Abstract
This study aimed to determine dolutegravir cerebrospinal fluid (CSF) diffusion in 13 patients with HIV-related cerebral impairment enrolled in a real-life observational study. Dolutegravir median (range) CSF concentration [9.6 (3.6–22.8) ng/mL] reached CSF therapeutic concentrations whatever the blood-brain barrier status and diffused in correlation with the albumin quotient (P = .0186).
Keywords: cerebrospinal fluid, central nervous system diffusion, central nervous system impairment, dolutegravir, pharmacokinetics
The major obstacle to a functional cure for human immunodeficiency virus (HIV) infection is the persistence of a latent HIV reservoir. This residual viral replication results partly from the existence of pharmacological sanctuaries largely impenetrable to drugs. A recent study showed that the virus can replicate in these sanctuaries, in which there is less antiviral pressure, contributing to the continual replenishment of viral reservoirs [1].
HIV is known to affect the blood-brain barrier (BBB) leading to intrathecal immunoactivation and neuronal injury [2]. It also may be the cause of neurocognitive disorders in a significant proportion of patients [3]. The role of the brain as a latent reservoir for HIV must be considered in the development of therapeutic strategies for eliminating HIV from infected subjects [4].
Dolutegravir (DTG) is increasingly being used in combination with nucleoside/nucleotide reverse transcriptase inhibitors. Recently, the World Health Organization (WHO) has even issued new antiretroviral (ART) treatment guidelines recommending DTG-based treatment as the preferred first-line treatment option for all adults, adolescents, and children, including women and adolescent girls who have access to consistent and reliable contraception [5]. DTG is well tolerated and rapidly inhibits viral replication [6].
Following on from the study that has characterized the cerebrospinal fluid (CSF) diffusion of DTG in antiretroviral therapy–naive, HIV-1–infected adults [7], we aimed to determine if DTG diffusion is also sufficient to reach effective concentrations in the CSF in treatment-experienced patients with HIV-related CNS (central nervous system) impairment (HCI).
The primary endpoint is the evaluation of DTG CSF concentration in treatment-experienced patients with HCI. Secondary endpoints are to evaluate the DTG diffusion and its correlation with the albumin quotient used to evaluate BBB status.
METHODS
Patients and Study Design
All of the patients included in this monocenter, prospective, single arm, open label, observational study were admitted to the Neuro-HIV Rehabilitation Care Unit (Assistance Publique-Hôpitaux de Paris, Hôpitaux Uuniversitaires Paris -Sud, Bicêtre Hospital, Le Kremlin-Bicêtre, France) between September 2015 and October 2016. Blood and CSF samples were collected simultaneously during routine care and at any time during the dosing interval. Plasma and CSF HIV-RNA levels were determined by reverse transcriptase-polymerase chain reaction (RT-PCR) (Abbott RealTime M2000, detection threshold = 40 copies/mL [Abbott Laboratories, Abbott Park, IL]). For this research, monitoring was conducted on a bank comprising routine blood and CSF samples. Therefore, patient care did not differ from usual care, thus the non-interventional nature of this research. French regulation did not require an ethic committee’s agreement for use of health data or biobank, nor a written consent. However, each patient admitted to the hospital received written information stating that medical records and biological samples drawn for care might be used for medical research. Each patient had the right to express opposition; none of the patients who participated in this study expressed opposition.
DTG Concentration and Diffusion
Bound and unbound plasma DTG were separated by ultrafiltration (Centrifree devices, cutoff, 30 kDa; Millipore, Molsheim, France). Total plasma, unbound plasma, and CSF DTG concentrations were determined with a quality control-validated method based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Acquity TQD-UPLC system, Waters Corp., Milford, MA ). Lower limits of quantification for total plasma concentration was 2 ng/mL or unbound or CSF DTG concentration was 0.5 ng/mL each.
