Abstract
Background
Estimation of left ventricular filling pressures (LVFP) is a determining factor in the follow-up of patients with cardiac amyloidosis (CA). Natriuretic peptides (NPs) and tissue Doppler imaging may be used to monitor LVFP in patients with CA. The aim of this study was to evaluate the value of NPs and Doppler parameters in estimating LVFP in patients with CA.
Methods
Fifty patients with biopsy-verified light chain (n=31), A protein amyloidosis (AA) (n=1), apoliporotein A2 (n=1) or bone scintigraphy-proven transthyretin (n=17) CA were retrospectively included. All patients underwent right heart catheterisation (RHC). Among them, 48 (96%) and 43 (86%) had assays of NPs (20 brain natriuretic peptide (BNP), 27 N-terminal pro-hormone brain natriuretic peptide (NT-proBNP) and 1 both) and transthoracic echocardiography performed within 24 hours of RHC, respectively.
Results
The median BNP and NT-proBNP levels were 1000 (243–1477) ng/L and 10 106 (2935–13 348) ng/L, respectively. Echocardiography demonstrated left atrial enlargement with a mean volume of 47±16 mL and low tissue Doppler lateral Ea of 5±2 cm/s. The mean early diastolic mitral inflow velocity on early lateral mitral annular diastolic velocity ratio (E/Ea) ratio was 18±7, and the mean pulmonary capillary wedge pressure (PCWP) by RHC was 18±8 mm Hg. There was no correlation between BNP (r=0.260, p=0.774) or NT-proBNP (r=−0.103, p=0.984) levels and PCWP. There was a slight correlation between E/Ea ratio and PCWP (r=0.337, p=0.029). E/Ea ratio >14 performed poorly in differentiating elevated and low LVFP.
Conclusion
In patients with CA, NPs do not accurately estimate PCWP. Tissue Doppler-derived mitral E/Ea ratio is correlated with PCWP, but the slight correlation requires to estimate LVFP in a broad clinical and imaging context to avoid diagnostic errors.
Keywords: cardiac amyloidosis, ventricular filling pressure, natriuretic peptides, echocardiography, tissue doppler imaging
Key questions.
What is already known about this subject?
Assessment of left ventricular filling pressure can be challenging in patients with cardiac amyloidosis.
Doppler parameters and natriuretic peptides are widely used and validated in this indication among patients with heart failure.
However, the extent of diastolic dysfunction and the amyloid fibrils-related toxicity in patients with cardiac amyloidosis raises questions about the validity of these parameters in this population.
What does this study add?
In the specific population of patients with cardiac amyloidosis, natriuretic peptides and Doppler parameters do not accurately estimate pulmonary capillary wedge pressure.
Natriuretic peptide levels are elevated, regardless of the pulmonary capillary wedge pressure.
The recommended early diastolic mitral inflow velocity on early lateral mitral annular diastolic velocity ratio (E/Ea) ratio cut-off performs poorly in differentiating elevated and low left ventricular filling pressure.
How might this impact on clinical practice?
In patients with cardiac amyloidosis, left ventricular filling pressure must be estimated in a broad clinical and imaging context to avoid diagnostic errors.
Introduction
Elevated left ventricular (LV) filling pressure is a cardinal feature of cardiac amyloidosis (CA), and the control of fluid retention remains generally the cornerstone of symptom management of CA. The increased myocardial stiffness induced by amyloid deposition leads to a shift in the diastolic pressure–volume relationship, with a rapid increase in pressure during diastole despite a small increase in LV chamber volume, leading to high LV end-diastolic pressure.1 Consequently, in patients with CA, filling is reliant on high left atrial pressure to push blood into the LV chamber. Amyloid deposition in the heart typically presents as restrictive cardiomyopathy, often with disproportionate signs of right ventricular failure (oedema, raised jugular venous pressure and congestive hepatomegaly), while low cardiac output and hypotension are features of advanced disease.2 In the later stages of CA, overdose of diuretics and low LV filling pressure can worsen hypotension, decrease cardiac output and lead to symptoms of fatigue, with signs of hypoperfusion. LV filling pressure assessment is therefore of major importance in this population.
Natriuretic peptides and tissue Doppler imaging may be used to monitor LV filling pressure in patients with CA. The aim of this study was to evaluate the value of natriuretic peptides and Doppler parameters in estimating LV filling pressure in patients with CA.
Methods
Study population
Fifty unselected patients with CA followed at Toulouse University Hospital, France, who underwent right heart catheterisation were retrospectively enrolled in this cross-sectional study. Patients who underwent transthoracic echocardiography and natriuretic peptides assays performed within 24 hours of right heart catheterisation were included in the analysis.
