cultured neurons that we previously reported (7.3 ± 0.8%, SNR = 20 ± 2.7).
DISCUSSION
In summary, we present the design, synthesis, and applications of a new class of sulfone carbofluorescein dyes towards voltage imaging. We show that inclusion of a sulfonic acid functional group prevents the spirocyclization typical of carbo- fluoresceins and develop a concise synthetic route to halogenated carbofluorescein precursors starting with readily available, inexpensive starting materials. We incorporate unsubstituted, difluoro-and novel dichloro-substituted sulfone carbofluoresceins into a PeT-based voltage-sensing scaffold and show that all of the new carboVF dyes are voltage-sensitive in mammalian cells. The best of these, carboVF2.1(OMe).Cl, possesses >30% ΔF/F response to 100 mV depolarizations in HEK cells, has excitation and emission spectra >560 nm, and readily reports on action potentials in mammalian neurons. The use of sulfone carbofluoresceins in the context of voltage sensing enables complementation with a growing toolkit of strategies for fluorogenic targeting.22–23 The resulting dye, carboVF-EX1, is the first long-wavelength voltage sensitive dye targeted to specific cells via enzyme-mediated fluorogenic activation, providing voltage imaging performance rivaling that of previous enzyme-activated voltage indicators, but at long wavelengths compatible with GFP. Future efforts will focus on improving the low contrast ratio between PLE-expressing cells and wildtype cells, by both decreasing the brightness of caged cVF-EXl-type dyes and improving singly-protected cVF-EXl-type dyes as substrates for PLE.
Supplementary Material
ACKNOWLEDGMENT
Research in the Miller Lab is supported in part by grants from the National Institutes of Health (R35GM119855, R01NS098088), National Science Foundation (NSF 1707350), and the Klingenstein Simons Foundation (40746). GO is a Gilliam Fellow of the Howard Hughes Medical Institute. PL is supported by an A*STAR graduate fellowship. We thank the Francis and Hammond labs for the use of a plate reader and the Catalysis Facility of Lawrence Berkeley National Laboratory, supported by the Director, Office of Science, of the US Department of Energy (contract no. DE-AC02-05CH11231) for the use of the preparative HPLC. We thank Dr. Jeff Pelton for assistance with the 900 MHz NMR spectrometer (NIH P41GM68933).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental details, synthetic procedures, imaging conditions, and supporting figures. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Li LS, Fluorescence probes for membrane potentials based on mesoscopic electron transfer. Nano Letters 2007, 7, 2981–2986. [DOI] [PubMed] [Google Scholar]
- 2.De Silva AP; Gunaratne HQN; Habibjiwan JL; Mccoy CP; Rice TE; Soumillion JP, New Fluorescent Model Compounds for the Study of Photoinduced Electron-Transfer -the Influence of a Molecular Electric-Field in the Excited-State. Angew Chem IntEd 1995, 34 (16), 1728–1731. [Google Scholar]
- 3.Miller EW, Small molecule fluorescent voltage indicators for studying membrane potential. Curr Opin Chem Biol 2016, 33, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni RU; Yin H; Pourmandi N; James F; Adil MM; Schaffer DV; Wang Y; Miller EW, A Rationally Designed, General Strategy for Membrane Orientation of Photoinduced Electron Transfer-Based Voltage-Sensitive Dyes. ACS Chem Biol 2017, 12 (2), 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orndorff WR; Vose RS, Sulfonefluorescein and dihydroxybenzoyl-benzene-ortho-sulfonic acid, and some of their derivatives. J Am Chem Soc 1924, 46, 1896–1912. [Google Scholar]
- 6.Gibbs RC; Shapiro CV, The absorption spectra of sulfonefluorescein and some of its derivatives. J Am Chem Soc 1928, 50 (6), 1755–1762. [Google Scholar]
- 7.Jiao GS; Han JW; Burgess K, Syntheses of regioisomerically pure 5- or 6-halogenated fluoresceins. J Org Chem 2003, 68 (21), 8264–7. [DOI] [PubMed] [Google Scholar]
- 8.Beier HT; Roth CC; Bixler JN; Sedelnikova AV; Ibey BL, Visualization of Dynamic Sub-microsecond Changes in Membrane Potential. Biophys J 2019, 116 (1), 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller EW; Lin JY; Frady EP; Steinbach PA; Kristan WB; Tsien RY, Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc Natl Acad Sci USA 2012, 109 (6), 2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deal PE; Kulkarni RU; Al-Abdullatif SH; Miller EW, Isomerically Pure Tetramethylrhodamine Voltage Reporters. J Am Chem Soc 2016, 138 (29), 9085–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YL; Walker AS; Miller EW, A Photostable Silicon Rhodamine Platform for Optical Voltage Sensing. J Am Chem Soc 2015, 137 (33), 10767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsien RY, The green fluorescent protein. Annu Rev Biochem 1998, 67, 509–44. [DOI] [PubMed] [Google Scholar]