The unbound fraction (FuDTG) was determined as the ratio of unbound to total plasma DTG concentrations. DTG diffusion was assessed by calculating the ratio of total CSF to total plasma DTG concentrations (QDTG), and BBB permeability was assessed by calculating the albumin quotient, as the ratio of CSF albumin (mg/L) to plasma albumin (g/L) concentrations [8]. A BBB was defined as damaged if QA was over 6.8 for patients younger than 45 years and over 10.2 for patients over 45 years of age.
Pharmacokinetic and Statistical Analysis
The correlations between total plasma DTG concentrations, unbound plasma DTG concentrations, and total CSF DTG concentrations were analyzed with Pearson’s correlation analysis. Similar analyses were performed to assess the correlation between the diffusion of DTG into the CSF and albumin quotient.
Wilcoxon rank sum tests were used to analyze CSF DTG concentrations as a function of CSF HIV-RNA levels (detectable versus undetectable).
All numerical variables are expressed as medians and ranges unless otherwise indicated. The data were analyzed with GraphPad Prism (version 6.00 for MacOS, GraphPad Software, San Diego, CA). Differences were considered significant if P < .05.
RESULTS
Characteristics of the Population
Thirteen patients (5 women and 8 men) were enrolled in the study. The median age of the patients was 46 years (range: 39–56 years). All patients had at least 1 HCI: HIV encephalitis (n = 6), progressive multifocal leukoencephalopathy (PML) (n = 4), cerebral toxoplasmosis (n = 2), or herpes simplex virus-2 encephalitis associated with PML (n = 1). The duration of the current ART regimen was 41 days (8–343 days) and median CD4 cell count was 258/µL (range: 52–646/µL). The other antiretroviral drugs administered together with DTG were the following: abacavir (ABC) + lamivudine (3TC) (n = 5); tenofovir DF (TDF) + emtricitabine (FTC) + darunavir/ritonavir (DRV/r) (n = 4); ABC + 3TC + DRV/r (n = 2); ABC + 3TC + maraviroc (MVC) (n = 1); or TDF + FTC + DRV/r + MVC (n = 1). Twelve patients received DTG once daily (50 mg/day); 1 patient received this drug twice daily (100 mg/day). Seven (54%) patients had an undetectable HIV plasma viral load and 8 (62%) had an undetectable HIV CSF viral load (<40 copies/mL). Only one patient had an undetectable HIV CSF viral load associated with a detectable HIV plasma viral load. For the patients with a detectable viral load, median plasma and CSF viral loads were 2.3 log10 copies/mL (range: 1.8–3.0 log10 copies/mL) and 2.8 log10 copies/mL (range: 1.7–4.8 log10 copies/mL), respectively.
DTG Concentrations
Total plasma, unbound plasma, and total CSF DTG concentrations are shown in Table 1. Median plasma DTG concentration was 1,675 ng/mL (range: 137–5,091 ng/mL). The median unbound DTG concentration was 9.2 ng/mL (range: 0.8–34.5 ng/mL) and was correlated with total plasma DTG concentration (Pearson’s correlation coefficient, r = 0.9677, P < .0001). Consequently, the median plasma FuDTG was 0.66% (range: 0.44%–0.94%), and FuDTG remained stable and was independent of total plasma DTG concentration.
Table 1.
Dolutegravir Concentrations in Plasma and Cerebrospinal Fluid
| Dolutegravir concentration | Median (range) |
|---|---|
| Total plasma – ng/mL | 1675 (137–5091) |
| Unbound plasma – ng/mL | 9.2 (0.8–34.5) |
| FuDTG – % | 0.66 (0.44–0.94) |
| Total CSF – ng/mL | 9.6 (3.6–22.8) |
| QDTG – % | 0.65 (0.19–5.11) |
| IQCSF | 48 (18–114) |
Abbreviations: CSF, cerebrospinal fluid; FuDTG, unbound fraction; IQCSF, CSF inhibitory quotient; QDTG, total CSF to total plasma dolutegravir concentrations ratio.