Diagnosis of CA was defined echocardiographically as end-diastolic thickness of the interventricular septum >12 mm in the absence of any other cause of ventricular hypertrophy3 and apex-to-base gradient in regional longitudinal strain (LS) >1.04 in the presence of systemic amyloidosis (for AL, AA and apolipoprotein A2 CA), or grade 2 or 3 cardiac uptake on a 99mTc-hydroxymethylene-diphosphonate scintigraphy and the absence of a detectable monoclonal protein (for transthyretin amyloidosis (TTR) CA).5 Systemic amyloidosis was defined by histological documentation of Congo Red staining and apple-green birefringence under cross-polarised light in at least one organ.6 For patients with TTR CA, familial TTR (mTTR) amyloidosis identification was based on identification of a pathogenic TTR gene mutation by DNA analysis.
Biochemistry
Blood chemistry parameters including creatinine and N-terminal pro-hormone brain natriuretic peptide (NT-proBNP) were measured by standard automated commercial techniques. Renal function was expressed as an estimated glomerular filtration rate (eGFR), which was calculated according to the modification of diet in renal disease formula. Plasma levels of brain natriuretic peptide (BNP) and NT-proBNP were quantified with an electrochemiluminescence immunoassay kit (Roche Diagnostics, Mannheim, Germany) from venous whole blood samples.
Echocardiography
Transthoracic echocardiography was performed with a commercially available ultrasound Vivid E9 System (GE Vingmed Ultrasound AS, Horten, Norway) using a 2.5 MHz transducer allowing a full-fledged analysis of archived sequences. A complete M-mode and two-dimensional (2D) greyscale echocardiography including the three standard apical views (four-chamber, three-chamber and two-chamber) using high frame rates (>60 frames/s) was performed for each patient.
Image analysis was independently performed by a single blinded observer unaware of clinical, biological and right heart catheterisation data (SB) using the offline EchoPAC V.110.1.2 software (Advanced Analysis Technologies; GE Medical Systems). The following measurements were performed according to the American Society of Echocardiography guidelines7: LV ejection fraction using the biplane Simpson’s method from apical two-chamber and four-chamber windows, left atrial volume index using a biplane area-length formula, end-diastolic interventricular septal and posterior wall thickness, and LV internal dimension in diastole. LV mass index was calculated based on modelling the LV as an ellipse. The mean LV wall thickness was calculated as (interventricular septum thickness + posterior wall thickness)/2. Diastolic parameters, including peak early (E) and late (A) diastolic mitral inflow velocity and its ratio (E/Ea), deceleration time, and average of the medial and lateral mitral annular diastolic velocities (Ea), were also measured according to the American Society of Echocardiography guidelines. All Doppler measurements were made over three cardiac cycles and averaged. In patients with atrial fibrillation, the data were averaged over five cardiac cycles.8 LS was calculated using speckle tracking from 2D greyscale images and the automated function imaging technique from the apical four-chamber, two-chamber and three-chamber views.9 Strain values from all segments were averaged to obtain a global LS value. The strain values for the six basal, six mid and six apical segments of the LV were averaged to obtain three regional LS values. The apex-to-base gradient in regional LS was calculated as: average apical LS/(average basal LS + mid-LS).4
Invasive measurements
A Swan-Ganz catheter guided by fluoroscopy was used for right heart catheterisation. At end expiration, pulmonary artery pressure and pulmonary capillary wedge pressure (PCWP) were measured using a haemodynamic monitoring system calibrated before each study. Cardiac output was calculated by thermodilution as a mean of three consecutive measurements not varying by more than 10%. All invasive parameters were measured with the patient in the supine position, at rest.
Statistical analysis
Continuous variables were expressed as mean±SD. Because NT-proBNP and furosemide dose were not normally distributed, results were presented as the median value and IQR. Nominal values were expressed as numbers and percentages. Association between the mean values of continuous variables was compared using the Mann-Whitney rank-sum test. Nominal variables were investigated by the Fisher’s exact test. Relationships between variables were assessed using Spearman correlation analysis and expressed by R. The accuracy of the mitral E/Ea ratio for predicting PCWP >15 mm Hg was assessed by computing the areas under the receiver operator characteristic (ROC) curves. Differences were considered statistically significant for p values of <0.05. All analyses were performed using standard statistical software, SPSS V.20.
Results
Studied sample
Fifty patients with CA were retrospectively enrolled in the study: 31 (62%) light chains amyloidosis (AL), 17 (34%) TTR, 1 (2%) AA and 1 (2%) hereditary apolipoprotein A2 amyloidosis. For AL, AA and apolipoprotein A2 CA (33 patients), organ involvement was proved by endomyocardial, kidney, lung, digestive, salivary gland and periumbilical fat biopsies in 13 (39%), 9 (27%), 1 (3%), 4 (12%), 3 (9%) and 1 (3%) patients, respectively. Among the 17 patients with TTR amyloidosis, 7 (41%) had TTR gene mutation (3 V30M, 2 V122I, 1 G26S and 1 S77T). All patients underwent right heart catheterisation. Among them, 48 (96%) and 43 (86%) had assays of natriuretic peptides and transthoracic echocardiography performed within 24 hours of right heart catheterisation, respectively. Forty-one (82%) had both natriuretic peptides assays and transthoracic echocardiography on the same day as right heart catheterisation. Among patients who had natriuretic peptides assays, 27 (56%), 20 (46%) and 1 (2%) underwent NT-proBNP, BNP, or both NT-proBNP and BNP, respectively.
