Abstract
Background:
Our purpose was to evaluate the prognostic impact of pathologically-confirmed esophageal adenosquamous carcinoma (ASC) and its association with HER2 status and clinicopathologic characteristics.
Methods:
Among 796 patients with esophageal or gastroesophageal junction (GEJ) adenocarcinoma who underwent curative resection, surgical pathology reports were reviewed, and suspected ASC was confirmed utilizing p63 and CK5/6 immunostaining. HER2 status was determined using immunohistochemistry and fluorescence in situ hybridization. Cox models were used to assess the impact of ASC on disease-specific survival (DSS) and overall survival (OS).
Results:
Overall, 2.0% (16/796) of patients had esophageal ASC, mostly demonstrating a close intermingling of squamous and adenocarcinoma cells within the same tumor. The percentage of squamous vs adenocarcinoma cells in the primary was generally recapitulated in nodal metastases, and intrapatient internodal heterogeneity was uncommon. Patients with esophageal ASC were statistically significantly more likely to be female (vs male), have normal (vs excess) body-mass index, and harbor HER2-negative (vs –positive) tumors, as compared to patients with adenocarcinoma-only. No ASC tumor was HER2-positive as compared to 16% of adenocarcinoma-only tumors (P =.018). Compared to patients with adenocarcinoma-only, those with ASC demonstrated profoundly worse DSS (5-year event-free rate: 34% vs 6%; multivariate hazard ratio 2.87 [95% confidence interval 1.59 to 4.76]; P=.0010) and OS (P=.0027) that was independent of known prognostic factors and HER2 status.
Conclusion:
Adenosquamous carcinoma identifies a rare aggressive HER2-negative subgroup of esophageal/GEJ adenocarcinoma.
Keywords: Esophageal adenosquamous carcinoma, prognosis, frequency, HER2/ERBB2, esophageal cancer, esophageal adenocarcinoma, gastric cancer, gastric adenocarcinoma, body-mass index, tumor heterogeneity
PRECIS
This study identifies esophageal adenosquamous carcinoma as a rare aggressive HER2-negative subtype with divergent clinicopathologic characteristics from pure esophageal adenocarcinoma. These data support further elucidation of its molecular landscape.
INTRODUCTION
Esophageal carcinoma mostly comprises two distinct histopathologic entities: adenocarcinoma (EAC) and squamous cell carcinoma (ESCC). Pure EAC typically originates from the distal esophagus or GEJ preceded by a premalignant inflammatory condition called Barrett’s metaplasia. EAC incidence has increased in Western countries, and major risk factors are gastroesophageal reflux and obesity.1 By contrast, ESCC typically originates from the mid- or proximal esophagus, and its incidence has stabilized in the last 30 years with ESCC now far less common in the U.S. than EAC. Smoking, alcohol, low intake of fresh fruits and vegetables, low socioeconomic status have been implicated as risk factors for ESCC.2 ESCCs have been reported in large surgical series to have a worse prognosis than EACs.3
A recent advance in the treatment of EAC was the validation of human epidermal growth factor receptor 2 (HER2/ERBB2) as a therapeutic target in esophagogastric adenocarcinoma.4 HER2 is a transmembrane tyrosine kinase receptor involved in controlling cell growth, survival, differentiation, and migration, and its expression/amplification has been detected in ~15% of EACs as we5, 6 and others7 have shown. Its frequency appears to be less frequent in ESCCs.8, 9
Adenosquamous carcinomas (ASCs) of the esophagus are uncommon tumors (0.37%-1% of esophageal carcinomas) in which the pathologic features of both adenocarcinoma and squamous cell carcinoma reside within the same tumor10–13. The prognostic impact, clinicopathologic and molecular characteristics of esophageal ASC are poorly understood. Barriers to further study include the lack of large cohorts of EAC with robust survival and clinicopathologic data. Here we examined the frequency, histologic characteristics, and survival of esophageal ASC in a large cohort of previously untreated patients who underwent surgical resection of their EAC. To better understand the pathogenesis of ASC as compared to EAC, we also compared ASCs and EACs in relation to smoking, BMI, and HER2 expression/amplification.
