ABSTRACT
Cymbidium aloifolium is known for its ornamental and medicinal values. It has been listed as threatened orchid species. In this study, in vitro propagated C. aloifolium plantlets were interacted with the Piriformospora indica. The growth assay was performed for 45 days; the plant growth pattern such as number and length of roots and shoots were measured. Microscopic study of the root section stained by trypan blue was done to detect the peloton formation. The methanol extracts of the fungal colonized plant as well as uncolonized (control) plant were prepared and various metabolites were identified by gas chromatography mass spectroscopy. Acclimatization was done in a substrate composition of coco peat: gravel: charcoal in ratio 2:2:1. P. indica-colonized plantlet showed the highest growth with the formation of clamdospore in the root section. The growth regulator such as auxin, ascorbic acid, andrographolide, hexadecanoic acid, and DL-proline were identified. After three months of field transfer, plantlet colonized by P. indica survived and remained healthy as compared to uncolonized control plantlet.
KEYWORDS: Piriformospora indica, Cymbidium aloifolium, acclimatization, symbiosis, bioactive compounds
Introduction
Cymbidium aloifolium is commonly known as aloe-leafed Cymbidium having both ornamental and medicinal values.1 It is widely distributed from dry lowland tropical to subtropical region of central and eastern Nepal.2 It has distinct characteristics that make them different from other orchid species. They have ovoid pseuodobulb, leathery bright leaves and basal inflorescence with persistent yellowish flower with reddish purple chestnut radial stripes.3 The persistent bright yellowish flower makes the C. aloifolium one of the highly traded flower plants.2 Moreover, the presence of the bioactive compounds such as dihyrophenanthrene, phenanthraquione, alkaloids, phenolic compounds and coumarin adds to its medicinal property.4,5 C. aloifolium is used in traditional medicine as tonic as well as in medicine to treat various human diseases and disorders.6,7 Therefore, this orchid species can be a potential candidate in modern medicine system for the development of drugs and therapeutic agent.8 Hence, the plants have a great potential to serve the mankind.
Orchid seed lack endosperm and are hard to germinate in nature. Only a few percent of the microscopic seeds can get a chance to germinate with the help of mycorrhizal association.9 This may also lead to a decline in the orchid population in natural habitat apart from human activities. However, in recent years, overexploitation of the forest and illegal trade have caused a serious threat to the existence of all orchid species.10
Plant tissue culture technique provides the alternate way to conserve the orchid species.11–13 However, in -vitro-grown plants typically orchids suffer both abiotic and biotic stresses during the hardening and field transfer process. In this regard, the P. indica can be used as biological tool for the growth and development, immunity during the process of hardening, and field transfer.14 As the C. aloifolium have become one of the threatened and valuable species for the commercial purpose, the present investigation develops the strategy to conserve the C. aloifolium by exploring the potential of P. indica to be used as a biological tool to enhance the acclimatization process. P. indica is well known for its plant root colonizing activities providing nutrient and defense mechanism to overcome the biotic and abiotic stress. Previous research work shows its ability to promote plant growth of a wide range of plant species belonging to monocot and dicot. Moreover, there are reports about its ability to colonize and promote the growth of the orchid species.15,16 Hence, the present study aims to achieve the interaction of in vitro cultured C. aloifolium with P. indica for the plant growth providing tolerance to overcome the abiotic and biotic stress when transfered to the natural environment.
Result
Plant growth assay
One-month-old C. aloifolium plantlets grown in in vitro condition were used for the interaction with P. indica. After 45 days of interaction, the significant differences in the growth pattern of the uncolonized and colonized plantlet were observed (Figure 1). The plantlet colonized by P. indica attained the highest growth in terms of root and shoot numbers as well as root and shoot length. (Figure 2). The histo-chemical assay of colonized root section showed the presence of clamdospore of the P. indica in the cortex region of the root tissue. Plant growth assay confirms the formation of mycorrhizal association between the fungus and plantlet (Figure 3).
Figure 1.