The baseline characteristics and treatment of the 50 patients enrolled are summarised in table 1. Thirty-seven (74%) were men and the mean age was 68±11 years. Most of the patients were symptomatic: 4 (8%), 21 (42%), 21 (42%) and 4 (8%) were classified as New York Heart Association class I, II, III and IV, respectively. The mean LV ejection fraction and global LS were 51%±13% and −10±4%, respectively.
Table 1.
Population demographics
| All | AL | TTR | Other | |
| n=50 | n=31 | n=17 | n=2 | |
| Age, years | 68±11 | 65±10 | 77±9 | 58±4 |
| Men, n (%) | 37 (74) | 19 (61) | 16 (94) | 0 |
| Weight, kg | 73±13 | 74±14 | 74±9 | 49±10 |
| Height, m | 1.69±0.08 | 1.69±0.08 | 1.71±0.06 | 1.56±0.09 |
| Body mass index, kg/m2 | 26±4 | 26±4 | 25±3 | 20±2 |
| Diabetes mellitus, n (%) | 6 (13) | 3 (10) | 3 (18) | 0 |
| Hypertension, n (%) | 23 (48) | 13 (42) | 9 (53) | 2 (100) |
| Hypercholesterolaemia, n (%) | 13 (27) | 7 (23) | 6 (35) | 0 |
| Smoking, n (%) | 5 (10) | 5 (10) | 0 | 0 |
| NYHA stage, n (%) | 48 (96) | 29 (94) | 16 (94) | 2 (100) |
| I | 1 (2) | 0 | 1 (6) | 0 |
| II | 21 (42) | 12 (39) | 7 (41) | 2 (100) |
| III | 21 (42) | 13 (42) | 8 (47) | 0 |
| IV | 4 (8) | 4 (13) | 0 | 0 |
| Systolic blood pressure, mm Hg | 118±20 | 112±17 | 128±16 | 120±28 |
| Diastolic blood pressure, mm Hg | 69±11 | 69±8 | 70±8 | 80±14 |
| Heart rate (beats/min) | 77±16 | 84±10 | 65±10 | 81±1 |
| Pacemaker, n (%) | 10 (20) | 3 (10) | 7 (41) | 0 |
| Atrial fibrillation, n (%) | 21 (44) | 10 (33) | 10 (59) | 1 (50) |
| Medication at inclusion | ||||
| ACEI/ARB, n (%) | 17 (35) | 11 (35) | 7 (42) | 1 (50) |
| Furosemide, n (%) | 43 (86) | 27 (87) | 16 (94) | 0 |
| Posology (mg/day) | 226 (80–250) | 243 (80–250) | 197 (80–229) | 0 |
| Mean eGFR (mL/min), n (%) | 46±21 | 48±30 | 44±18 | 38±30 |
| eGFR <30 mL/min | 13 (26) | 6 (19) | 6 (35) | 1 (50) |
| 30≤ eGFR <45 mL/min | 12 (24) | 9 (29) | 4 (23) | 0 |
| 45≤ eGFR <60 mL/min | 9 (18) | 5 (16) | 3 (18) | 0 |
| eGFR ≥60 mL/min | 16 (32) | 11 (35) | 4 (23) | 1 (50) |
ACEI, ACE inhibitor;AL, light chains amyloidosis; ARB, angiotensin II receptor antagonist;eGFR, estimated glomerular filtration rate;NYHA, New York Heart Association; TTR, transthyretin amyloidosis.
Invasive haemodynamic measurements, natriuretic peptides assays and echocardiographic data
Adequate mitral inflow and tissue Doppler signals were collected in all patients enrolled in the echocardiographic group. Haemodynamic, peptide natriuretic and echocardiographic measurements for all patients stratified according to the type of amyloidosis are presented in table 2.
Table 2.