METHODS
Study population
In the current study we analyzed data from the Mayo Esophageal Cancer Outcome Database, which was previously described.14 Briefly, adult patients (N = 796) were diagnosed with tissue-confirmed adenocarcinoma of the esophagus, gastroesophageal junction or gastric cardia (Siewert type I or II) and consecutively underwent surgery with curative intent at Mayo Clinic in Rochester, Minnesota (January 1, 1980 to December 31, 1997). Subcardial tumors and tumors lacking an adenocarcinoma component were excluded. Data on clinicopathologic characteristics, exposures, and patient survival were systematically collected from individual medical records. Smoking data were collected pre-surgery via self-reported written institutional questionnaires and verified during a visit with their primary care physician at Mayo. Height and weight were measured at the same time of surgery.
Identification of ASC
Twenty cases of ASC were selected as diagnosed in the original pathology reports. The glass slides were then retrieved and reviewed by a GI pathologist (T-T.W) and the paraffin blocks pulled in order to cut fresh sections. The new sections were stained with antibodies against p63 and cytokeratin CK5/6 to identify the squamous cell carcinoma component. Stains for chromogranin and synaptophysin to exclude a neuroendocrine component were also performed. In this fashion ASC was confirmed in 16 patients. Thirteen ASC had node-positive disease, and the percentage of squamous vs adenocarcinoma cells in both primary and nodes was evaluable in 9 patients. The correlation in the percentage of tumor cells staining positive for p63 and CK5/6 was very high (data not shown); data for p63 are shown.
HER2
HER2 expression by immunohistochemistry (IHC; HercepTest [Dako]) and HER2 gene amplification by fluorescence in situ hybridization (PathVysion) were assessed using gastroesophageal-specific criteria, as previously described.5, 6, 15 For IHC, each case was scored by two pathologists (T-T.W., W.R.S.), as follows: high (IHC3+), strong intensity in 10% or more of cancer cells; medium (IHC2+), weak-moderate intensity in 10% or more; low (IHC1+), faint intensity in 10% or more; absent (IHC0). A specimen with an HER2/CEP17 ratio of 2.0 or more in invasive cells was classified as HER2-amplified. HER2-positive was defined as IHC3+ or IHC2+ with gene amplification, consistent with guidelines.15
Statistical Analysis
Statistical significance of the results was determined by chi-square. Agreement in the percentage of SCC in the primary vs nodes was determined using the intraclass correlation coefficient (ICC) with values <.40, 0.40-0.75, and >.75 considered poor, fair-to-good, and excellent, respectively.16 Univariate analysis of survival was performed using the Kaplan Meier method. Cox proportional hazards models were used to estimate the univariate and multivariate association between predictor variables and outcomes. Overall survival was calculated as the time from surgery to death from any cause. Disease-specific survival was calculated as the time from surgery to death due to index cancer. Events beyond 5 years were censored. P <.05 was considered significant. Data were collected using REDCap electronic data capture tools and statistical analysis was performed using JMP (JMP Pro 10.0.0 SAS Institute Inc. 2012) and MedCalc (version 14.12.0) software.
RESULTS
Study Population
Table 1 shows the baseline characteristics of the study population (N = 796). All patient tumors contained adenocarcinoma, and ASC was confirmed in 16 patients. None of the 16 patients with ASC had been diagnosed with a second primary at the time of surgery. Neoadjuvant chemotherapy with concurrent radiotherapy, chemotherapy alone, or radiotherapy alone was administered in 7 patients (0.9%), 1 patient (0.1%), and 1 patient (0.1%), respectively; no patient with ASC received neoadjuvant therapy. Adjuvant chemotherapy with concurrent radiotherapy, chemotherapy alone, or radiotherapy alone was administered in 53 patients (7%), 28 patients (4%), and 26 patients (3%), respectively; among patients with ASC, 2 patients received RT alone and 2 patients received chemoradiation.
Table 1.
