Abstract
Recombinant adeno-associated viral (rAAV) vectors are a promising tool for therapeutic gene delivery to the brain. However, the delivery of rAAVs across the blood-brain barrier (BBB) and entry into the brain remains a major challenge for rAAV-based gene therapy. To circumvent this limitation, transcranial MRI-guided focused ultrasound (MRIgFUS) combined with intravenously injected microbubbles has been used to transiently and reversibly increase BBB permeability in targeted brain regions. Systemic administration of rAAVs at the time of sonication with focused ultrasound (FUS) facilitates the passage of rAAVs through the BBB and into the brain parenchyma. We and others have demonstrated that FUS-mediated rAAV delivery to the brain results in efficient transduction and transgene expression in vivo. Using this approach, the dose of intravenously injected rAAV variants that can cross the BBB can be reduced by 100 times, achieving significant transgene expression in the brain parenchyma with reduced peripheral transduction. Moreover, this strategy can be used to deliver rAAV variants that do not cross the BBB from the blood to selected brain regions. Here, we provide a detailed protocol for FUS-induced BBB permeability for targeted rAAV delivery to the brain of adult mice and rats.
Keywords: Adeno-associated virus, Gene therapy, Focused ultrasound, Blood-brain barrier, Gene therapy, Viral vector delivery
1. Introduction
Strategies are being developed to deliver therapeutic genes to the brain with the potential to treat patients affected by neurological disorders [1]. Extensive evidence from preclinical research and clinical studies suggests that targeted gene therapy is a promising approach to enhance treatment efficacy and reduce the toxicity of therapeutic drugs for a wide variety of neurological conditions including neurodegenerative diseases, brain tumors, stroke, and epilepsy [2, 3]. Recombinant adeno-associated virus (rAAV) vectors have emerged as an attractive gene delivery vehicle for the brain [1–3]. Recent developments in gene therapy [4–6] include rAAVs that, when given at high dosages, have the capability to cross the blood-brain barrier (BBB) [7–11]. For example, rAAV9 was recently used in the first life-saving treatment ofneurodegeneration in infants with spinal muscular atrophy [12]. For neurodegenerative disorders of the brain, such as Alzheimer’s disease (AD), the delivery of rAAVs and their genetic cargo has to date been achieved by invasive intraparenchymal injection to desired brain regions [13, 14]. In recent years, several companies have joined forces to develop innovative gene-based immunotherapy approaches for AD. These partenerships could be transformative, surmounting the limitations of current systemic biweekly injections of therapeutic antibodies targeting AD pathology, in which only a small amount penetrates the brain. A single treatment with gene therapy can lead to long-term production of therapeutic transgenes and provide sustained clinical benefits.
Current limitations in the design of rAAV-based gene therapy for diseases of the central nervous system (CNS) include the restricted access of rAAVs to the brain and spinal cord when administered systemically [2]. This can be overcome, when appropriate, with high dosages of rAAVs that are intrinsically capable of crossing the BBB. An alternative approach is to use focused ultrasound (FUS) disruption of the BBB, thereby enhancing delivery of intravenously injected rAAV vectors to the CNS (Fig. 1). Focused ultrasound, combined with microbubbles and guided by magnetic resonance imaging (MRI), has been applied to open the BBB in a safe, noninvasive, and reversible manner in targeted areas ofbrain in animal models [15] and, most recently, in AD patients [16]. MRI-guided FUS (MRIgFUS)-mediated delivery of rAAVs to targeted areas of the brain and spinal cord can be achieved with systemic doses which are up to 100 times lower than those conventionally used with rAAVs capable of crossing the BBB [11, 15, 17–20]. Intravenous injections of rAAVs, with and without FUS, can lead to peripheral transduction, limiting CNS delivery and treatment efficacy and potentially generating adverse side effects. Strategies for mitigating peripheral rAAVs transduction [21] and expression [22] can be deployed as required.
Fig. 1.
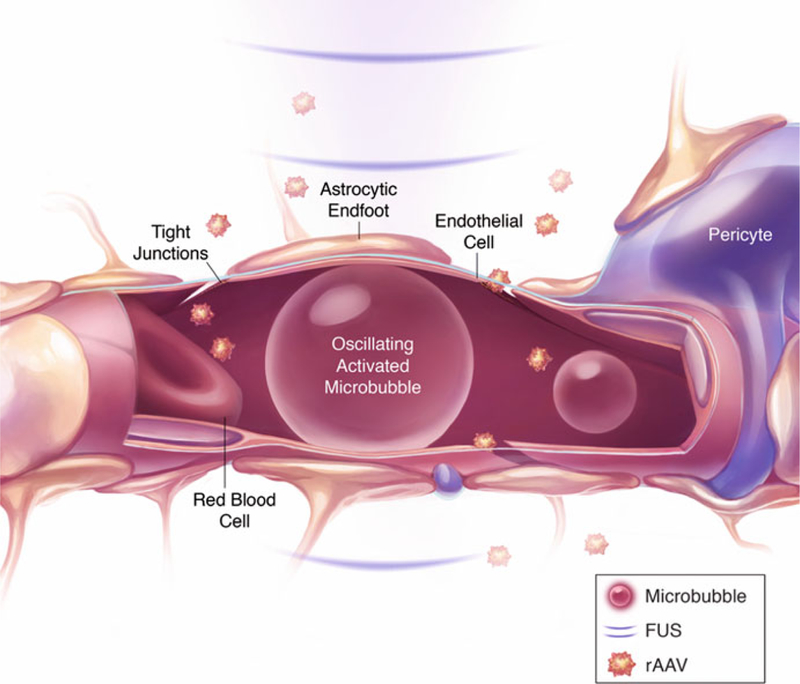
FUS-mediated delivery of rAAVs across the BBB. Circulating microbubbles in the brain vasculature are activated and oscillate in response to ultrasonic energy delivered by FUS. The interaction of FUS with microbubbles leads to an increase in BBB permeability, which is localized and temporary, allowing rAAVs injected intravenously to enter FUS-targeted brain regions. Reproduced with permission from Sunnybrook Research Institute
FUS, combined with intravenously injected microbubble contrast agents circulating in the vasculature, is a noninvasive drug delivery strategy that results in local, reversible, and safe increases in BBB permeability [23]. Under these conditions, circulating microbubbles are stimulated by ultrasonic energy, generating stable oscillations. These oscillations induce mechanical stress and also transient downregulation of the tight junction proteins between endothelial cells that play a role in BBB permeability [24, 25]. MRI-guided FUS is used to achieve precise targeting of focal spots and to visualize the induction of BBB permeability using MRI contrast agents [26]. FUS-induced BBB permeability in the presence of microbubbles has been used to deliver a wide variety of therapeutic agents in preclinical animal models, such as drugs, antibodies, cells, plasmid DNA, viral vectors, and other drug vehicles [15, 27, 28]. Using MRI-guided FUS, our group has demonstrated targeted gene delivery to the mouse brain after a single systemic injection of rAAV9 vectors. Efficient transduction of neurons and glia in the brain was reported using a low systemic dose of 2.5 × 109 vector genomes (vg) per g [20]. Another study reported efficient rAAV2 brain transduction using a systemic dose of 1 × 109 vg/g [29]. In addition to FUS-facilitated viral vector delivery for gene therapy, this technique has also been developed for optogenetic applications. Recombinant AAVs encoding light-activated channels delivered by this approach allow for noninvasive manipulation of neuronal activity, thereby avoiding damage caused by direct brain infusion. No inflammatory response or long-term tissue damage was reported following FUS-facilitated delivery of rAAV9 encoding the light-sensitive protein Channelrhodopsin-2 (ChR2) to the mouse brain [30].