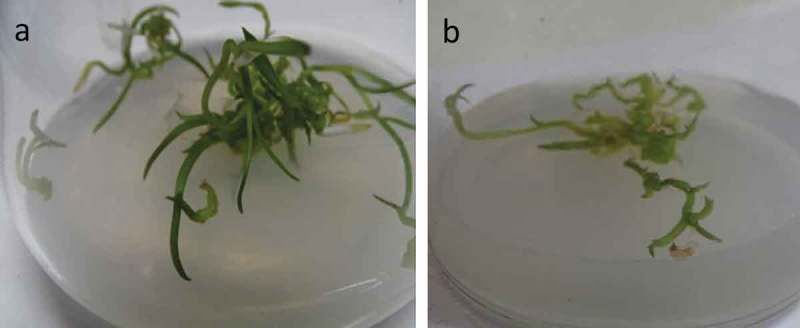
In-vitro plant growth assay of Cymbidium aloifolium colonized by P. indica (a) compared to uncolonized plantlets, control (b) for 45 days of growth assay.
Figure 2.
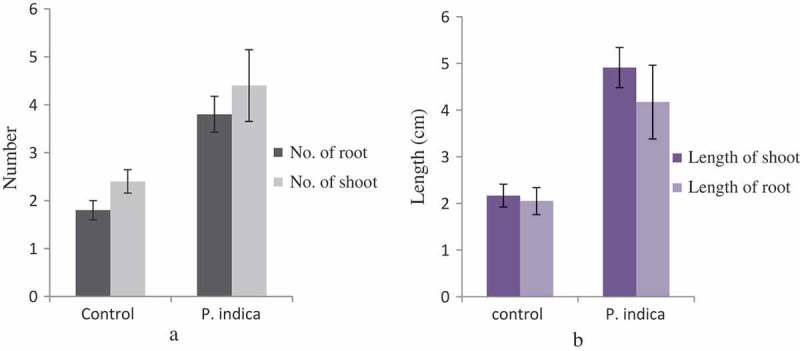
Morphological changes in growth pattern of the P. indica-colonized plantlet versus uncolonized plantlet in terms of mean of roots and shoots number (a) as well as mean of root and shoot length (b). Bar represents mean ± SE (n = 15). The data is significant at the level of P ≤ 0.05.
Figure 3.
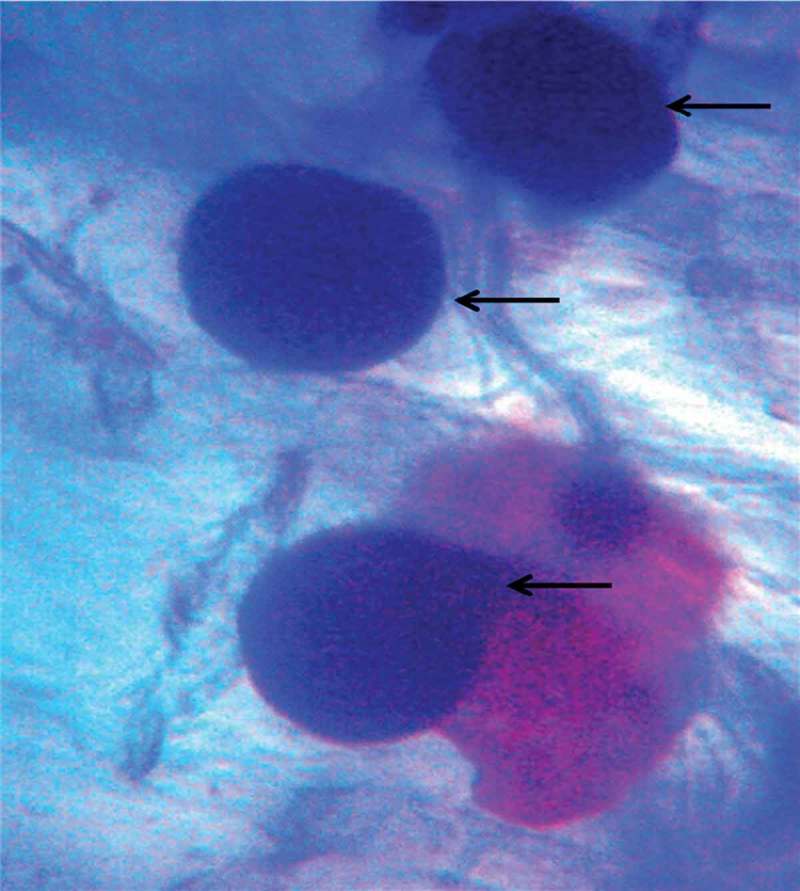
Microscopic view showing the formation of clamdospore in the cortical section of root tissue (arrow indicates the clamdospore formation inside the cortex region of root section).