Invasive haemodynamic measurements, natriuretic peptides assays and echocardiographic data
| All | AL | TTR | Other | |
| Right h eart c atheteri ation | n = 50 | n = 31 | n = 17 | n = 2 |
| CO, L/min | 4±1 | 4.1±1.2 | 3.9±0.7 | 3±1.4 |
| CI, L/min/m2 | 2.2±0.6 | 2.3±0.6 | 2.2±0.4 | 2.1±0.9 |
| Systolic PAP | 40±14 | 39±13 | 43±15 | 27±4 |
| Mean PAP | 25±8 | 24±9 | 26±8 | 17±3 |
| PCWP | 18±8 | 17±8 | 19±6 | 9±1 |
| Natriuretic peptides | n=48 | n=30 | n=16 | n=2 |
| BNP | 1000 (243–1477) | 858 (227–1477) | 721 (252–1255) | 4798 |
| NT-proBNP | 10 106 (2935–13 348) | 13 855 (3315–18 846) | 3346 (2587–3496) | 3456 |
| Echocardiography | n=43 | n=27 | n=15 | n=1 |
| LV septum, mm | 19±2 | 20±2 | 19±5 | 15±7 |
| LV posterior, mm | 20±2 | 21±3 | 19±6 | 10±1 |
| LVEDD, mm | 51±5 | 55±7 | 43±10 | 50±14 |
| LV mass (Penn) | 345±162 | 299±114 | 441±210 | 297±98 |
| LV mass (ASE) | 287±130 | 250±91 | 365±169 | 248±78 |
| LVEDV, mL | 91±34 | 89±32 | 95±40 | 95±22 |
| LVEF, % | 51±13 | 50±11 | 54±14 | 38±28 |
| GLS, % | −10±4 | −10±4 | −9±4 | −11 |
| LA volume, mL | 47±16 | 47±17 | 46±14 | 41±13 |
| E velocity, cm/s | 92±27 | 90±29 | 95±23 | 87±2 |
| DT, ms | 174±59 | 167±58 | 176±44 | 257±138 |
| E/A | 1.8±0.9 | 1.8±0.9 | 1.8±0.8 | 0.5±0.7 |
| Ea lateral, cm/s | 5±2 | 5±2 | 5±2 | 6±1 |
| E/Ea lateral | 18±7 | 17±7 | 19±8 | 22±1 |
ASE, American Society of Echocardiography;BNP, brain natriuretic peptide;CI, cardiac index;CO, cardiac output;DT, deceleration time;E/A, ratio of early (E) and late (A) diastolic mitral inflow velocity; Ea, early lateral mitral annular diastolic velocity; GLS, global longitudinal strain;LA, left atrial;LV, left ventricular;LVEDD, left ventricular end-diastolic diameter;LVEDV, left ventricular end-diastolic volume;LVEF, left ventricular ejection fraction;NT-proBNP, N-terminal pro-hormone brain natriuretic peptide;PAP, pulmonary arterial pressure;PCWP, pulmonary capillary wedge pressure; TTR, transthyretin amyloidosis.
The relationships between PCWP and different echocardiographic parameters are shown in table 3. A weak but statistically significant positive correlation was found between PCWP and mitral E/Ea ratio. This positive correlation was still persistent for patients with AL amyloidosis but no longer for TTR amyloidosis. A trend for a negative correlation was found between PCWP and mitral deceleration time and Ea lateral velocity in the whole population. This trend was no longer observed after subgroup analysis by type of amyloidosis.
Table 3.
Correlation coefficients of echocardiographic variables with PCWP
| All | AL | TTR | ||||
| n= 43 | n= 27 | n= 15 | ||||
| r | P value | r | P value | r | P value | |
| LV mass | 0.021 | 0.898 | 0.218 | 0.295 | −0.561 | 0.058 |
| LVEDV | 0.012 | 0.941 | 0.215 | 0.281 | −0.307 | 0.307 |
| Left atrial volume index | 0.180 | 0.279 | 0.190 | 0.351 | −0.196 | 0.588 |
| E velocity | 0.364 | 0.015 | 0.301 | 0.120 | 0.616 | 0.014 |
| DT | −0.289 | 0.064 | −0.289 | 0.144 | −0.080 | 0.795 |
| Ea lateral | −0.215 | 0.071 | −0.313 | 0.105 | 0.376 | 0.206 |
| E/Ea lateral | 0.337 | 0.029 | 0.460 | 0.016 | −0.092 | 0.755 |
DT, deceleration time;E, early diastolic mitral inflow velocity; Ea, early lateral mitral annular diastolic velocity; LV, left ventricular;LVEDV, left ventricular end-diastolic volume; PCWP, pulmonary capillary wedge pressure; TTR, transthyretin amyloidosis.
For patients in sinus rhythm (n=30), there was a good positive correlation between mitral E/A ratio and PCWP (r=0.664, p<0.001). This positive correlation was still persistent for patients with AL amyloidosis (n=20, r=0.755, p<0.001) but no longer for TTR amyloidosis (n=10, r=0.159, p=0.683).