FUS-mediated gene delivery to the CNS is a promising tool for basic neuroscience research and for providing effective and long-lasting treatments for neurological disorders. Here, we provide a detailed method for the efficient delivery of systemically administered rAAV vectors to targeted brain areas in rodents using MRIgFUS-mediated BBB disruption.
2. Materials
2.1. Animals
Researchers must comply with institutional animal use and other applicable policies, guidelines, and regulations (see Note 1).
Adult mice or rats balanced for sex and weight across experimental groups (see Note 2).
Housing for both mice and rats: individual micro-isolator cages on HEPA filtered vented racks with bed-o-cob bedding and enrichment material including nestlets, paper-based material, and tunnels.
Commercially available irradiated laboratory rodent diet, soft food (food pellet placed in water), and Nutra-Gel diet (Bio-Serv).
Chlorinated RO water supplied through an automated watering system on the rack or in autoclaved water bottles.
A thermal blanket (e.g. circulating water warming pad) or forced warm air (e.g. Bair Hugger animal warming unit) for thermoregulation.
2.2. rAAV Preparation
rAAV solution diluted in sterile 0.9% NaCl (see Note 3).
A 250 μL Hamilton syringe with a 22G needle (Hamilton #1725RN).
1% bleach solution for disinfection of materials.
Class IIA2 biosafety cabinet.
2.3. FUS-Induced BBB Disruption
22G angiocatheter.
1 mL insulin syringes with 27G × ½” needle.
0.9% NaCl sterile IV solution.
Depilatory cream.
Cotton swabs.
Alcohol pads.
Gauze.
Surgical tape.
Sterile surgical instruments.
Ophthalmic ointment.
Small animal hair clipper.
An anesthesia machine with a rodent circuit including an induction chamber and anesthesia nose cone for induction and maintenance of anesthesia during catheterization. Use isoflurane with 50% medical air and 50% oxygen as carrier gases.
A standard animal 7T MRI scanner (Bruker) is used for imaging. Standard clinical 1.5T or 3T scanners can also be used for these studies.
MRI body coil (Bruker, 86 mm Quad SN37).
MRI surface coil (Bruker, 86 mm Quad Receive).
MR-compatible isoflurane anesthesia machine including an anesthesia nose cone for isoflurane anesthesia during MRI with 100% medical air carrier gas (see Note 4).
- MR-Compatible Focused Ultrasound (FUS) system. The FUS Instruments RK-300 system, which is designed to work with the small-bore Bruker 7T MR scanner, includes the following components:
-
(a)Spherically focused transducer. In rats, FUS-induced BBB permeability is achieved using a 551.5 kHz transducer (focal number = 0.8, external diameter = 75 mm, internal diameter = 20 mm). A 1.68 MHz transducer is used for experiments carried out in mice (focal number = 0.8, external diameter = 75 mm, internal diameter = 20 mm).
-
(b)Function generator (Agilent 33220A).
-
(c)Scope card (14 bit Alazar Tech ATS460).
-
(d)Radio frequency amplifier (NPTECH NP2519 50W).
-
(e)Power meter.
-
(f)Transducer matching circuit.
-
(g)Three-axis transducer positioning system [31].
-
(h)Polyvinylidene difluoride (PVDF) or lead zirconate tita-nate (PZT) hydrophone.
-
(i)Acoustic pressure feedback controller [32].
-
(j)Plastic tank filled with degassed, deionized water.
-
(k)MR-compatible plastic sled with anesthesia nose cone. The sled is used to transfer the animal between the MR scanner for imaging and the FUS system to induce BBB permeability. The relative position ofthe sled between the MR and FUS system is registered in the calibration stage (see Subheading 3.3.2). The sled contains a fixture, comprising two Kapton polyimide membranes filled with degassed, deionized water, which sits in the degassed, deionized water tank to provide good ultrasound coupling to the animal brain.
-
(a)
Ultrasound gel (Wavelength CL, National Therapy Products Inc.).
Ultrasound phantom.
DEFINITY microbubbles (0.02 mL/kg, Lantheus Medical Imaging) stored at 4 °C. For BBB disruption, similar results can be achieved with other microbubble contrast agents (see Note 5).
Gadolinium-based MRI contrast agent, Gadovist (0.1 mL/kg, Schering AG), stored at room temperature.
1 mL insulin syringes with 27G × V” needles and 18G blunt-end needles.
Sterile 0.9% NaCl. Mice receive a 0.2 mL saline flush and rats receive a 0.5 mL saline flush after each injection of rAAV, microbubbles, or MRI contrast agent.
2.4. Animal Recovery
1 mL insulin syringes with 27G × ½” needles.
Enrofloxacin antibiotic (2.5 mg/kg Baytril).
Opioid analgesic (0.05 mg/kg Buprenorphine).
Silver sulfadiazine cream (Flamazine).
Recovery cages with paper bedding.
Small animal heat lamp.
3. Methods
3.1. Animal Preparation
It is recommended that group-housed rodents be separated to individual cages for at least 1 week prior to treatment.
Rodents should be handled at least 3 days prior to treatment. Handling twice a day for 5 min decreases anxiety levels and improves the animal’s response to the experimental procedure.
Inject 1 mL of sterile saline subcutaneously the day before sonication and less than 45 min before sonication to prevent dehydration.