Baseline characteristics (N = 796)
| Characteristic | Adenocarcinoma n = 780 |
Adenosquamous carcinoma n = 16 |
|
|---|---|---|---|
| N (%) | N (%) | P | |
| Host characteristics | |||
| Age | |||
| Median, y | 65 | 61 | 0.1826 |
| Gender | |||
| Male | 695 (89%) | 10 (63%) | 0.0061 |
| Female | 85 (11%) | 6 (37%) | |
| Body mass index | |||
| Normal | 263 (34%) | 10 (63%) | 0.0203 |
| Excess | 517 (66%) | 6 (37%) | |
| Smoking | |||
| Ever | 193 (25%) | 4 (25%) | 0.9813 |
| Never | 587 (75%) | 12 (75%) | |
| Tumor characteristics | |||
| T stage | |||
| T1-T2 | 288 (37%) | 4 (25%) | 0.3027 |
| T3-T4 | 486 (63%) | 12 (75%) | |
| Missing | 4 | 0 | |
| No. metastatic LNs | |||
| Median | 2 | 2.5 | 0.1731 |
| Histologic grade | |||
| 1 to 3 | 469 (61%) | 8 (50%) | 0.3785 |
| 4 | 300 (39%) | 8 (50%) | |
| Missing | 11 | 0 | |
| Adjacent Barrett’s | |||
| Yes | 277 (36%) | 5 (31%) | 0.7216 |
| No | 503 (64%) | 11 (69%) | |
| Tumor location | |||
| Esophagus | 277 (36%) | 6 (38%) | 0.8729 |
| GEJ or cardia | 502 (64%) | 10 (63%) | |
| HER2 statusᵅ | |||
| Positive | 121 (17%) | 0 | 0.0175 |
| Negative | 577 (83%) | 15 (100%) | |
| Missing | 82 | 1 | |
HER2-positive defined as strong protein expression (IHC 3+) or equivocal expression (IHC 2+) with gene amplification
P <.05 is bolded.
IHC, immunohistochemistry.
ASC in primary tumor and nodes
In most patients with ASC (93% [15/16]) squamous and adenocarcinoma cells were closely intermingled within the same primary tumor, although in one tumor (7% [1/15]) two distinct subpopulations of squamous vs adenocarcinoma cells were identified (Figure 1). Across all ASC tumors, SCCs comprised a median 40% of the tumor cells, although considerable variability was observed (range 1-100%; interquartile range 16%-58%). Invasive depth of the SCC component was T3 in most ASC tumors (ie, 10 of 14 tumors with evaluable subpopulation depth), T2 in one tumor, and T1b in three tumors. Invasive depth of the adenocarcinoma component was likewise T3 in most ASC tumors (9 of 13 tumors with evaluable subpopulation depth), T4 in one tumor, T2 in one tumor, and T1b in two tumors. In the majority of ASC tumors (62% [8/13]) the invasive depth of the SCC component was the same as that of the adenocarcinoma component.
Figure 1:
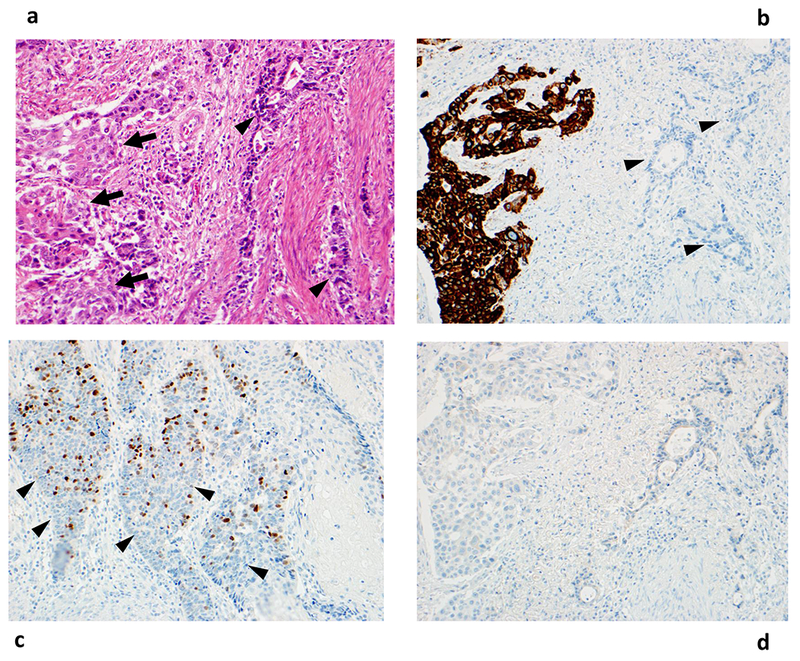
Histologic features of adenosquamous carcinoma. Panel A shows discrete subpopulations of squamous cell carcinoma and adenocarcinoma in a hematoxylin and eosin-stained image. Panel B confirms the presence of squamous carcinoma cells through cytokeratin 5/6 staining in the same tumor. By contrast, Panel C shows close intermingling of squamous cell carcinoma (demonstrated by p63 staining) and adenocarcinoma cells from a different tumor. Panel D shows that the adenosquamous carcinoma is negative for chromogranin stain. Arrows denote squamous cell carcinoma cells, and arrowheads denote adenocarcinoma cells. All images are 200×.