PCWP and mitral E/Ea ratio
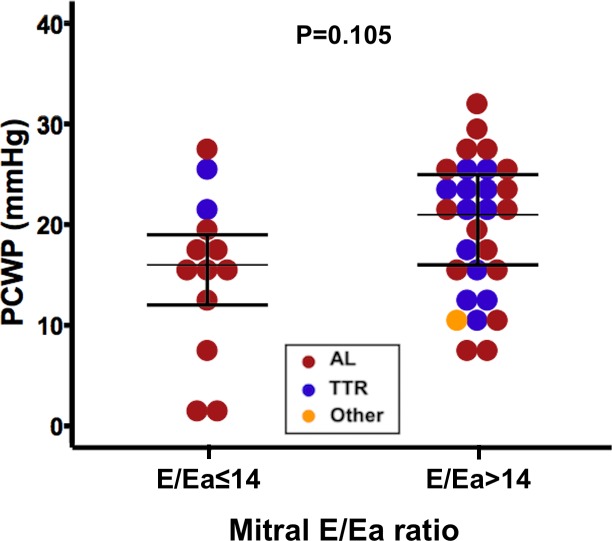
A weak but statistically significant positive correlation was found between PCWP and mitral E/Ea ratio in the lateral annulus (figure 1). However, as illustrated in figure 2, even a low mitral E/Ea ratio could be associated with an elevated PCWP and vice versa.
Figure 1.
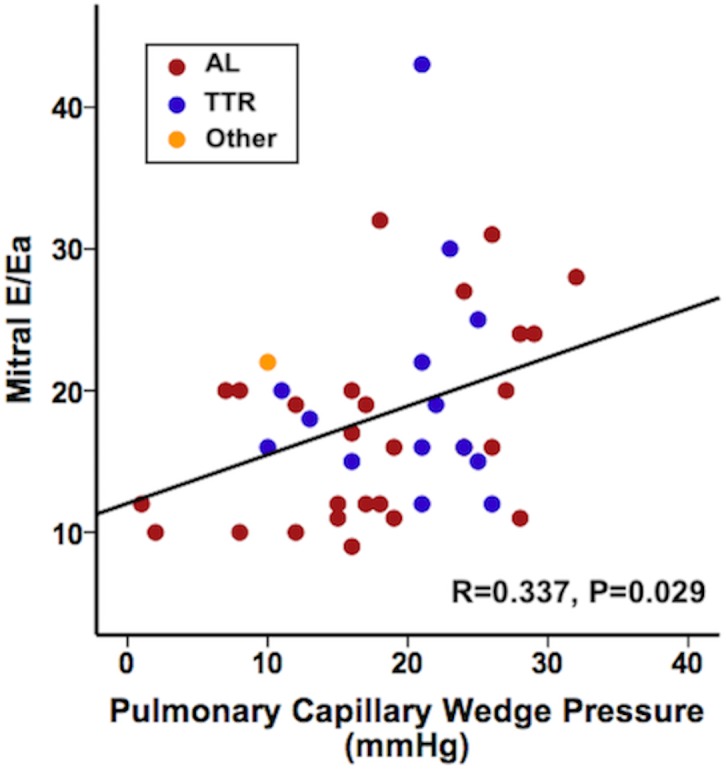
Relation between mitral E/Ea ratio and pulmonary capillary wedge pressure. E, early diastolic mitral inflow velocity; Ea, average of the medial and lateral mitral annular diastolic velocities; TTR, transthyretin amyloidosis.
Figure 2.
PCWP by mitral E/Ea ratio groups using the recommended cut-off of 14. E, early diastolic mitral inflow velocity; Ea, average of the medial and lateral mitral annular diastolic velocities; PCWP, pulmonary capillary wedge pressure; TTR, transthyretin amyloidosis.
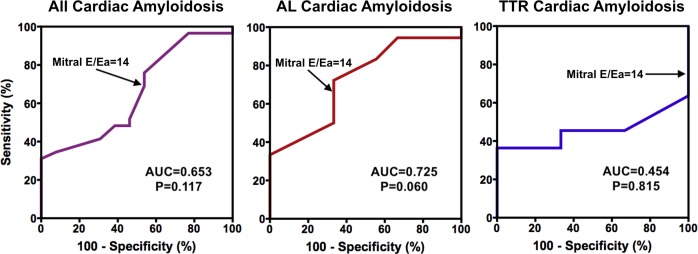
Clinical accuracy of mitral E/Ea ratio to predict PCWP
As illustrated in figure 3, mitral E/Ea ratio was not suitable for diagnostic elevated PCWP as its ROC curve area was not significantly greater than 0.50. Sensitivity and specificity for mitral E/Ea ratio >14 to identify a PCWP >15 mm Hg were 74% and 46%, respectively. For patients with AL amyloidosis, they were 72% and 66%, respectively. For patients with TTR amyloidosis, they were 73% and 0%, respectively.
Figure 3.
Receiver operating characteristic curves for the prediction of pulmonary capillary wedge pressure >15 mm Hg for mitral E/Ea ratio. AUC, area under the curve; E, early diastolic mitral inflow velocity; Ea, average of the medial and lateral mitral annular diastolic velocities; TTR, transthyretin amyloidosis.
