Fig. 1 |. Generation of a personal neoantigen-targeting vaccine for newly diagnosed patients with glioblastoma that had unmethylated MGMT promoters.
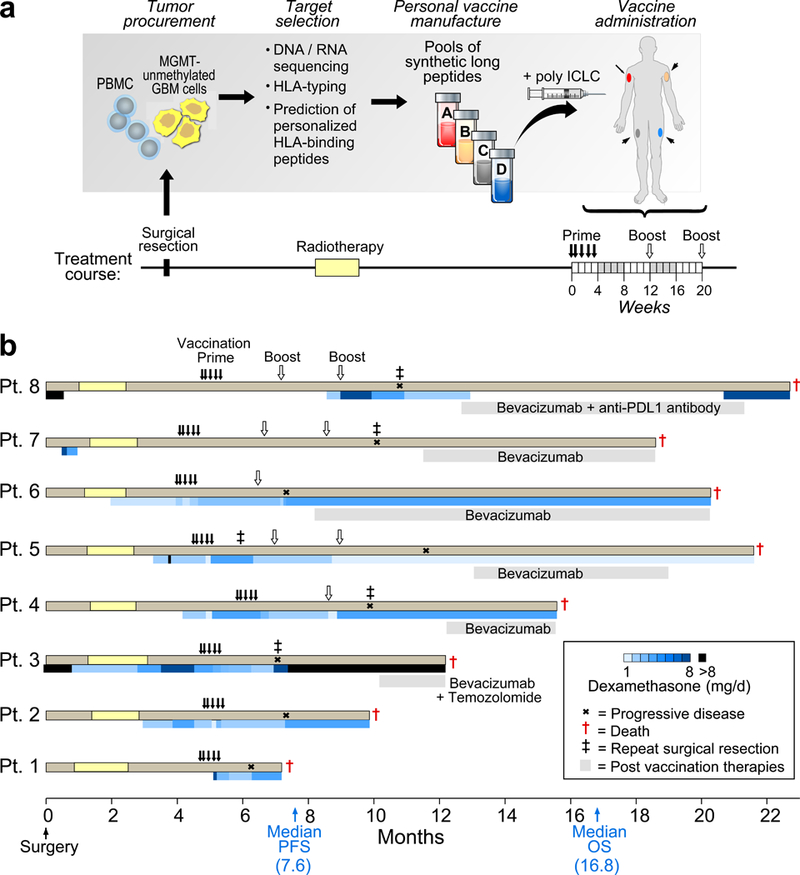
a, Somatic mutations were identified by Clinical Laboratory Improvement Amendments (CLIA)-certified whole-exome sequencing of DNA from surgically resected glioblastoma and matched normal cells (PBMCs) and their expression was confirmed by tumour RNA-seq. Immunizing peptides were selected based on HLA class I binding predictions (Methods). Each patient was vaccinated with up to 20 long peptides, administered in non-rotating pools of 3–5 peptides. b, Clinical event timeline for the eight patients who received at least one vaccine dose, from surgery until time of death due to progressive disease. Blue bars, dexamethasone dose and duration. Grey bars, salvage therapy administered following progression. Median progression-free survival (PFS) and overall survival (OS) was 7.6 months (90% confidence interval, 6.2–9.5) and 16.8 months (90% confidence interval, 9.6–21.3), respectively.