As shown in Figure 2, paired regional nodes were examined in nine primary ASC cases with node-positive disease that was evaluable for ASC (22 nodes in total) (see Methods). SCC was identified in at least one node in most patients (78% [7/9]), and in those nodes SCC comprised at least 10% of tumor cells. The percentage of squamous vs adenocarcinoma cells in the primary showed good agreement with the corresponding percentage in the nodes (ICC =.73 [95% CI 0.19, 0.93]), although discrepant cases were observed (eg, Patients 3-5). For most patients with multiple nodes examined, the percentage of SCC cells in a node showed minimal variation between nodes.
Figure 2:
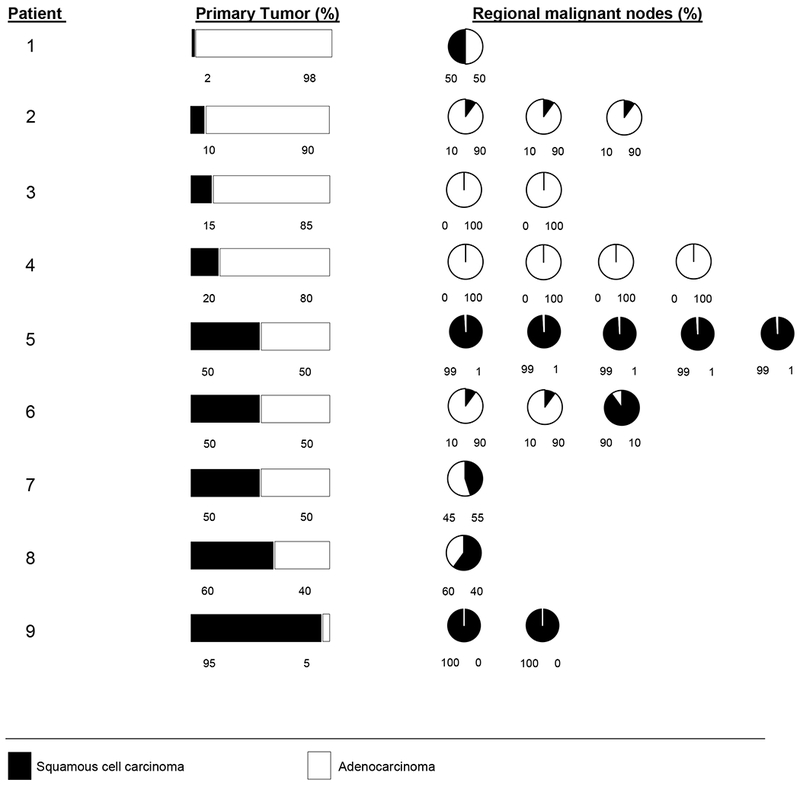
Proportion of squamous carcinoma cells in primary adenosquamous carcinomas and paired regional malignant nodes.
Clinicopathologic characteristics of ASC
Patients with esophageal ASC showed a statistically significant higher likelihood of being female (vs male), having normal body-mass index (vs excess), and having a tumor that was HER2-negative (vs HER2-positive), as compared to patients whose esophageal tumors showed adenocarcinoma-only (Table 1). Other characteristics, including the presence of adjacent intestinal metaplasia, were not noticeably different between ASC and adenocarcinoma-only patients.