Comparative characterization of bioactive compounds of uncolonized and colonized plants
The metabolites present in the methanol extract of the uncolonized and colonized C. aloifolium are depicted with their reported biological function in Tables 1 and 2, respectively. The plant extracts were prepared in methanol and filtered by Whatman filter paper. The bioactive compounds present in colonized plant methanol extract as well as in uncolonized plant methanol extract were identified by GC-MS technique. The presence of bioactive compounds in the uncolonized and colonized plant extracts differed significantly. The compounds such as indole acetic acid, ascorbic acid, DL-proline, and andrographolide were detected in the colonized plant extract. These bioactive compounds were responsible to promote the plant growth and development. Ethyl iso-allocholate, mannitol, and hexadecanoic acid were detected in the extracts of uncolonized plants.
Table 1.
Bioactive compound present in methanolic extracts of the control plantlets (uncolonized).
| Peak | Retention time | Name | Mass peak | Base peak | Reported biological function |
|---|---|---|---|---|---|
| 1. | 10.410 | Hexadecanoic acid, methyl ester | 640 | 74.10 | Antimicrobial activity17 |
| 2. | 12.930 | Hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | 588 | 57.15 | Antimicrobial activity17 |
| 3. | 13.580 | D-Mannitol, 1-O-(22-hydroxydocosyl)- | 613 | 73.10 | Role in abiotic and biotic stress18 |
| 4. | 17.850 | Ethyl iso-allocholate | 685 | 55.10 | Apoptotic activity19 |
| 5. | 25.975 | Beta-carotene | 820 | 55.10 | Plant biological proecess such as photosynthesis and antioxidant acitvitities |
Table 2.
Bioactive compound present in methanolic extracts of the P. indica-colonized plantlets.
| Peak | Retention time | Compound name | Mass peak | Base peak | Reported biological function |
|---|---|---|---|---|---|
| 1. | 6.333 | DL-proline, 5-0xo-,methyl ester | 604 | 84.10 | Drought-stress tolerant20,21 |
| 2. | 7.400 | Hexadecanoic acid, methyl ester | 601 | 74.10 | Antimicrobial activity17 |
| 3. | 9.985 | 1H-Indole-3-acetic acid, methyl ester | 644 | 130.15 | Plant growth and development, root initiation and elongation14,15,22 |
| 4. | 10.715 | l-(+)-Ascorbic acid | 637 | 73.10 | Important factor in biochemical pathway, biological processes such as photosynthesis23,24 |
| 5. | 19.895 | Andrographolide | 702 | 107.15 | Metabolites having anticancer and antioxidant activities.25–27 |
Hardening and acclimatization of uncolonized and colonized plantlet
Pre-acclimatization procedures and pot assay were carried out to measure the survival of the colonized plants compared to uncolonized plants. In this regard, different compositiond of cocopeat, gravel and charcoal were considered. The plantlets colonized by P. indica were able to adapt to the natural environment condition overcoming all the abotic and biotic stresses. In the present study, five individual colonized plants and five uncolonized plants (control) were transfered to the field. The substrate compositions used were cocopeat (P): gravel (G): charcoal (C) (PGC) in the ratio of 2:2:1. New roots were formed during hardening, and root and shoot lengths attained higher growth compared to control (Figure 4). The growth of the control plantlet was not significant, and leaves and roots decayed during acclimatization.