3.2. rAAV Preparation
Prepare the viral working solution on the same day or 1 day in advance of FUS treatment. Handle the viral solution on ice or store at 4 °C.
rAAV preparation must be carried out in a class IIA2 biosafety cabinet. Clean the workstation with 1% bleach solution prior to all procedures.
Stock rAAV solution should be diluted such that the maximum volume of all intravenous injectables does not exceed 25 mL/kg for the heaviest mouse in the experimental cohort. Similar calculations should be performed for dilution of stock rAAV solution in rat studies using a maximal dose volume of 20 mL/kg of body weight (see Note 6).
Fill the Hamilton syringe with rAAV solution. Use a different syringe for each rAAV vector if multiple vectors are being tested.
All materials that come into contact with rAAV solution (e.g., tips, tubes) should be placed in 1% bleach solution then disposed in biohazard containers as appropriate. Clean instruments with 1% bleach solution, rinse with distilled water, then rinse with alcohol (see Note 7).
3.3. FUS-Induced BBB Disruption
When using the RK-300 FUS system, which is designed to work with the small-bore Bruker 7T MRI, the FUS procedure is performed outside the MR room. An MR-compatible, spatially registered sled is used to transfer the animal between the MR room and the FUS system (see Note 8).
3.3.1. Ultrasound System Setup
The experimental system setup is presented in Fig. 2a.
The ultrasound transducer should be placed within a plastic tank of degassed, deionized water, and mounted in a mechanical arm holder. Movement of the transducer arm is controlled by a motorized positioning system.
Position the MRI-compatible sled on the plate covering the water tank.
Fig. 2.
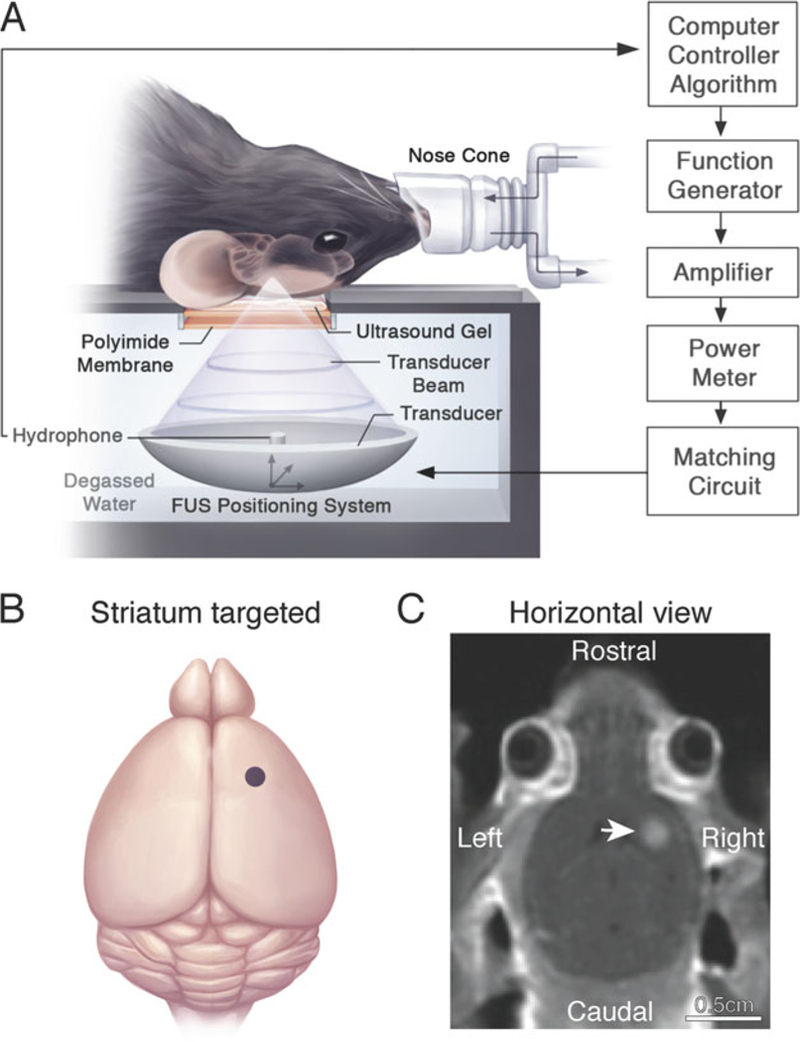
MRIgFUS-mediated BBB permeability in the unilateral mouse striatum. (a) The animal is placed in the supine position on an MRI-compatible sled. The skull is coupled to a polyimide membrane with ultrasound gel. A second polyimide membrane is coupled to a degassed, deionized water tank containing the transducer and hydrophone positioned in the center of the transducer. Sonication parameters and procedures are described in Subheading 3.3. (b) Relative neuroanatomical location of the FUS focal spot targeted to the striatum (indicated by a purple dot). Target locations in the brain are selected on axial T2-weighted MR images. (c) Post-sonication, a contrast-enhanced T1-weighted MR image is used to visualize the entry of an MRI contrast agent, Gadovist, in the sonicated mouse striatum (indicated by a white arrow) and confirm BBB opening in the targeted location. Increased voxel intensity in the sonicated region is proportional to the extent of BBB permeability and amount of virus delivered into the brain. Reproduced with permission from Sunnybrook Research Institute
3.3.2. Focus Finding
Register the position of the transducer focal spot to the MRI-coordinate system. Align the transducer focal spot directly underneath the polyimide membrane fixture on the MRI-compatible sled using the motorized positioning system.
At a low power level (0.5 W), aim the ultrasound beam at the water surface. The focal spot be visible as a fountain in the water.
Secure a plastic focus finding marker at the focal spot (directly above the fountain in the water) on the sled.
Remove the sled from the ultrasound setup and place on the MRI bed for imaging.
Perform a tri-pilot imaging sequence to image the focus finder marker.
Record the MRI coordinates of the marker (Left/Right, Anterior/Posterior, and Superior/Inferior). Use this location as a reference for targeting the brain.
Using an ultrasound phantom positioned on the sled and coupled with ultrasound gel, ensure that the reflected power is less than 10% on the power meter reading.
Analyze the fast Fourier transform (FFT) of the hydrophone data captured at 20 MHz sampling frequency. No significant peaks other than the driving frequency and its harmonics should be visible, and the baseline noise floor should be lower than the pre-calibrated level.