ASC and patient survival
Median follow-up duration for surviving patients was 12.8 years. In univariate analysis ASC (vs adenocarcinoma-only) was associated with shorter DSS (5-year DSS rate = 6% vs 34%, respectively; P <.0001 log-rank) and OS (5-year OS rate = 6% vs 31%, respectively; P=.0001 log-rank) (Table S1, Figure 3). As expected, increasing age, T stage, number of malignant nodes, and tumor grade were also associated with worse survival, supporting the generalizability of our cohort (Table S1). After adjustment for all covariates, ASC (vs adenocarcinoma-only) remained statistically significantly associated with shorter DSS (HR 2.87 [95% CI 1.59-4.76]; P =.0010) and OS (HR 2.57 [95% CI 1.43-4.26]; P =.0027) (Table 2). Sensitivity analyses in multivariable models excluding patients who received neoadjuvant and/or adjuvant therapy revealed stable results (Table S2).
Figure 3.
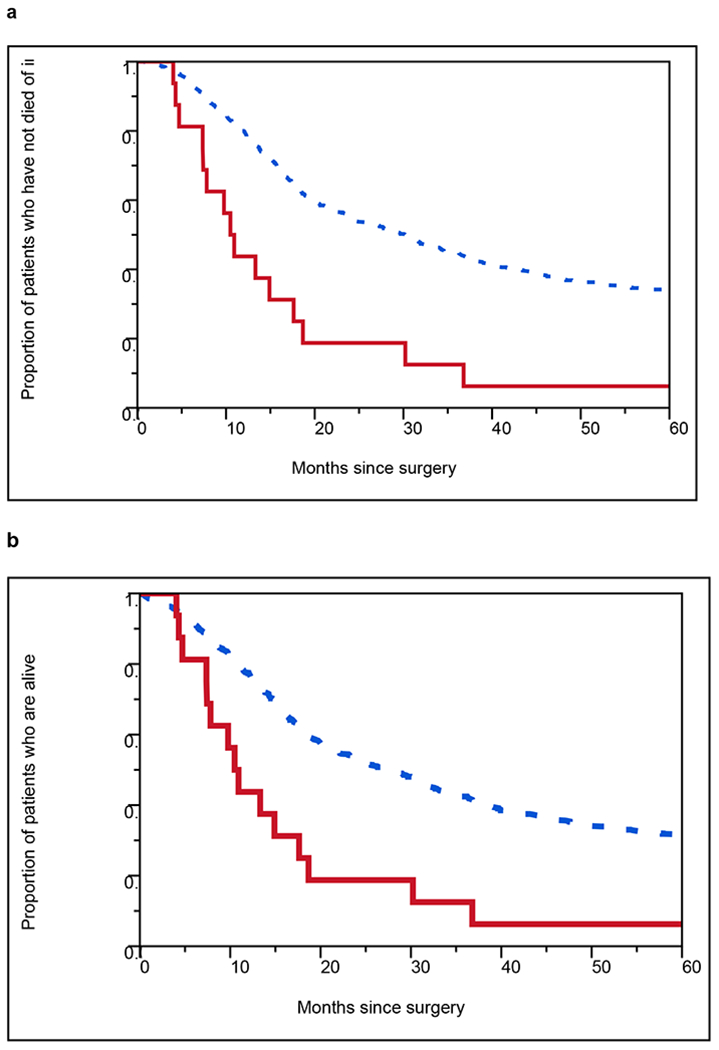
Kaplan-Meier curves showing (a) disease-specific survival and (b) overall survival after surgery in patients with adenosquamous carcinoma vs adenocarcinoma of the esophagus or gastroesophageal junction after surgery. HRs from univariate Cox models are shown with log-rank P values. CI, confidence interval; HR, hazard ratio.
- Red solid line should be labeled as “Adenosquamous Carcinoma” inside the graph.