Discussion
P. indica is known to show a broad spectrum of root colonizing capacity that includes both dicot and monocot plant species.28,29 P. indica provides resistance toward abiotic and biotic stress to the colonized plant species.30,31 Previous reports clearly explain its unique ability to colonize the roots of several plant species and help in the production of plant phytohormone and growth regulator such as auxin, cytokinin and jasmonic acid.30–32 P. indica promote orchid seed germination as well help in growth and development. The reports on seed germination of Dactylorhiza majalis by P. indica15 and Oncidium plantlet growth promotion activity are the fine examples of P. indica interaction with orchid species.16 There are several examples of the P. indica being a root-colonizing endophytic fungus. The major goal of the present study was to investigate its ability to colonize the in-vitro-grown C. aloifolium with P. indica, contributing to fitness, plant growth, and development during the field trial. The formation of the clamadospore in the cortical cells of root section showed its ability to colonize the plantlet. The ability to colonize the root is beneficial for plants for nutrient uptake. This has been proven in plant growth assay (Figures 1 and 2). The plant growth assay showed its ability to promote the plant growth and development significantly as compared to the control. The present result shows similarity with the previous study of Chinese cabbage plantlet colonized by P. indica.14 The overall metabolomic study of P. indica colonized Chinese cabbage demonstrated the number of metabolites, intermediate product, and their precursor. These metabolites were GABA, oxylipin-family compounds, polysaturated fatty acid, auxin, and its intermediate such as L-tryptophan, indoleacetamide, and indoleacetonitrile. Moreover, they were successful to show the increase in saturated fatty acids and the decreased in unsaturated fatty acids, which is an important phenomenon to combat abiotic and biotic stress.33–35 Whereas the present study shows the ability of the P. indica to produce auxin on MS media supplemented with auxin precursor tryptophan. It indicates the role of P. indica to produce auxin from tryptophan supplement and helps the colonized plant to grow by providing hormones and nutrients effectively. Apart from its ability to synthesize auxin molecules, Table 2 suggests its ability to produce bioactive compound, mainly ascorbic acid, which plays an important role in photosynthesis, biochemical pathway, as well as possesses antioxidant property.23,24 Detection of DL-proline and hexadecanoic acid in the P. indica colonized C. aloifolium shows the ability of the P. indica to provide resistantance against abiotic and biotic stress.17,20 Moreover, P. indica also enhances the medicinal values of the colonized plant. Bioactive compound andrographolide known for its anticancer property was detected in the colonized plant. These evidences show that the P. indica provides fitness in in-vitro plant, promotes plant growth and development, as well as adds medicinal values to the C. aliofolium. Metabolites such as DL-proline and hexadecanoic acid present in P. indica colonized C. aloifoium confirms the ability of P. indica to show drought, fungal infection resistance during hardening, and acclimatization process (Figure 4). The P. indica colonized plantlets were able to adapt easily as compared to control attaining high growth (Figure 5). Apart from this, C. aliofolium has medicinal values, the detection of the ethyl iso-allocholate compound in the extract of in-vitro-grown uncolonized plantlet extract. The presences of ethyl iso-allocholate in the uncolonized plant extract also explains the ability of the plant without the involvement of microbes.
Figure 4.

Acclimatization and hardening of the P. indica-colonized plants and uncolonized plants for 3 months using different compositions of substrate at different ratios. (a) Control. (b)Treated. (c) Control. (d) Treated. (e) Control. (f) Treated. (g) Control. (h) Treated.
Figure 5.
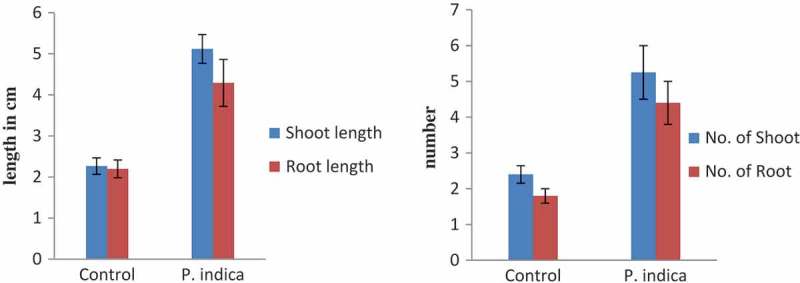
Morphological changes in growth pattern of P. indica-colonized plantlet versus uncolonized plantlet (3-month-old) after acclimatization in terms of mean of roots and shoots number (a) as well as mean of root and shoot length (b). Bar represents mean ± SE (n = 15). The data is significant at the level of P ≤ 0.05.