3.3.3. Tail Vein Catheter Insertion
Move the animal to a procedure room located adjacent to the MRI suite to reduce transit time for the anesthetized animal. Tail vein catheterization should be performed aseptically in a Class IIA2 biosafety cabinet.
Provide external warmth to the animal cages for 30 min before starting the procedure using forced warm air at 40 °C. Cover the cages with a large sheet to help maintain heat inside the cages (see Note 9).
Weigh the animal to determine the volume of injectables that it will receive during the experiment.
Place the animal inside the anesthesia induction chamber and adjust the isoflurane concentration to 5%, with 50% medical air and 50% oxygen as carrier gas. When the animal is anesthetized, remove it from the induction chamber.
Insert the nose of the animal into the anesthesia nose cone and reduce the isoflurane concentration to 2% or to effect.
Place the animal on a circulating warm water blanket to maintain body temperature at 37 °C.
Apply ophthalmic ointment to the eyes.
Shave the fur on top of the skull, spanning an area from the neck region (between the ears) to the frontal portion of the head (between the eyes), using a small animal clipper followed by application of depilatory cream. Rinse off the depilatory cream thoroughly with sterile saline. Clean the shaved area with gauze and an alcohol wipe (see Note 10).
Swab the tail with an alcohol wipe. This also increases visibility of the tail vein.
Locate the tail vein and insert the angiocatheter into the vein. If the needle is in the vein, blood will be visible in the hub of the catheter when the needle is removed.
Remove the needle from catheter and leave the plastic hub inside the tail.
Secure the catheter hub to the tail using surgical tape. Firmly tape the wood stick of a cotton swab applicator to the tail near the catheter hub to facilitate multiple injections during the experiment.
3.3.4. Animal Positioning
Bring the anesthetized animal to the MRI suite, and place the animal on the MRI-compatible sled in a supine position such that head of the animal contacts the Kapton polyimide film.
Position the anesthetized animal on the sled by placing its teeth over the incisor bar and sliding the adaptor forward until the nose cone is stabilized on the animal’s nose. The nose cone should be attached to tubing that leads to an anesthesia machine, delivering 2% isoflurane with 100% medical air as carrier gas (see Note 4). Maintain anesthesia at this level for the duration of the experiment. The nose cone should be connected to tubing in such a way that the entire sled can be easily connected and separated while the head position remains fixed.
Apply ultrasonic gel between the head and the membrane on the sled.
Firmly attach the head and body of the animal to the sled with surgical tape.
Cover the animal with a warming bag.
Place the animal (attached to the sled) into the MRI sliding table and connect the nose cone to an isoflurane anesthesia machine. Slide the table slowly into the MRI scanner.
Perform a tri-pilot scan to ensure good head positioning and water coupling (see Note 11). The specific acquisition parameters for all MRI scans are listed in Table 1.
Perform an axial T2-weighted FSE sequence.
Perform a baseline axial Tl-weighted FSE sequence.
Remove the animal (attached to the sled) from the MRI scanner and position the sled on top of the ultrasound setup. Reconnect the nose cone to an isoflurane anesthetic machine.
Select the ultrasound target locations from the axial T2-weighted FSE scan (see Note 12). To target a single focal spot in the striatum, we selected coordinates as shown in Fig. 2b, c. For unilateral treatments, ensure that the orientation of the axial MR image used for targeting corresponds to desired brain side to be treated. Multiple sonication spots should be at least 1 mm apart so that the focal spots do not overlap.
Table 1.
Parameters used for MR imaging
| MRI Parameters | Rat |
Mouse |
||||
|---|---|---|---|---|---|---|
| Tri-pilot | T1w | T2w | Tri-pilot | T1w | T2w | |
| Sequence type | Flash | RARE | RARE | Flash | RARE | RARE |
| Echo time (ms) | 3 | 10 | 75 | 3 | 10 | 75 |
| Repetition time (ms) | 200 | 500 | 4000 | 200 | 500 | 4000 |
| RARE factor | n/a | 2 | 10 | n/a | 2 | 10 |
| Averages | 1 | 3 | 2 | 1 | 3 | 4 |
| Field of view (mm) | 60 | 50 | 50 | 60 | 40 | 40 |
| Matrix size | 256 | 150 | 200 | 256 | 160 | 160 |
| Slice thickness (mm) | 1 | 1.5 | 1.5 | 1 | 1.5 | 1.5 |
3.3.5. Sonication
Start the sonication using the following parameters: 10 ms burst length, 1 Hz pulse repetition, and 120 s sonication duration. A complete list of all sonication parameters is provided in Table 2.
Increase the applied acoustic pressure in a stepwise manner with each burst. For the 551 kHz transducer, use an initial acoustic pressure of 0.128 MPa and increase incrementally by0.008 MPa after each burst. For the 1.68 MHz transducer, use a starting pressure of 0.25 MPa and increase incrementally by 0.25 MPa. Acoustic emissions are recorded by the hydrophone.
Acoustic emissions recorded during each burst using the hydrophone are processed with a real-time algorithm that detects subharmonic or ultraharmonic frequencies to adjust treatment pressures. Once these emissions are detected, the acoustic pressure is reduced to 50% for the duration of the sonication. Use ultraharmonic emissions as a threshold for the 551 kHz transducer, and use subharmonic emissions as a threshold for the 1.68 MHz transducer.
Table 2.
Parameters used for FUS-induced BBB permeability
| Parameters | Rat | Mouse |
|---|---|---|
| Frequency (MHz) | 0.55 | 1.68 |
| Length of sonication (s) | 120 | 120 |
| Duty cycle (%) | 1 | 1 |
| Pulse repetition frequency (Hz) | 1 | 1 |
| Burst duration (ms) | 10 | 10 |
| Microbubble type | DEFINITY | DEFINITY |
| Microbubble dose (mL/kg) | 0.02 | 0.02 |
| Microbubble size (pm) and dispersion | 1.1–3.3 | 1.1–3.3 |
| Passive cavitation detection | PVDF hydrophone | PZT hydrophone |
| Sampling rate (MHz) | 20 | 20 |
| Detection frequency (kHz) | 825 | 840 |
| Emissions target (pressure ratio) | 0.5 | 0.5 |
| Starting acoustic pressure (MPa) | 0.128 | 0.25 |
| Acoustic pressure increment (MPa) | 0.008 | 0.025 |
| Contrast enhancement (imaging detection) | Gadovist (0.1 mL/kg) | Gadovist (0.1 mL/kg) |
3.3.6. Microbubble Administration
Immediately prior to sonication, activated DEFINITY microbubbles must be diluted with saline to evenly distribute microspheres. Slowly withdraw activated DEFINITY microbubbles from the stock suspension using an 18G blunt needle attached to a 1 mL syringe. For rats, dilute 0.1 mL of DEFINITY stock with 0.9 mL of saline, and inject 0.2 mL/kg of the diluted solution (equivalent to 0.02 mL/kg of undiluted DEFINITY) via the tail vein. For mice, dilute 0.02 mL of DEFINITY stock in 0.98 mL of saline and inject 1 mL/kg of diluted DEFINITY solution (equivalent to 0.02 mL/kg of undiluted DEFINITY) intravenously.