DTG concentration was lower in the CSF than in plasma, but in the same range as the unbound plasma DTG concentration. The median CSF DTG concentration was 9.6 ng/mL (range: 3.6–22.8 ng/mL). QDTG was 0.65% (range: 0.19%–5.11%). All patients had CSF DTG concentrations above the wild-type 50% inhibitory concentration in vitro (IC50CNS = 0.2 ng/mL) [9] and above the CSF therapeutic concentration (estimated at 2.4 ng/mL) [10]. The median CSF inhibitory quotient (IQCSF) (ratio of CSF DTG concentration to IC50CNS) was 48 (range: 18–114).
CSF DTG concentration was not correlated with total plasma DTG concentration (Pearson’s correlation coefficient, r = 0.5102, P = .0748) and there was no correlation between CSF DTG concentration and unbound DTG concentration (Pearson’s correlation coefficient, r = 0.4748, P = .1011).
DTG Diffusion into the CSF
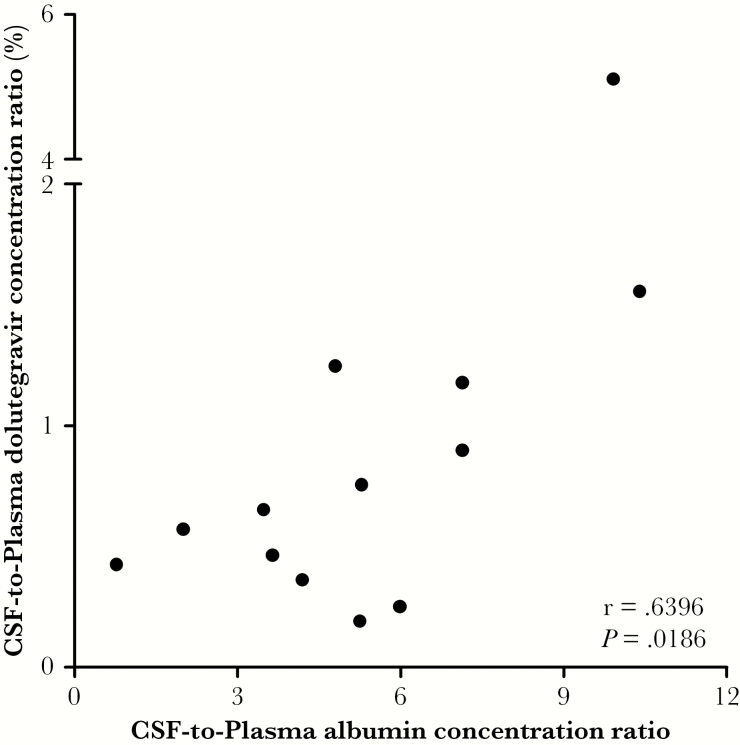
The median albumin quotient was 5.3 (range: 0.8–10.4). The CSF diffusion of DTG (QDTG) was significantly correlated with albumin quotient (Pearson’s correlation coefficient, r = 0.6396, P = .0186; Figure 1). Patients with damaged BBB (n = 3, 23%) have the higher CSF DTG concentrations.
Figure 1.
Evaluation of dolutegravir cerebrospinal fluid (CSF) diffusion. The CSF diffusion of dolutegravir was significantly correlated with albumin quotient.
CSF DTG concentrations did not differ significantly between patients with and without a detectable CSF viral load (P = .8835).
DISCUSSION
In this study, we evaluated if the diffusion of DTG is sufficient to obtain therapeutic concentrations in the CSF of HIV-1 patients. Not only is this a real-life study, but, moreover, it included patients who were treatment-experienced and presented at least 1 HIV-related CNS impairment.
IQCSF were consistent with those reported in another previous study (median [range]), 48 (18–114) versus 66 (19–92) [7], suggesting high levels of antiretroviral activity. Whatever the BBB status, DTG seems to have a sufficient capacity of diffusion and, therefore, to have a benefit in medical management of HCI.