Conclusion
The data and evidence that we collected from our experiment suggest that P. indica has the ability to colonize the roots of Cymbidium aloifolium, a valuable orchid species, and promotes its growth and development. The bioactive compounds identified in colonized plant play a role in providing tolerance against drought and pathogen attack during field transfer. Therefore, P. indica can be used as a biological means for hardening the in-vitro-grown orchid plantlets to restore the ecology of the threatened orchid species.
Material and method
Establishment of in vitro cultured C. aloifolium
The mature green capsule of C. aloifolium was collected from the Makwanpur district of Nepal. The pod was surface sterilized. The seeds from the pod were then transferred to the well-standardized protocol for its growth on different strengths of Murashige and Skoog (MS) basal media for its germination.
Plant growth assay with P. indica
For the plant growth assay with P. indica, one-month-old plantlets were taken. The small plug of mycelium agar disc of P. indica was introduced near to the seedling on MS media flask supplemented with tryptophan. The growth and development activity of the seedling was monitored for 45 days. The five independent plantlets with three replicates were carried out with respect to the five independent P. indica-untreated seedling (control) to observe the significant colonization and seedling growth promotion activities of the P. indica. The plant growth assay was performed under aseptic conditions for 45 days under a 16 h photoperiod, 25 ± 2°C.
Histochemical analysis of the root sections
Ten random root sections from the fungus colonized plant was taken to investigate the pattern of colonization by fungus as per the method used in the previous work. The root sections were treated with 10% KOH solution and heated at 60°C for 15 mins followed by treatment of 1% HCl solution. Thereafter, the root sections were stained by 0.05% of trypan blue solution for 1 h followed by counterstaining with lacto phenol.36 The root sections were observed under a light microscope.
Comparison of bioactive compound in colonized and uncolonized plant methanolic extract
The in-vitro-grown plantlets uncolonzsed as well as colonized by P. indica were taken after 45 days of their growth. The colonized and uncolonized (control) plantlets were separately ground with the help of mortar and pestle. Thereafter, it was suspended in High Performance Liquid Chromatography (HPLC) grade methanol (Fisher Scientific) for a day to extract bioactive compounds. The methanol extract was then further filtered by whatman filter paper Grade 1:11 µm and taken for chemical profiling by GC-MS technique.37 The compounds were separated by GC technique and identified by MS. The instrument used for the experiment was GC-MS-QP2010 Ultra (Shimadzu Europa GmbH, Germany) instrument fitted with RTX-5MS (30 × 0.25 × 0.10 m) column. All the parameters were subjected. The initial and final temperatures of the instrument were set to 100°C and 250°C, respectively. Helium flow rate was 1 ml min−1 at 0.80 KV of ionization voltage. The splitless mode was set for the sample injection. Mass spectral scan range was set from 30 to 600 (m/z). The obtain chromatogram was then compared with the library of National Institute of Standard and Technology, NIST, US, to identify the bioactive metabolite.
Hardening and acclimatization of uncolonized and colonized plantlets
The transfer of in-vitro-grown plantlet to the natural environment is a very important step in which plantlets suffer high mortality. For orchids, different strategies were followed as previously described.38–42 Pre-acclimatization of the in-vitro-grown plantlet was done in series of pre-acclimatization steps that were followed. The humidity inside the vessel of the in-vitro plantlets was reduced by making holes in the lid and left for 15 days.
For acclimatization, substrates such as cocopeat (P), charcoal (C), and gravel (G) were autoclaved and used. The different composition of the mixtures that were used was as follows.
Cocopeat (P): gravel (G): charcoal (C) = 2:2:1
The five plantlets colonized by P. indica and five uncoloniszd plantlets in three independent replicates were taken and growth was observed for 3 months in natural environmental conditions. In this process, the plantlets were transfered to the each sterilized pot containing the substrate.