The acoustic feedback controller records baseline emissions for 10 s. Therefore, DEFINITY microbubbles should be injected 10 s after the start of sonication.
Inject saline to flush the microbubbles through the catheter (0.2 mL for mice, 0.5 mL for rats).
For additional sonications (in another anatomical location), wait 5 min between microbubble injections. This ensures that the previously injected microbubbles are cleared from the bloodstream.
3.3.7. rAAV Delivery
Keep the Hamilton syringe with rAAV solution on ice for the duration of the experiment.
Administer rAAV immediately after microbubble injection. For multiple sonication locations, we recommend administering rAAV after the first microbubble injection, and with the start of the first sonication.
Place Hamilton syringe into the tail vein catheter hub and inject the viral solution slowly.
Inject saline to flush rAAV through the catheter (0.2 mL for mice, 0.5 mL for rats).
3.3.8. Confirm FUS-Induced BBB Permeability
For rats, inject 0.1 mL/kg of gadolinium-based MRI contrast agent (Gadovist) at stock concentration through the tail vein catheter hub. For mice, dilute 0.1 mL of Gadovist stock in 0.9 mL of saline in a 1 mL syringe and inject 1 mL/kg of the diluted solution (equivalent to 0.1 mL/kg of undiluted Gadovist).
Inject saline to flush the contrast agent through the catheter (0.2 mL for mice, 0.5 mL for rats).
Move the animal to the MRI scanner and acquire a T1-weighted FSE image.
The increase in signal intensity indicates influx of the MRI contrast agent at sonicated regions, and is proportional to both the degree of BBB opening and the amount of rAAV delivered into the brain (Fig. 2c).
3.3.9. Recovery
Detach the rodent from the sled and return it to the animal procedure room.
Let the animal recover from anesthesia in a recovery cage on paper-based bedding and position the heat lamp nearby. The recovery cages should be placed on a circulating water blanket.
Gently remove the tail vein catheter and apply pressure with gauze until blood stops flowing from the puncture site.
Reapply ophthalmic ointment, clean the shaved head with an alcohol wipe, and apply Flamazine.
Inject the animal subcutaneously with enrofloxacin (2.5 mg/kg, Baytril) and Buprenorphine (0.05 mg/kg).
The animal must be continuously monitored until locomotion, breathing rate, and behavioral activities are normal.
Once the animal is fully recovered, return the animal to the animal housing room and provide soft food and Nutra-Gel.
3.4. Post-FUS Handling
Provide soft food and Nutra-Gel for 2 days posttreatment (see Note 13).
Inject Buprenorphine for 2 days post-treatment to reduce pain and discomfort from the procedure.
Inject 1 mL of saline subcutaneously for at least 2 days post-treatment to prevent dehydration.
Give antibiotics (enrofloxacin) for at least 2 days post-treatment to prevent infection.
Weigh the animals and monitor their nesting activity in their home cage post-experiment. These observations can also provide information about health and welfare of the animals.
Assess transgene expression after FUS-mediated rAAV delivery (see Notes 14 and 15).
4. Notes
All animal work was approved by the Sunnybrook Research Institute Animal Care Committee and was performed in compliance with the Canadian Council on Animal Care Policies & Guidelines and the regulatory requirements of the Animals for Research Act of Ontario.
It is important to include sex-matched experimental groups in gene therapy studies. Emerging evidence suggests there are sex differences with respect to systemic AAV gene transfer efficiency in the rodent brain [33, 34]. For example, Maguire et al. reported increased transgene expression in adult female mice compared to male mice after intravenous injection of AAV9 encoding the firefly luciferase (Fluc) and green fluorescent protein (GFP) reporter genes [33].
It is recommended to prepare a stock solution of rAAV from which three or more concentrations can be tested, in order to establish the dose required for transgene expression in FUS-treated areas [20]. We recommend first testing three doses (a given concentration of rAAV, 10 × more, and 10 × less), and then testing additional doses as needed.
During MRIgFUS BBB disruption, animals are anesthetized using isoflurane gas with 100% medical air. Isoflurane anesthesia with oxygen carrier gas has been shown to significantly reduce the amount of BBB opening, evaluated using contrast-enhanced MRI, when compared with medical air [35, 36]. Previous studies demonstrate that the circulation time for perfluo-rocarbon microbubbles is greatly decreased when oxygen is used as the carrier gas relative to medical air [37, 38]. As an alternative, intraperitoneal injections of ketamine and xylazine can be used for anesthesia.
There are several commercially available microbubble contrast agents approved for clinical use that have been applied for safe and localized induction of BBB permeability. The most widely used microbubble contrast agents are composed of either lipid (DEFINITY) or human serum albumin (Optison, GE Health-care) shells filled with the perfluorocarbon gas Perflutren. DEFINITY and Optison microbubbles produce nearly equivalent results with respect to the extent of BBB permeability [39]. It is also important to note that the safety profile of FUS-induced BBB permeability is dependent on the micro-bubble dose administered. A range of DEFINITY doses have been described as safe and effective for BBB opening. For clinical imaging using DEFINITY as a contrast agent, 20 μL/kg is the maximal dose recommended by the manufacturer [40], which has been shown to successfully increase BBB permeability following FUS in rodents [39, 41]. The first clinical trial in AD patients used DEFINITY at a dose of 4 μL/kg for FUS-mediated BBB opening in humans [16]. Using a clinical imaging dose of DEFINITY microbubbles for FUS-mediated BBB permeability, McMahon et al. reported no acute inflammatory response, hemorrhage, or edema following treatment. In contrast, using a higher microbubble dose (tenfold greater than the dose applied for clinical imaging), significant neuroinflammation and tissue damage including edema, hemorrhage, neutrophil infiltration, and cell death were observed following FUS, as reported in previous studies [42–44].