- Black dotted line should be labeled as “Adenocarcinoma” inside the graph.
| N (events) | 5-year event-free rate | HR | 95% CI | P log-rank | |
|---|---|---|---|---|---|
| Adenosquamous Carcinoma | 16 (15) | 6% | 2.94 | 1.68 to 4.74 | <.0001 |
| Adenocarcinoma | 780 (491) | 34% | Reference | ||
| N (events) | 5-year event-free rate | HR | 95% CI | P log-rank | |
|---|---|---|---|---|---|
| Adenosquamous Carcinoma | 16 (15) | 6% | 2.66 | 1.52 to 4.29 | .0001 |
| Adenocarcinoma | 780 (529) | 31% | Reference | ||
Table 2.
Multivariable Cox Proportional Hazards Models Examining Esophageal Adenosquamous Carcinoma in Relation to Patient Survival (N = 796)a
| Variable | Disease-specific survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Cancer Subtype | ||||||
| Adenosquamous carcinoma | 2.87 | 1.59, 4.76 | .0010 | 2.57 | 1.43, 4.26 | .0027 |
| Adenocarcinoma | ref | ref | ||||
| Age, y | ||||||
| Each additional year | 1.01 | 1.00, 1.023 | .0030 | 1.02 | 1.01, 1.03 | <.0001 |
| Gender | ||||||
| Male | 1.26 | 0.94, 1.73 | .1221 | 1.30 | 0.97, 1.76 | 0.0751 |
| Female | ref | ref | ||||
| T stage | ||||||
| T3-4 | 1.87 | 1.50, 2.35 | <.0001 | 1.80 | 1.46, 2.24 | <.0001 |
| T1-2 | ref | ref | ||||
| No. metastatic nodes | ||||||
| Each additional node | 1.10 | 1.09, 1.13 | <.0001 | 1.10 | 1.08, 1.12 | <.0001 |
| Tumor Grade | ||||||
| 4 | 1.30 | 1.08, 1.57 | .0066 | 1.30 | 1.08, 1.55 | 0.0052 |
| 1 to 3 | ref | ref | ||||
| HER2 status | ||||||
| Positive | 1.00 | 0.77, 1.28 | 1.00 | 0.96 | 0.74, 1.22 | 0.7205 |
| Negative | ref | ref | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio; ref; reference.
HRs are adjusted for all variables shown. Values >1 denote a higher risk of adverse survival compared to the reference level.
DISCUSSION
To our knowledge, we report the first data on the prognostic impact of pathologically confirmed ASC in a large cohort of EAC in association with HER2 status and major risk factors. We found that ASCs comprised 2% of non-metastatic esophageal/GEJ adenocarcinomas, and this rare histologic subtype was associated with a statistically significant >2-fold worsening of DSS and OS after adjustment for covariates. In addition, we found that patients with ASC were statistically significantly more likely to be female, to have normal weight, and to harbor HER2-negative tumors, as compared with patients with EAC only. Together, these data indicate that ASC is a distinct aggressive subtype of EAC that warrants further investigation.
Close intermingling of SCC and adenocarcinoma cells was observed in most primary esophageal ASCs, consistent with prior pathologic descriptions of ASCs in ESCC populations.13, 17 The percentage of squamous vs adenocarcinoma cells in the primary showed good agreement with the corresponding percentage in paired nodes, with minimal internodal variation in the same patient, suggesting the intratumor heterogeneity of the primary ASC is generally recapitulated in metastatic lesions, although discrepancies between primary and node were also observed. While the precise molecular characterization of this heterogeneity remains to be elucidated, it is increasingly accepted that clonal diversity in primary cancers may increase the risk of subsequent progression due to a broader assortment of subclones which allow for the selection and rapid progression of specific tumor subclones.18
Consistent with the theoretically greater tumor aggressiveness conferred by intratumor heterogeneity, we found that patients with esophageal ASC had a significantly worse survival compared to those with adenocarcinoma only, even after adjustment for known prognostic variables. Our data are supported by two recent reports from population-based studies in the U.S. which found that esophageal ASCs are associated with worse OS as compared to EAC-only.10, 11 Advantages of the current study over prior reports include the expert pathologic confirmation of ASC, as well as the availability of data on treatment, BMI, smoking, and HER2 status. Interestingly, multiple prior studies have determined that the prognosis of esophageal ASCs were not significantly different from ESCCs.10, 11, 13, 17, 19, 20