- 13.Hinner MJ; Hbener G; Fromherz P, Enzyme-induced staining of biomembranes with voltage-sensitive fluorescent dyes. J Phys ChemB 2004, 108 (7), 2445–53. [DOI] [PubMed] [Google Scholar]
- 14.Ng DN; Fromherz P, Genetic targeting of a voltagesensitive dye by enzymatic activation of phosphonooxymethylammonium derivative. ACS Chem Biol 2011, 6 (5), 444–51. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni RU; Vandenberghe M; Thunemann M; James F; Andreassen OA; Djurovic S; Devor A; Miller EW, In Vivo Two-Photon Voltage Imaging with Sulfonated Rhodamine Dyes. ACS Cent Sci 2018, 4 (10), 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakeri B; Fierer JO; Celik E; Chittock EC; Schwarz-Linek U; Moy VT; Howarth M, Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci USA 2012, 109 (12), E690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grenier V; Daws BR; Liu P; Miller EW, Spying on Neuronal Membrane Potential with Genetically Targetable Voltage Indicators. J Am Chem Soc 2019, 141 (3), 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keppler A; Gendreizig S; Gronemeyer T; Pick H; Vogel H; Johnsson K, A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol 2003, 21 (1), 86–89. [DOI] [PubMed] [Google Scholar]
- 19.Los GV; Encell LP; McDougall MG; Hartzell DD; Karassina N; Zimprich C; Wood MG; Learish R; Ohana RF; Urh M; Simpson D; Mendez J; Zimmerman K; Otto P; Vidugiris G; Zhu J; Darzins A; Klaubert DH; Bulleit RF; Wood KV, HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 2008, 3 (6), 373–82. [DOI] [PubMed] [Google Scholar]
- 20.Calloway NT; Choob M; Sanz A; Sheetz MP; Miller LW; Cornish VW, Optimized fluorescent trimethoprim derivatives for in vivo protein labeling. Chembiochem 2007, 8 (7), 767–74. [DOI] [PubMed] [Google Scholar]
- 21.Sundukova M; Prifti E; Bucci A; Kirillova K; Serrao J; Reymond L; Umebayashi M; Hovius R; Riezman H; Johnsson K; Heppenstall PA, A Chemogenetic Approach for the Optical Monitoring of Voltage in Neurons. Angew Chem Int Ed 2019, 58 (8), 2341–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grenier V; Walker AS; Miller EW, A Small-Molecule Photoactivatable Optical Sensor of Transmembrane Potential. J Am Chem Soc 2015, 137 (34), 10894–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P; Grenier V; Hong W; Muller VR; Miller EW, Fluorogenic Targeting of Voltage-Sensitive Dyes to Neurons. J Am Chem Soc 2017, 139 (48), 17334–17340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urano Y; Kamiya M; Kanda K; Ueno T; Hirose K; Nagano T, Evolution of fluorescein as a platform for finely tunable fluorescence probes. J Am Chem Soc 2005, 127 (13), 4888–94. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T; Urano Y; Kamiya M; Ueno T; Kojima H; Nagano T, Highly Activatable and Rapidly Releasable Caged Fluorescein Derivatives. J Am Chem Soc 2007, 129 (21), 6696–6697. [DOI] [PubMed] [Google Scholar]
- 26.Grimm JB; Sung AJ; Legant WR; Hulamm P; Matlosz SM; Betzig E; Lavis LD, Carbofluoresceins and carborhodamines as scaffolds for high-contrast fluorogenic probes. ACS Chem Biol 2013, 8 (6), 1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimm JB; Gruber TD; Ortiz G; Brown TA; Lavis LD, Virginia Orange: A Versatile, Red-Shifted Fluorescein Scaffold for Single- and Dual-Input Fluorogenic Probes. Bioconjug Chem 2016, 27 (2), 474–80. [DOI] [PubMed] [Google Scholar]
- 28.Diwu Z; Guo H; Peng R; Zhao Q; Liu J; Liao J Carbofluorescein lactone metal ion indicators and their applications. US20140378344A1, 2014. [Google Scholar]
- 29.Miller SC, Profiling Sulfonate Ester Stability: Identification of Complementary Protecting Groups for Sulfonates. J Org Chem 2010, 75 (13), 4632–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonhardt H; Gordon L; Livingston R, Acid-base equilibriums of fluorescein and 2’,7’-dichlorofluorescein in their ground and fluorescent states. J Phys Chem 1971, 75 (2), 245–249. [Google Scholar]
- 31.Hinner MJ; Hubener G; Fromherz P, Genetic targeting of individual cells with a voltage-sensitive dye through enzymatic activation of membrane binding. Chembiochem 2006, 7 (3), 495–505. [DOI] [PubMed] [Google Scholar]
- 32.Tian L; Yang Y; Wysocki LM; Arnold AC; Hu A; Ravichandran B; Sternson SM; Looger LL; Lavis LD, Selective esterase-ester pair for targeting small molecules with cellular specificity. Proc Natl Acad Sci USA 2012, 109 (13), 4756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medof ME; Walter EI; Roberts WL; Haas R; Rosenberry TL, Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry 1986, 25 (22), 6740–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