- For intravenous injections, the maximum injectable volume for a mouse is 25 mL/kg per day administered slowly [45]. During the MRIgFUS experiment, DEFINITY, Gadovist, and saline injections are all administered through the tail vein. Thus, for a 30 g mouse, this leaves a volume of about 90 μL for virus injection. If the injection volume of the viral working solution is less than 20 μL, catheter-associated variability may affect the delivered dosage in the bloodstream. For example, in a recent study using mice, working viral solutions were calculated based on the following values:
-
(a)rAAV stock titer: 1.4 × 1010 vg/μL
-
(b)Intravenous injection dose: 3.0 × 109 vg/g
-
(c)Number of mice: 6
-
(d)Average weight: 27 g
-
(e)Heaviest weight: 30 gInjecting the heaviest mouse with the maximum volume (90 μL) at a dose of 3.0 × 109 vg/g required a virus concentration of 1.0 × 109 vg/μL, and thus the stock solution was diluted 14-fold in sterile 0.9% NaCl to prepare the working solution. 500 μL of working solution was prepared for this experiment. Similar calculations can be applied to prepare diluted virus solution for rat studies. The maximum injectable volume for a rat is 20 mL/kg daily [45].
-
(a)
If exposure to bleach is not recommended for disinfecting instruments by the manufacturer, clean supplies with 100% acetone, rinse with distilled water, and then rinse with 100% ethanol.
For FUS systems designed to work with whole-body MR scanners, such as the FUS Instruments RK-100, the FUS procedure can be carried out directly inside the MR scanner. This procedure is described in an earlier published protocol [26].
Alternatively, heaters, recirculating warm water blankets, or heat lamps (at a safe distance from the animal) are all effective means of providing warmth. It is important to keep animals warm throughout the procedure. This improves blood circulation, which helps to visualize the tail vein and install the catheter. It also improves tolerance to anesthesia during the procedure and recovery from anesthesia. Increasing blood flow is also important for efficient transport of injectables from the bloodstream to the brain vasculature.
Proper animal preparation is a critical aspect of this procedure. The hair on the animal’s head must be removed completely to avoid attenuating the ultrasound beam.
Reposition the head if necessary and repeat the localizer scan to provide optimal acoustic coupling between the transducer and desired target brain region.
Previous studies have demonstrated the feasibility of rAAV delivery using FUS targeted to the cortex, hippocampus, and striatum in the rat and mouse brain. The focal sizes of BBB opening generated by the two transducers typically used in our experiments are described here. In water, for 550 kHz, the full width at half maximum intensity (FWHM) of pressure is approximately 3 mm in diameter laterally and 15 mm along the beam. For 1.6 MHz, FWHM is less than 1 mm laterally and 3–4 mm along the beam. For FUS-induced BBB permeability, we use only peak pressure, and thus the size of the actual focal opening in the lateral dimension is normally smaller than the FWHM. Along the beam, however, FUS-induced BBB disruption tends to be wider due to standing wave formation in close proximity to the rodent skull. The spatial resolution of FUS-induced BBB opening in the axial and lateral dimensions using the 1.6 MHz transducer can be visualized by the distribution of GFP expression after targeted delivery of rAAV9-CMV-GFP to the mouse striatum using MRIgFUS as shown in Fig. 3. In contrast to a single focal spot, FUS has also been applied across several spots of the brain and spinal cord for broad CNS AAV delivery [19, 20].
In some instances, this feeding period with soft food and Nutra-Gel may need to be extended, such as for rodent strains that have low body weight and for animals that lose more than 20% of body weight after the procedure. If substantial weight loss is observed, we recommend providing soft food and Nutra-Gel for a week or the entire survival duration after treatment.
The survival time of animals after treatment is variable and depends on several factors including the nature of the rAAV vector, the target cells, and the objectives of the study. The duration of time necessary to detect rAAV-mediated gene expression is highly dependent on the type of virus used. Some rAAVs provide fast, robust expression within 1 week, while others require 4 weeks to reach maximum expression in brain [20, 29, 30, 46]. FUS-mediated gene delivery studies detected efficient transgene expression in targeted regions using rAAV9 after 12–15 days [20, 30]. Dose-dependent GFP expression levels following targeted delivery of rAAV9-CMV-GFP to the striatum 12 days after FUS is illustrated in Fig. 3. FUS-facilitated rAAV2 delivery provided detectable GFP expression within the first week, but the optimum expression was achieved 21 days following FUS [29]. As an alternative, luciferase can be used as a transgene, thereby allowing for in vivo imaging of transgene expression levels at multiple time-points in the same animal [47]. Luciferase activity can be measured after tail vein injection of its substrate (luciferin) and measurements can be conducted sequentially in individual animals.
In addition to increasing the efficiency of gene expression, MRIgFUS-mediated rAAV delivery into the brain may also alter viral tropism. rAAV tropism after intraparenchymal injection has been well described for a variety of serotypes. However, MRIgFUS delivery exposes astrocytes surrounding the BBB as the primary entry route, thereby creating alternative accessibility to different cell types and locations in the brain [11,48]. For example, Foust et al. compared rAAV9 tropism in the brain after intravenous and intracranial injections into the striatum and hippocampus. In adult mice, intravascular injection of rAAV9 produced predominantly glial transgene expression in the brain, whereas intraparenchymal injection resulted in almost exclusively neuronal expression [11]. Similarly, MRIgFUS-mediated delivery of intravenously injected rAAV2 to the brain transduced both astrocytes (41%) and neurons (12%), in contrast to the typical neuronal-biased transduction after direct injection of the rAAV2 serotype [29]. Thus, it is important to take into account the impact of the delivery method on serotype tropism when targeting cell-specific gene expression.
Fig. 3.