PCWP and natriuretic peptides
Among the 48 patients who had natriuretic peptide assays, 20 (42%) patients had BNP assay, 27 (56%) patients had NT-proBNP assay and 1 (2%) patient had both BNP and NT-proBNP assays. As illustrated in figure 4, there was no correlation between PCWP and peptide natriuretic levels. This lack of correlation was similar when patients with chronic kidney disease were excluded from the analysis (r=−0.538, p=0.271 for BNP and r=−0.363, p=0.303 for NT-proBNP). There was no correlation between PCWP and eGFR (r=−0.177, p=0.224). After stratification according to the type of amyloidosis, among patients with AL amyloidosis, BNP was positively correlated with PCWP (figure 4, bottom), whereas there was no correlation between NT-proBNP and PCWP, nor among patients with TTR amyloidosis.
Figure 4.
Relation between natriuretic peptides and pulmonary capillary wedge pressure. BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro-hormone brain natriuretic peptide; TTR, transthyretin amyloidosis.
Discussion
This study demonstrates that daily used biological and echocardiographic parameters for the estimation of LV filling pressure have a poor accuracy in estimating PCWP in patients with CA. Natriuretic peptide levels are elevated in CA, regardless of the PCWP, and echocardiographic mitral E/Ea ratio has a significant, but modest, correlation with PCWP. Consequently, the recommended E/Ea ratio cut-off performs poorly in differentiating elevated and low LV filling pressure.
Natriuretic peptides
Natriuretic peptides are currently used for diagnosis, prognosis and assessment of the response to treatment in both AL and TTR CA. They are released in response to ventricular pressure and stretch.10 A study has postulated that, in CA, both cardiomyocyte compression and stretching by amyloid deposits and elevated ventricular filling pressures by diastolic dysfunction may be responsible for peptide natriuretic elevation.11 Plasma natriuretic peptides are frequently elevated in patients with CA, even in the absence of heart failure,12 and increased NT-proBNP is associated with early cardiac involvement in patients with mTTR amyloidosis.13
In patients with AL CA, natriuretic peptide synthesis has been shown to be modulated by cardiotoxic light chain amyloid precursor and is a marker of amyloid-related toxicity.14 Several reports, especially in the AL-related forms, have brought the use of natriuretic peptides changes as an index of response to therapy. Thus, despite similar LV mass and renal function, patients with TTR CA showed lower levels of natriuretic peptides than patients with AL amyloidosis.15 However, in our study, there appears to be a correlation between BNP levels and PCWP. However, given the physiology of the BNP in patients with AL amyloidosis and with regard to the dispersion of the values, it seems that this correlation is fortuitous, which will however have to be confirmed by future studies. The fortuitous nature of this correlation is consistent with regard to the inverse correlation between BNP levels and PCWP found in patients with amyloid TTR.
NT-proBNP has a longer half-life than BNP and is subject to renal clearance.16 Renal failure is a common feature of AL amyloidosis and is not infrequent in elderly patients with wild-type TTR amyloidosis. However, our results show that regardless of renal function, NT-proBNP levels are not correlated with LV filling pressures.
Consequently, our results, with regard to previous reports, suggest that increased natriuretic peptides levels are a consequence of cardiac involvement in amyloidosis,14 17 and not only the consequence of the extent of diastolic dysfunction and the increase of LV filling pressures.
Mitral E/Ea ratio
Mitral E/Ea ratio is recommended in daily practice for evaluation of LV filling pressures.8 It has been validated in general population of patients with and without heart failure, and guidelines have been adjusted for patients with LV hypertrophy, who typically represent patients with heart failure and preserved ejection fraction. However, in patients with heart failure and preserved ejection fraction, E/Ea ratio has been shown to neither accurately estimate PCWP nor identify patients with elevated PCWP.18 Heart failure with preserved ejection fraction encompasses a wide variety of aetiologies, and it has been shown that ~15% of patients with heart failure and preserved ejection fraction have CA.19 20 It is legitimate to suppose that in patients with CA, Ea wave by tissue Doppler imaging does not reflect relaxation capacities of the myocardium by itself but is impacted and decreased by myocardial infiltration by amyloid deposition. This could explain the modest correlation between E/Ea ratio and PCWP and the lack of efficiency to differentiate elevated and low LV filling pressure. Consequently, E/Ea cannot be recommended to evaluate LV filling pressures in patients with CA.
Other echocardiographic parameters
In patients with normal LV ejection fraction, E/Ea ratio, Ea velocities and left atrial volume index are recommended for the diagnosis of LV diastolic dysfunction, and by extension the estimation of LV filling pressure.8 However, in our study, although there was a trend for some of these parameters, only the E/Ea ratio was slightly correlated with PCWP.