Statistical analysis
The plant growth pattern in terms of shoot and root number as well as their length were calculated. The bar diagrams with standard mean and standard error were taken. One-way analysis of variance was done at the significant level of P ≤ 0.05.
Funding Statement
This work was supported by the University Grants Commission, Nepal (NP) [PhD-72/73-S&T-Y-04].
Acknowledgments
The research work was financially supported by University Grants Commission (UGC), Nepal. The authors are thankful to National Centre for Microbial Resource-National Centre for Cell Science, Pune, India, and Centre for Co-operation in Science & Technology among Developing Societies (CCSTDS), India, for facilitating GCMS instrumentation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Author’s contribution
SS conceptualized the research work, performed P. indica and C. aloifolium interaction, GCMS analysis, histochemical microscopy of P. indica-colonized root section and prepared the manuscript. BBT did hardening and acclimatization as well as data recording. KC, PJ, and LS provided in-vitro C. aloifolium protocorms, BP and SP established the plant tissue culture protocol for C. aloifolium. AMS and AV provided P. indica for the experiment. BP overall supervised the entire research work. BP and SP did manuscript editing and revising.
References
- 1.Pant B, Raskoti BB.. Medicinal orchids of Nepal. Himalayan Map House Pvt. Ltd. (Publisher); 2013. http://www.nhbs.com/medicinal_orchids_of_nepal_tefno_189971.html
- 2.Pant B, Paudel MR, Chand MB, Wagner SW.. Treasure troves orchids in Central Nepal. Kirtipur (Kathmandu): Central Department of Botany, Tribhuvan University; 2016. [Google Scholar]
- 3.Pant B, Paudel MR, Chand MB, Pradhan S, Malla BB, Raskoti BB. Orchid diversity in two community forests of Makawanpur district, central Nepal. J Threat Taxa. 2018;10:12523–12530. [Google Scholar]
- 4.Barua AK, Ghosh BB, Ray S, Patra A. Cymbinodin-A, a phenanthraquinone from Cymbidium aloifolium. Phytochemistry. 1990;29(9):3046–3047. doi: 10.1016/0031-9422(90)87138-K. [DOI] [Google Scholar]
- 5.Hossain MM, Sharma M, Pathak P. Cost effective protocol for in vitro mass propagation of Cymbidium aloifolium (L.) Sw.–a medicinally important orchid. Eng Life Sci. 2009;9(6):444–453. doi: 10.1002/elsc.200900015. [DOI] [Google Scholar]
- 6.Medhi RP, Chakrabarti S. Traditional knowledge of NE people on conservation of wild orchids. Indian J Tradit Know. 2009;8:11–16. [Google Scholar]
- 7.Howlader MA, Alam M. Central nervous system depressant effects of the ethanolic extract of Cymbidium aloifolium (L.). J Appl Pharm Sci. 2011;01:60–62. [Google Scholar]
- 8.Shrestha R, Shah S, Pant B. Identification of endophytic fungi from roots of two Dendrobium species and evaluation of their antibacterial property. Afr J Microbiol Res. 2018;12(29):697–704. doi: 10.5897/AJMR2018.8924. [DOI] [Google Scholar]
- 9.Pant B, Shah S, Shrestha R, Pandey S, Joshi PR. An overview on orchid endophytes In: Varma A, Prasad R, Tuteja N, editors. Mycorrhiza - nutrient uptake, biocontrol, ecorestoration. Cham (Switzerland): Springer; 2017. p. 503–524. [Google Scholar]
- 10.Pant B, Poudel MR, Chand MB, Pradhan S, Malla B, Raskoti R. Orchid diversity in two community forests of Makawanpur district, central Nepal. J Threat Taxa. 2018;10:12523–12530. [Google Scholar]
- 11.Pant B. Medicinal orchids and their uses: tissue culture a potential alternative for conservation. Afr J Plant Sci. 2013;7:448–467. doi: 10.5897/AJPS. [DOI] [Google Scholar]
- 12.Pradhan S, Tiruwa B, Subedee BR, Pant B. In vitro germination and propagation ofthreatened medicinal orchid, Cymbidium aloifolium (L.) Sw. through artificial seed. Asian Pac J Trop Biomed. 2014;4:971–976. doi: 10.12980/APJTB.4.2014APJTB-2014-0369. [DOI] [Google Scholar]