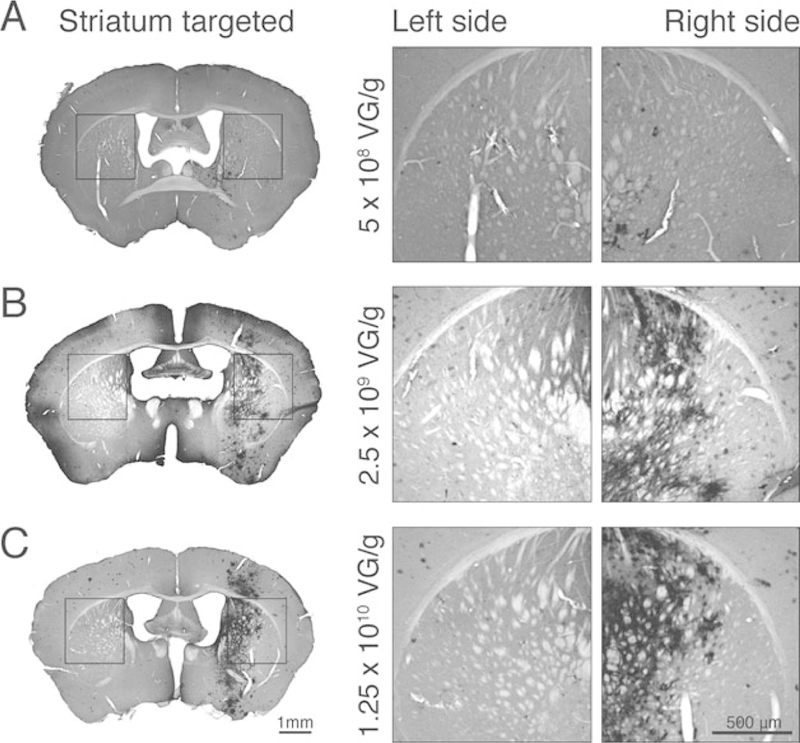
MRIgFUS for targeted delivery of rAAV9-CMV-GFP to the unilateral mouse striatum. (a–c) GFP expression was assessed by immunohistochemical DAB staining in the contralateral hemisphere (left) and in FUS-targeted regions (striatum right hemisphere), 2 weeks after treatment. GFP expression was examined after three different doses of intravenous rAAV9-CMV-GFP delivered by MRIgFUS to the right striatum: (a) 5 × 108 vg/g, (B) 2.5 × 109 vg/g, and (c) 1.25 × 1010 vg/g. Strong GFP expression was detected in the FUS-treated striatum (right) compared to the contralateral untreated hemisphere (left) at 2.5 × 109 vg/g and 1.25 × 1010 vg/g (b, c). The expression of GFP in FUS-treated animals at 5 × 108 vg/g was low-to-undetectable (a)
Acknowledgments
We would like to acknowledge Dr. Emmanuel Thevenot for his work, generating animals and images used to create Fig. 3. We thank Hang Yu Lin for her assistance in generating the graphical illustrations, and Sanjana Seerala, Kristina Mikloska, and Dr. Badru Moloo for editing the manuscript. Dr. Melitta Schachner contributed to the initial discussion of the project. This work was supported by the Canadian Institutes of Health Research (KX, Doctoral Scholarship; IA and KH: FRN137064), the Weston Brain Institute (IA, KH), and the Alzheimer Society of Canada (ZN, Doctoral Scholarship).
References
- 1.Kumar SRP, Markusic DM, Biswas M, High KA, Herzog RW (2016) Clinical development of gene therapy: results and lessons from recent successes. Mol Ther Methods Clin Dev 3:16034 10.1038/mtm.2016.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costantini LC, Bakowska JC, Breakefield XO, Isacson O (2000) Gene therapy in the CNS. Gene Ther 7:93 10.1038/sj.gt.3301119 [DOI] [PubMed] [Google Scholar]
- 3.Lim ST, Airavaara M, Harvey BK (2010) Viral vectors for neurotrophic factor delivery: a gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol Res 61 (1):14–26. 10.1016/j.phrs.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy J (2018) Neurodegeneration: the first mechanistic therapy and other progress in 2017. Lancet Neurol 17(1):3–5. 10.1016/S1474-4422(17)30414-3 [DOI] [PubMed] [Google Scholar]
- 5.Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M (2018) Gene therapy comes of age. Science 359(6372):eaan4672. 10.1126/science.aan4672 [DOI] [PubMed] [Google Scholar]
- 6.Wilson JM (2017) 2017 is the year we have been waiting for. Hum Gen Ther Clin Dev 28(4):165–166. 10.1089/humc.2017.29030.wil [DOI] [PubMed] [Google Scholar]
- 7.Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sanchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradi-naru V (2017) Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20 (8):1172–1179. 10.1038/nn.4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsayeth JR, Bankiewicz KS (2011) AAV9: over the fence and into the woods. Mol Ther 19(6):1006–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manfredsson FP, Rising AC, Mandel RJ (2009) AAV9: a potential blood-brain barrier buster. Mol Ther 17(3):403–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AH, Kaspar BK (2010) Rescue of the spinal muscular atrophy pheno-type in a mouse model by early postnatal delivery of SMN. Nat Biotechnol 28(3):271–274 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK (2009) Intravascular AAV9 preferentially targets neo-natal neurons and adult astrocytes. Nat Bio-technol 27(1):59–65. 10.1038/nbt.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, Lowes L, Alfano L, Berry K, Church K, Kissel JT, Nagendran S, L’ltalien J, Sproule DM, Wells C, Cardenas JA, Heitzer MD, Kaspar A, Corcoran S, Braun L, Likhite S, Miranda C, Meyer K, Foust KD, Burghes AHM, Kaspar BK (2017) Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 377(18):1713–1722. 10.1056/NEJMoa1706198 [DOI] [PubMed] [Google Scholar]
- 13.Rafii MS, Tuszynski MH, Thomas RG, Barba D, Brewer JB, Rissman RA, Siffert J, Aisen PS, Team ANS (2018) Adeno-associated viral vector (serotype 2)-nerve growth factor for patients with alzheimer disease: a randomized clinical trial. JAMA Neurol 75 (7):834–841. 10.1001/jamaneurol.2018.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuszynski MH, Yang JH, Barba D, U HS, Bakay RA, Pay MM, Masliah E, Conner JM, Kobalka P, Roy S, Nagahara AH (2015) Nerve growth factor gene therapy: activation of neuronal responses in Alzheimer disease. JAMA Neurol 72(10):1139–1147. 10.1001/jamaneurol.2015.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timbie KF, Mead BP, Price RJ (2015) Drug and gene delivery across the blood-brain barrier with focused ultrasound. J Control Release 219:61–75. 10.1016/j.jconrel.2015.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, Smith GS, Hynynen K, Black SE (2018) Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun 9(1):2336 10.1038/s41467-018-04529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ (2011) Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 19:1058–1069. 10.1038/mt.2011.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, Fyfe J, Moullier P, Colle MA, Barkats M (2009) Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther 17(7):1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber-Adrian D, Thtivenot E, O’Reilly MA, Oakden W, Akens MK, Ellens N, Markham-Coultes K, Burgess A, Finkelstein J, Yee AJ, Whyne CM, Foust KD, Kaspar BK, Stanisz GJ, Chopra R, Hynynen K, Aubert I (2015) Gene delivery to the spinal cord using MRI-guided focused ultrasound. Gene Ther 22(7):568–577. 10.1038/gt.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thevenot E, Jordao JF, O’Reilly MA, Markham K, Weng YQ, Foust KD, Kaspar BK, Hynynen K, Aubert I (2012) Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther 23(11):1144–1155. 10.1089/hum.2012.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korbelin J, Dogbevia G, Michelfelder S, Rid-der DA, Hunger A, Wenzel J, Seismann H, Lampe M, Bannach J, Pasparakis M, Kleinsch-midt JA, Schwaninger M, Trepel M (2016) A brain microvasculature endothelial cell-specific viral vector with the potential to treat neuro-vascular and neurological diseases. EMBO Mol Med 8(6):609–625. 10.15252/emmm.201506078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kugler S (2016) Tissue-specific promoters in the CNS. Methods Mol Biol 1382-(Chapter 6):81–91. 10.1007/978-1-4939-3271-9_6 [DOI] [PubMed] [Google Scholar]