Due to the low frequency of esophageal ASC, its clinicopathologic features are not well established. In our study patients with esophageal ASCs did not have significant differences from patients with EAC-only in terms of smoking history, depth of tumor invasion, pathologic tumor grade, or the number of malignant nodes. However, patients with esophageal ASC (vs EAC-only) were less likely to have excess BMI, which is a known risk factor for EAC, but not ESCC.21 In addition, no ASC demonstrated HER2 protein expression or gene amplification, in contrast to 17% of EAC-only tumors. Interestingly, esophageal ASCs did not show a significant difference from pure EACs with regard to their tumor location or the presence of adjacent Barrett’s metaplasia, suggesting that esophageal ASC may be as likely to arise from Barret’s as pure EAC. Our finding is consistent with data from rat models with surgically induced gastroduodenal reflux which have shown that ASCs arise in esophageal mucosa characterized by chronic squamous esophagitis22 and glandular metaplasia,23 suggesting that alkaline reflux esophagitis contributes to the genesis of not only EAC, but also ASC.22
Esophageal or gastric ASCs are believed to develop from an unidentified common progenitor cell in some cases24, 25 or through collision of two individual tumors in others.26 Preliminary data indicate that EAC and ESCC components of esophageal ASC tumors share patterns of allelic loss at multiple chromosomal locations, TP53 mutation, and/or aberrant expression of p53, p16, and RB in some tumors,27–29 while other esophageal ASC tumors exhibit significantly divergent alterations in microsatellites and beta-catenin and EGFR expression.28, 30 In lung xenograft models, recent data suggest that ASCs originate from cancer stem like cells that differentiate to multi-lineage structures with branching lung morphology expressing bronchial, alveolar and neuroendocrine markers in vitro. Our findings provide rationale for examining esophageal ASC as a distinct entity in ongoing and future comprehensive molecular characterizations.
Strengths of our study include the large size and extensive clinicopathologic annotation of our cohort, knowledge of treatment received, and long duration of survival follow-up. Novel clinicopathologic annotations include BMI, adjacent Barrett’s, smoking history, and HER2 expression and amplification using modern disease-specific methods. In addition, unlike prior studies,10, 13, 17, 19, 20 the presence of ASC was confirmed by expert pathologic examination including the use of new immunostains. Limitations of our study include the retrospective nature of data collection and the relatively small number of ASC cases.
In conclusion, our study identifies esophageal ASC as a rare aggressive HER2-negative subtype with divergent clinicopathologic characteristics from pure EAC, highlighting the importance of further elucidating the molecular landscape of ASC.
Supplementary Material
Acknowledgements:
The authors are grateful to Raquel Ostby for providing administrative assistance.
Funding sources: Grant No. K12 CA90628-10U to H.H.Y.; Genentech to H.H.Y, Mayo Clinic Center for Clinical and Translational Science (CCaTS) (UL1TR002377)
Footnotes
Conflicts of interest: None
REFERENCES
- 1.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013;381(9864): 400–12. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterology Clinics of North America 2009;38(1): 27–57, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Annals of surgery 2001;234(3): 360–7; discussion 68–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376(9742): 687–97. [DOI] [PubMed] [Google Scholar]
- 5.Yoon HH, Shi Q, Sukov WR, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research 2012;18(2): 546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon HH, Sukov WR, Shi Q, et al. HER-2/neu gene amplification in relation to expression of HER2 and HER3 proteins in patients with esophageal adenocarcinoma. Cancer 2014;120(3): 415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang JX, Zhao K, Lin M, et al. HER2 gene amplification in esophageal squamous cell carcinoma is less than in gastroesophageal junction and gastric adenocarcinoma. Oncology letters 2013;6(1): 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato H, Arao T, Matsumoto K, et al. Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. International journal of oncology 2013;42(4): 1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yendamuri S, Malhotra U, Hennon M, et al. Clinical characteristics of adenosquamous esophageal carcinoma. Journal of gastrointestinal oncology 2017;8(1): 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans M, Liu Y, Chen C, et al. Adenosquamous Carcinoma of the Esophagus: An NCDB-Based Investigation on Comparative Features and Overall Survival in a Rare Tumor. Oncology 2017. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HD, Chen CG, Gao YY, et al. Primary esophageal adenosquamous carcinoma: a retrospective analysis of 24 cases. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / I.S.D.E 2013. [DOI] [PubMed] [Google Scholar]