In the earlier stages of CA, diastolic function can vary from grade 1 diastolic dysfunction with impaired relaxation and normal LV filling pressures to grade 2 (pseudonormalisation). In later stages, grade 3 diastolic dysfunction occurs when LV relaxation is impaired along with markedly elevated LV filling pressures.21 Restrictive cardiomyopathy and elevated LV filling pressure are associated with elevated mitral E/A ratio, short deceleration time and decreased mitral annular velocity. Our study confirms that E/A ratio is probably the best echocardiographic parameter for the estimation of LV filling pressure. However, the positive correlation between mitral E/A ratio and PCWP was no longer found in patients with TTR amyloidosis. Furthermore, atrial fibrillation prevalence has been reported to be 15% in patients with CA.22 In our study, it reached 40%. All these findings limit the use of mitral E/A ratio for the estimation of LV filling pressure in the whole population of patients with CA.
A recent study shows that follow-up care directed by echocardiography and natriuretic peptides improves survival in ambulatory patients with heart failure and reduced or mildly reduced LV ejection fraction with respect to patients followed by conventional clinical parameters.23 However, as most previous studies, this study did not include patients with CA and is not applicable to this specific population. Nevertheless, the combination of parameters probably remains the best way to follow patients with heart failure and particularly CA, rather than relying on a single parameter.
Limitations
Our study has all the limitations associated with retrospective, single-site and limited sample studies. However, while not providing any definitive conclusions, this study suggests vigilance in the interpretation of the daily used parameters for the estimation of LV filling pressure in the specific population of patients with CA.
The patients were evaluated during resting conditions. However, a recent study evaluated changes in LV filling pressure during exercise in patients with CA: a pronounced increase in LV filling pressure was demonstrated with exercise, which limits the value of resting right heart catheterisation in this population.24 It is possible that the value of natriuretic peptides reflects both resting and exercise conditions, whereas right heart catheterisation only reflects resting conditions.
As mentioned above, BNP levels appear to be correlated with PCWP in patients with AL amyloidosis, which is not concordant with the whole results of the study. We think that this correlation is fortuitous. However, these findings have to be confirmed by future studies.
Finally, Ea septal annulus and E/Ea ratio using septal annulus are missing. Guidelines recommend to average Ea septal and lateral annulus to calculate the E/Ea ratio.8 Ea septal annulus is not used in daily practice, which explained these missing data in our retrospective study. However, Ea septal annulus has been shown to correlate well with Ea lateral annulus. Consequently, it should be surprising that Ea septal annulus assessment changes the results of our study.
Conclusion
Daily used biological and echocardiographic parameters for the estimation of LV filling pressure have a poor accuracy in estimating PCWP in patients with CA. Natriuretic peptide levels are elevated, regardless of the PCWP, and echocardiographic parameters have a modest correlation with PCWP. The recommended E/Ea ratio cut-off performs poorly in differentiating elevated and low LV filling pressure. Consequently, LV filling pressure must be estimated in a broad clinical and imaging context to avoid diagnostic errors in patients with CA.
Footnotes
Contributors: SB and EC collected, analysed and interpreted the clinical, biological and echographic data. PF collected and analysed the haemodynamic data. AH performed the statistical analysis. DC and MG critically contributed to the manuscript. SF contributed to the discussion and review. DR led the study, revised the manuscript and gave final approval of the version to be published. OL designed the study and wrote the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The investigation conforms to the principles outlined in the Declaration of Helsinki. All patients were informed at admission that their clinical data could be used for research purpose and they gave their consent. The study was approved by the French Data Protection Authority (Commission Nationale de l’Informatique etdes Libertés, #2 171 433 v0).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information.