- 23.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220(3):640–646. 10.1148/radiol.2202001804 [DOI] [PubMed] [Google Scholar]
- 24.Hosseinkhah N, Goertz DE, Hynynen K (2015) Microbubbles and blood-brain barrier opening: a numerical study on acoustic emissions and wall stress predictions. IEEE Trans Biomed Eng 62(5):1293–1304. 10.1109/tbme.2014.2385651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheikov N, McDannold N, Sharma S, Hynynen K (2008) Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol 34(7):1093–1104. 10.1016/j.ultrasmedbio.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Hynynen K (2011) MR-guided focused ultrasound for brain ablation and blood-brain barrier disruption. Methods Mol Biol 711:579–593. 10.1007/978-1-61737-992-5_30 [DOI] [PubMed] [Google Scholar]
- 27.Burgess A, Ayala-Grosso CA, Ganguly M, Jor-dao JF, Aubert I, Hynynen K (2011) Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One 6(11):e27877. 10.1371/journal.pone.0027877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen WB (2017) Magnetic enhancement of stem cell-targeted delivery into the brain following MR-guided focused ultrasound for opening the blood-brain barrier. Cell Transplant 26(7):1235–1246. 10.1177/0963689717715824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu P-H, Wei K-C, Huang C-Y, Wen C-J, Yen T-C, Liu C-L, Lin Y-T, Chen J-C, Shen C-R, Liu H-L (2013) Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One 8(2):e57682. 10.1371/journal.pone.0057682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Kugelman T, Buch A, Herman M, Han Y, Karakatsani ME, Hussaini SA, Duff K, Konofagou EE (2017) Non-invasive, focused ultrasound-facilitated gene delivery for optogenetics. Sci Rep 7:39955 10.1038/srep39955. https://www.nature.com/articles/srep39955#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chopra R, Curiel L, Staruch R, Morrison L, Hynynen K (2009) An MRI-compatible system for focused ultrasound experiments in small animal models. Med Phys 36:1867–1874. 10.1118/1.3115680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Reilly MA, Hynynen K(2012) Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology 263 (1):96–106. 10.1148/radiol.11111417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maguire CA, Crommentuijn MH, Mu D, Hudry E, Serrano-Pozo A, Hyman BT, Tannous BA (2013) Mouse gender influences brain transduction by intravascularly administered AAV9. Mol Ther 21(8):1470–1471. 10.1038/mt.2013.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson KL, Dayton RD, Klein RL (2015) AAV9 supports wide-scale transduction of the CNS and TDP-43 disease modeling in adult rats. Mol Ther Methods Clin Dev 2:15036 10.1038/mtm.2015.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDannold N, Zhang Y, Vykhodtseva N (2011) Blood-brain barrier disruption and vascular damage induced by ultrasound bursts combined with microbubbles can be influenced by choice of anesthesia protocol. Ultrasound Med Biol 37(8):1259–1270. 10.1016/j.ultrasmedbio.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDannold N, Zhang Y, Vykhodtseva N (2017) The effects of oxygen on ultrasound-induced blood-brain barrier disruption in mice. Ultrasound Med Biol 43(2):469–475. 10.1016/j.ultrasmedbio.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullin L, Gessner R, Kwan J, Kaya M, Borden MA, Dayton PA (2011) Effect of anesthesia carrier gas on in vivo circulation times of ultrasound microbubble contrast agents in rats. Contrast Media Mol Imaging 6(3):126–131. 10.1002/cmmi.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itani M, Mattrey RF (2012) The effect of inhaled gases on ultrasound contrast agent longevity in vivo. Mol Imaging Biol 14(1):40–46. 10.1007/s11307-011-0475-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDannold N, Vykhodtseva N, Hynynen K (2007) Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption: a feasibility study. Ultrasound Med Biol 33(4):584–590. 10.1016/j.ultrasmedbio.2006.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DEFINITY® (2017) Lantheus Medical Imaging, Inc., North Billerica, MA [Google Scholar]
- 41.O’Reilly MA, Hynynen K (2018) Ultrasound and microbubble-mediated blood-brain barrier disruption for targeted delivery of therapeutics to the brain In: Sirianni RW, Behkam B (eds) Targeted drug delivery: methods and protocols. Springer, New York, NY, pp 111–119. 10.1007/978-1-4939-8661-3_9 [DOI] [PubMed] [Google Scholar]
- 42.McMahon D, Hynynen K (2017) Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics 7(16):3989–4000. 10.7150/thno.21630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, Bresler M, Burks SR, Frank JA (2017) Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. PNAS 114(1):E75–E84. 10.1073/pnas.1614777114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K (2007) Targeted delivery ofdoxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultra-sound. Int J Cancer 121(4):901–907. 10.1002/ijc.22732 [DOI] [PubMed] [Google Scholar]
- 45.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C (2001) A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21(1):15–23 [DOI] [PubMed] [Google Scholar]
- 46.Mason MRJ, Ehlert EME, Eggers R, Pool CW, Hermening S, Huseinovic A, Timmermans E, Blits B, Verhaagen J (2010) Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther 18(4):715–724. 10.1038/mt.2010.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE (2007) Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 16(6):1073–1080 [DOI] [PubMed] [Google Scholar]
- 48.Pardridge WM (2012) Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32(11):1959–1972. 10.1038/jcbfm.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]