- 13.Pradhan S, Regmi T, Ranjit M, Pant B. Production of virus-free orchid Cymbidiumk aloifolium (L.) Sw. by various tissue culture techniques. Heliyon. 2016;2:e00176. doi: 10.1016/j.heliyon.2016.e00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua MDS, Kumar RS, Shyur LF, Cheng YB, Tian Z, Oelmüller R, Yeh KW. Metabolomic compounds identified in Piriformospora indica-colonized Chinese cabbage roots delineate symbiotic functions of the interaction. Sci Rep. 2017;7:9291. doi: 10.1038/s41598-017-08715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varma A, Fekete A, Srivastava A, Saxena AK, Frommberger M, Li D, Tripathi S. Piriformospora indica. Soil Biol. 2013;33:201–219. [Google Scholar]
- 16.Ye W, Shen CH, Lin Y, Chen PJ, Xu X, Oelmüller R, Lai Z. Growth promotion-related miRNAs in Oncidium orchid roots colonized by the endophytic fungus Piriformospora indica. PLoS One. 2014;9(1):0084920. doi: 10.1371/journal.pone.0084920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhary K, Kaushik N. Fungal endophyte diversity and bioactivity in the Indian medicinal plant Ocimum sanctum Linn. PLoS One. 2015;10:e0141444. doi: 10.1371/journal.pone.0141444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoop MHJ, Williamson JD, Mason Pharr D. Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci. 1996;1(5):139–144. doi: 10.1016/S1360-1385(96)80048-3. [DOI] [Google Scholar]
- 19.Malathi K, Ramaiah S. Ethyl Iso-allocholate from a medicinal rice karungkavuni inhibits dihydropteroate synthase in escherichia coli: a molecular docking and dynamics study. Indian J Pharm Sci. 2017;78:780–788. [Google Scholar]
- 20.Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: A review. Plant Signal Behav. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trovato M, Mattioli R, Costantino P. Multiple roles of proline in plant stress tolerance and development. Rend Lincei Sci Fis. 2008;19:325–346. doi: 10.1007/s12210-008-0022-8. [DOI] [Google Scholar]
- 22.Robinson M, Riov J, Sharon A. Indole-3-Acetic Acid Biosynthesis in Colletotrichum gloeosporioides f. sp. aeschynomene. Appl Environ Microbiol. 1998;64:5030–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallie DR. L-Ascorbic acid: a multifunctional molecule supporting plant growth and development. Scientifica. 2013;2013:24. doi: 10.1155/2013/795964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noctor G, Veljovic-Jovanovic S, Foyer CH, Grace S. Peroxide processing in photosynthesis: antioxidant coupling and redox signaling. Philos Trans R Soc B. 2000;355:1465–1475. doi: 10.1098/rstb.2000.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vakil MMA, Mendhulkar VD. Enhanced synthesis of andrographolide by Aspergillus niger and Penicillium expansum elicitors in cell suspension culture of Andrographis paniculata (Burm. f.) Nees. Bot Stud. 2013;54:49. doi: 10.1186/1999-3110-54-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alzaharna M, Alqouqa I, Cheung H-Y, Bhutia SK. Taxifolin synergizes Andrographolide-induced cell death by attenuation of autophagy and augmentation of caspase dependent and independent cell death in HeLa cells. PLoS One. 2017;12:2–e0171325. doi: 10.1371/journal.pone.0171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Zhang J, Yuan L, Lay Y, Wong Y, Lim T, Ong C, Lin Q, Wang J, Hua Z. Andrographolide suppresses MV4-11 cell proliferation through the inhibition of FLT3 signaling, fatty acid synthesis and cellular iron uptake. Molecules. 2017;22:1444. doi: 10.3390/molecules22091444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varma A, Verma S, Sudha SN, Bütehorn B, Franken P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol. 1999;65:2741–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varma A, Sherameti I, Tripathi S, Prasad R, Das A, Sharma M, Bakshi M, Johnson JM, Bhardwaj S, Arora M, et al. The symbiotic fungus Piriformospora indica: review In: Hock B, editor. Fungal associations, the mycota IX. 2nd Berlin (Heidelberg): Springer; 2012. p.231–254. [Google Scholar]