The CSF-to-plasma DTG concentration ratio (median, 0.65%) is highly consistent with the size of the FuDTG in plasma (median, 0.66%). CSF diffusion of DTG was strongly correlated with albumin quotient (r = 0.6396, P = .0186) suggesting that the CSF diffusion of DTG depends at least partly on the physical integrity of the BBB, with greater damage to the BBB associated with higher levels of DTG in the CSF. This good diffusion probably may explain part of the neuropsychiatric adverse effects observed in some patients with DTG [11].
The novelty of this study is our finding that increasing the BBB permeability is associated to an increase of CSF diffusion of DTG in treatment-experienced patients with HCI. Given the observational nature of this study, ART regimens and sampling times were heterogeneous, leading to the wide range of plasma DTG concentrations. However, this allows to be confident about the DTG diffusion whatever the treatment backbone and over a wide range of time delay between sampling and drug administration. This good ability to diffuse also is consistent with the good placental transfer [12], which gives rise to questions in light of the last WHO statement on potential safety issue related to neural tube defects in infants born to women who were taking DTG at the time of conception and during early pregnancy [13].
This study has 2 main limitations due to real-life study. The number of patients included is small, but this is an obvious consequence of the need for lumbar puncture samples collected during routine care. Furthermore, heterogeneous ART backbone regimens could influence the viral load level.
Taken together, our findings indicate that DTG is present at therapeutic concentrations over the dosing interval in the CSF of all treatment-experienced patients with HCI, even if the BBB is intact and despite high levels of binding to plasma proteins. Therefore, DTG may have a real benefit in the control of HIV replication in the CNS. This control should help to limit the local inflammatory response and HIV-induced neurotoxicity, thereby protecting individuals against HCI and favoring neurocognitive recovery.
Acknowledgments
We gratefully acknowledge all of the patients participating in this study. Also, we are grateful for the active cooperation of the teams within the involved departments of Bicêtre Hospital.
Financial support. J.G. reports grants from Janssen and ViiV as well as personal fees from Gilead. A.B.T. reports personal fees from Gilead, ViiV, Janssen, Roche, Abbvie, and MSD. A.C. reports grants from Merck, Janssen, ViiV, and personal fees from Gilead and Janssen. All other authors: none to declare.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lorenzo-Redondo R, Fryer HR, Bedford T, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016; 530:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anesten B, Yilmaz A, Hagberg L, et al. Blood-brain barrier integrity, intrathecal immunoactivation, and neuronal injury in HIV. Neurol Neuroimmunol Neuroinflamm 2016; 3:e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010; 24:1243–50. [DOI] [PubMed] [Google Scholar]
- 4. González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol 2005; 5:69–81. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidance. (WHO/CDS/HIV/18.51). Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 6. Molina JM, Clotet B, van Lunzen J, et al. ; FLAMINGO study team Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV 2015; 2:e127–36. [DOI] [PubMed] [Google Scholar]
- 7. Letendre SL, Mills AM, Tashima KT, et al. ; extended ING116070 study team ING116070: a study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV-1-infected, antiretroviral therapy-naive subjects. Clin Infect Dis 2014; 59:1032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. II. Relation of the concentration of the proteins in serum and cerebrospinal fluid. Scand J Clin Lab Invest 1977; 37:391–6. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi M, Yoshinaga T, Seki T, et al. In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 2011; 55:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS 2011; 25:1737–45. [DOI] [PubMed] [Google Scholar]
- 11. Hill AM, Mitchell N, Hughes S, Pozniak AL. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: meta-analysis of randomized trials. Curr Opin HIV AIDS 2018; 13:102–11. [DOI] [PubMed] [Google Scholar]
- 12. Rimawi BH, Johnson E, Rajakumar A, et al. Pharmacokinetics and placental transfer of elvitegravir, dolutegravir, and other antiretrovirals during pregnancy. Antimicrob Agents Chemother 2017; 61: pii:e02213–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. Statement on Dolutegravir: Potential Safety Issue Affecting Women Living With HIV Using Dolutegravir at the Time of Conception. Geneva, Switzerland: World Health Organization; 2018. Available at: https://www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf. [Google Scholar]