- 13.Chen SB, Weng HR, Wang G, et al. Primary adenosquamous carcinoma of the esophagus. World Journal of Gastroenterology 2013;19(45): 8382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon HH, Khan M, Shi Q, et al. The prognostic value of clinical and pathologic factors in esophageal adenocarcinoma: a mayo cohort of 796 patients with extended follow-up after surgical resection. Mayo Clinic Proceedings 2010;85(12): 1080–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartley AN, Washington MK, Ventura CB, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Archives of Pathology and Laboratory Medicine 2016;140(12): 1345–63. [DOI] [PubMed] [Google Scholar]
- 16.Oremus M, Oremus C, Hall GB, McKinnon MC. Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa Scales. BMJ open 2012;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yachida S, Nakanishi Y, Shimoda T, et al. Adenosquamous carcinoma of the esophagus. Clinicopathologic study of 18 cases. Oncology 2004;66(3): 218–25. [DOI] [PubMed] [Google Scholar]
- 18.Kleppe M, Levine RL. Tumor heterogeneity confounds and illuminates: assessing the implications. Nature Medicine 2014;20(4): 342–4. [DOI] [PubMed] [Google Scholar]
- 19.Ni PZ, Yang YS, Hu WP, Wang WP, Yuan Y, Chen LQ. Primary adenosquamous carcinoma of the esophagus: an analysis of 39 cases. Journal of thoracic disease 2016;8(10): 2689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang HD, Chen CG, Gao YY, et al. Primary esophageal adenosquamous carcinoma: a retrospective analysis of 24 cases. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus 2014;27(8): 783–9. [DOI] [PubMed] [Google Scholar]
- 21.Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miwa K, Sahara H, Segawa M, et al. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. International journal of cancer. Journal international du cancer 1996;67(2): 269–74. [DOI] [PubMed] [Google Scholar]
- 23.Pera M, Brito MJ, Poulsom R, et al. Duodenal-content reflux esophagitis induces the development of glandular metaplasia and adenosquamous carcinoma in rats. Carcinogenesis 2000;21(8): 1587–91. [PubMed] [Google Scholar]
- 24.Kanazawa H, Ebina M, Ino-Oka N, et al. Transition from squamous cell carcinoma to adenocarcinoma in adenosquamous carcinoma of the lung. The American journal of pathology 2000;156(4): 1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang SM, Kang HJ, Shin JH, et al. Identical epidermal growth factor receptor mutations in adenocarcinomatous and squamous cell carcinomatous components of adenosquamous carcinoma of the lung. Cancer 2007;109(3): 581–7. [DOI] [PubMed] [Google Scholar]
- 26.Mather JP, Roberts PE, Pan Z, et al. Isolation of cancer stem like cells from human adenosquamous carcinoma of the lung supports a monoclonal origin from a multipotential tissue stem cell. PloS one 2013;8(12): e79456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rees BP, Rouse RW, de Wit MJ, et al. Molecular evidence for the same clonal origin of both components of an adenosquamous Barrett carcinoma. Gastroenterology 2002;122(3): 784–8. [DOI] [PubMed] [Google Scholar]
- 28.Milne AN, Carvalho R, van Rees BP, van Lanschot JJ, Offerhaus GJ, Weterman MA. Do collision tumors of the gastroesophageal junction exist? A molecular analysis. The American journal of surgical pathology 2004;28(11): 1492–8. [DOI] [PubMed] [Google Scholar]
- 29.Lee WA, Woo DK, Kim YI, Kim WH. p53, p16 and RB expression in adenosquamous and squamous cell carcinomas of the stomach. Pathology, research and practice 1999;195(11): 747–52. [DOI] [PubMed] [Google Scholar]
- 30.Pandilla R, Kotapalli V, Gowrishankar S, et al. Distinct genetic aberrations in oesophageal adeno and squamous carcinoma. European journal of clinical investigation 2013;43(12): 1233–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