- 30.Xu L, Wu C, Oelmüller R. Zhang W. Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: more questions than answers. Front Microbiol. 2018;9:1646. doi: 10.3389/fmicb.2018.01646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill SS, Gill R, Trivedi DK, Anjum NA, Sharma KK, Ansari MW, Ansari A, Johri A, Prasad R, Pereira E, et al. Piriformospora indica: potential and significance in plant stress tolerance. Front Microbiol. 2016;7:332. doi: 10.3389/fmicb.2016.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirrenberg A, Göbel C, Grond S, Czempinski N, Ratzinger A, Karlovsky P, Santos P, Feussne I, Pawlowski K. Piriformospora indica affects plant growth by auxin production. Physiol Plant. 2007;131:581–589. doi: 10.1111/j.1399-3054.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 33.Falcone DL, Ogas JP, Somerville CR. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 2004;4:17. doi: 10.1186/1471-2229-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu XZ, Huang BR. Changes in fatty acid composition and saturation in leaves and roots of creeping bent grass exposed to high soil temperature. J Am Soc Hortic Sci. 2004;129:795–801. doi: 10.21273/JASHS.129.6.0795. [DOI] [Google Scholar]
- 35.Williams JP, Khan MU, Mitchell K, Johnson G. The effect of temperature on the level and biosynthesis of unsaturated fatty-acids in diacylglycerols of Brassica-Napus leaves. Plant Physiol. 1988;87:904–910. doi: 10.1104/pp.87.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayan OP, Verma N, Singh AK, Oelmüller R, Kumar M, Prasad D, Kapoor R, Dua M, Johri AK. Antioxidant enzymes in chickpea colonized by Piriformospora indica participate in defense against the pathogen Botrytis cinerea. Sci Rep. 2017;7:13553. doi: 10.1038/s41598-017-12944-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah S, Shrestha R, Maharjan S, Selosse M-A, Bijaya PB. Isolation and characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants. 2019;8:5. doi: 10.3390/plants8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherif NA, Benjamin JHF, Kumar TS, Rao MV. Somatic embryogenesis, acclimatization and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl., an endangered terrestrial jewel orchid. Plant Cell Tissue Organ Cult. 2018;132:303–316. doi: 10.1007/s11240-017-1330-4. [DOI] [Google Scholar]
- 39.Da Silva JAT, Hossain MM, Sharma M, Dobránszki J, Cardoso JC, Songjun ZENG. Acclimatization of in vitro-derived Dendrobium. Hortic Plant J. 2017;3:110–124. doi: 10.1016/j.hpj.2017.07.009. [DOI] [Google Scholar]
- 40.Venturieri GA, Arbieto EAMD. Ex-vitro establishment of Phalaenopsis amabilis seedlings in different substrates. Acta Sci-Agron. 2011;33(3):495–501. doi: 10.4025/actasciagron.v33i3.3950. [DOI] [Google Scholar]
- 41.Cha-Um S, Puthea O, Kirdmanee C. An effective in-vitro acclimatization using uniconazole treatments and ex-vitro adaptation of Phalaenopsis orchid. Sci Hortic. 2009;121:468–473. doi: 10.1016/j.scienta.2009.02.027. [DOI] [Google Scholar]
- 42.Dutra D, Johnson TR, Kauth PJ, Stewart SL, Kane ME, Richardson L. Asymbiotic seed germination, in vitro seedling development, and greenhouse acclimatization of the threatened terrestrial orchid Bletia purpurea. Plant Cell Tissue Organ Cult. 2008;94:11–21. doi: 10.1007/s11240-008-9382-0. [DOI] [Google Scholar]