Contributor Information
Toulouse Amyloidosis Research Network collaborators:
Daniel Adoue, Laurent Alric, Léonardo Astudillo, Pauline Bernadet Monrozies, Dominique Chauveau, Pascal Cintas, Magali Colombat, Audrey Delas, Delphine Dupin-Deguine, Joelle Guitard, Bénédicte Puissant, Grégory Pugnet, Grégoire Prévot, Murielle Roussel, and Laurent Sailler
References
- 1. Borlaug BA, Kass DA. Invasive hemodynamic assessment in heart failure. Cardiol Clin 2011;29:269–80. 10.1016/j.ccl.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 2. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. The Lancet 2016;387:2641–54. 10.1016/S0140-6736(15)01274-X [DOI] [PubMed] [Google Scholar]
- 3. Gertz MA, Comenzo R, Falk RH, et al. . Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on amyloid and amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol;2005:319–28. [DOI] [PubMed] [Google Scholar]
- 4. Phelan D, Collier P, Thavendiranathan P, et al. . Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012;98:1442–8. 10.1136/heartjnl-2012-302353 [DOI] [PubMed] [Google Scholar]
- 5. Gillmore JD, Maurer MS, Falk RH, et al. . Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–12. 10.1161/CIRCULATIONAHA.116.021612 [DOI] [PubMed] [Google Scholar]
- 6. Arbustini E, Verga L, Concardi M, et al. . Electron and immuno-electron microscopy of abdominal fat identifies and characterizes amyloid fibrils in suspected cardiac amyloidosis. Amyloid 2002;9:108–14. 10.3109/13506120208995243 [DOI] [PubMed] [Google Scholar]
- 7. Gottdiener JS, Bednarz J, Devereux R, et al. . American Society of echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr 2004;17:1086–119. 10.1016/j.echo.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 8. Nagueh SF, Smiseth OA, Appleton CP, et al. . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. 10.1093/ehjci/jew082 [DOI] [PubMed] [Google Scholar]
- 9. Voigt J-U, Pedrizzetti G, Lysyansky P, et al. . Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1–11. 10.1093/ehjci/jeu184 [DOI] [PubMed] [Google Scholar]
- 10. Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology 1993;132:1961–70. 10.1210/endo.132.5.8477647 [DOI] [PubMed] [Google Scholar]
- 11. Takemura G, Takatsu Y, Doyama K, et al. . Expression of atrial and brain natriuretic peptides and their genes in hearts of patients with cardiac amyloidosis. J Am Coll Cardiol 1998;31:754–65. 10.1016/S0735-1097(98)00045-X [DOI] [PubMed] [Google Scholar]
- 12. Nordlinger M, Magnani B, Skinner M, et al. . Is elevated plasma B-natriuretic peptide in amyloidosis simply a function of the presence of heart failure? Am J Cardiol 2005;96:982–4. 10.1016/j.amjcard.2005.05.057 [DOI] [PubMed] [Google Scholar]
- 13. Damy T, Deux J-F, Moutereau S, et al. . Role of natriuretic peptide to predict cardiac abnormalities in patients with hereditary transthyretin amyloidosis. Amyloid 2013;20:212–20. 10.3109/13506129.2013.825240 [DOI] [PubMed] [Google Scholar]
- 14. Merlini G, Lousada I, Ando Y, et al. . Rationale, application and clinical qualification for NT-proBNP as a surrogate end point in pivotal clinical trials in patients with al amyloidosis. Leukemia 2016;30:1979–86. 10.1038/leu.2016.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perfetto F, Bergesio F, Grifoni E, et al. . Different NT-proBNP circulating levels for different types of cardiac amyloidosis. J Cardiovasc Med 2016;17:810–7. 10.2459/JCM.0000000000000349 [DOI] [PubMed] [Google Scholar]
- 16. Emdin M, Passino C, Prontera C, et al. . Comparison of brain natriuretic peptide (BNP) and amino-terminal proBNP for early diagnosis of heart failure. Clin Chem 2007;53:1289–97. 10.1373/clinchem.2006.080234 [DOI] [PubMed] [Google Scholar]
- 17. Klaassen SHC, Tromp J, Nienhuis HLA, et al. . Frequency of and prognostic significance of cardiac involvement at presentation in hereditary Transthyretin-Derived amyloidosis and the value of N-terminal pro-B-type natriuretic peptide. Am J Cardiol 2018;121:107–12. 10.1016/j.amjcard.2017.09.029 [DOI] [PubMed] [Google Scholar]
- 18. Santos M, Rivero J, McCullough SD, et al. . E/e’ ratio in patients with unexplained dyspnea: lack of accuracy in estimating left ventricular filling pressure. Circ Heart Fail 2015;8:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohammed SF, Mirzoyev SA, Edwards WD, et al. . Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014;2:113–22. 10.1016/j.jchf.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bennani Smires Y, Victor G, Ribes D, et al. . Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging 2016;32:1403–13. 10.1007/s10554-016-0915-z [DOI] [PubMed] [Google Scholar]
- 21. Klein AL, Hatle LK, Burstow DJ, et al. . Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol 1989;13:1017–26. 10.1016/0735-1097(89)90254-4 [DOI] [PubMed] [Google Scholar]
- 22. Longhi S, Quarta CC, Milandri A, et al. . Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid 2015;22:147–55. 10.3109/13506129.2015.1028616 [DOI] [PubMed] [Google Scholar]
- 23. Bajraktari G, Pugliese NR, D’Agostino A, et al. . Echo- and B-type natriuretic Peptide-Guided follow-up versus Symptom-Guided follow-up: comparison of the outcome in ambulatory heart failure patients. Cardiol Res Pract 2018;2018:1–8. 10.1155/2018/3139861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clemmensen TS, Mølgaard H, Sörensen J, et al. . Inotropic myocardial reserve deficiency is the predominant feature of exercise haemodynamics in cardiac amyloidosis. Eur J Heart Fail 2017;19:1457–65. 10.1002/ejhf.899 [DOI] [PubMed] [Google Scholar]